Abstract
Background
Although implicated in the pathogenesis of several chronic inflammatory disorders and hematologic malignancies, telomerase mutations have not been thoroughly characterized in human cancers. The present study was performed to examine the frequency and potential clinical relevance of telomerase mutations in esophageal carcinomas.
Methods
Sequencing techniques were used to evaluate mutational status of telomerase reverse transcriptase (TERT) and telomerase RNA component (TERC) in neoplastic and adjacent normal mucosa from 143 esophageal cancer (EsC) patients. MTS, flow cytometry, time lapse microscopy, and murine xenograft techniques were used to assess proliferation, apoptosis, chemotaxis, and tumorigenicity of EsC cells expressing either wtTERT or TERT variants. Immunoprecipitation, immunoblot, immunofluorescence, promoter-reporter and qRT-PCR techniques were used to evaluate interactions of TERT and several TERT variants with BRG-1 and β-catenin, and to assess expression of cytoskeletal proteins, and cell signaling. Fluorescence in-situ hybridization and spectral karyotyping techniques were used to examine telomere length and chromosomal stability.
Results
Sequencing analysis revealed one deletion involving TERC (TERC del 341-360), and two non-synonymous TERT variants [A279T (2 homozygous, 9 heterozygous); A1062T (4 heterozygous)]. The minor allele frequency of the A279T variant was five-fold higher in EsC patients compared to healthy blood donors (p<0.01). Relative to wtTERT, A279T decreased telomere length, destabilized TERT-BRG-1-β-catenin complex, markedly depleted β-catenin, and down-regulated canonical Wnt signaling in cancer cells; these phenomena coincided with decreased proliferation, depletion of additional cytoskeletal proteins, impaired chemotaxis, increased chemosensitivity, and significantly decreased tumorigenicity of EsC cells. A279T expression significantly increased chromosomal aberrations in mouse embryonic fibroblasts (MEFs) following Zeocin™ exposure, as well as Li Fraumeni fibroblasts in the absence of pharmacologically-induced DNA damage.
Conclusions
A279T induces telomere dysfunction and inhibits non-canonical telomerase activity in esophageal cancer cells. These findings warrant further analysis of A279T expression in esophageal cancers and premalignant esophageal lesions.
Introduction
Telomeres are highly evolved nucleoprotein structures, which function to maintain and protect chromosomal ends [1]. Telomeric DNA contains long tandem hexameric repeats (TTAGGG), capped by shelterin proteins (TRF1, TRF2, RAP1, TPP1, POT1, TIN2), which prevent activation of DNA double strand break repair at chromosomal ends [2], [3]. With each cell replication, telomere length decreases until a critical point is reached (Hayflick limit), whereby further telomere attrition induces replicative senescence or apoptosis [4]. Via repeat addition processivity mechanisms, human telomerase ribonucleoprotein complex successively adds hexameric repeats to chromosomal ends [5], [6], thereby slowing telomere attrition; this complex is composed of two copies of telomerase reverse transcriptase (TERT), and two copies of its RNA template (TERC), as well as additional proteins such as N0P10, NHP2, GAR and dyskerin, which bind to TERC to stabilize the complex [2].
Increasing evidence indicates that telomere dysfunction contributes to the pathogenesis of a variety of human cancers by mechanisms, which have not been fully elucidated [2], [7]–[10]. Recently a patient with a history of Barrett's esophagus presented to the National Cancer Institute for treatment of a locally advanced esophageal adenocarcinoma. Additional evaluation revealed pancytopenia, the etiology of which could not be ascertained despite extensive evaluation, and liver cirrhosis without portal hypertension. The family history was notable for anemia, biliary cirrhosis, and esophageal cancer. The patient underwent esophagectomy with final pathology revealing T3N0M0 (Stage IIB) adenocarcinoma. Post-operatively, the patient developed progressive hepatic insufficiency, and died approximately four months later. Subsequent analysis revealed a germ-line deletion in telomerase RNA component (TERC del 341–360) [11]; this loss-of-function mutation was also identified in the proband's son, who at 30 years of age exhibited premature aging, mild anemia, and early cirrhosis. The present study was undertaken to examine the frequency and potential clinical relevance of telomerase complex mutations in sporadic esophageal cancers.
Materials and Methods
Ethics Statement
All human tissues were procured on IRB-approved protocols. All mouse experiments were approved by the National Cancer Institute Animal Care and Use Committee, and were in accordance with the NIH Guide for Care and Use of Laboratory Animals.
Patient samples
Genomic DNA was isolated as described [12] from snap-frozen esophageal cancers and adjacent normal mucosa from 80 patients undergoing potentially curative resections at the National Cancer Institute, University of Michigan, and Dalhousie University. In addition, genomic DNA was extracted from formalin-fixed paraffin embedded (FFPE) tissues from 63 esophageal cancer patients from Cornell University Medical Center, using PicoPure DNA Extraction Kit (Qiagen; Valencia, CA), and later purified with DNeasy Blood & Tissue Kit (Qiagen). PCR products from snap-frozen tissues were purified with a QIAquick PCR purification kit (Qiagen), followed by direct sequencing as described [13]. PCR products from FFPE samples were analyzed by pyrosequencing techniques using primers listed in Table S1.
Cell lines and reagents
Esophageal adenocarcinoma lines, NCI-SB-EsC1 (EsC1) and NCI-SB-EsC2 (EsC2) were established from two patients with Stage IV esophageal adenocarcinoma. These cell lines exhibit HLA and cytokeratin expression profiles identical to the respective primary tumors, and have been continuously passaged for >4 years. The TERT/TERC deficient VA-13 lung fibroblast line [11] was provided by Dr. Neal Young (NIH). HCT116, HeLa, and mouse embryonic fibroblast (MEF) cell lines were obtained from American Type Culture Collection (Manassas, VA). All cells were maintained in RPMI 1640 media at 37°C in 5% CO2. Li Fraumeni fibroblasts (MDAH087) were generously provided by Michael Tainsky (Karmanos Cancer Institute, Detroit, MI), and were cultured as described [14]. The proteasome inhibitors, MG132 and ALLN were obtained from Sigma (Allentown, PA), reconstituted in DMSO, and stored at −20°C. Cisplatin and paclitaxel were purchased from the Clinical Center Pharmacy at the NCI.
Cell Proliferation Assays
EsC1 cells (4×103 cells per well) and EsC2 cells (8×103 cells per well) were plated in 96-well plates in 100 µL media. Cell viability was quantitated by MTS colorimetric techniques using the Cell Titer 96 Aqueous One Solution Cell Proliferation Assay (Promega; Madison, WI). For chemosensitivity experiments, responses to cisplatin or paclitaxel were plotted as fractions of viable cells relative to untreated controls. Each experiment was performed in triplicate at least twice.
Annexin V-FITC assay
Apoptosis was assessed using the Annexin V-FITC kit (Abcam; Cambridge, MA) according to vendor protocols.
Telomerase activity by telomerase repeats amplification protocol
Two micrograms of pcDNA3-Flag-TERC or -Terc 341–360 del were co-transfected with either 2 µg of pcDNA3-Flag vector, pcDNA3-Flag- wtTERT, or -A279T into VA-13 cells at 60 percent confluency in 6-well polystyrene dishes using Superfect Transfection Reagent (Qiagen), according to manufacturer's instructions. Telomerase activity in transfected cells was determined using the fluorescent telomerase repeat amplification kit (TRAPeze XL; Chemicon; Temecula, CA) as previously described [11].
Telomere length assay
Mean telomere length in esophageal cancer cells constitutively expressing wtTERT, A279T, or vector control sequences were analyzed by quantitative polymerase chain reaction (qPCR) techniques. PCR was conducted in triplicate in a Rotor-Gene Q real-time instrument with the Rotor-Gene SYBR Green Kit (Qiagen). The telomere length for each sample was determined using the telomere to single copy gene ratio (T/S ratio) with the calculation of the DCt[Ct(telomere)/Ct(single gene)]. The T/S ratio for each sample was normalized to the mean T/S ratio of reference sample [2_(DCtx_DCtr) 1/4 2_DDCt], which was used for the standard curve, both as a reference sample and as a validation sample [15].
Generation of TERT and mutant stable cells
pLenti4/TO/V5-hTERT and pLentiviral4/TO/V5-A279T were generated using reagents and protocols provided by Invitrogen (Carlsbad, CA), and primers listed in Table S1. The only difference between wtTERT and A279T sequences is a single nucleotide (G to A) change, resulting in the substitution of threonine for alanine at codon 279. Empty pcDNA 3.0 vector as well as pcDNA3 vectors expressing wtTERT, A279T TERT, G260D TERT, A1062T TERT, or TERC del 341–360 were provided by Neal Young. These vectors were used to transduce/transfect EsC1, EsC2, HeLa or HCT116 cells followed by selection with Zeocin for lentiviral transduced cells, or G418 for cells transfected with pcDNA vectors. Constitutive expression of TERT or A279T was assayed by real time PCR using primers listed in Table S1. Genotyping of transfected/transduced cells was confirmed by sequencing and PyroMark techniques. Unless otherwise mentioned, stable transductants/transfectants were used for all experiments. Target gene expression was confirmed by qRT-PCR and immunoblot techniques.
PCR Superarray and quantitative reverse transcription-PCR (qRT-PCR)
Effects of wtTERT and A279T expression on Wnt, tumor suppressor and stem cell gene expression were analyzed using human Q-PCR arrays (SA Bioscience; Frederick, MD). Confirmatory quantitative RT-PCR experiments were performed using primers listed in Table S1.
Immunoblot, Immunoprecipitation, and Immunofluorescence
Total cellular proteins were extracted using the RIPA buffer lysis kit (Millipore; Billerica, MA) supplemented with 1X protease inhibitor (Roche, Inc., Indianapolis, IN), and 1 mmol/L phenylmethylsulfonyl fluoride. Cell lysates were resolved on 4–20% Tris glycine gels (Invitrogen), transferred to PVDF membranes, and incubated overnight with primary antibodies listed in Table S2. HeLa cells were transiently transfected with wtTERT, A279T and G260D mutants and immunoprecipitated with anti-TERT (Rocklands), BRG-1 (Millipore) and β-catenin (Abcam; Cambridge, MA). Immunoblot signals were detected using appropriate horseradish peroxidase–conjugated secondary antibodies, and SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology; Rockford, IL).
For immunofluorescence experiments, 1×105 cells were grown on LAB-TEK II slides and fixed for 5 min with ice-cold ethanol. Slides were blocked with 1% BSA in PBS for 30 min. Cells were incubated for one hour in blocking solution with primary antibodies listed in Table S2, washed and then incubated for 30 min with appropriate secondary antibodies. Immunofluorescence analysis of F-actin and vinculin was performed using the Actin Cytoskeleton/Focal Adhesion Staining Kit (Millipore) and secondary antibody (Goat anti-Mouse IgG, (H+L) FITC Conjugated;Millipore) according to vendor protocols. Slides were mounted in VECTASHIELD Mounting Medium with DAPI. A Zeiss LSM 710 confocal microscope (25x) was used to evaluate all slides except for vinculin images, which were recorded using a Nikon A1 Confocal Microscope with the objective of Plan Apo 20X VC 0.75NA. Images were acquired under the same conditions and displayed at the same scale for comparison.
Luciferase Promoter-Reporter Transient Transfection Experiments
1×105 HeLa cells were plated per well in 24-well plates. After 24 hours, cells were transiently co-transfected with empty vector, wtTERT and A279T with the T-cell factor (TCF) responsive vector TOPFlash and the TCF mutant vector FOPFlash (Millipore) using Lipofectamine 2000 (Invitrogen). Approximately 24 hours later, cells were lysed and assayed for luciferase activity using the dual luciferase reporter assay (Promega) according to vendor instructions. Renilla luciferase activity was used as a control to normalize inter-sample variability.
Chemotaxis and Time-lapse Video Microscopy
Chemotaxis of EsC1and ExC2 cells was performed as described [16] with minor modifications. Briefly, EsC1 and EsC2 cells (106/ml) were plated in serum free RPMI-1640 media on collagen type IV-coated microslides (Ibidi; Prospect, IL), and left to adhere for 4 hours at room temperature. Microslide reservoirs were then filled with serum free media, and 18 µl of chemoattractant (10% FBS) was added; 15 minutes later, cancer cell migration was monitored using a Zeiss LSM 510 or 710 NLO confocal microscope. AIM or ZEN Imaging software (Zeiss) was used for time-lapse imaging. Phase-contrast images were captured every 15 minutes. Image J Plugin was used to manually track cells, and characterize chemotaxis from the captured images. Average total movement of all cells within the experimental time was defined as the center of mass. Rayleigh test for inhomogeneity of cell distribution was determined with Ibidi Chemotaxis Tool software.
Murine Xenograft Experiments
EsC2- wtTERT and EsC2-A279T cells were trypsinized, washed in HBSS, suspended in sterile PBS at a concentration of 1×106 cells per 100 µL, and inoculated in contralateral flanks of athymic nude mice. Tumor size and take were recorded biweekly. Tumors were excised, weighed, and processed for additional studies.
Telomere-Specific FISH Analysis
FFPE tissue sections (5 µm thickness) from paired tumors were placed on the same slide to ensure that FISH conditions were identical for paired samples. Deparaffinized slides were hydrated, steamed for 20 minutes in citrate buffer, dehydrated and hybridized with a Cy3-labeled peptide nucleic acid (PNA) probe complementary to the mammalian telomere repeat sequence ([N-terminus to C-terminus]). As a positive control for hybridization efficiency, a FITC-labeled PNA probe having specificity for human centromeric DNA repeats (CENP-B binding sequence) was also included in the hybridization solution. Confocal images were sequentially acquired with Zeiss ZEN 2009 software on a Zeiss LSM 710 Confocal System (Carl Zeiss Inc, Thornwood, NY) with a Zeiss Observer Z1 inverted microscope and Chameleon IR laser tuned to 760 nm, a 25 mW Argon visible laser tuned to 488 nm and a 15 mW DPSS laser tuned to 561 nm. A 63x Plan-Apochromat 1.4 NA oil immersion objective was used, and digital images were 512×512 pixels with 0.264 µm pixel size. Emission signals after sequential excitation of DAPI, GFP, and Rhodamine by the 760 nm, 488 nm or 561 nm laser lines were collected with a BP 419–485 nm filter, BP 495–534 nm filter, and BP 568–624 nm filter, respectively, using individual photomultipliers. Images were acquired under the same conditions and displayed at the same scale for comparison.
Spectral Karyotype (SKY) Analysis
SKY probes were prepared as described [17], [18]. Parental murine MEF-1 cells and human Li Fraumeni cells or respective cells stably transfected with control vectors, wtTERT, or A279T were grown in normal media (DMEM for MEF-1 cells and MEM for Li Fraumeni cells), and metaphases were arrested by overnight incubation with Colcemid prior to harvest. MEF-1 cells were also treated with Zeocin™ (100 µg/ml) for three days as described [19] to induce double strand breaks. Thereafter, debris was removed, and viable cells were washed with HBSS, and incubated in normal media overnight. The following day, metaphases were arrested, and SKY analysis of mouse and human chromosomes was performed. The images of MEF-1 cells were acquired with a spectral cube system (Applied Spectral Imaging, Migdal Haemek, Israel) attached to a fluorescence microscope (DMRXA, Leica, Wetzlar, Germany), and the emission spectrum was measured with a custom – made triple-band-pass filter (Chroma Technology, Bellows Falls, VT). Spectral images of the hybridized metaphases with Li Fraumeni cells were acquired using a SD300 SpectraCubeTM system (Applied Spectral Imaging Inc., CA) mounted on top of an epifluorescence microscope Axioplan 2 (Zeiss). Approximately 10–15 metaphase spreads per sample were analyzed, and scored for numerical and structural aberrations. Human cells were analyzed following the nomenclature rules presented in ISCN (2009). For mouse cells, chromosome analysis followed established nomenclature rules: http://www.informatics.jax.org/mgihome/nomen/gene.shtml.
Statistical analysis
Differences in the frequencies of coding-sequence variations between samples from patients and those from controls were evaluated by means of Fisher's exact test, considering a p value <0.05 as statistically significant. T test was used to analyze results from all other experiments except chemotaxis assays described above.
Results
Frequency of TERC and TERT Mutations in Esophageal Cancers
Except for the one observed in the proband, no TERC mutations were identified among 54 patients. Direct sequencing analysis revealed two non-synonymous TERT variants (A279T and A1062T) among these 54 patients; one homozygous and 4 heterozygous A279T variants were detected, whereas one heterozygous A1062T variant was identified. To confirm and extend these observations, pyrosequencing techniques were used to analyze the frequency of A279T and A1062T in 89 additional esophageal carcinoma specimens. The previous homozygous A279T variant was confirmed with this approach (Figure S1). Several additional A279T and A1062T variants were detected in these specimens. In all cases in which a TERT variant was identified in esophageal cancer, the same variant was detected in matched normal esophageal mucosa.
The overall frequencies of A279T and A1062T variants identified in 143 esophageal cancers are summarized in Table 1. The minor allele frequency (mAF) of A279T [SNP database rs61748181] in esophageal cancers (∼5%) was significantly higher than that previously observed in a large number of healthy adult blood donors (0.9%), or individuals with aplastic anemia [20]; [21], and was comparable to that previously reported for patients with bone marrow failure and dyskeratosis congenita (DC) [22]. In contrast, the mAF of A1062T in esophageal cancers was not significantly different than that previously observed in healthy blood donors [21].
Table 1. Mutations of TERT in Esophageal Cancers among 143 Cases.
| Esophageal CA | Healthy controls (21) | Aplastic anemia (21) | Bone marrow failure (22) | |||
| Adenocarcinoma | Squamous cell carcinoma | Dyskeratosis congenita | ||||
| Total | 117 | 26 | 528 | 200 | 80 | |
| A279T† | Homozygous | 2 | 0 | 0 | 0 | 1 |
| Heterozygous | 6 | 3 | 10 | 6 | 5 | |
| mAF* | 4.27% | 5.77% | 0.90% | 1.50% | 4.37% | |
| p value** | <0.01 | <0.05 | NS*** | |||
| A1062T‡ | Homozygous | 0 | 0 | 0 | ||
| Heterozygous | 3 | 1 | 7 | |||
| mAF* | 1.28% | 1.92% | 0.66% | |||
| p value** | NS*** | |||||
codon 279 GCC/ACC (Ala/Thr).
codon 1062 GCC/ACC (Ala/Thr)].
*mAF (minor allele frequency): frequency of the less frequent allele in a given population.
**p value: compared to both esophageal cancers combined. Fisher's exact test.
***NS: not significant.
Effects of A279T on Proliferation of Esophageal Cancer Cells
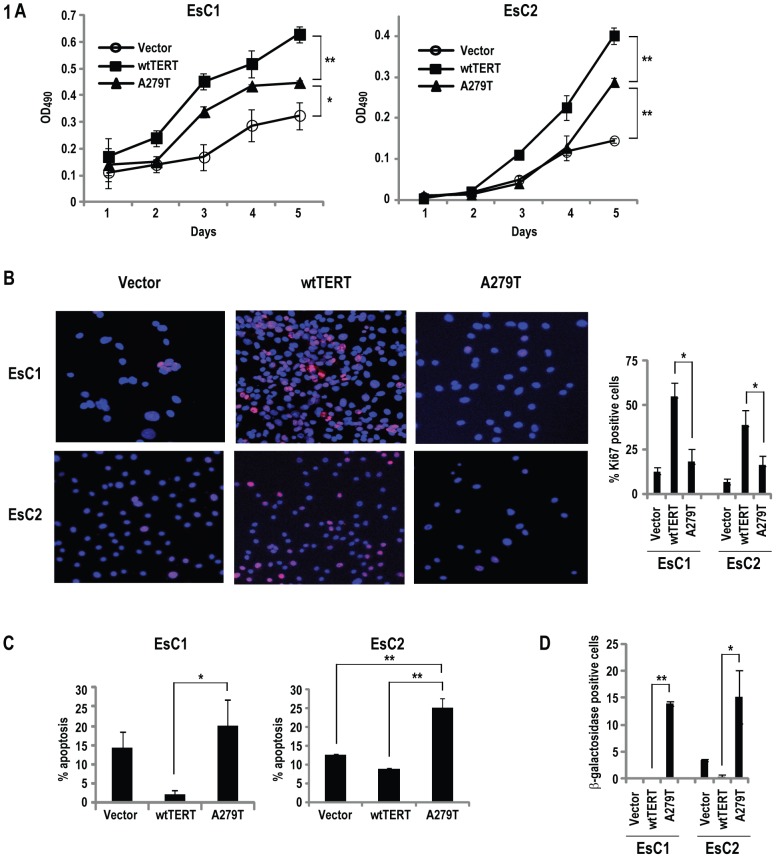
The fact that the mAF of A279T in esophageal cancers was approximately five-fold higher than that observed in peripheral blood from healthy donors suggested that this variant might contribute to the pathogenesis of these malignancies. As such, a series of experiments were performed to examine if A279T expression modulated the malignant phenotype of esophageal cancer cells. EsC1 and EsC2 cells, which exhibit low level wtTERT and TERC expression (Table S3), were stably transduced with lentiviral vectors encoding A279T or wtTERT, or control sequences. MTS assays revealed that EsC1 and EsC2 cells expressing A279T (EsC1-A279T and EsC2-A279T, respectively) grew significantly slower than cells constitutively expressing wtTERT (EsC1-TERT, and EsC2-TERT, respectively), yet faster than vector controls (Figure 1A). Immunofluorescence experiments demonstrated that Ki67 levels in EsC1-TERT and EsC2-TERT cells were significantly higher than those observed in respective vector controls, consistent with increased proliferation mediated by TERT over-expression. In contrast Ki67 levels in EsC1-A279T and ESC2-A279T were modestly but insignificantly higher than those in vector controls, and significantly lower than those observed in respective TERT-over-expressers. Annexin V experiments demonstrated a significant increase in apoptotic index in EsC1-A279T and EsC2-A279T cells relative to respective cells over-expressing wtTERT (Figure 1C). Subsequent immunohistochemistry experiments demonstrated that β-galactosidase levels were significantly higher in A279T-transduced esophageal cancer cells relative to respective TERT-transduced or vector control cells (Figure 1D). These preliminary findings suggested that the A279T amino acid substitution simultaneously induced apoptosis and senescence, which attenuated the proliferative effects of telomerase over-expression in esophageal cancer cells.
Figure 1. A279T inhibits proliferation of esophageal cancer cells.
(*p<0.05; **p<0.01). A. MTS assay demonstrating inhibition of EsC1 (left) and EsC2 (right) proliferation by A279T relative to wtTERT. B. Immunofluorescence analysis (left panel) with corresponding summary (right panel) of Ki67 expression in esophageal cancer cells expressing wtTERT or A279T (Red: Ki67; blue: DAPI). EsC1 and EsC2 cells expressing A279T exhibit decreased Ki67 levels relative to respective cells expressing wtTERT. C. Annexin V-FITC assay demonstrating A279T-induces apoptosis in EsC2 but not EsC1 cells. Results are expressing as mean ± SD of triplicate experiments. D. Graphic summarization of immunofluorescence analysis of β-galactosidase expression in EsC1 and EsC2 following constitutive expression of wtTERT or A279T. Red: β-galactosidase; blue: DAPI.
Effects of A279T on Telomerase Activity and Telomere Length
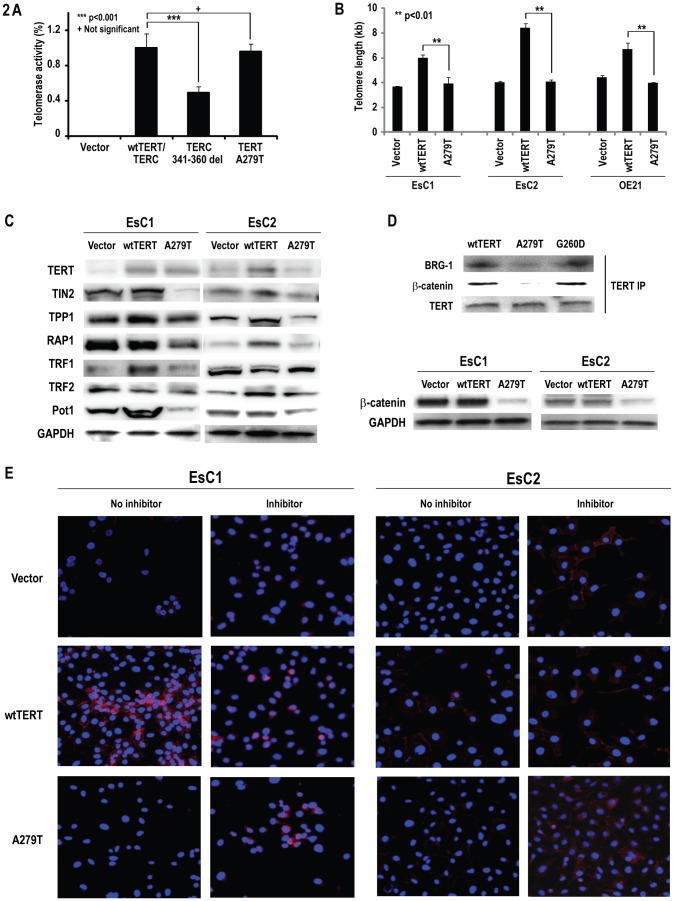
Additional experiments were performed to examine if A279T expression modulated telomerase catalytic activity and telomere length in esophageal cancer cells. In initial experiments, vectors containing A279T or wtTERT were co-transfected with either TERC del 341–360 (TERCdel) or wtTERC into TERT/TERC-deficient VA-13 cells; telomerase catalytic activity was measured in cell lysates. Results of this analysis are depicted in Figure 2A. Consistent with previous observations [11], TERCdel significantly reduced telomerase enzymatic activity relative to wild-type TERC. In contrast, A279T did not appear to significantly diminish telomerase catalytic activity under these experimental conditions.
Figure 2. A279T down-regulates β-catenin independent of telomerase activity.
A. Telomerase enzymatic activity of TERT and TERC mutations in VA13 cells, measured by TRAPeze assay. Telomerase activity is defined as 100% of wtTERT. B. Quantitative PCR analysis of telomere lengths in EsC1, EsC2 and OE21 esophageal cancer cells transfected with empty vector, wtTERT, or A279T-TERT. C. Immunoblot analysis of TERT and related shelterin protein levels in EsC1 and EsC2 cells transduced with wtTERT, A279T-TERT or empty vector. Expression of A279T depletes several shelterin proteins in esophageal cancer cells. D. Upper panel: Immunoprecipitation experiments demonstrating that A279T disrupts TERT-BRG-1-β-catenin complex. This phenomenon was not seen in cells expressing G260D. Lower panel: immunoblot experiments demonstrating decreased β-catenin levels in EsC1 and EsC2 expressing A279T. E. Representative immunofluorescence analysis of β-catenin expression in EsC1 and EsC2 cells cultured in normal media in the presence or absence of proteasome inhibitors (red: β-catenin; blue: DAPI).
In subsequent experiments, quantitative PCR techniques were used to examine mean telomere lengths in EsC1 and EsC2 cells stably transduced (>1 year) with wtTERT, A279T, or control vectors. Parental EsC1 and EsC2 exhibit moderate levels of TERC and relatively low endogenous levels of TERT (Table S3). Results of this analysis are depicted in Figure 2B. Mean telomere lengths in A279T-transduced EsC cells were significantly shorter than those observed in wtTERT-transduced cells, and were, in fact, similar to those observed in respective vector controls. Immunoblot analysis using an antibody that recognized wtTERT as well as A279T, demonstrated that the differences in mean telomere lengths observed in A279T-relative to wtTERT transduced esophageal cancer cells were not attributable to consistent differences in telomerase protein levels (Figure 2C). Additional immunoblot experiments (Figure 2C) demonstrated that relative to EsC1-TERT and EsC2-TERT, or respective vector controls, EsC1-A279T and EsC2-A279T cells exhibited decreased levels of several shelterin proteins including POT1, which binds to single stranded telomeric 3′ overhangs, as well as TIN2, which together with TPP1, connects POT1 to TRP1 to regulate telomere length, and prevent telomeres from activating non-homologous end joining (NHEJ) or other DNA double strand break repair pathways [3]. Collectively, these results suggest that A279T-TERT disrupts primary as well as secondary/tertiary telomere structure in esophageal cancer cells.
Effects of A279T on Non-canonical TERT Activities
Recent studies have demonstrated that in addition to TERC dependent (canonical) elongation of telomeres, TERT enhances cell proliferation and immortalization by non-canonical mechanisms including direct interactions with BRG-1 and β-catenin [23]. As such, additional studies were undertaken to ascertain if the TERT A279T variant affected non-canonical TERT activity in esophageal cancer cells. Briefly, HeLa cells were transiently transfected with control vectors, wtTERT, or A279T; immunoprecipitation techniques were then utilized to examine interactions of TERT with BRG-1 and β-catenin. HeLa cells were chosen for these experiments because relative to EsC1 or EsC2, these cells exhibit high transfection efficiency, as well as abundant levels of endogenous BRG-1 [24]. Results of these experiments are shown in Figure 2D, upper panel. Compared to wtTERT-transfected HeLa (TERT-HeLa) cells, immunoprecipitates from A279T-HeLa cells had much lower levels of BRG-1 and β-catenin following pull-down with an anti-TERT antibody. Similarly, TERT and BRG-1 levels were lower in β-catenin immunoprecipitates from A279T-HeLa relative to TERT-HeLa cells. Lastly, β-catenin and TERT levels were lower in BRG-1 immunoprecipitates from A279T-HeLa cells relative to TERT-HeLa cells. These results were not observed in HeLa cells transfected with G260D, a TERT variant frequently detected in hematologic malignancies [25], [26], which is in the same region of TERT where A279T occurs (Figure 2D). Furthermore, these results were not observed in HeLa cells transfected with A1062T, another TERT variant associated with hematologic disorders [25] (Figure S2). Consistent with these findings, immunoblot experiments demonstrated markedly decreased β-catenin levels in EsC1-A279T as well as EsC2-A279T cells relative to respective vector controls, or EsC cells constitutively expressing wtTERT (Figure 2D; lower panel). Quantitative RT-PCR experiments demonstrated that changes in β-catenin levels mediated by A279T in these cells did not coincide with consistent alterations in β-catenin mRNA levels (data not shown).
Since free intracellular β-catenin levels are tightly regulated by the cytoplasmic APC/Axin destruction complex [27], additional experiments were performed to examine the effects of proteosome inhibitors in esophageal cancer cells constitutively expressing A279T or wtTERT. Immunofluorescence analysis demonstrated that MG132 and ALLN attenuated A279T-mediated decreases in β-catenin levels in EsC1 and EsC2 cells (Figure 2E). Collectively, these findings suggest that A279T destabilizes the BRG-1-TERT-β-catenin complex, resulting in depletion of β-catenin via proteosomal degradation in esophageal cancer cells.
Effects of A279T on Canonical Wnt Signaling
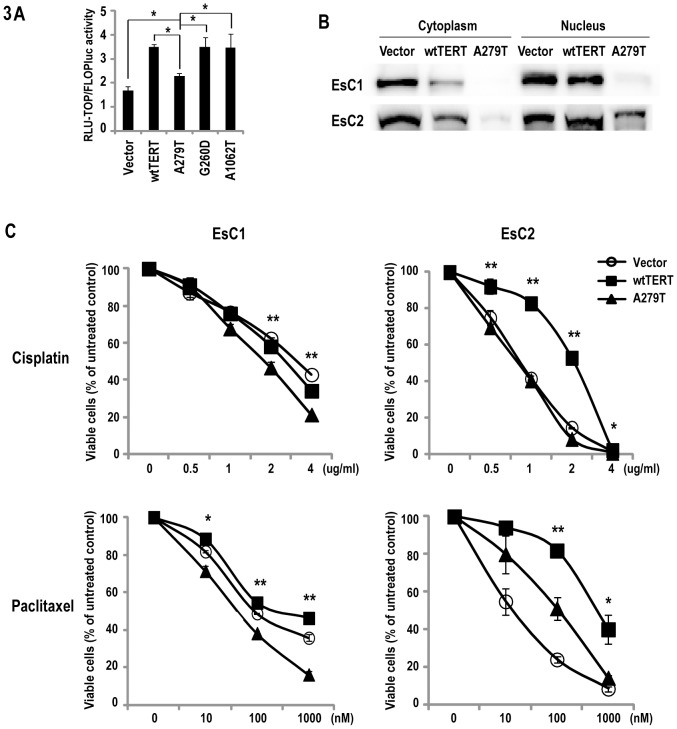
β-catenin is a critical mediator of canonical Wnt signaling [28], translocating from the plasma membrane to the nucleus to activate target genes [29]. Therefore, additional experiments were performed to examine if A279T modulated Wnt activity in cancer cells. Briefly, HeLa cells transiently expressing either control vector, wtTERT, A279T, G260D or A1062T TERT sequences were transfected with either TOP-FLASH or FOP-FLASH promoter reporters. Once again, HeLa cells were chosen for these experiments due to high transduction efficiency. Results of these experiments are summarized in Figure 3A. As expected, TCF luciferase activity was significantly increased in cells transfected with wtTERT relative to control vector. Comparable increases in luciferase activities were observed in HeLa cells expressing G260D as well as A1062T telomerase variants. In contrast, whereas A279T-HeLa cells also exhibited higher TCF promoter activity compared to vector controls, luciferase levels in A279T-HeLa cells were significantly lower than those observed in wtTERT, G260D or A1062T transfectants.
Figure 3. Effects of A279T on Wnt signaling and chemosensitivity in cancer cells.
*p<0.05, **p<0.01. A. TOP-flash promoter-reporter assay demonstrating that relative to wtTERT, A279T inhibits Wnt signaling in HeLa cells. This phenomenon was not seen in cells expressing G260D or A1062T. B. Immunoblot of β-catenin levels in cytoplasmic and nuclear extracts of EsC1 and EsC2 cells following constitutive expression of wtTERT or A279T-TERT. C. MTS assay demonstrating that relative to EsC1 and EsC2 cells expressing wtTERT, EsC1 and EsC2 cells expressing A279T are more sensitive to cisplatin (2 day treatment) and paclitaxel (3 hour treatment) measured on day 3.
To extend these observations, immunoblot and qRT-PCR array experiments were performed to examine the effects of A279T expression on Wnt signaling and associated pathways in esophageal cells. Immunoblot experiments demonstrated depletion of β-catenin in nuclear as well as cytoplasmic extracts from EsC1-A279T and to a lesser extent EsC2-A279T cells relative to respective TERT-transduced cells, or vector controls (Figure 3B). Focused qRT-PCR arrays and confirmatory qRT-PCR experiments (Table 2) demonstrated that relative to wtTERT, A279T mediated repression of several Wnt-related genes in EsC1 and/or EsC2 cells including cyclin D1, a well-established target of canonical Wnt signaling [30]. Furthermore, consistent with recent observations that β-catenin directly regulates TERT expression [31], [32], endogenous TERT mRNA levels were lower in A279T-EsC1 and A279T-EsC2 cells relative to EsC1 and EsC2 cells over-expressing wtTERT. Additional experiments revealed that a variety of mediators of DNA damage response and apoptosis/senescence including BRCA1, BRCA2, p57, caspase 8, TNF, FAS, IL-6 and IL-8 were induced, whereas JunB was repressed in EsC-1 and/or EsC2 cells expressing A279T relative to wtTERT.
Table 2. Gene expression levels of A279T-transduced cells normalized to wtTERT-transduced cells.
| EsC1-A279T | EsC2-A279T | |||||
| Mean | SEM | p value | Mean | SEM | p value | |
| TERT | 0.1 | 0.01 | 5.0E-05 | 0.4 | 0.05 | 5.5E-03 |
| CCND1 | 0.1 | 0.01 | 7.5E-06 | ND* | ||
| JUNB | 0.1 | 0.01 | 1.5E-03 | 0.7 | 0.08 | 3.3E-02 |
| Dkk-1 | 381.8 | 175.96 | 9.8E-02 | 2.8 | 0.38 | 1.9E-02 |
| CASP8 | 1.1 | 0.26 | 8.5E-01 | 3.5 | 0.42 | 6.4E-03 |
| p57 | 2.2 | 0.19 | 1.0E-02 | 1.7 | 0.15 | 1.9E-02 |
| IL-6 | 9.6 | 1.00 | 1.0E-03 | 25.7 | 1.53 | 8.6E-05 |
| IL-8 | 1.3 | 0.06 | 5.4E-03 | 5.6 | 0.22 | 3.6E-05 |
| BRCA1 | 4.4 | 0.13 | 5.0E-05 | 1.8 | 0.24 | 4.6E-02 |
| BRCA2 | 4.9 | 0.33 | 5.1E-04 | 2.4 | 0.32 | 1.5E-02 |
| JUNB | 0.1 | 0.01 | 1.5E-03 | 0.7 | 0.08 | 3.3E-02 |
| TNF | 0.3 | 0.16 | 5.6E-02 | 28.3 | 2.10 | 2.0E-04 |
*ND: Not detected.
Effects of A279T on Chemosensitivity of Esophageal Cancer Cells
Because telomerase activity, telomere length, and Wnt/β-catenin signaling appear to modulate chemoresistance in cancer cells [33]–[36], additional experiments were performed to ascertain if A279T affected sensitivity of esophageal cancer cells to cisplatin and paclitaxel, two agents typically used to treat esophageal carcinomas in clinical settings. Preliminary experiments were undertaken to optimize drug exposure conditions and timing of viability assays. As shown in Figure 3C, cisplatin as well as paclitaxel mediated dose-dependent cytotoxicity in EsC1 as well as EsC2 cells. Relative to cells expressing wtTERT, EsC1-A279T and EsC2-A279T appeared more sensitive to cisplatin and paclitaxel. This phenomenon was more impressive in EsC2 cells; A279T abolished TERT-mediated resistance to cisplatin, and significantly diminished TERT-mediated resistance to paclitaxel.
Effects of A279T on Cytoskeletal Integrity and Chemotaxis in Cancer Cells
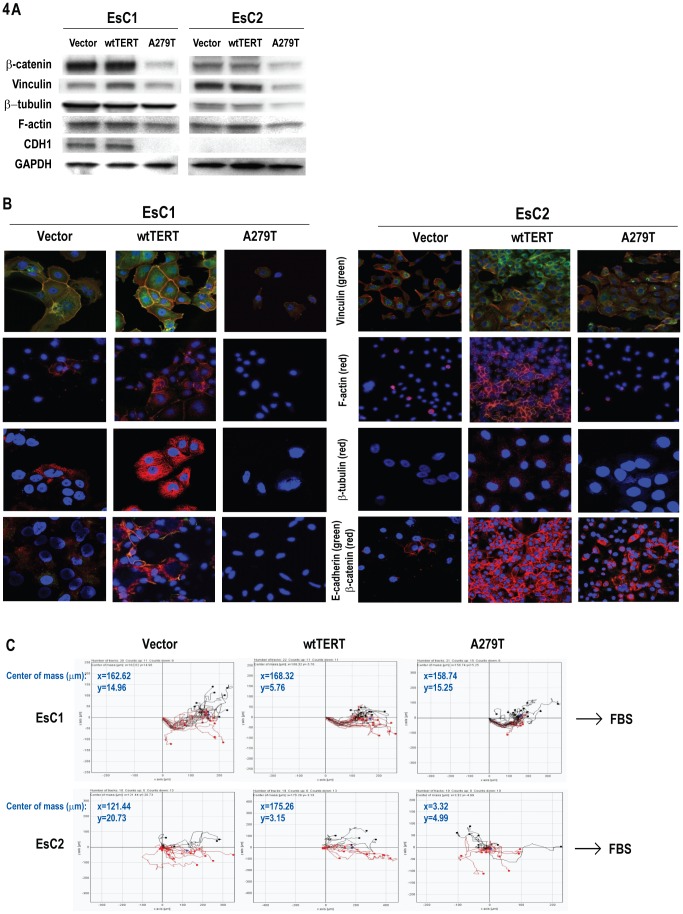
β-catenin, α-catenin and p120 interact with the intracellular domain of E-cadherin at the plasma membrane, thereby stabilizing adherens junctions, and connecting the cadherin-catenin complex to microtubules, as well as actin and actin-associated proteins such as F-actin, vinculin, and formin-1 [37]. As such, additional experiments were performed to ascertain if expression of A279T affected cytoskeletal organization in cancer cells. Although some variability was noted between lines, immunoblot experiments (Figure 4A) revealed that relative to cells constitutively expressing wtTERT or control vectors, EsC1- and EsC2- A279T cells not only had decreased β-catenin levels, but also exhibited reduced expression of vinculin, β-tubulin, F-actin, and CDH1. Immunofluorescence experiments (Figure 4B) confirmed results of immunoblot analyses.
Figure 4. A279T depletes cytoskeletal proteins and impairs chemotaxis in esophageal cancer cells.
A. Immunoblot analysis depicting down-regulation of β-catenin, vinculin, β-tubulin, F-actin and CDH1 in EsC1 and EsC2 cells expressing A279T-TERT relative to wtTERT or control vectors. B. Immunofluorescence analysis demonstrating that A279T disrupts cytoskeletal integrity in EsC1 and EsC2 cells as evidenced by decreased vinculin, F-actin, β-tubulin, E-cadherin and β-catenin expression. (Blue: DAPI). C. Results of time-lapse video microscopy demonstrating that EsC1 and EsC2 cells expressing A279T exhibit impaired chemotaxis relative to parental cells, or cells expressing wtTERT.
Because A279T appeared to disrupt cytoskeletal organization, additional studies were undertaken to directly examine if A279T affected cell motility. Briefly, EsC1 and EsC2 cells constitutively expressing control vector, wtTERT, or A279T were placed in chamber slides and time lapse microscopy techniques [16] were used to evaluate chemotaxis in response to mitogen. Representative results are depicted in Figure 4C. EsC1-TERT and EsC2-TERT, as well as respective vector controls exhibited chemotaxis in response to FBS. In contrast, chemotaxis was significantly impaired in EsC1-A279T cells, and was completely abolished in EsC2-A279T cells (p<0.05 for A279T vs. wtTERT).
Effects of A279T on Tumorigenicity of Esophageal Cancer Cells
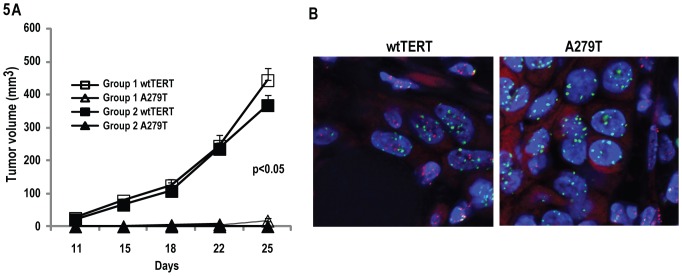
Additional experiments were performed to ascertain if expression of A279T affected tumorigenicity of esophageal cells. Briefly, EsC2 cells constitutively expressing wtTERT or A279T were inoculated subcutaneously into athymic nude mice. Representative results of two independent experiments are depicted in Figure 5A. EsC2-A279T cells exhibited only 60% tumor take compared to 100% for EsC2-TERT cells. Furthermore, volumes and masses of EsC2-A279T xenografts were significantly less than EsC2-TERT tumors (p<0.05). Similar experiments using EsC1 cells were not possible since parental EsC1 cells are not tumorigenic in nude mice (data not shown).
Figure 5. Effects of A279T on tumorigenicity and telomere length of esophageal cancer cells in vivo.
A. A279T significantly inhibits growth of subcutaneous EsC2 xenografts in athymic nude mice. B. Representative results of telomere-specific FISH analysis of ExC2 xenografts depicting shortened telomere length in xenografts from EsC2-A279T cells compared to those derived from EsC2-TERT cells; Red: telomere; green: centromere; blue: DAPI.
Fluorescence in-situ hybridization (FISH) experiments were performed to examine if the effects of A279T on tumorigenicity coincided with decreased telomere length in esophageal cancer cells. Representative results of these telomere FISH experiments are depicted in Figure 5B. Murine chromosomes in stromal cells exhibited relatively weak centromeric signals (green) due to the fact that the probe set used for FISH had greater affinity for human centromeric repeats. On the other hand, chromosomes in mouse stromal cells exhibited intense red staining due to very long telomeres [38]. EsC2-TERT cells exhibited strong green centromeric signals as well as bright red telomeric staining. Whereas EsC2-A279T xenografts also exhibited strong centromeric signals, these cells lacked red telomeric staining, indicative of short telomeres.
Effects of A279T on Chromosomal Integrity in Normal Cells
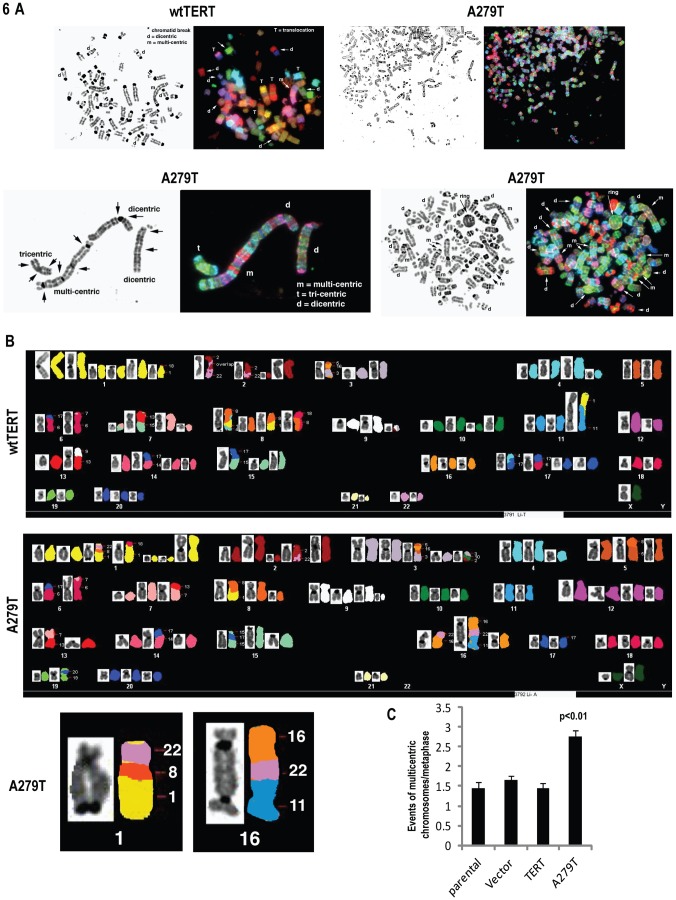
Results of experiments described above strongly suggested that A279T expression inhibits the malignant phenotype of esophageal cancer cells. On the other hand, the fact that the MAF of A279T was significantly higher in esophageal cancer patients relative to healthy blood donors suggested that expression of this telomerase variant predisposes to malignancy. In order to reconcile these discrepant observations, experiments were undertaken to examine if A279T affected chromosome integrity in normal cells. In initial experiments, mouse embryonic fibroblasts (MEF) stably transfected with control vectors, wtTERT, or A279T were cultured for 72 h in normal media with or without Zeocin to induce double strand breaks. Cells were then evaluated by spectral karyotyping (SKY) techniques. Representative results of these experiments are depicted in Figure 6A. Structural aberrations included translocations (t), deletions (del), dicentric (d), and multi-centric (m; three or more centromeres) chromosomes, rings, and other chromosome-breakage exchanges. In all untreated cells, ring chromosomes and evidence of chromosome-breakage were rarely detected. Untreated parental, vector-control and wtTERT-transfected MEFs consisted predominantly of hypo-tetraploid cells (<4 n) exhibiting few clonal numerical and structural aberrations. There were more translocation events and more hyper-tetraploid cells (>4 n) in A279T-MEF cells, but the difference between A279T and control MEF cells was not statistically significant, possibly due to the low number of cells analyzed (average of 15 cells per sample), as well as the fact that murine chromosomes have very long telomeres [38]. In contrast, A279T-MEFs treated with Zeocin exhibited significantly higher numbers of structural aberrations relative to Zeocin treated parental, vector control, or wtTERT transfected cells (p = 0.000249, p = 0.001, p = 0.0105, respectively); Zeocin-treated A279T- MEFs had approximately twice the number of rings and multi-centric chromosomes, with virtually every chromosome involved in translocations.
Figure 6. Effects of A279T on genomic stability in normal cells.
A. SKY assay demonstrating that A279T-induces genomic instability in Zeocin™-treated MEF-1 cells. Upper panel: translocations, dicentric, and rearranged chromosomes are present in cells expressing A279T compared to wtTERT. Lower left panel: a multi-centric chromosome observed in cells harboring A279T. Lower right panel: a ring chromosome is formed and every chromosome is rearranged in cells transfected with A279T. See text for additional details. B. Upper panel: representative results of SKY analysis Li Fraumeni fibroblasts constitutively expressing wtTERT or A279T-TERT. Lower panel: close-up of chromosomes 1 and 16. C. Summary of results of two independent experiments demonstrating that A279T expression increases genomic instability in Li Fraumeni cells.
To further examine this issue, additional SKY experiments were performed using Li Fraumeni fibroblasts stably transfected with control vectors, wtTERT, or A279T. Results of two independent experiments, which were performed without Zeocin, are depicted in Figure 6B. The numbers of chromosomal abnormalities in Li Fraumeni fibroblasts constitutively expressing wtTERT were not significantly different than those observed in parental cells or vector controls. In contrast, Li Fraumeni fibroblasts constitutively expressing A279T exhibited approximately two-fold higher numbers of multicentric chromosomes, with numerous translocations (p<0.01), indicative of genomic instability.
Discussion
Mutations or sequence variants within telomerase complex genes have been linked to a variety of benign inflammatory conditions such as pulmonary fibrosis [2] and biliary cirrhosis [11], inherited bone marrow failure syndromes [2], as well as aging [39] and cancer [2], [40]. Telomere dysfunction evidenced by loss of telomere length has been identified in myelodysplasia [15] as well as premalignant lesions in breast, pancreas, prostate, lung, colon and esophagus [7]. In malignancy, telomere attrition induces telomere recombination [8] and chromosomal rearrangements through breakage/fusion/bridge mechanisms [9], as well as tetraploidization [10], resulting in activation of DNA damage response and early crisis. Inactivation of Rb and p53 tumor suppressor pathways enables preneoplastic cells with telomere dysfunction to emerge from crisis [4]; subsequent activation of TERT by a variety of mechanisms prevents further telomere shortening during late stages of malignant transformation, and in established cancers [31], [32], [41], [42]. Approximately 10–15% of human cancers lack detectable telomerase activity; in these neoplasms telomere length is maintained by telomerase independent, alternative lengthening of telomeres (ALT) mechanisms [43]. Although frequently observed in sarcomas and CNS malignancies, ALT appears to be quite uncommon in epithelial malignancies [44].
In the present study we sought to examine the frequency and potential clinical relevance of telomerase complex mutations in sporadic esophageal carcinomas after identifying a unique germline TERC deletion in a patient with Barrett's adenocarcinoma [11]. Although we observed no additional TERC mutations, our analysis identified a telomerase variant (A279T) that occurred nearly five-fold more frequently in esophageal cancer patients compared to healthy blood donors; the frequency of A279T variant expression in esophageal cancers exceeds that of recently described ALK mutations in non-small cell lung cancers [45]. The fact that A279T was observed in tumor as well as corresponding normal esophageal mucosa strongly suggests that this was a germline variant; however, because we did not have corresponding peripheral blood samples to analyze, our results cannot exclude the possibility that A279T was a mutation acquired during field cancerization [46]. Additional experiments revealed that A279T decreased telomere length and destabilized the BRG1-TERT-β-catenin complex, depleting β-catenin in esophageal cancer cells. Relative to wtTERT, A279T mediated growth inhibition and apoptosis/senescence in-vitro, disrupted cytoskeletal integrity, markedly impaired chemotaxis, increased chemosensitivity and significantly reduced tumorigenicity of esophageal cancer cells. To the best of our knowledge, these experiments are the first to identify a telomerase variant in a human malignancy that simultaneously disrupts canonical as well as non-canonical telomerase activities.
Whereas perpetual replicative capacity is directly linked to canonical telomerase activities [47], other aspects of cancer cell biology appear attributable to telomerase independent functions of TERT, including transcriptional modulation of Wnt β-catenin signaling [23], or RNA polymerase activity when TERT is complexed with the RNA component of mitochondrial RNA processing endoribonuclease (RMRP) [48]. Indeed, recent observations that constitutive expression of β-catenin increases cell cycle progression, and promotes full malignant transformation in TERT-immortalized human fetal hepatocytes, with up-regulation of genes mediating invasion and angiogenesis [49], attest to the significance of non-canonical telomerase activities during initiation and progression of cancer. In our study we observed that esophageal cancer cells expressing A279T had short telomeres relative to cells constitutively expressing wtTERT; these findings are consistent with observations by Vulliamy et al [22] that leukocytes from individuals with A279T genotype have short telomeres. However, our current results have not precisely defined the mechanisms by which A279T induces telomere dysfunction in esophageal carcinomas. A279T occurs in a region of TERT that is not essential for in-vitro activity of telomerase [22], which may explain our inability to observe effects of A279T on telomerase catalytic activity using TRAPeze assays. In this regard our findings are consistent with previously published studies demonstrating no significant decrease in telomerase catalytic activity by TRAPeze or direct primer extension assays [50], [51]. Conceivably, deficient repeat addition processivity [5], [6] could contribute to inhibition of telomere length in esophageal cancer cells expressing A279T; however, recent studies by Zaug et al [52] using well-established rabbit reticulocyte lysate experiments have demonstrated no effect of this TERT variant on processivity functions of telomerase. Alternatively, A279T may destabilize interactions of TERT with other telomerase complex proteins, and as suggested by our immunoblot experiments, impair chromosomal capping by shelterin proteins [26], [53]. Studies are in progress to further characterize the effects of A279T on telomere biology in normal and cancer cells.
The fact that TERT not only interacts with and stabilizes β-catenin [23], [54], but also is a direct target of β-catenin signaling [31], [32], indicates that highly complex and interdependent regulatory networks mediate canonical and non-canonical telomerase activities in cancer cells. As such, precisely ascribing various phenotypic alterations in esophageal cancer cells to effects of A279T on canonical versus non-canonical telomerase activities may be quite difficult, particularly in light of recent observations that TERT over-expression increases growth of primary epithelial cells via processes, which are independent of TERT catalytic activity, chromosomal capping, or Wnt β-catenin signaling [55]. For instance, we observed that β-catenin was markedly depleted in cancer cells expressing A279T, and that A279T attenuated TERT-mediated chemoresistance in esophageal cancer cells. Whereas inhibition of Wnt/β-catenin signaling has been shown to sensitize oropharyngeal and prostate cancer cells to cisplatin and paclitaxel, respectively [36], [56], more recent studies [33], [34] suggest that telomere length determines chemosensitivity in cancer cells. Collectively, these findings, together with recent observations that telomerase regulates heterochromatin structure within centromeres and transposons via interactions with BRG-1 and nucleostemin [57], suggest that the effects of A279T expression in cancer cells are pleiotropic and highly complex, and in all likelihood contingent on genetic/epigenetic landscapes. Current efforts are focused on identification of cancer cell lines endogenously expressing A279T to further examine these issues.
Recent elegant experiments have demonstrated that telomere dysfunction disrupts alternative splicing of multiple genes- some of which encode cytoskeletal proteins, and induces senescence in normal fibroblasts [58]. These observations together with our findings that A279T induced senescence, disrupted cytoskeletal integrity and markedly impaired chemotaxis in esophageal cancer cells raise the possibility that esophageal cancers expressing A279T might have low metastatic potential, hence more favorable clinical behavior. Unfortunately, the relatively small sample size and incomplete data regarding stage, response to therapy and survival of the patients whose tissues were used for this study precluded any assessment of the prognostic or predictive significance of A279T expression in esophageal cancers. Such analysis using a larger sample size and tissues linked to complete clinical databases should be undertaken if possible to confirm our initial observations, and define the clinical relevance of A279T expression in esophageal carcinomas.
Telomere shortening correlates with genomic instability [59] and progression to adenocarcinoma [60] in Barrett's esophagus- a chronic condition in which the squamous epithelia in the distal esophagus is replaced by proliferating, intestinal-type columnar epithelial cells in the context of gastro-esophageal reflux [61]. These findings suggest that telomere dysfunction occurs early during esophageal carcinogenesis. In our study we observed that relative to wtTERT, A279T significantly increased chromosomal aberrations in MEFs with wt p53 following exposure to Zeocin; A279T induced chromosomal instability in p53 deficient Li Fraumeni-fibroblasts in the absence of DNA damage; similar chromosomal aberrations have been identified during oncogene-mediated immortalization of human esophageal epithelial cells [62]. As such, our findings provide a potential mechanism (genomic instability) by which A279T could facilitate esophageal carcinogenesis- particularly in the context of p53 mutations, which are frequently observed in esophageal cancers and their precursor lesions [63]. Consistent with this notion, we have recently detected p53 mutations in 3 of 4 esophageal cancer samples expressing A279T. Insufficient genomic DNA prevented us from fully evaluating p53 status relative to A279T expression in the remaining tissue samples. These issues are the focus of ongoing studies in our laboratory.
Several recent studies suggest that leukocyte telomere length is an indicator of total body aging, and that decreased leukocyte telomere length coincides with predisposition to cancer [64], [65]. Of particular relevance regarding our current study are observations by Risques and colleagues [66] that patients with Barrett's esophagus with leukocyte telomeres that are short relative to their age have significantly increased risk of esophageal adenocarcinomas. These findings suggest that germline mutations involving telomerase complex predispose to esophageal cancers. Unfortunately, we were unable to access samples from the Risques study to determine if germline A279T expression correlates with esophageal cancer risk. Such studies should be undertaken if possible to ascertain if A279T is a potential biomarker of progression to cancer in patients with Barrett's esophagus.
It is counter-intuitive that A279T-mediated perturbations of telomerase appear to be oncogenic in non-transformed cells, yet tumor suppressive in esophageal cancer cells. However, these paradoxical observations are consistent with recent studies demonstrating that constitutive telomerase dysfunction inhibits metastatic progression in murine breast and prostate cancer models [67], [68].
Because this was not a case control study, it is possible that our analysis over-estimated the apparent enrichment of A279T in esophageal cancer patients. Indeed, depending on which database is queried, the mAF of A279T ranges from 0.9% in a large pool of healthy adult blood donors (528 individuals) including Caucasians, Blacks, Latinos and Asians [21], to 2.2% in patients with diverse pathologic conditions including idiopathic pulmonary fibrosis, aplastic anemia, acute myeloid leukemia, and dyskeratosis congenita in the NHLBI Exome Sequencing Project [52]. Despite these limitations, our findings that A279T modulates canonical as well as non-canonical telomerase activities highlight the complexity of telomerase expression in normal cellular homeostasis and human diseases. Whereas the mechanisms underlying our observations have not been fully delineated, our current findings support additional larger, case control studies to define the frequency and clinical significance of A279T expression in esophageal carcinomas and related preneoplastic lesions.
Supporting Information
Sanger's sequencing (left panel) and pyrosequencing (right panel) of homozygous, heterozygous, and wild type A279T.
(TIF)
Immunoblot demonstrating no appreciable decrease in β-catenin levels in HeLa cells expressing TERT variant A1062T.
(TIF)
Primers and PCR Conditions.
(DOCX)
Real-Time Quantitative RT-PCR Primers and Antibodies.
(DOCX)
Real-Time PCR Analysis of TERT and TERC Expression in Cell Lines. (relative copy/# β-actin x e4).
(DOCX)
Funding Statement
This work was supported by a NIH intramural grant. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Podlevsky JD, Chen JJ (2012) It all comes together at the ends: Telomerase structure, function, and biogenesis. Mutat Res 730: 3–11 S0027-5107(11)00292-2 [pii]; 10.1016/j.mrfmmm.2011.11.002 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calado RT, Young NS (2009) Telomere diseases. N Engl J Med 361: 2353–2365 361/24/2353 [pii]; 10.1056/NEJMra0903373 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brandsma I, Gent DC (2012) Pathway choice in DNA double strand break repair: observations of a balancing act. Genome Integr 3: 9 2041-9414-3-9 [pii]; 10.1186/2041-9414-3-9 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shay JW, Wright WE (2011) Role of telomeres and telomerase in cancer. Semin Cancer Biol 21: 349–353 S1044-579X(11)00064-2 [pii]; 10.1016/j.semcancer.2011.10.001 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xie M, Podlevsky JD, Qi X, Bley CJ, Chen JJ (2010) A novel motif in telomerase reverse transcriptase regulates telomere repeat addition rate and processivity. Nucleic Acids Res 38: 1982–1996 gkp1198 [pii]; 10.1093/nar/gkp1198 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berman AJ, Akiyama BM, Stone MD, Cech TR (2011) The RNA accordion model for template positioning by telomerase RNA during telomeric DNA synthesis. Nat Struct Mol Biol 18: 1371–1375 nsmb.2174 [pii]; 10.1038/nsmb.2174 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rampazzo E, Bertorelle R, Serra L, Terrin L, Candiotto C, et al. (2010) Relationship between telomere shortening, genetic instability, and site of tumour origin in colorectal cancers. Br J Cancer 102(8): 1300–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morrish TA, Greider CW (2009) Short telomeres initiate telomere recombination in primary and tumor cells. PLoS Genet 5: e1000357 10.1371/journal.pgen.1000357 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murnane JP (2011) Telomere dysfunction and chromosome instability. Mutat Res. S0027-5107(11)00091-1 [pii]; 10.1016/j.mrfmmm.2011.04.008 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davoli T, Denchi EL, de Lange T (2010) Persistent telomere damage induces bypass of mitosis and tetraploidy. Cell 141: 81–93 S0092-8674(10)00066-8 [pii]; 10.1016/j.cell.2010.01.031 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Calado RT, Regal JA, Kleiner DE, Schrump DS, Peterson NR, et al. (2009) A spectrum of severe familial liver disorders associate with telomerase mutations. PLoS One 4: e7926 10.1371/journal.pone.0007926 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller CT, Moy JR, Lin L, Schipper M, Normolle D, et al. (2003) Gene amplification in esophageal adenocarcinomas and Barrett's with high-grade dysplasia. Clin Cancer Res 9: 4819–4825. [PubMed] [Google Scholar]
- 13. Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, et al. (2005) Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med 352: 1413–1424 352/14/1413 [pii]; 10.1056/NEJMoa042980 [doi] [DOI] [PubMed] [Google Scholar]
- 14. Gollahon LS, Kraus E, Wu TA, Yim SO, Strong LC, et al. (1998) Telomerase activity during spontaneous immortalization of Li-Fraumeni syndrome skin fibroblasts. Oncogene 17: 709–717 10.1038/sj.onc.1201987 [doi] [DOI] [PubMed] [Google Scholar]
- 15. Calado RT, Cooper JN, Padilla-Nash HM, Sloand EM, Wu CO, et al. (2011) Short telomeres result in chromosomal instability in hematopoietic cells and precede malignant evolution in human aplastic anemia. Leukemia. leu2011272 [pii]; 10.1038/leu.2011.272 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zantl R, Horn E (2011) Chemotaxis of slow migrating mammalian cells analysed by video microscopy. Methods Mol Biol 769: 191–203 10.1007/978-1-61779-207-6_13 [doi] [DOI] [PubMed] [Google Scholar]
- 17. Padilla-Nash HM, Barenboim-Stapleton L, Difilippantonio MJ, Ried T (2006) Spectral karyotyping analysis of human and mouse chromosomes. Nat Protoc 1: 3129–3142 nprot.2006.358 [pii]; 10.1038/nprot.2006.358 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schrock E, du Manoir S, Veldman T, Schoell B, Wienberg J, et al. (1996) Multicolor spectral karyotyping of human chromosomes. Science 273: 494–497. [DOI] [PubMed] [Google Scholar]
- 19. Davoli T, Denchi EL, de Lange T (2010) Persistent telomere damage induces bypass of mitosis and tetraploidy. Cell 141: 81–93 S0092-8674(10)00066-8 [pii]; 10.1016/j.cell.2010.01.031 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walne AJ, Vulliamy T, Beswick R, Kirwan M, Dokal I (2008) TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes. Blood 112: 3594–3600 blood-2008-05-153445 [pii]; 10.1182/blood-2008-05-153445 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, et al. (2005) Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med 352: 1413–1424 352/14/1413 [pii]; 10.1056/NEJMoa042980 [doi] [DOI] [PubMed] [Google Scholar]
- 22. Vulliamy TJ, Walne A, Baskaradas A, Mason PJ, Marrone A, et al. (2005) Mutations in the reverse transcriptase component of telomerase (TERT) in patients with bone marrow failure. Blood Cells Mol Dis 34: 257–263 S1079-9796(05)00030-6 [pii]; 10.1016/j.bcmd.2004.12.008 [doi] [DOI] [PubMed] [Google Scholar]
- 23. Park JI, Venteicher AS, Hong JY, Choi J, Jun S, et al. (2009) Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 460: 66–72 nature08137 [pii]; 10.1038/nature08137 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. He H, Luo Y (2012) Brg1 regulates the transcription of human papillomavirus type 18 E6 and E7 genes. Cell Cycle 11: 617–627 19115 [pii]; 10.4161/cc.11.3.19115 [doi] [DOI] [PubMed] [Google Scholar]
- 25. Calado RT, Regal JA, Hills M, Yewdell WT, Dalmazzo LF, et al. (2009) Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proc Natl Acad Sci U S A 106: 1187–1192 0807057106 [pii]; 10.1073/pnas.0807057106 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Calado RT, Regal JA, Kajigaya S, Young NS (2009) Erosion of telomeric single-stranded overhang in patients with aplastic anaemia carrying telomerase complex mutations. Eur J Clin Invest 39: 1025–1032 ECI2209 [pii]; 10.1111/j.1365-2362.2009.02209.x [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, et al. (2012) Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell 149: 1245–1256 S0092-8674(12)00530-2 [pii]; 10.1016/j.cell.2012.05.002 [doi] [DOI] [PubMed] [Google Scholar]
- 28. Grossmann TN, Yeh JT, Bowman BR, Chu Q, Moellering RE, et al. (2012) Inhibition of oncogenic Wnt signaling through direct targeting of beta-catenin. Proc Natl Acad Sci U S A 109: 17942–17947 1208396109 [pii]; 10.1073/pnas.1208396109 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clevers H, Nusse R (2012) Wnt/beta-catenin signaling and disease. Cell 149: 1192–1205 S0092-8674(12)00586-7 [pii]; 10.1016/j.cell.2012.05.012 [doi] [DOI] [PubMed] [Google Scholar]
- 30. Katoh M (2008) WNT signaling in stem cell biology and regenerative medicine. Curr Drug Targets 9: 565–570. [DOI] [PubMed] [Google Scholar]
- 31. Zhang Y, Toh L, Lau P, Wang X (2012) Human telomerase reverse transcriptase (hTERT) is a novel target of the Wnt/beta-catenin pathway in human cancer. J Biol Chem 287: 32494–32511 M112.368282 [pii]; 10.1074/jbc.M112.368282 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoffmeyer K, Raggioli A, Rudloff S, Anton R, Hierholzer A, et al. (2012) Wnt/beta-catenin signaling regulates telomerase in stem cells and cancer cells. Science 336: 1549–1554 336/6088/1549 [pii]; 10.1126/science.1218370 [doi] [DOI] [PubMed] [Google Scholar]
- 33. Smith V, Dai F, Spitz M, Peters GJ, Fiebig HH, et al. (2009) Telomerase activity and telomere length in human tumor cells with acquired resistance to anticancer agents. J Chemother 21: 542–549. [DOI] [PubMed] [Google Scholar]
- 34. Uziel O, Beery E, Dronichev V, Samocha K, Gryaznov S, et al. (2010) Telomere shortening sensitizes cancer cells to selected cytotoxic agents: in vitro and in vivo studies and putative mechanisms. PLoS One 5: e9132 10.1371/journal.pone.0009132 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cerone MA, Londono-Vallejo JA, Autexier C (2006) Telomerase inhibition enhances the response to anticancer drug treatment in human breast cancer cells. Mol Cancer Ther 5: 1669–1675 5/7/1669 [pii]; 10.1158/1535-7163.MCT-06-0033 [doi] [DOI] [PubMed] [Google Scholar]
- 36. Ohigashi T, Mizuno R, Nakashima J, Marumo K, Murai M (2005) Inhibition of Wnt signaling downregulates Akt activity and induces chemosensitivity in PTEN-mutated prostate cancer cells. Prostate 62: 61–68 10.1002/pros.20117 [doi] [DOI] [PubMed] [Google Scholar]
- 37. Benjamin JM, Nelson WJ (2008) Bench to bedside and back again: molecular mechanisms of alpha-catenin function and roles in tumorigenesis. Semin Cancer Biol 18: 53–64 S1044-579X(07)00073-9 [pii]; 10.1016/j.semcancer.2007.08.003 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wright WE, Shay JW (2000) Telomere dynamics in cancer progression and prevention: fundamental differences in human and mouse telomere biology. Nat Med 6: 849–851 10.1038/78592 [doi] [DOI] [PubMed] [Google Scholar]
- 39. Nicholls C, Li H, Wang JQ, Liu JP (2011) Molecular regulation of telomerase activity in aging. Protein Cell 2: 726–738 10.1007/s13238-011-1093-3 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rafnar T, Sulem P, Stacey SN, Geller F, Gudmundsson J, et al. (2009) Sequence variants at the TERT-CLPTM1L locus associate with many cancer types. Nat Genet 41: 221–227 ng.296 [pii]; 10.1038/ng.296 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, et al. (2013) Highly recurrent TERT promoter mutations in human melanoma. Science 339: 957–959 science.1229259 [pii]; 10.1126/science.1229259 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arita H, Narita Y, Fukushima S, Tateishi K, Matsushita Y, et al. (2013) Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 10.1007/s00401-013-1141-6 [doi] [DOI] [PubMed] [Google Scholar]
- 43. Shay JW, Reddel RR, Wright WE (2012) Cancer. Cancer and telomeres–an ALTernative to telomerase. Science 336: 1388–1390 336/6087/1388 [pii]; 10.1126/science.1222394 [doi] [DOI] [PubMed] [Google Scholar]
- 44. Heaphy CM, Subhawong AP, Hong SM, Goggins MG, Montgomery EA, et al. (2011) Prevalence of the Alternative Lengthening of Telomeres Telomere Maintenance Mechanism in Human Cancer Subtypes. Am J Pathol 179: 1608–1615 S0002-9440(11)00635-3 [pii]; 10.1016/j.ajpath.2011.06.018 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dai Z, Kelly JC, Meloni-Ehrig A, Slovak ML, Boles D, et al. (2012) Incidence and patterns of ALK FISH abnormalities seen in a large unselected series of lung carcinomas. Mol Cytogenet 5: 44 1755-8166-5-44 [pii]; 10.1186/1755-8166-5-44 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kammori M, Poon SS, Nakamura K, Izumiyama N, Ishikawa N, et al. (2007) Squamous cell carcinomas of the esophagus arise from a telomere-shortened epithelial field. Int J Mol Med 20: 793–799. [PubMed] [Google Scholar]
- 47. Taboski MA, Sealey DC, Dorrens J, Tayade C, Betts DH, et al. (2012) Long telomeres bypass the requirement for telomere maintenance in human tumorigenesis. Cell Rep 1: 91–98 S2211-1247(11)00014-3 [pii]; 10.1016/j.celrep.2011.12.004 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maida Y, Yasukawa M, Furuuchi M, Lassmann T, Possemato R, et al. (2009) An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA. Nature 461: 230–235 nature08283 [pii]; 10.1038/nature08283 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wege H, Heim D, Lutgehetmann M, Dierlamm J, Lohse AW, et al. (2011) Forced activation of beta-catenin signaling supports the transformation of hTERT-immortalized human fetal hepatocytes. Mol Cancer Res 9: 1222–1231 1541-7786.MCR-10-0474 [pii]; 10.1158/1541-7786.MCR-10-0474 [doi] [DOI] [PubMed] [Google Scholar]
- 50. Du HY, Pumbo E, Ivanovich J, An P, Maziarz RT, et al. (2009) TERC and TERT gene mutations in patients with bone marrow failure and the significance of telomere length measurements. Blood 113: 309–316 blood-2008-07-166421 [pii]; 10.1182/blood-2008-07-166421 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, et al. (2008) Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A 105: 13051–13056 0804280105 [pii]; 10.1073/pnas.0804280105 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zaug AJ, Crary SM, Jesse FM, Campbell K, Cech TR (2013) Many disease-associated variants of hTERT retain high telomerase enzymatic activity. Nucleic Acids Res 41: 8969–8978 gkt653 [pii]; 10.1093/nar/gkt653 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Martinez P, Blasco MA (2010) Role of shelterin in cancer and aging. Aging Cell 9: 653–666 ACE596 [pii]; 10.1111/j.1474-9726.2010.00596.x [doi] [DOI] [PubMed] [Google Scholar]
- 54. Chiodi I, Mondello C (2012) Telomere-independent functions of telomerase in nuclei, cytoplasm, and mitochondria. Front Oncol 2: 133 10.3389/fonc.2012.00133 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mukherjee S, Firpo EJ, Wang Y, Roberts JM (2011) Separation of telomerase functions by reverse genetics. Proc Natl Acad Sci U S A. 1112414108 [pii]; 10.1073/pnas.1112414108 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gosepath EM, Eckstein N, Hamacher A, Servan K, von Jonquieres G, et al. (2008) Acquired cisplatin resistance in the head-neck cancer cell line Cal27 is associated with decreased DKK1 expression and can partially be reversed by overexpression of DKK1. Int J Cancer 123: 2013–2019 10.1002/ijc.23721 [doi] [DOI] [PubMed] [Google Scholar]
- 57. Maida Y, Yasukawa M, Okamoto N, Ohka S, Kinoshita K, et al. (2014) Involvement of TERT in heterochromatin maintenance. Mol Cell Biol. MCB.00093-14 [pii]; 10.1128/MCB.00093-14 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cao K, Blair CD, Faddah DA, Kieckhaefer JE, Olive M, et al. (2011) Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J Clin Invest 121: 2833–2844 43578 [pii]; 10.1172/JCI43578 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shiraishi H, Mikami T, Aida J, Nakamura K, Izumiyama-Shimomura N, et al. (2009) Telomere shortening in Barrett's mucosa and esophageal adenocarcinoma and its association with loss of heterozygosity. Scand J Gastroenterol 44: 538–544 908749582 [pii]; 10.1080/00365520902718705 [doi] [DOI] [PubMed] [Google Scholar]
- 60. Xing J, Ajani JA, Chen M, Izzo J, Lin J, et al. (2009) Constitutive short telomere length of chromosome 17p and 12q but not 11q and 2p is associated with an increased risk for esophageal cancer. Cancer Prev Res (Phila) 2: 459–465 1940-6207.CAPR-08-0227 [pii]; 10.1158/1940-6207.CAPR-08-0227 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Streppel MM, Montgomery EA, Maitra A (2013) New Advances in the Pathogenesis and Progression of Barrett's Esophagus. Curr Mol Med. CMM-EPUB-54165 [pii] [DOI] [PubMed] [Google Scholar]
- 62. Zhang H, Jin Y, Chen X, Jin C, Law S, et al. (2006) Cytogenetic aberrations in immortalization of esophageal epithelial cells. Cancer Genet Cytogenet 165: 25–35 S0165-4608(05)00407-3 [pii]; 10.1016/j.cancergencyto.2005.07.016 [doi] [DOI] [PubMed] [Google Scholar]
- 63. Jenkins GJ, Doak SH, Griffiths AP, Tofazzal N, Shah V, et al. (2003) Early p53 mutations in nondysplastic Barrett's tissue detected by the restriction site mutation (RSM) methodology. Br J Cancer 88: 1271–1276 10.1038/sj.bjc.6600891 [doi];6600891 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ma H, Zhou Z, Wei S, Liu Z, Pooley KA, et al. (2011) Shortened telomere length is associated with increased risk of cancer: a meta-analysis. PLoS One 6: e20466 10.1371/journal.pone.0020466 [doi];PONE-D-11-04747 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA (2011) The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev 20: 1238–1250 1055-9965.EPI-11-0005 [pii]; 10.1158/1055-9965.EPI-11-0005 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Risques RA, Vaughan TL, Li X, Odze RD, Blount PL, et al. (2007) Leukocyte telomere length predicts cancer risk in Barrett's esophagus. Cancer Epidemiol Biomarkers Prev 16: 2649–2655 16/12/2649 [pii]; 10.1158/1055-9965.EPI-07-0624 [doi] [DOI] [PubMed] [Google Scholar]
- 67. Jaskelioff M, Song W, Xia J, Liu C, Kramer J, et al. (2009) Telomerase deficiency and telomere dysfunction inhibit mammary tumors induced by polyomavirus middle T oncogene. Oncogene 28: 4225–4236 onc2009268 [pii]; 10.1038/onc.2009.268 [doi] [DOI] [PubMed] [Google Scholar]
- 68. Ding Z, Wu CJ, Jaskelioff M, Ivanova E, Kost-Alimova M, et al. (2012) Telomerase reactivation following telomere dysfunction yields murine prostate tumors with bone metastases. Cell 148: 896–907 S0092-8674(12)00144-4 [pii]; 10.1016/j.cell.2012.01.039 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sanger's sequencing (left panel) and pyrosequencing (right panel) of homozygous, heterozygous, and wild type A279T.
(TIF)
Immunoblot demonstrating no appreciable decrease in β-catenin levels in HeLa cells expressing TERT variant A1062T.
(TIF)
Primers and PCR Conditions.
(DOCX)
Real-Time Quantitative RT-PCR Primers and Antibodies.
(DOCX)
Real-Time PCR Analysis of TERT and TERC Expression in Cell Lines. (relative copy/# β-actin x e4).
(DOCX)