Abstract
We report the first cases of Edwardsiella ictaluri causing epizootics in laboratory populations of Zebrafish Danio rerio. Edwardsiella ictaluri is primarily recognized as a disease of catfish species and is known to cause an economically important bacterial disease of farm-raised catfish in the USA and abroad; however, it has been isolated on occasion from 10 other genera of nonictalurid fishes. We isolated E. ictaluri from moribund Zebrafish held in quarantine at two different universities in two states and from a research facility in a third state between February 23 and December 6, 2011. Edwardsiellosis in Zebrafish can be described as a severe systemic disease characterized by tissue necrosis and the presence of large numbers of extracellular and intracellular bacteria, often within macrophages. The kidneys (pronephros and mesonephros), spleen, nares, and forebrain were the most commonly and severely affected tissues. In outbreaks, mortality was acute and numerous fish died over a 1–2 week period. Mortality continued until the majority of the population was lost, at which time the remaining fish were euthanized. In addition to these cases, four cultures of bacteria isolated from Zebrafish by another diagnostic laboratory were submitted to the Louisiana Aquatic Diagnostic Laboratory for identification and were confirmed as E. ictaluri. In total, eight cultures of E. ictaluri from Zebrafish from Louisiana, Massachusetts, Pennsylvania, and Florida were identified. The isolates were confirmed as E. ictaluri by biochemical phenotype, API 20E (bioMérieux), and amplification and sequencing of a portion of the 16S rRNA gene. Edwardsiella ictaluri isolates from Zebrafish are believed to comprise a unique group and were differentiated from catfish isolates by exhibiting weaker motility, autoaggregation in broth, a different plasmid profile (two plasmids of 4.0 and 3.5 kb), a different API 20E code (4204000), and lack of lipopolysaccharide recognition with Mab Ed9.
In recent years there has been a dramatic increase in the use of Zebrafish Danio rerio in biomedical research. Zebrafish are raised indoors in research laboratories, in either recirculating or flow-through water systems with ultraviolet (UV) sterilizers (Harper and Lawrence 2011). Fish stocks are generally housed in separate tanks according to genetic background (wild-type, mutant, and transgenic lines) and by generation. The closed nature of these systems facilitates tracking of morbidities and mortalities as well as disease monitoring. The source of Zebrafish for research laboratories ranges from pond-reared fish for the aquarium trade to laboratories such as the Zebrafish International Resource Center (ZIRC), University of Oregon, in which pathogens are documented and controlled. Most research facilities introduce new fish into their main facilities as second generations derived from eggs that are surface disinfected with chlorine (Westerfield 2007; Kent et al. 2009).
Two of us (M. Kent and K. Murray) have been providing diagnostic services through the ZIRC to the Zebrafish community since 1999, and the diseases of laboratory Zebrafish have been documented and described (http://zebrafish.org/zirc/health/diseaseManual.php). The most common bacterial infections diagnosed in Zebrafish are chronic or asymptomatic infections by Mycobacterium spp., most often M. chelonae (Whipps et al. 2008; Murray et al. 2011). Although species like M. marinum and M. haemophilum have been associated with outbreaks of morbidity and mortality, these outbreaks are generally protracted and the onset of mortalities is not acute (Watral and Kent 2007; Whipps et al. 2007). Acute mortalities in Zebrafish facilities are more likely to be associated with minimal or un-compensated physiologic stress, often manifested as an acute stress response, due to perturbations in environmental (water) quality. Opportunistic pathogens may then be responsible for environmental gill disease or septicemia caused by secondary Gram-negative bacterial infections (e.g., Aeromonas spp., Pseudomonas spp., Plesiomonas spp.). Here we describe the unique presentation of acute mortalities associated with Edwardsiella ictaluri infections in laboratory Zebrafish facilities (Table 1).
TABLE 1.
Summary of outbreaks of edwardsiellosis in Zebrafish colonies in 2011. +/+ indicates that both tissue histology and bacterial cultures were conducted.
| Location | Case number | Date | Facility | Source | Histology/culture |
|---|---|---|---|---|---|
| Louisiana State University | LADL11-100 | Jun 7 | Quarantine | Commercial | +/+ |
| Louisiana State University | LADL11-104 | Jun 14 | Quarantine | Commercial | +/+ |
| University of Pittsburg | ZIRC D11-13 | Feb 11 | Quarantine | Commercial | +/+ |
| University of Pittsburg | ZIRC D11-81 | Aug 1 | Quarantine | Commercial | +/+ |
| University of Massachusetts Amherst | ZIRC D11-112 | Nov 17 | Main facility | In house | +/+ |
Enteric septicemia of catfish (ESC) (Hawke 1979) is considered the most economically important cause of disease on catfish farms in the USA and is responsible for the majority of disease-related mortality annually (USDA 2003a, 2003b). The causative agent of ESC was identified and described as a new species named E. ictaluri (Hawke et al. 1981) and is a member of the Class Gammaproteobacteria, Order Enterobacteriales, and Family Enterobacteriaceae. Mortality rates in pond-raised Channel Catfish Ictalurus punctatus can range from 10% to 50% depending on the age and immune status of susceptible fish in the population (Hawke and Khoo 2004). Initially E. ictaluri was considered to be a host-specific pathogen of catfish species in the United States such as the Channel Catfish, the White Catfish Ameiurus catus, and the Brown Bullhead A. nebulosus (Hawke 1979; Hawke et al. 1981; Waltman et al. 1985); however, in recent years the bacterium has been identified as the cause of disease in other species of catfish internationally including Walking Catfish Clarius batrachus in Thailand (Kasornchandra et al. 1987), the Vietnamese freshwater catfish Pangasius hypophthalmus (Crumlish et al. 2002), Chinese Yellow Catfish Pelteobagrus fulvidraco (Liu et al. 2010) and the Japanese Ayu Plecoglossus altevelis (Sakai et al. 2008). Other susceptible catfish species in the United States are the Blue Catfish I. furcatus (Wolters and Johnson 1994) and the Tadpole Madtom Noturus gyrinus (Klesius et al. 2003). Infections have also been observed in noncatfish species, including the Green Knifefish Eigemannia virescens (Kent and Lyons 1982), the Devario Devario (Danio) devario (Waltman et al. 1985), the Rosy Barb Puntius conchonius (Humphrey et al. 1986), and the Nile Tilapia Oreochromis niloticus (Soto et al. 2012). In addition, experimental infection of noncatfish species has been achieved in Rainbow Trout Oncorhynchus mykiss, Chinook Salmon O. tshawytscha (Baxa et al. 1990), and Blue Tilapia Oreochromis aureus (Plumb and Sanchez 1983). Experimental infection of Zebrafish with a catfish isolate was achieved by injection and immersion (Petrie-Hanson et al. 2007), and recently this protocol was used to experimentally infect Zebrafish in studies on adhesion protection by probiotic bacteria (Rendueles et al. 2012). Prior to our account, epizootics caused by Edwardsiella ictaluri have not been reported in pond populations or research laboratory populations of Zebrafish.
Edwardsiella ictaluri is most closely related to E. tarda (56–62% DNA homology) and is typically identified using a combination of staining characteristics, cell morphology, and biochemical and physiologic tests (Hawke et al. 1981). The bacterium is a Gram-negative rod, motile by peritrichous flag-ella (0.75 µm in width × 1.25 µm in length), oxidase negative, and fermentative in O/F glucose or glucose motility deeps (GMD). The triple sugar iron (TSI) slant reaction is K/A with negative H2S and the indole test is negative. Catfish strains of E. ictaluri consistently produce the code number 4004000 in the API 20E system (bioMérieux, Durham, North Carolina). More recently, molecular methods of identification have been utilized by diagnostic laboratories such as species-specific real-time PCR (Bilodeau et al. 2003), PCR amplification of the 16S rRNA gene with universal primers and sequencing of the amplicons followed by basic local alignment search tool (BLAST) analysis (Janda and Abbott 2007), and detection of the organism in water by real-time PCR (Griffin et al. 2011). The species has traditionally been regarded as homogeneous with catfish strains from different geographic locations being practically identical in biochemical phenotype (Plumb and Vinitnantharat 1989), isozymes (Starliper et al. 1988), plasmids (Newton et al. 1998), and serology (Chen and Light 1994). By means of genomic fingerprinting, as many as four different patterns were identified using arbitrarily primed PCR (AP-PCR) (Bader et al. 1998); however, Griffin (2011) found a high level of homogeneity in the electrophoretic profiles generated from 19 U.S. catfish isolates of E. ictaluri when they were analyzed by repetitive-element PCR amplification utilizing ERIC and BOX primer sets.
METHODS
Necropsy and bacterial isolation
Standard necropsy procedures included the following: observation of fish externally and internally for gross clinical signs, observation of gill clippings and skin scrapings microscopically in wet mounts for bacteria and parasites, aseptic dissection and collection of tissues (spleen, anterior kidney, and liver), and streaking tissue homogenates on trypticase soy agar with 5% sheep blood (SBA) (Remel, Lenexa, Kansas) for bacterial isolation. Primary isolation plates were incubated at 28°C for 48 h. Isolated colonies were subcultured to fresh SBA plates for maintenance of stock cultures prior to inoculation of test media.
Biochemical identification of bacterial isolates
For presumptive identification, bacteria were evaluated by the Gram stain, cytochrome oxidase test, catalase test, glucose fermentation in GMD, esculin hydrolysis on bile esculin slants, H2S production in TSI slants, and citrate utilization using standard bacteriological test tube methods (Hawke et al. 1981). Motility was assessed in wet mounts and in GMD. Inoculation of API 20E and API 50 CH strips (bioMérieux) and interpretation of results was done according to manufacturer’s instructions for Enterobacteriaceae; however, incubation of the strips was at 28°C for 48 h.
DNA extraction
Representative isolates recovered from fish in the different cases or submitted to the Louisiana Aquatic Diagnostic Laboratory (LADL) for identification were subjected to molecular identification procedures. For each isolate, the bacteria were suspended in 500 µL of sterile phosphate-buffered saline (PBS) at a density equivalent to a McFarland 4 equivalence turbidity standard (Remel) (Lorian 1986). The cells were centrifuged at 3,000 × g for 5 min, washed 2×, and resuspended in 200 µL of PBS. Bacterial suspensions were subjected to DNA extraction and purification as per the manufacturer’s protocol using the High Pure PCR Template Preparation Kit (Roche). The DNA was stored at 4°C until further use.
PCR and 16S rRNA sequencing
The 16S rRNA encoding gene was amplified by PCR using the primers SSU27 (5′-AGAGTTTGATCMTGGCTCAG-3′) and SSU1492 (5′-TACGGYTACCTTTGTTACGACTT-3′) and Phusion high fidelity(HF) DNA polymerase (Finnzymes, Thermo Scientific, Vantaa, Finland) by the method of Pombert et al. (2009). The 50 µL universal eubacterial 16S rRNA PCR reaction was composed of 0.5 µm of each primer, 0.25 mm of dNTPs, 1.0 U of Phusion DNA polymerase, 5× Phusion HF buffer (Finnzymes), and approximately 50–250 ng of template DNA. Cycling conditions consisted of an initial denaturation step of 30 s at 98°C, followed by 35 cycles of 10 s at 98°C, 30 s at 56.2°C, and 30 s at 72°C, with a final extension step of 10 min at 72°C in a Perkin Elmer GeneAmp PCR System 2400. The PCR products were subjected to electrophoresis on a 1% agarose gel and stained with SYBR Safe DNA gel stain (Invitrogen). Amplicons for sequencing were purified with the QiaQuick PCR Cleanup Kit (Qiagen) as directed by the manufacturer and were sequenced on an Applied Biosystems 3130 Genetic Analyzer using PCR primers (F11–F5) and (F1–R13). The sequences were compared with those stored in GenBank using the BLASTN program from the National Center for Biotechnology Information (NCBI).
Histopathology
Whole fish were fixed in neutral-buffered 10% formalin or Dietrich’s fixative, dehydrated in ethanol, and embedded in paraffin using standard procedures (Luna 1968). Tissues were sectioned at 5 µm thickness, mounted on slides, stained with hematoxylin and eosin, and examined by light microscopy.
Auto-aggregation
Auto-aggregation was examined by comparing isolates from Channel Catfish with isolates from Zebrafish following growth in brain–heart infusion (BHI) broth. Auto-aggregation was demonstrated by inoculating 5 mL of BHI broth in a test tube with 50 µL of a bacterial suspension in PBS equivalent to a McFarland 0.5 turbidity standard. The broth culture was incubated for 18 h in a roller apparatus (Cell-Gro Tissue Culture Rotator, Barnstead/Lab Line Products, Dubuque, Iowa) at 80 rpm overnight to a final cell density of approximately 109 CFU/mL. The tubes were then placed vertically in a rack on the bench top at room temperature (21°C) for 6 h with no shaking and examined for auto-aggregation hourly. A tube was scored positive for auto-aggregation if bacteria in suspension began clumping and settling to the bottom of the tube leaving a clear liquid in 90% of the tube within 1–2 h. Strains of E. ictaluri tested from Channel Catfish were LADL93-146, ATCC 33202, LADL88-108, LADL91-581, S07-794, and S05-518. Strains from Zebrafish were LADL11-100, LADL11-104, 11TAL197, 11TAL204, LADL11-194, and 11TAL232.
Plasmid analysis
Plasmids were isolated from cultures using the Spin Miniprep kit (Qiagen,, Valencia, California). Supercoiled plasmids were separated by 0.6% agarose gel electrophoresis with supercoiled ladder (New England Biolabs., Ipswich, Massachusetts) as the size standard. Plasmids were digested with either EcoRI or BstZ17I and were separated by 0.6% agarose gel electrophoresis with 1-kb ladder (New England Biolabs) as the size standard.
Bacterial LPS recognition with Mab Ed9
Bacterial cultures were grown to late log phase and were pelleted by centrifugation, followed by two washes with PBS at pH 7.4. Pellets were resuspended in 1× lithium dodecyl sulfate (LDS) sample buffer (Life Technologies, Grand Island, New York) and boiled for 10 min. The lysate was cleared by centrifugation at 16,100 × g for 20 min, the lysate was diluted 1:100 in LDS buffer, and separated on a NuPAGE Novex 12% bis-tris gel (Life Technologies). Separated samples were transferred to a polyvinylidene fluoride membrane using the iBlot Dry Transfer System (Life Technologies). The membrane was incubated with the Ed9 lipopolysaccharide (LPS) monoclonal antibody raised against the ATCC 33202 isolate of E. ictaluri, donated by Dr. Jerold Ainsworth, Mississippi State University (Ainsworth et al. 1986), diluted 1:8 in tris-buffered saline containing 0.1% Tween-20 (Bio-Rad Laboratories, Hercules, California). Horseradish peroxidase-conjugated goat anti-mouse antibody (Thermo-Fisher Scientific,× Rock-ford, Illinois) was used to label the Ed9 antibody with Super-Signal West Pico chemiluminescent substrate (Thermo-Fisher Scientific).
Antibiotic-susceptibility testing
Antibiotic susceptibility was determined by both disk diffusion and minimal inhibitory concentration (MIC) by broth microdilution using quality control methods outlined in the M42 and M49 documents of the Clinical Laboratory Standards Institute (CLSI 2006a, 2006b). For disk diffusion susceptibility tests, suspensions equivalent to a McFarland 0.5 turbidity standard were made of each culture to be tested in 5 mL of sterile 0.85% saline. Using a cotton-tipped applicator, Mueller-Hinton agar plates with 5% sheep blood were inoculated by streaking the entire surface with the applicator saturated with the bacterial suspension. Paper disks impregnated with the antibiotics: oxytetracycline (T30), enrofloxacin (ENO15), florfenicol (FFC 10), and Romet (25), were spaced evenly on the surface of the plate immediately after it was inoculated. A control plate inoculated with Escherichia coli ATCC 25922 served as the quality control standard. Plates were incubated at 28°C and zones of diffusion were measured at 24 and 48 h. Broth microdilution tests were performed using the Sensititre Avian 1F plates (Trek Diagnostic Systems, West Sussex, UK). The plates were inoculated according to the manufacturer’s instructions but were incubated at 28°C for 24 and 48 h. A control plate was inoculated with E. coli ATCC 25922.
Fullfillment of Koch’s postulates
Edwardsiella ictaluri strain LADL11-100 was selected as a representative from Louisiana State University (LSU) outbreaks and was used in an immersion challenge to fulfill Koch’s postulates. Four 20-L tanks were each stocked with 10 Zebrafish (average weight, 0.5 g) and challenged by immersion in 107 CFU/mL for 2 h. After immersion of fish, the bacteria were flushed out of the tank by gradual water replacement in a flow-through system, and flow-through conditions were maintained for the duration of the 10-d trial. Mortality was recorded daily and moribund or dead fish were necropsied and bacterial cultures, taken from the liver and brain, were grown on tryptic soy agar with 5% sheep blood (TSAB) (Remel).
RESULTS
Disease Outbreaks in Zebrafish
LSU Department of Biological Sciences
Two populations of Zebrafish (approximately 1 year of age) were obtained from a commercial source on June 7, 2011, (group 1, 468 fish) and on June 14, 2011, (group 2, 281 fish) and held in separate groups of quarantine tanks in the fish holding facilities of the Life Sciences Building, Department of Biological Sciences, LSU, for use in unrelated experiments. Upon arrival the fish exhibited no signs of transport stress or mortality. At 24 h after arrival, the fish in the first group began to exhibit clinical signs that included hemorrhage in the skin near the eyes and opercula, base of fins, and ventral surface of the abdomen. By day 3, mortalities were occurring in the population and fish exhibiting clinical signs were submitted to the LADL at the LSU School of Veterinary Medicine where fish were subjected to a standard necropsy. Specimens showing clinical signs and others showing lethargic behavior were negative for parasites on the gills or skin. The gills were pale and internally the liver was pale and the spleen was swollen. Skin ulceration was noted on a few fish (Figure 1A). Other specimens exhibited clinical signs that included abdominal swelling due to accumulation of ascites (Figure 1B) and hemorrhaging in the skin near the eyes and opercula, base of fins, or ventral surface of the abdomen (Figure 1C, D). Mortality continued for 10 d until cumulative mortality had reached 280 fish and the decision was made to euthanize the remainder of the population. A second group of fish received from the same source was also diagnosed as positive for a Gram-negative bacterial septicemia and were euthanized after quarantine. In total, 750 Zebrafish were lost to the infection or euthanasia. Pure cultures recovered from the internal organs and brains of the moribund specimens were identified as E. ictaluri. A third group of Zebrafish was received and quarantined by LSU Department of Biological Sciences on June 17, 2011, from a different vendor. Fish in this group cultured negative for bacterial disease, postshipping mortality was minimal, and the fish performed well in the experiments.
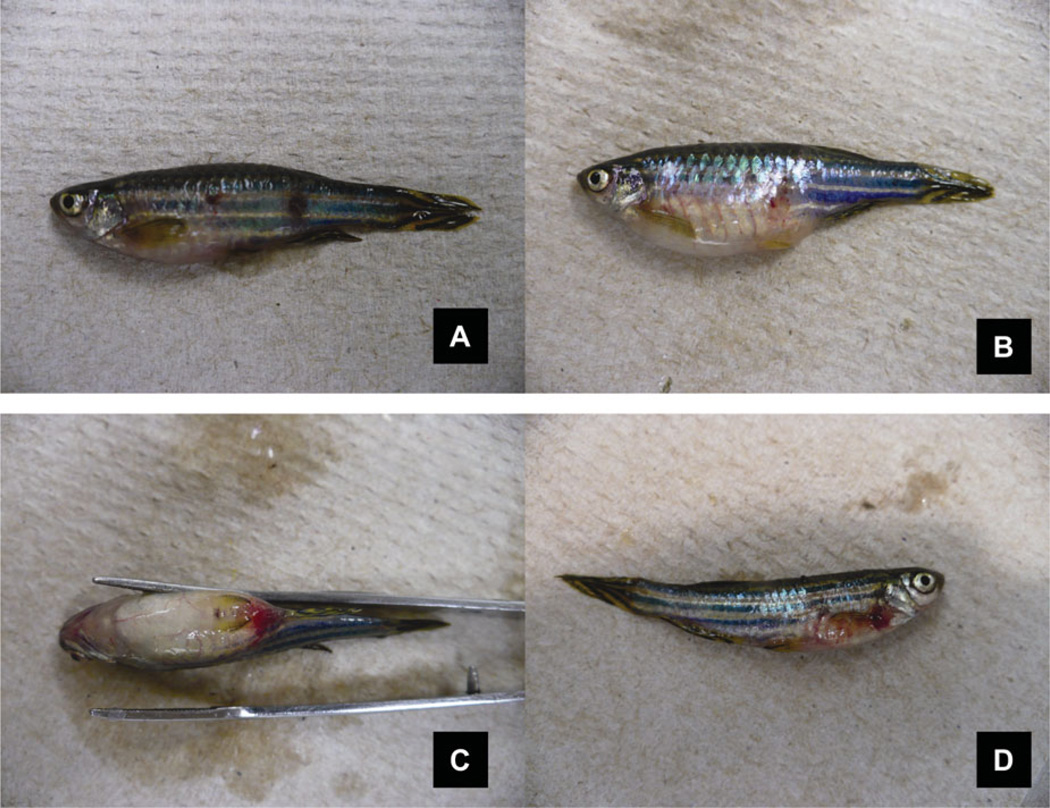
FIGURE 1.
Clinical signs of edwardsiellosis in Zebrafish. (A) Hemorrhage and ulceration in the skin. (B) Swollen abdomen due to accumulation of ascites. (C) Swollen abdomen and hemorrhage ventrally. (D) Hemorrhage at the base of the fins.
University of Pittsburgh
The University of Pittsburgh purchased 610 fish from a commercial source on January 18, 2011. The fish (approximately 1 year of age) were quarantined in 9-L tanks at a density of approximately 10 fish/L. A few mortalities (approximately 10 fish) occurred in the first week and were believed to be related to transport stress. After 2 weeks of acclimation in quarantine, 11 fish died in one tank in 1 d and in the following days more mortality occurred in this tank. Clinical signs included lethargy, raised scales, and hemorrhage in the skin of the operculum and abdomen. Preliminary diagnostic tests were negative for internal and external parasites and only one of four fish yielded a positive kidney culture of Plesiomonas shigelloides after 48 h incubation at 28°C. At this time, a total of 40 fish had died and the decision was made to treat the remaining fish in the tank with medicated feed containing the antibiotic florfenicol based on its efficacy in treating bacterial infections in Channel Catfish (Gaunt et al. 2004). The individual affected tank was separated from the rack on February 9, 2011, but on February 10 two fish had died in two other tanks. The principal investigator was asked to sacrifice all the remaining fish on the rack. The total number of fish sacrificed was 630: 550 fish from the new batch, 20 spinalized fish (fish with experimental spinal lesions), and 60 fish from a previous shipment. Normally fish in quarantine that show an outbreak of an infectious disease are euthanized and not treated to avoid putting the primary fish facility at risk, but in this case the high value of the current fish population prompted an attempt to reduce the mortality rate of affected fish. A 660-mg dosage of florfenicol (Intervet/Schering-Plow Animal Health, Roseland, New Jersey) was incorporated into 880 g of feed and fed to the fish ad libitum for 7 d. In the following days no additional fish died in the treated tank. The fish were sacrificed 3 d after treatment ended when the experimental endpoint was reached.
Three moribund specimens submitted to the ZIRC on August 1, 2011, for histopathologial examination were reported to have “severe encephalitis, nephritis, and splenitis associated with acute bacterial disease.” Acid-fast stains were negative and a viral assay (Ambrose and Clewley 2006) performed by Dr. M. J. Crim, University of Missouri, designed to amplifly viral DNA from a wide variety of viruses by PCR, yielded no positives. The brains of sentinel fish from the rack system in the first outbreak that were previously frozen and maintained at the University of Pittsburg and brains of fish in the present outbreak were cultured on SBA at 25°C. The cultures were examined by the ZIRC and forwarded to the LADL at LSU for identification. The two bacteria identified from the cultures were identified as E. ictaluri and Pseudomonas fluorescens; however, the Pseudomonas was deemed to be secondary because the bacterium seen in histology slides and histopathology was indicative of E. ictaluri.
University of Massachusetts at Amherst
This laboratory Zebrafish facility submitted fish to the ZIRC diagnostic service after a rapid increase in mortality. The facility runs a recirculating water system with UV sterilizers. The fish colony is maintained by in-house breeding and only surface-sanitized embryos are put in the system. In July 2011, after an accidental 50% system water exchange, a large number of mortalities occurred resulting in a loss of approximately 70% of the population. Bacterial cultures of pooled heart blood samples revealed Aeromonas hydrophila and an unidentified Gram-negative bacterium. A small group of symptomatic tanks were treated with enrofloxacin, although mortalities had already begun to subside. A second spike in mortality occurred in November 2011. Approximately 15% of the population (600 fish) died during a 2-week period. Fish appeared lethargic, hovered in the water column, and developed hemorrhagic and ulcerative skin lesions. Three 4-month-old Zebrafish, one transgenic and two heterozygous mutant carriers, were preserved in 10% formalin and submitted to the ZIRC for histopathology. Additional affected moribund fish specimens were placed on ice and sent directly to the LADL for necropsy and bacterial culture. Edwardsiella ictaluri was isolated from these specimens.
Bacterial Isolation and Identification
Following necropsy, pure cultures of a Gram-negative bacterium were isolated from the organs and tissues of most of the fish submitted to diagnostic laboratories. Cultures from the liver, kidney, spleen, and brain of moribund specimens on TSAB produced predominant, slow-growing, grey, nonhemolytic colonies after 48 h incubation at 28°C. Occasionally secondary bacteria such as Aeromonas hydrophila, Pseudomonas fluorescens, and Plesiomonas shigelloides were isolated but their occurrence was not consistent. When these organisms were present they would overgrow the E. ictaluri due to their faster growth rate and larger colonies. Edwardsiella ictaluri was determined to be the primary causative agent of the disease in the Zebrafish based on histopathology, consistency of isolation from multiple tissues and organs, and presumptive identification by biochemical tests and API 20E. The isolates were confirmed as E. ictaluri by conducting PCR, sequencing the 16S rRNA amplicons, and performing a search for matches in GenBank using the BLASTN program from the NCBI
The causative bacterium in all cases was shown to be a Gram-negative rod, 0.75 × 1.5 µm in size, oxidase negative, fermentative in glucose, and negative for bile esculin hydrolysis. The TSI reaction was K/A with no gas and no H2S produced. The isolates from Zebrafish differed from typical E. ictaluri isolates from catfish by being very weakly motile at 28°C, as determined in wet mount and GMD, and were positive for citrate in the API 20E system. Citrate was negative when tested in the tube test (Simmon’s citrate test). The resulting code in the API 20E was 4204000 which varies from the typical code of 4004000 of catfish isolates. In the API 50CH system Zebrafish and Channel Catfish isolates give identical results in producing acid from carbohydrates, glycerol, d-ribose, d-galactose, d-glucose, d-fructose, d-mannose, N-acetylglucosamine, d-maltose, and potassium gluconate. Zebrafish strains of E. ictaluri are differentiated from Channel Catfish strains by being positive for citrate utilization in the API 20E and 50CH, and having very weak motility and autoagglutination. Edwardsiella ictaluri is differentiated from E. tarda by failing to grow at 35°C and having weaker motility, weaker gas production in carbohydrate media, negative H2S production, negative indole reaction, slower growth rate, and smaller colony morphology.
Four additional cultures from Zebrafish isolated by Dr. Roy Yanong at the University of Florida, Tropical Aquaculture Laboratory, were submitted to the LADL and were identified as E. ictaluri. These isolates—11TAL132, 11TAL197, 11TAL193, and 11TAL232—were identical biochemically and gave the same API 20E code 4204000 as the other Zebrafish isolates. Sequencing of the 16S rRNA amplicons and analysis using the BLASTN program identified all isolates from Zebrafish to be E. ictaluri with 99% maximum identity using both forward and reverse primers. The representative bacterial isolates used in various testing procedures in this study are listed in Table 2.
TABLE 2.
Bacterial strains used for biochemical identification, molecular identification, plasmid profiles, auto-aggregation, LPS specificity with Mab, and antibiotic-susceptibility testing.
| Strain | Host | Source or location |
|---|---|---|
| ATCC 33202 | Channel Catfish | ATCC (Georgia) |
| LADL93-146 | Channel Catfish | Louisiana |
| LADL88-108 | Channel Catfish | Louisiana |
| LADL91-581 | Channel Catfish | Louisiana |
| S05-518 | Channel Catfish | Mississippi |
| S07-794 | Channel Catfish | Mississippi |
| LADL11-100 | Zebrafish | Louisiana (LSU) |
| LADL11-142 | Zebrafish | University of Pittsburg |
| 11TAL132(LADL11-149) | Zebrafish | Florida |
| 11TAL197(LADL11-184) | Zebrafish | Florida |
| 11TAL204(LADL11-193) | Zebrafish | Florida |
| LADL11-194 | Zebrafish | University of Massachusetts Amherst |
| 11TAL232(LADL11-203) | Zebrafish | Florida |
Histopathology
In general, edwardsiellosis in the Zebrafish from all four cases described in this paper presented as a severe, multifocally extensive to diffuse, systemic disease characterized by tissue necrosis and large numbers of bacteria often within macrophages (Figure 2). The kidneys (pronephros and mesonephros) and spleen were most commonly and severely affected. Within affected tissues, notably the kidney, spleen, and olfactory rosettes, there were florid inflammatory cell infiltrates composed of macrophages, neutrophils, eosinophilic granular cells, and fewer lymphocytes (Figure 3B). In specimens from multiple laboratories there was diffuse and severe inflammation and necrosis of the nasal pits. In the area of the nares, locally extensive sheets of necrotizing chronic inflammation extended through the olfactory rosettes, obliterating them. The inflammation continued along the olfactory nerve into the olfactory bulbs, telencephalic ventricle, and telencephalon (Figure 3A, C). Spleens were enlarged, and more than 95% of the parenchyma was necrotic with numerous aponecrotic macrophages and innumerable rod-shaped bacteria (1 × 5 µm) within and between cells (Figure 4A). In kidneys, areas of inflammation and necrosis were evident as discrete expansive foci, which with progression of severity would preferentially affect hematopoietic cords prior to nephron necrosis (Figure 4B). The liver often contained small foci of necrosis and inflammation, often comprising less than 5% of the organ (Figure 4C). The nares, brain, and endomeninges were often affected, in some cases with marked expansion of the mesencephalic ventricles by necrotic leukocytes and bacteria (Figure 4D). Other less common lesions included skeletal muscle necrosis with liquefaction and dermatitis with epidermal ulceration. Lesions were consistent with those described by Petrie-Hanson et al. (2007) in Zebrafish challenged by immersion with a catfish isolate of E. ictaluri. Infection of the olfactory sac, olfactory nerve, and olfactory lobe of the brain, leading to meningoencephalitis, has been described in the chronic form of the disease in Channel Catfish (Shotts et al. 1986; Newton et al. 1989; Morrison and Plumb 1994).
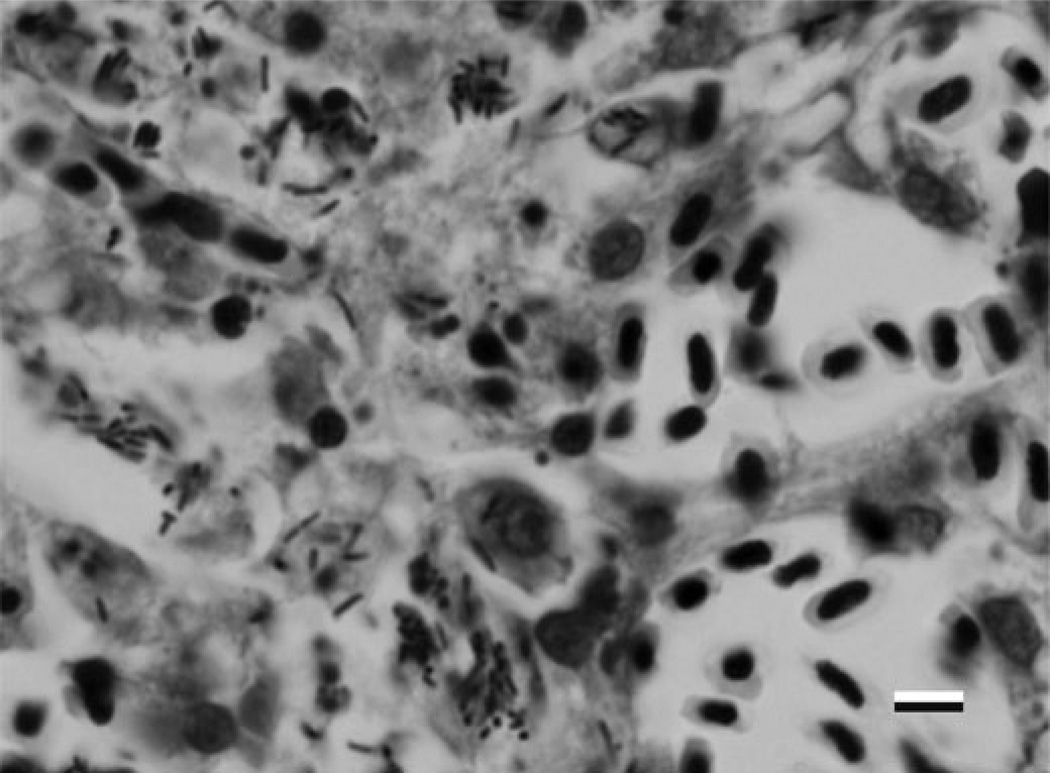
FIGURE 2.
Anterior kidney of Zebrafish with Edwardsiella ictaluri visible within macrophages (600×).
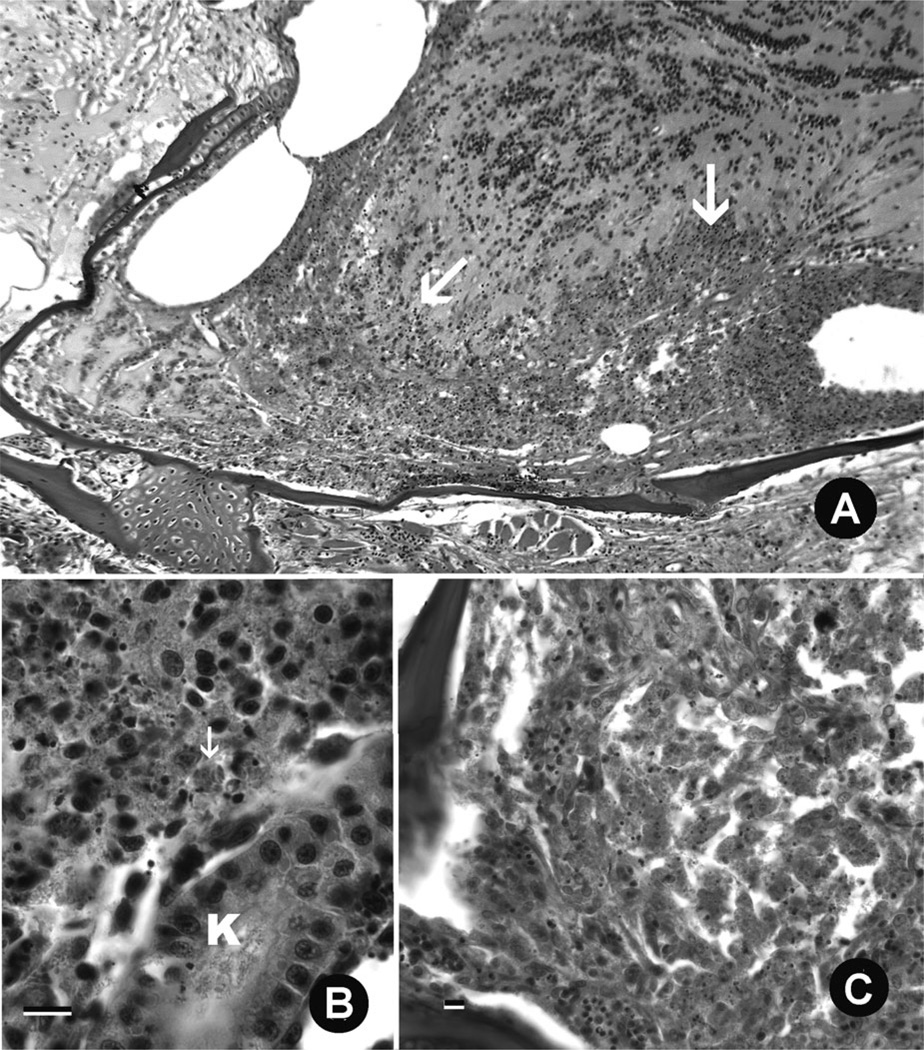
FIGURE 3.
Histological sections of Zebrafish infected with Edwardsiella ictaluri (hematoxylin and eosin stain). (A) Severe, chronic inflammation of the forebrain (arrow), extending into the olfactory bulb. (B). Severe, diffuse necrosis of kidney interstitium, with bacteria in phagocytes (arrow). K = kidney tubule. Bar = 10 µm. (C) Severe, diffuse, chronic inflammation and necrosis obliterating the olfactory rosettes within the nares. Bar = 10 µm.
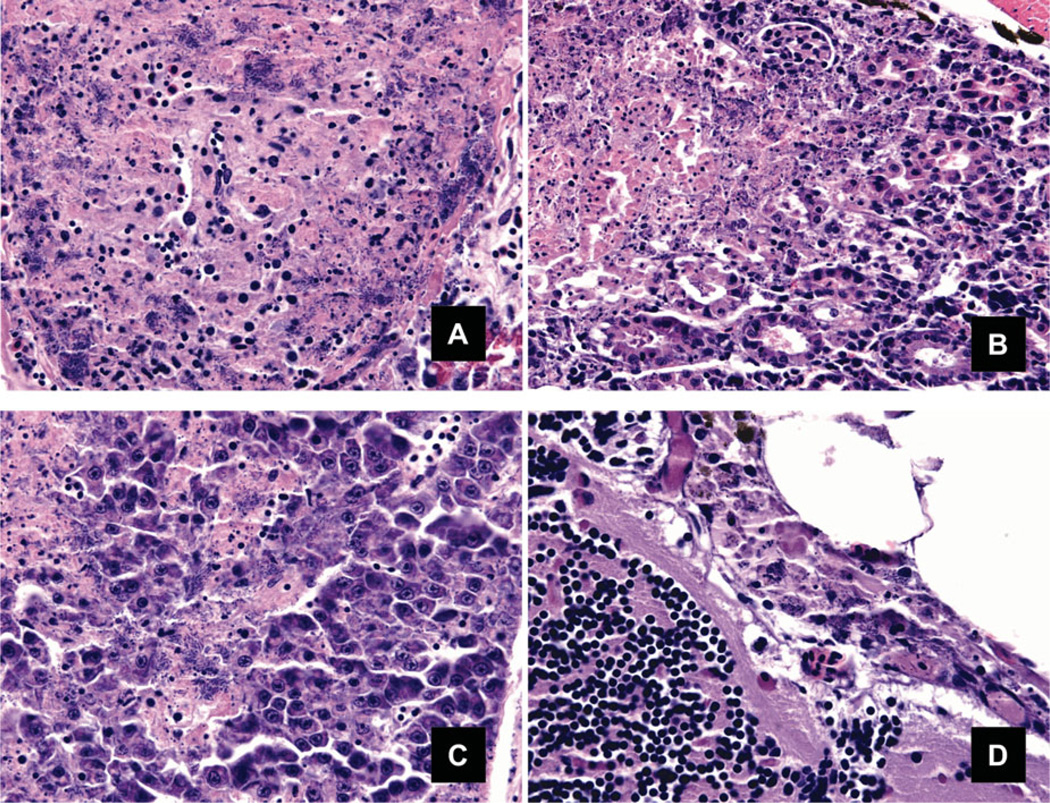
FIGURE 4.
(A) The spleen exhibits necrosis in more than 95% of the parenchyma with numerous necrotic macrophages and innumerable rod-shaped bacteria within and between cells. Pancreatic exocrine tissue is present at lower right. (B) Areas of inflammation and necrosis were evident in the kidney as discrete expansive foci with progression of severity preferentially affecting hematopoietic cords prior to nephron necrosis. In the left of the image many small shrunken pyknotic cells and karyorrhectic debris (necrosis) are evident admixed with many bacteria. (C) The liver contains several foci of necrosis with numerous bacteria. (D) The endomeninges are expanded two to three times their normal size by aponecrotic leukocytes and bacteria. The brain is at lower left of the image, pericephalic adipose at upper right (400×).
Auto-aggregation
Auto-aggregation occurred within 1 h with strains from Zebrafish: LADL11-100, LADL11-142, 11TAL197, 11TAL204, LADL11-194, and 11TAL232, but not with strains from Channel Catfish: ATCC 33202, LADL88-108, LADL91-581, S07-794, and S05-518.
Plasmid Profiles
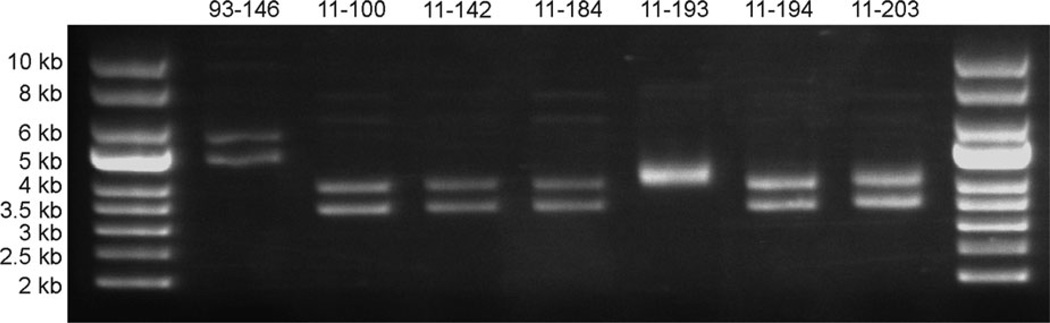
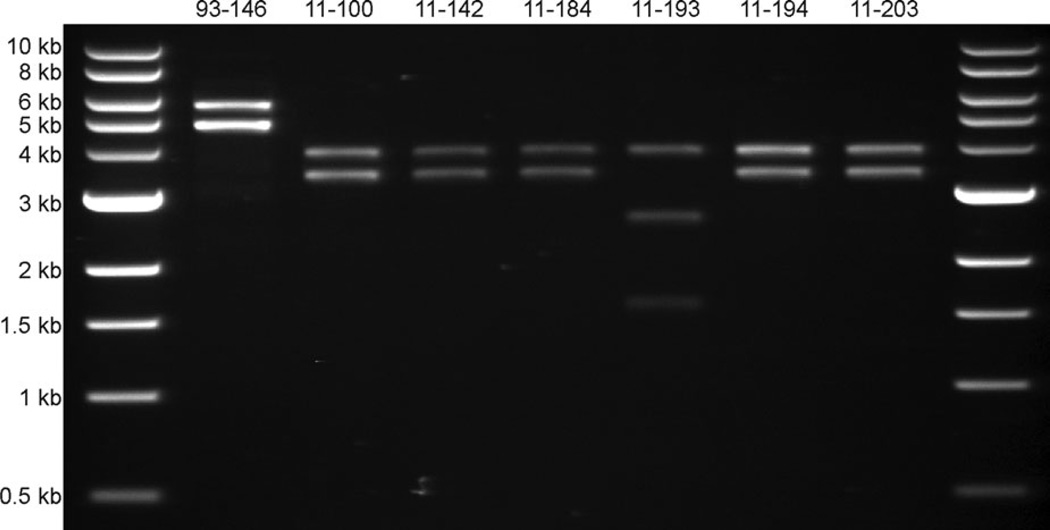
The Zebrafish isolates of E. ictaluri present a different plasmid profile than is commonly seen in E. ictaluri isolated from Channel Catfish (Figure 5) (Lobb and Rhoades 1987; Speyerer and Boyle 1987; Newton et al. 1988; Fernandez et al. 2001). Channel Catfish isolates typically carry two plasmids, pEI1 and pEI2, that are approximately 4.8 and 5.6 kb, respectively (Fernandez et al. 2001). Six of the Zebrafish isolates carry two plasmids of approximately 3.5 and 4 kb, while the seventh isolate, LADL11-193 (11TAL204), carries two plasmids that are both about 4–4.5 kb and appear as a single band when supercoiled. Restriction enzyme digestion with EcoRI linearizes pEI1 and pEI2 (Figure 6), but only cuts the 4-kb band of the Zebrafish isolates (data not shown). Digestion with BstZ17I, however, linearizes both of the plasmids from Zebrafish isolates, but only cuts pEI1 of Channel Catfish E. ictaluri (data not shown). The BstZ17I-digested plasmids are approximately 3.5 and 4 kb for the six isolates, matching the size observed in supercoiled samples. Digestion of the LADL11-193 plasmids with BstZ17I linearizes a 4-kb plasmid that is possibly similar to that size of plasmid from the other Zebrafish isolates. The second plasmid of LADL11-193 cuts at least twice by BstZ17I, resulting in two visible fragments at approximately 1.7 and 2.8 kb. It is suspected that the 4-kb plasmids carried by the Zebrafish isolates are genetically related to pEI1 of Channel Catfish E. ictaluri based on previous hybridization data between 4- and 4.8-kb plasmids (Reid and Boyle 1989). It is unknown if the 3.5- or 4.5-kb plasmids carried by Zebrafish isolates contain similar DNA sequences as those carried by Channel Catfish isolates. Further work is required to sequence plasmids carried by the Zebrafish E. ictaluri isolates.
FIGURE 5.
Supercoiled plasmid profiles of Edwardsiella ictaluri isolates. Plasmid DNA was harvested from bacterial cultures and separated by 0.6% agarose gel electrophoresis using supercoiled DNA ladder as the size standard. Lane 1: supercoiled DNA ladder; Lane 2: LADL93-146 (Channel Catfish); Lane 3: LADL11-100 (Zebrafish, Louisiana [LSU]); Lane 4: LADL11-142 (Zebrafish, Pennsylvania); Lane 5: LADL11-184 (Zebrafish, Florida); Lane 6: LADL11-193 (Zebrafish, Florida); Lane 7: LADL11-194 (Zebrafish, Massachusetts); Lane 8: LADL11-203 (Zebrafish, Florida); Lane 9: supercoiled DNA ladder.
FIGURE 6.
Linearized plasmid profiles of Edwardsiella ictaluri isolates. Plasmid DNA from E. ictaluri isolated from Channel Catfish or Zebrafish was digested with EcoRI or BstZ17I, respectively, and separated by 0.6% agarose gel electrophoresis using a 1-kb DNA ladder as the size standard. Lane 1: 1-kb DNA ladder; Lane 2: LADL93-146 (Channel Catfish); Lane 3: LADL11-100 (Zebrafish, Louisiana [LSU]); Lane 4: LADL11-142 (Zebrafish, Pennsylvania); Lane 5: LADL11-184 (Zebrafish, Florida); Lane 6: LADL11-193 (Zebrafish, Florida); Lane 7: LADL11-194 (Zebrafish, Massachusetts); Lane 8: LADL11-203 (Zebrafish, Florida); Lane 9: 1-kb DNA ladder.
LPS recognition with MabEd9
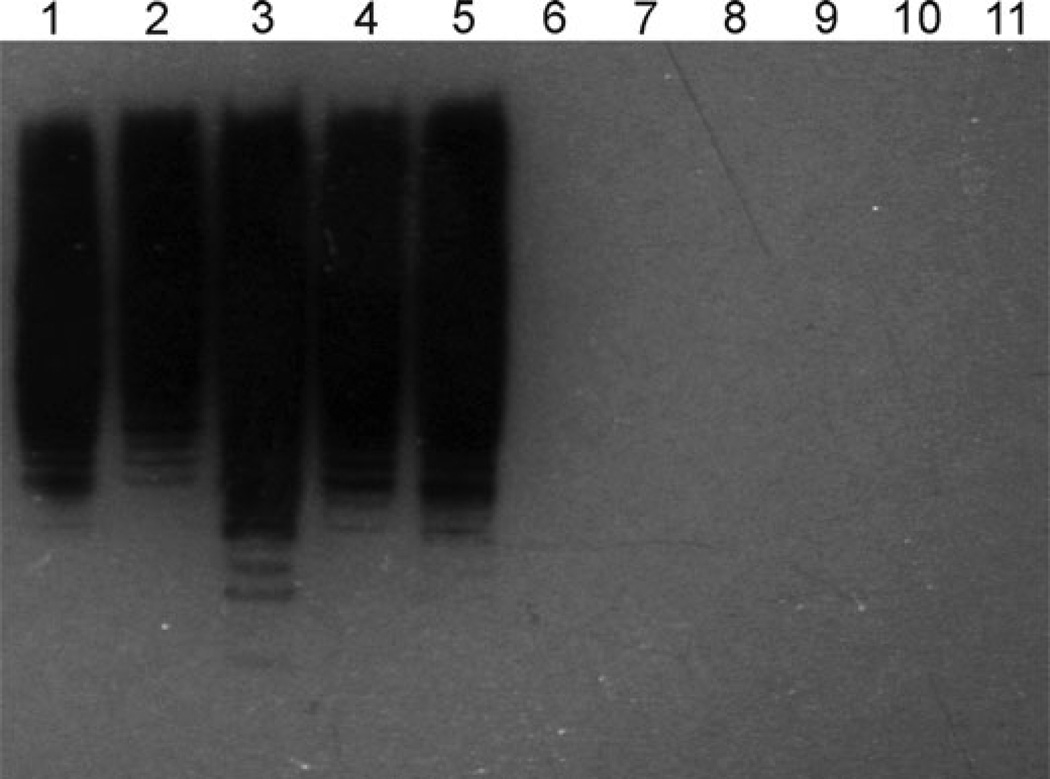
The monoclonal antibody Ed9 raised against the ATCC 33202 strain of E. ictaluri from Channel Catfish (Ainsworth et al. 1986) is believed to be specific for E. ictaluri LPS (Lawrence et al. 2001), but failed to react with the LPS of the Zebrafish isolates (Figure 7). This demonstrates a difference in the structure of LPS between the Channel Catfish and Zebrafish isolates. The importance of this difference, however, is unknown due to the single epitope recognition of the monoclonal antibody. Early serological studies found little heterogeneity between E. ictaluri isolates (Plumb and Klesius 1988; Bertolini et al. 1990). However, Lobb et al. (1993) found that E. ictaluri isolated from Channel Catfish was serologically different from the strain isolated from the Green Knifefish, indicating serological heterogeneity may exist among E. ictaluri isolates from different species. Further analysis of the LPS moieties is required to determine the differences between the LPS of Zebrafish and Channel Catfish E. ictaluri isolates and whether these differences influence relative virulence.
FIGURE 7.
Heterogeneity of LPS from Channel Catfish and Zebrafish Edwardsiella ictaluri isolates. Bacterial whole-cell lysates were probed with the Ed9 mouse monoclonal antibody raised against the LPS of the ATCC 33202 E. ictaluri isolate from Channel Catfish. Goat anti-mouse conjugated to horseradish peroxidase was used to label Ed9 bound to LPS on the membrane, followed by chemiluminescent detection. Channel Catfish isolates: Lane 1: LADL93-146; Lane 2: LADL88-108; Lane 3: LADL91-581; Lane 4: S05-518; Lane 5: S07-794. Zebrafish isolates: Lane 6: LADL11-100; Lane 7: LADL11-142; Lane 8: LADL11-184; Lane 9: LADL11-193; Lane 10: LADL11-194; Lane 11: LADL11-203.
Antibiotic Susceptibility
Antibiotic susceptibility as disk diffusion zones are listed in Table 3. The Sensititre Avian 1F plates were found to be useful in determining the MIC for tetracycline and oxytetracycline for Zebrafish E. ictaluri strains, but failed to show endpoints for florfenicol (< 1.0 µg), enrofloxacin (<0.12 µg), and Romet as measured by SXT (<0.5/9.5 µg). In general, from these results the Zebrafish isolates were considered susceptible to antibiotics Romet, oxytetracycline, florfenicol, and enrofloxacin based on data available for human and veterinary bacterial isolates; however, clinical breakpoints for these drugs have not been determined for Zebrafish.
TABLE 3.
Antibiotic susceptibility of Zebrafish isolates of Edwardsiella ictaluri by disk diffusion. Values in table are diffusion zone diameters in millimeters.
| Antibiotic | ||||
|---|---|---|---|---|
| Strain | Oxytetracycline T30 | Romet SOR25 | Florfenicol FFC30 | Enrofloxacin ENO5 |
| LADL11-100 | 36 | 26 | 40 | 34 |
| LADL11-104 | 36 | 38 | 42 | 34 |
| LADL11-142 | 30 | 30 | 36 | 34 |
| LADL11-194 | 34 | 32 | 38 | 38 |
| 11TAL132 | 34 | 36 | 40 | 40 |
| 11TAL197 | 36 | 30 | 40 | 36 |
| 11TAL204 | 36 | 32 | 40 | 40 |
| 11TAL232 | 30 | 28 | 40 | 34 |
Fulfillment of Koch’s Postulates
Exposure to the challenge dose of 107 CFU/mL by immersion for 30 min resulted in 100% mortality of Zebrafish at 10 d postexposure. Clinical signs and pathology were consistent with fish in naturally occurring infections.
DISCUSSION
Edwardsiella ictaluri is known as the causative agent of an economically important bacterial disease of farm-raised catfish in the USA and abroad; however, the bacterium has only rarely been reported from hosts other than catfish (Kent and Lyons 1982; Waltman et al. 1985; Humphrey et al. 1986; Soto et al. 2012). In this report we described the first cases of E. ictaluri causing naturally occurring epizootics in laboratory populations of Zebrafish. We also proposed the utility of quarantine in preventing the introduction and spread of this contagious pathogen into a laboratory with valuable Zebrafish colonies. Three different populations of Zebrafish, obtained from commercial sources and held in quarantine at three different research laboratories in three different states, experienced acute disease resulting from E. ictaluri infection, and a fourth case was presumptively identified by similar histopathological lesions. In most cases the decision was made to euthanize the remaining fish in the quarantined population. Euthanasia was chosen over antibiotic therapy because, although the bacteria were considered to be sensitive to several antibiotics, it was uncertain whether antibiotic therapy would eradicate the pathogen, and the risk of spread to other Zebrafish colonies in the laboratory was too great. Two laboratories did choose to use medicated feeds for short-term control of the infection until experiments could be completed and the remaining fish euthanized. Florfenicol was used as a medicated feed at the University of Pittsburg and enrofloxacin was used as a medicated feed at the University of Massachusetts at Amherst and, in each case, mortality was curtailed. The carrier status of survivors was not assessed. In many research laboratories, the high value of the fish in the colonies makes it advantageous to develop management strategies that will make it possible to control or eradicate the pathogen.
After the diagnosis of E. ictaluri infection in the LSU groups, which resulted in isolation of two strains (LADL11-100 and LADL11-104), an additional sample of diseased fish (University of Massachusetts) was submitted for necropsy and resulted in isolation of a third strain, LADL11-194. Five additional cultures from Zebrafish in two other states, Pennsylvania and Florida, were submitted to the LADL for identification. In total, eight isolates from Zebrafish were identified as E. ictaluri by biochemical testing, API 20E and API 50 CH, and 16S rRNA sequencing. All Zebrafish E. ictaluri isolates were identical in biochemical and molecular tests for identification by partial amplification and sequencing of the 16S rRNA gene using specific primers. Edwardsiella ictaluri isolates from Zebrafish were differentiated from Channel Catfish isolates by exhibiting weaker motility and a different plasmid profile (two plasmids of 4.0 and 3.5 kb) with the exception of one isolate, 11TAL204, which had two plasmids of approximately 4 kb. The Zebrafish isolates also exhibited failure to react with Mab Ed9 in Western blot analysis, possibly indicating a different LPS O side-chain and a different serotype. Zebrafish isolates of E. ictaluri consistently produced a different API 20E code (4204000) from Channel Catfish isolates, were nonmotile to weakly motile in GMD, and auto-aggregated in BHI broth. All Zebrafish isolates were considered to be susceptible to the antibiotics Romet, oxytetracycline, florfenicol, and enrofloxacin, although clinical breakpoints for these drugs have not been determined for Zebrafish.
Fish from all cases showed similar histological changes, characterized by numerous rod-shaped bacterial cells in macrophages and chronic necrotizing inflammation in various visceral organs. This was consistent with the findings of Petrie-Hanson et al. (2007) in experimentally infected Zebrafish. The occurrence of pathologic changes in the nares and forebrain was very characteristic of E. ictaluri infections in other species (Shotts et al. 1986).
A fourth Zebrafish research laboratory, with a history of morbidity and mortality in their fish, submitted fish to the ZIRC diagnostic service in September 2011. The facility operates a recirculating water system with UV sterilizers. Fish were purchased from a pet store and were moved into the system. Beginning in April 2011, the facility experienced 4 months of recurrent outbreaks of morbidity and mortality in the Zebrafish population. Signs of disease included color change, hemorrhage or redness, hydrocoelom, protruding scales, and skin ulcers. Morbidity and mortality recurred when new fish were added to the system. The facility had counted over 125 disease-associated mortalities when specimens were sent to the ZIRC. Five adult Zebrafish were fixed in preservative and submitted to the ZIRC for histopathology. The type of fixative used and age and genetic strain of the fish were not specified. Histopathology was consistent with E. ictaluri infections in other Zebrafish cases. Hence, we believe that fish from the fourth laboratory, in which bacterial culture was not conducted, were also infected with E. ictaluri. Because Zebrafish in this case were obtained from a pet store we reemphasize the need for routine quarantine procedures and screening of new fish for E. ictaluri.
Edwardsiella ictaluri is known to be an obligate pathogen that does not persist in the environment for very long and must be transmitted from fish to fish by close contact, via the water, by fecal shedding, or orally. We believe it is important for Zebrafish-rearing facilities and laboratories to be aware of this emerging disease problem and take proper precautionary measures to prevent contamination of valuable genetic stocks of this important laboratory fish.
ACKNOWLEDGMENTS
We thank Jerold Ainsworth of the College of Veterinary Medicine, Mississippi State University, for donating the Ed9 monoclonal antibodies used in the study, Patricia Gaunt of the College of Veterinary Medicine, Mississippi State University, for providing Romet antibiotic susceptibility disks, Roy Yanong of the University of Florida, Tropical Aquaculture Laboratory, Ruskin, Florida, for supplying additional Zebrafish isolates of E. ictaluri, and Judy Bennet of the University of Massachusetts at Amherst for additional Zebrafish specimens.
Footnotes
Publisher's Disclaimer: Taylor & Francis makes every effort to ensure the accuracy of all the information (the “Content”) contained in the publications on our platform. However, Taylor & Francis, our agents, and our licensors make no representations or warranties whatsoever as to the accuracy, completeness, or suitability for any purpose of the Content. Any opinions and views expressed in this publication are the opinions and views of the authors, and are not the views of or endorsed by Taylor & Francis. The accuracy of the Content should not be relied upon and should be independently verified with primary sources of information. Taylor and Francis shall not be liable for any losses, actions, claims, proceedings, demands, costs, expenses, damages, and other liabilities whatsoever or howsoever caused arising directly or indirectly in connection with, in relation to or arising out of the use of the Content.
This article may be used for research, teaching, and private study purposes. Any substantial or systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any form to anyone is expressly forbidden. Terms & Conditions of access and use can be found at http://www.tandfonline.com/page/terms-and-conditions
Contributor Information
John P. Hawke, Department of Pathobiological Sciences, School of Veterinary Medicine, Louisiana State University, 1909 Skip Bertman Drive, Baton Rouge, Louisiana 70803, USA.
Michael Kent, Department of Microbiology and Biomedical Sciences, College of Veterinary Medicine, Oregon State University, 202 Nash Hall, Corvallis, Oregon 97331, USA.
Matt Rogge, Department of Biology, University of Wisconsin at Stevens Point, 800 Reserve Street, Stevens Point, Wisconsin 54481, USA.
Wes Baumgartner, Department of Pathobiology and Population Medicine, College of Veterinary Medicine, Mississippi State University, 240 Wise Center Drive, Mississippi State, Mississippi 39762, USA.
Judy Wiles, Department of Pathobiological Sciences, School of Veterinary Medicine, Louisiana State University, 1909 Skip Bertman Drive, Baton Rouge, Louisiana 70803, USA.
Johnny Shelley, Department of Pathobiological Sciences, School of Veterinary Medicine, Louisiana State University, 1909 Skip Bertman Drive, Baton Rouge, Louisiana 70803, USA.
L. Christine Savolainen, Department of Biological Sciences, Louisiana State University, 103 Life Sciences, South Campus Drive, Baton Rouge, Louisiana 70803, USA.
Robert Wagner, Division of Laboratory Animal Resources, University of Pittsburg, 4200 Fifth Avenue, Pittsburg, Pennsylvania 15261, USA.
Katy Murray, Zebrafish International Resource Center, University of Oregon, 1100 Johnson Lane, Eugene, Oregon 97403, USA.
Tracy S. Peterson, Department of Microbiology and Biomedical Sciences, College of Veterinary Medicine, Oregon State University, 202 Nash Hall, Corvallis, Oregon 97331, USA.
REFERENCES
- Ainsworth AJ, Capley G, Waterstreet P, Munson D. Use of monoclonal antibodies in the indirect fluorescent antibody technique (IFA) for the diagnosis of Edwardsiella ictaluri. Journal of Fish Diseases. 1986;9:439–444. [Google Scholar]
- Ambrose HE, Clewley JP. Virus discovery by sequence-independent genome amplification. Reviews in Medical Virology. 2006;16:365–383. doi: 10.1002/rmv.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader JA, Shoemaker CA, Klesius PH, Connolly MA, Barbaree JM. Genomic subtyping of Edwardsiella ictaluri isolated from diseased Channel Catfish by arbitrarily primed polymerase chain reaction. Journal of Aquatic Animal Health. 1998;10:22–27. [Google Scholar]
- Baxa DV, Groff JM, Wishkovsky A, Hedrick RP. Susceptibility of nonictalurid fishes to experimental infection with Edwardsiella ictaluri. Diseases of Aquatic Organisms. 1990;8:113–117. [Google Scholar]
- Bertolini JM, Cipriano RC, Pyle SW, McLaughlin JA. Serological investigation of the fish pathogen Edwardsiella ictaluri, cause of enteric septicemia of catfish. Journal of Wildlife Diseases. 1990;26:246–252. doi: 10.7589/0090-3558-26.2.246. [DOI] [PubMed] [Google Scholar]
- Bilodeau AL, Waldbieser GC, Terhune JS, Wise DJ, Wolters WR. A real-time polymerase chain reaction assay of the bacterium Edwardsiella ictaluri in Channel Catfish. Journal of Aquatic Animal Health. 2003;15:80–86. [Google Scholar]
- Chen MF, Light TS. Specificity of the Channel Catfish antibody to Edwardsiella ictaluri. Journal of Aquatic Animal Health. 1994;6:266–270. [Google Scholar]
- CLSI (Clinical and Laboratory Standards Institute) Methods for antimicrobial disk susceptibility testing of bacteria isolated from aquatic animals; approved guideline. Wayne, Pennsylvania: CLSI, Document M42-A; 2006a. [Google Scholar]
- CLSI (Clinical and Laboratory Standards Institute) Methods for broth dilution susceptibility testing of bacteria isolated from aquatic animals; approved guideline. Wayne, Pennsylvania: CLSI, Document M49-A; 2006b. [Google Scholar]
- Crumlish M, Dung TT, Turnbull JF, Ngoc NTN, Ferguson HW. Identification of Edwardsiella ictaluri from diseased freshwater catfish, Pangasius hypophthalmus (Sauvage), cultured in the Mekong Delta, Vietnam. Journal of Fish Diseases. 2002;25:733–736. [Google Scholar]
- Fernandez DH, Pittmann-Cooley L, Thune RL. Sequencing and analysis of the Edwardsiella ictaluri plasmids. Plasmid. 2001;45:52–56. doi: 10.1006/plas.2000.1499. [DOI] [PubMed] [Google Scholar]
- Gaunt PS, Endris RG, Khoo L, Howard R, McGinnis AL, Santucci TD, Katz T. Determination of dose rate of florfenicol in feed for control of mortality in Channel Catfish Ictalurus punctatus (Rafinesque) infected with Edwardsiella ictaluri, etiological agent of enteric septicemia. Journal of the World Aquaculture Society. 2004;35:257–267. [Google Scholar]
- Griffin MJ, Mauel MJ, Greenway TE, Khoo LH, Wise DJ. A real-time polymerase chain reaction assay for quantification of Edwardsiella ictaluri in catfish pond water and genetic homogeneity of diagnostic case isolates from Mississippi. Journal of Aquatic Animal Health. 2011;23:178–188. doi: 10.1080/08997659.2011.637006. [DOI] [PubMed] [Google Scholar]
- Harper C, Lawrence C. The laboratory Zebrafish. Boca Raton, Florida: CRC Press; 2011. [Google Scholar]
- Hawke JP. A bacterium associated with disease of pond cultured Channel Catfish, Ictalurus punctatus. Journal of the Fisheries Research Board of Canada. 1979;36:1508–1512. [Google Scholar]
- Hawke JP, Khoo LH. Infectious diseases. In: Tucker CS, Hargreaves JA, editors. Biology and culture of Channel Catfish. Amsterdam: Elsevier; 2004. pp. 387–443. [Google Scholar]
- Hawke JP, McWhorter AC, Steigerwalt AG, Brenner DJ. Edwardsiella ictaluri sp. nov., the causative agent of enteric septicemia of catfish. International Journal of Systematic Bacteriology. 1981;31:396–400. [Google Scholar]
- Humphrey JD, Lancaster C, Gudkovs N, McDonald W. Exotic bacterial pathogens Edwardsiella tarda and Edwardsiella ictaluri from imported ornamental fish Betta splendens and Puntius conchonius, respectively: isolation and quarantine significance. Australian Veterinary Journal. 1986;63:369–371. doi: 10.1111/j.1751-0813.1986.tb02900.x. [DOI] [PubMed] [Google Scholar]
- Janda JM, Abbott SL. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. Journal of Clinical Microbiology. 2007;45:2761–2764. doi: 10.1128/JCM.01228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasornchandra J, Rogers WA, Plumb JA. Edwardsiella ictaluri from Walking Catfish, Clarias batrachus L., in Thailand. Journal of Fish Diseases. 1987;10:137–138. [Google Scholar]
- Kent ML, Feist SW, Harper C, Hoogstraten-Miller S, Law JM, Sanchez-Morgado JM, Tanguay RL, Sanders GE, Spitsbergen JM, Whipps CM. Recommendations for control of pathogens and infectious diseases in fish research facilities. Comparative Biochemistry and Physiology C (Toxicology and Pharmacology) 2009;149:240–248. doi: 10.1016/j.cbpc.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent ML, Lyons JM. Edwardsiella ictaluri in the Green Knifefish, Eigemannia virescens. Fish Health News. 1982;11:ii. [Google Scholar]
- Klesius P, Lovy J, Evans J, Washuta E, Arias C. Isolation of Edwardsiella ictaluri from Tadpole Madtom in a southwestern New Jersey river. Journal of Aquatic Animal Health. 2003;15:295–301. [Google Scholar]
- Lawrence ML, Banes MM, Williams ML. Phenotype and virulence of a transposon-derived lipopolysaccharide O side-chain mutant strain of Edwardsiella ictaluri. Journal of Aquatic Animal Health. 2001;13:291–299. [Google Scholar]
- Liu JY, Li AH, Zhou DR, Wen ZR, Ye XP. Isolation and characterization of Edwardsiella ictaluri strains as pathogens from diseased Yellow Catfish Pelteobagrus fulvidraco (Richardson) cultured in China. Aquaculture Research. 2010;41:1835–1844. [Google Scholar]
- Lobb CJ, Ghaffari SH, Hayman JR, Thompson DT. Plasmid and serological differences between Edwardsiella ictaluri strains. Applied and Environmental Microbiology. 1993;59:2830–2836. doi: 10.1128/aem.59.9.2830-2836.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb CJ, Rhoades M. Rapid plasmid analysis for identification of Edwardsiella ictaluri from infected Channel Catfish (Ictalurus punctatus) Applied and Environmental Microbiology. 1987;53:1267–1272. doi: 10.1128/aem.53.6.1267-1272.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorian V, editor. Antibiotics in laboratory medicine. 2nd edition. Baltimore, Maryland: Williams and Wilkins; 1986. [Google Scholar]
- Luna LG. Manual of histologic staining methods of the Armed Forces Institute of Pathology. New York: McGraw-Hill; 1968. [Google Scholar]
- Morrison EE, Plumb JA. Olfactory organ of Channel Catfish as a site of experimental Edwardsiella ictaluri infection. Journal of Aquatic Animal Health. 1994;6:101–109. [Google Scholar]
- Murray KN, Bauer J, Tallen A, Matthews JL, Westerfield M, Varga ZM. Characterization and management of asymptomatic Mycobacterium infections at the Zebrafish International Resource Center. Journal of the Association for Laboratory Animal Science. 2011;50:675–679. [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Iwamoto E, Sakai T, Arima T, Tensha K, Iida Y, Iida T, Nakai T. Characterization of Edwardsiella ictaluri isolated from wild Ay u Plecoglossus altivelis in Japan. Fish Pathology. 2008;43:158–163. [Google Scholar]
- Newton JC, Bird RC, Blevins WT, Wilt GR, Wolfe LG. Isolation, characterization, and molecular cloning of cryptic plasmids isolated from Edwardsiella ictaluri. American Journal of Veterinary Research. 1988;49:1856–1860. [PubMed] [Google Scholar]
- Newton JC, Wolfe LG, Grizzle JM, Plumb JA. Pathology of experimental enteric septicaemia in Channel Catfish, Ictalurus punctatus (Rafinesque), following immersion-exposure to Edwardsiella ictaluri. Journal of Fish Diseases. 1989;12:335–347. [Google Scholar]
- Petrie-Hanson L, Romano CL, Mackey RB, Khosravi P, Hohn CM, Boyle CR. Evaluation of Zebrafish Danio rerio as a model for enteric septicemia of catfish (ESC) Journal of Aquatic Animal Health. 2007;19:151–158. doi: 10.1577/H06-026.1. [DOI] [PubMed] [Google Scholar]
- Plumb JA, Klesius P. An assessment of the antigenic homogeneity of Edwardsiella ictaluri using monoclonal antibody. Journal of Fish Diseases. 1988;11:499–509. [Google Scholar]
- Plumb JA, Sanchez DJ. Susceptibility of five species of fish to Edwardsiella ictaluri. Journal of Fish Diseases. 1983;6:261–266. [Google Scholar]
- Plumb JA, Vinitnantharat S. Biochemical, biophysical, and serological homogeneity of Edwardsiella ictaluri. Journal of Aquatic Animal Health. 1989;1:51–56. [Google Scholar]
- Reid WS, Boyle JA. Plasmid homologies in Edwardsiella ictaluri. Applied and Environmental Microbiology. 1989;55:3253–3255. doi: 10.1128/aem.55.12.3253-3255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendueles O, Ferriéres L, Frétaud M, Bégaud E, Herbomel P, Levraud JP, Ghigo JM. A new Zebrafish model of oro-intestinal pathogen colonization reveals a key role for adhesion in protection byprobiotic bacteria. PLoS (Public Library of Science) Pathogens [online serial] 2012;8(7):e1002815. doi: 10.1371/journal.ppat.1002815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotts EB, Blazer VS, Waltman WD. Pathogenesis of experimental Edwardsiella ictaluri infections in Channel Catfish (Ictalurus punctatus) Canadian Journal of Fisheries and Aquatic Sciences. 1986;43:36–42. [Google Scholar]
- Soto E, Griffin M, Arauz M, Riofrio A, Martinez A, Cabrejos ME. Edwardsiella ictaluri as the causative agent of mortality in cultured Nile Tilapia. Journal of Aquatic Animal Health. 2012;24:81–90. doi: 10.1080/08997659.2012.675931. [DOI] [PubMed] [Google Scholar]
- Speyerer PD, Boyle JA. The plasmid profile of Edwardsiella ictaluri. Journal of Fish Diseases. 1987;10:461–469. [Google Scholar]
- Starliper CE, Schill WB, Shotts EB, Waltman WD. Isozyme analysis of Edwardsiella ictaluri. Microbios Letters. 1988;37:81–87. [Google Scholar]
- USDA (U.S. Department of Agriculture) USDA, Animal and Plant Health Inspection Service, Veterinary Services, National Animal Health Monitoring System, Report N406.1103. Fort Collins, Colorado: 2003a. Catfish 2003—part I: reference of fingerling catfish health and production practices in the United States, 2003. Available: www.aphis.usda.gov/animal_health/nahms/aquaculture/downloads/catfish03/Cat03_dr_PartI.pdf (November 2012). [Google Scholar]
- USDA (U.S. Department of Agriculture) USDA, Animal and Plant Health Inspection Service, Veterinary Services, National Animal Health Monitoring System, Report N407.1103. Fort Collins, Colorado: 2003b. Catfish 2003—part II: reference of foodsize catfish health and production practices in the United States, 2003. Available: www.aphis.usda.gov/animal_health/nahms/aquaculture/downloads/catfish03/Cat03_dr_PartII.pdf (November 2012). [Google Scholar]
- Waltman WD, Shotts EB, Blazer VS. Recovery of Edwardsiella ictaluri from danio (Danio devario) Aquaculture. 1985;46:63–66. [Google Scholar]
- Watral V, Kent ML. Pathogenesis of Mycobacterium spp. in Zebrafish (Danio rerio) from research facilities. Comparative Biochemistry and Physiology C (Toxicology and Pharmacology) 2007;145:55–60. doi: 10.1016/j.cbpc.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish book: a guide for the laboratory use of Zebrafish (Brachydanio rerio) 5th edition. Eugene: University of Oregon Press; 2007. [Google Scholar]
- Whipps CM, Dougan ST, Kent ML. Mycobacterium haemophilum infections of Zebrafish (Danio rerio) in research facilities. FEMS Microbiology Letters. 2007;270:21–26. doi: 10.1111/j.1574-6968.2007.00671.x. [DOI] [PubMed] [Google Scholar]
- Whipps CM, Matthews JL, Kent ML. Distribution and genetic characterization of Mycobacterium chelonae in laboratory Zebrafish Danio rerio. Diseases of Aquatic Organisms. 2008;82:45–54. doi: 10.3354/dao01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters WR, Johnson MR. Enteric septicemia resistance in Blue Catfish and three Channel Catfish strains. Journal of Aquatic Animal Health. 1994;6:329–334. [Google Scholar]