Abstract
Two decades ago, lymphatic mapping of sentinel lymph nodes (SLN) was introduced into surgical cancer management and was termed sentinel node navigated surgery. Although this technique is now routinely performed in the management of breast cancer and malignant melanoma, it is still under investigation for use in other cancers. The radioisotope technetium (99mTc) and vital blue dyes are among the most widely used enhancers for SLN mapping, although near-infrared fluorescence imaging of indocyanine green is also becoming more commonly used. 99mTc-tilmanocept is a new synthetic radioisotope with a relatively small molecular size that was specifically developed for lymphatic mapping. Because of its small size, 99mTc-tilmanocept quickly migrates from its site of injection and rapidly accumulates in the SLN. The mannose moieties of 99mTc-tilmanosept facilitate its binding to mannose receptors (CD206) expressed in reticuloendothelial cells of the SLN. This binding prevents transit to second-echelon lymph nodes. In Phase III trials of breast cancer and malignant melanoma, and Phase II trials of other malignancies, 99mTc-tilmanocept had superior identification rates and sensitivity compared with blue dye. Trials comparing 99mTc-tilmanocept with other 99mTc-based agents are required before it can be routinely used in clinical settings.
Keywords: lymphatic mapping, sentinel lymph node, new tracer
The role of lymph node mapping in cancer management
The sentinel node concept is based on the orderly spreading of tumor cells from a primary tumor to a defined lymph node in the relevant nodal basin. Therefore, this concept only applies to tumors in which these cells are spread via the lymphatic system. Lymphatic mapping has two major objectives: to reduce morbidity associated with lymph node assessment and to improve the accuracy of nodal assessment.1
Lymph node mapping of the sentinel lymph node (SLN) by radiologic lymphography was first introduced for penile carcinoma by Cabanas in 1977.2 It was not until the early 1990s that this concept was applied for malignant melanoma. In 1992, Morton et al described the use of lymphatic mapping with a vital dye for early stage melanoma in 194 patients.3 One year later, Krag et al reported for the first time the feasibility of radioguided lymphatic mapping with 99mtechnetium (99mTc)-sulfur colloid in 22 breast cancer patients.4 In 1994, Giuliano et al reported blue dye-based mapping for SLN biopsy (SLNB) in a cohort of 174 patients with early breast cancer.5
Many clinical investigators worldwide have evaluated this new concept in patients with breast cancer, in single-center and multicenter studies.6–11 In these studies, the identification rate ranged from 85% to 97% while sensitivity ranged from 90% to 100% when SLNB was followed by axillary lymph node dissection (ALND). Sophisticated pathologic examination of the SLN has also improved the staging of axillary nodal disease.12
Ultrastaging enables pathologists to identify much smaller metastatic deposits in lymph nodes with improved sensitivity. Isolated tumor cells and micrometastases are now coded according to the American Joint Committee on Cancer staging systems as substages of N0 according to the tumor-node-metastasis staging for breast and colon cancer. Ultrastaging uses combinations of three complementary techniques: serial sectioning; immunohistochemistry; and reverse-transcriptase polymerase chain reaction.13
The sentinel technique was established shortly thereafter and SLNB has become the gold standard for axillary staging of early breast cancer (unifocal, T1–T2, clinically node-negative).14,15 Several recent large-scale multicenter trials have confirmed that SLNB is equivalent to ALND in terms of correct staging but is associated with less-extensive morbidity than ALND.16–18
Some clinicians have extended the use of SLNB to a variety of specific situations. Lymphatic mapping is also safe in patients with multicentric disease when administering the agent via periareolar injection.19 Combining SLNB with preoperative chemotherapy has also been a focus of intensive research. Although SLNB can be safely performed before preoperative chemotherapy, it requires an additional operation and patients with initial node-positive disease cannot benefit from downstaging by preoperative chemotherapy.20–22 However, SLNB performed after preoperative chemotherapy is associated with higher false-negative rates, especially in patients with originally node-positive cancer.23–25
In 40% of cases, the SLN is the only involved axillary node.26 Therefore, it is unclear whether ALND is necessary for all node-positive breast cancers. Several retrospective studies where ALND was omitted after detecting micro-or macrometastases in the SLN showed very low rates of axillary recurrence.27–29 Two prospective randomized trials confirmed these results for micrometastases and up to two macrometastases. However, no difference was found in terms of the locoregional disease and survival rates.30–32
It is now possible to omit ALND in patients with clinically node-negative breast cancer if one or two SLNs are histologically positive and if the patient receives breast-conserving therapy and radiotherapy.
SLNB is increasingly being applied to malignant melanoma in clinical practice. For melanomas of the trunk, lymphatic mapping can reveal which regions are drained by the tumor.
SLNB is also recommended for intermediate melanomas with a Breslow thickness of 1–4 mm. In routine use, SLNB can provide accurate staging in this population, with high identification rates and sensitivity. Although relatively few studies have focused on patients with thick melanomas (T4; Breslow thickness >4 mm), SLNB may also be recommended for staging purposes and to facilitate regional disease control in this population. However, there is insufficient evidence to support routine SLNB for patients with thin melanomas (T1; Breslow thickness <1 mm).33
The 10-year results of the Multicenter Selective Lymphadenectomy Trial confirmed that disease-free survival was significantly longer if SLNB was followed by lymphadenectomy for nodal involvement compared with nodal observation and lymphadenectomy on demand. A significant improvement in melanoma-specific overall survival was additionally observed for intermediate-thickness melanomas (1.20–3.50 mm thick).34,35 By contrast, the effects of SLNB on survival in patients with nonmelanoma skin cancer are still controversial.36
The SLN concept has also been applied to colorectal cancer in the last 2 decades. In colorectal cancer, sentinel node navigated surgery (SNNS) is not intended to reduce the surgical extension. However, it should detect additional lymph nodes located beyond the regional lymph nodes that are targeted for resection. Intensive work-up of the identified SLN improves the accuracy of staging of nodal disease,37 which is important because node-positive patients with colorectal cancer require adjuvant chemotherapy. In 22% of cases, SLNB could change the extent of resection.38 In a recent meta-analysis conducted by van der Zaag et al, the pooled identification rate was 90% and the pooled sensitivity was 70%.39 Of note, the identification rate increased if >100 patients were investigated, if the lymphatic mapping was performed ex vivo, and if the patients’ body mass index was low.40 The sensitivity increased if more than four SLNs were removed, if the tumors were small (T1 or T2 versus T3 or T4), and in colon cancers relative to rectal cancers. The large number of false-negative results was due to aberrant drainage sites and skip lesions caused by obstruction of the lymphatic system.41 The extent of the pathological work-up is another crucial factor in predicting the outcome of SLNB.42 Because of these limitations, SLNB is not yet routinely applied to colorectal cancer.
Although data are limited, lymphatic mapping and SLNB of squamous cell carcinoma of the anus had high identification rates (47%–100%) and low false-negative rates (0%–14%) for the majority of patients.43 Therefore, SLNB can be recommended for patients with squamous cell carcinoma of the anus.
The SLN concept has also been evaluated in several trials of esophageal cancer. The identification rates and the sensitivity were promising in patients with T1 or T2 tumors without clinical lymph node involvement.44,45 Further prospective trials with larger numbers of patients are required to confirm these findings.
In gastric cancer, SLNB had identification rates of 80%–100% and accuracy of 90%–100%.46 However, in the multicenter Japan Clinical Oncology Group study 0302, one serious limitation was the high false-negative rate of 46% for intraoperative frozen-section analyses of the SLN.47 Therefore, lymphatic mapping may be unsuitable for gastric cancer.
Although SNNS is a controversial procedure in patients with cervical cancer and ovarian cancer, it has been extensively applied to squamous cell carcinoma of the vulva.48 In this setting, the identification rate ranged from 92% to 96% and the sensitivity ranged from 90% to 95%.49 In addition, the false-negative rate was <2% for tumors of ≤4 cm in diameter.50 These results suggest that lymphatic mapping is suitable for midline tumors with clinically negative inguinal lymph nodes.
Regarding urologic tumors, perhaps the most experience of SNNS has been gained for penile carcinoma, for which the pooled identification rate was 88% and the pooled sensitivity was 88%.51 These values were increased by using blue dye in combination with a radiotracer.51
There are several reports of lymphatic mapping in patients with early head and neck squamous cell carcinoma, for which the identification rate was 95%, but the sensitivity was relatively poor at 86%.52
SNNS is now under investigation for application to other malignancies, including non-small-cell lung cancer and prostate cancer (Table 1).53,54
Table 1.
Lymphatic mapping in cancer management
| Type of cancer | Evidence | Clinical use | Features |
|---|---|---|---|
| Breast cancer | High | Gold standard | |
| Skin malignancies | |||
| Melanoma | High | Gold standard | Addition look for lymph basin |
| Nonmelanoma skin cancer | Poor | Experimental | |
| Gastrointestinal malignancies | |||
| Esophageal cancer | Poor | Experimental | |
| Gastric cancer | Poor | Experimental | Low sensitivity/frozen section |
| Colorectal cancer | Good | Experimental | Aberrant lymph drainage |
| Anal cancer | Good | Established | |
| Head and neck malignancies | Poor | Experimental | |
| Squamous cell cancer | Good | Established | |
| Thyroid cancer | Poor | Experimental | |
| Parathyroid cancer | Poor | Experimental | |
| Gynecological cancers | |||
| Vulva cancer | Good | Established | |
| Other gynecological cancers | Poor | Experimental | |
| Urological cancers | |||
| Prostate cancer | Good | Experimental | |
| Penile cancer | Good | Established | |
| Other urological cancers | Poor | Experimental | |
| Lung cancer | Poor | Experimental | |
Lymph node mapping techniques
Several substances that are transported by lymph vessels have been developed for use in lymphatic mapping.
There is abundant evidence supporting the use of radioisotopes containing 99mTc. For example, the common use of filtered or unfiltered 99mTc-sulfur colloid in the United States of America, albumin-based colloids in Europe, and 99mTc antimony trisulfide colloid in Australia. The identification rate for 99mTc radioisotopes ranges from 86% to 99%.55,56 The use of radioisotopes requires a nearby nuclear medicine unit for the preoperative injection, which is given 2–14 hours before imaging, and a γ detector in the operating room. Preoperative scintigraphy may be helpful but is not essential.
The injection of blue dyes, such as isosulfan blue or patent blue, can identify the sentinel node in 68%–86% of cases.10,55,57 Blue dyes are used worldwide for lymphatic mapping in surgical cancer management. The blue dye is injected in the operation room about 10–15 minutes before the biopsy. Allergic reactions, one of the main adverse events associated with blue dye injection, occur in <1% of cases.58
Because injection of a radioisotope in combination with a blue dye can achieve high identification rates of 89%–97%, this combination is widely used.11,57,58 The false-negative rate of this combination ranges from 6 % to 10%, which is similar to the rate obtained with radioguided SLNB alone. However, the false-negative rate is higher if blue dye is used alone.59
The subareolar technique has equivalent value to peritumoral injection for lymphatic mapping in breast cancer.60 Subareolar injection is preferred in the majority of institutions for several practical reasons, which include the need for single injection for multicentric disease and because radiologically guided injection (stereotactic- or ultrasonographic-guided) is not necessary for nonpalpable tumors.
Since 1999, when Motomura et al first described lymphatic mapping with fluorescent indocyanine green dye and visualization using a near-infrared camera, this technique has become widely used, especially in Eastern Asia.61 Indocyanine green has similar identification rates and sensitivity to those of radioguided or blue-dye-based mapping. The main advantage of indocyanine green is that it allows percutaneous visualization of the afferent lymph vessels and lymph nodes. However, the operating room must be equipped with an infrared camera to enable this procedure.62–65
SentiMag® (Endomagnetics, Cambridge, UK) is a new radiation-free technique that was recently introduced. It consists of a magnetometer and a magnetic tracer, which can be visualized through the skin by its brown color. This system showed similar identification rates to radioisotopes and blue dye.66
Mechanism of action of 99mTc-tilmanocept in lymph node mapping
99mTc-tilmanocept (Lymphoseek®; Navidea Biopharmaceuticals, Dublin, OH, USA) is a synthetic radioisotope that was designed for use in SNNS. 99mTc-tilmanocept consists of multiple diethylenetriaminepentaacetic acid (DTPA) and mannose residues linked to a dextran frame. 99mTc is attached to DTPA while the mannose residues bind in a multivalent manner to mannose receptors (CD206) expressed on the surface of reticuloendothelial cells in lymph nodes (Figure 1).67 Because of its small molecular size (molecular weight: 16.7 kDa) and its small diameter (7.1 nm), 99mTc-tilmanocept is quickly transported through the afferent lymph vessels. The mannose residues bind to the reticuloendothelial cells in the SLN with a residence time of about 30 hours. Several Phase I and Phase II trials have confirmed that 99mTc-tilmanocept does not escape from the SLN to the second echelon lymph nodes.68–70
Figure 1.
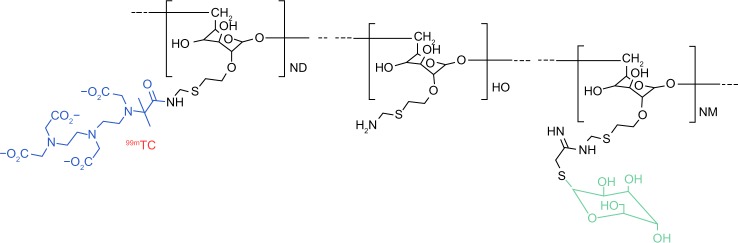
99mTc-tilmanosept molecule.
Abbreviation: 99mTc, 99mtechnetium.
Safety and effectiveness of 99mTc-tilmanocept
The first report describing the use of 99mTc-tilmanocept in SNNS was published in 2001.71 Animal models confirmed that 99mTc-tilmanocept was rapidly cleared from the subcutaneous injection site and that it accumulated in the proximal lymph node, but not in distal lymph nodes. Similar data were also obtained in a pig model of the stomach and colon, in comparison with blue dye.72,73 The first Phase I trial of 12 breast cancer patients showed that 99mTc-tilmanocept was cleared from the injection site more quickly than 99mTc-sulfur colloid by peritumoral injection, but the accumulation of both compounds in the axillary lymph nodes was equal. The median number of hot lymph nodes was lower for 99mTc-tilmanocept.67 Similar results were obtained in the first Phase I trial of 24 melanoma patients.69 The rapid clearance of 99mTc-tilmanocept from its injection site was also observed following intradermal injection in 12 patients with breast cancer.70
In a multicenter Phase II trial, 78 patients (47 with melanoma and 31 with breast cancer) were injected with 50 μg of 99mTc-tilmanocept.68 Lymphatic mapping was possible in 52/55 patients (94.5%) who underwent lymphoscintigraphy. During surgery, the identification rate was 96%. Metastatic disease was found in 13.7% of patients.68
A multicenter Phase III trial compared 99mTc-tilmanocept and blue dye in 148 patients with breast cancer.74 99mTc-tilmanocept detected 207/209 nodes that were detected by blue dye, resulting in a concordance rate of 99%. However, 99mTc-tilmanocept detected the SLN in significantly more patients than did the blue dye (146 versus 131; P<0.0001). In 129/131 patients, all of the blue-stained nodes were hot. 99mTc-tilmanocept identified 31/33 positive nodes, whereas blue dye only detected 25/33 positive nodes (P=0.0312).74
Similar data were reported for malignant melanoma in a multicenter Phase III trial involving 154 patients.75 The concordance rate was 98.7% (232/235 blue nodes were detected by 99mTc-tilmanocept). 99mTc-tilmanocept also identified at least one SLN in 150 patients compared with 138 patients with the blue dye (P=0.002). Overall, 4/34 node-positive patients were diagnosed by 99mTc-tilmanocept alone.75
In both trials, patients were injected with 50 μg of 99mTc-tilmanocept. Patients who were scheduled for surgery on the same day received around 0.5 mCi of 99mTc-tilmanocept. Patients scheduled for a 2-day procedure received 1.0–2.0 mCi of 99mTc-tilmanocept. 99mTc-tilmanocept was only injected intradermally for patients with melanoma, while in patients with breast cancer, the injection site (intradermal, subareolar, or peritumoral) was at the surgeon’s preference.
There were no allergic reactions described for 99mTc-tilmanocept in either trial. Furthermore, there were no serious adverse events that were considered clinically relevant to the administration of 99mTc-tilmanocept.
There is currently only one indirect comparison between 99mTc-tilmanocept and the 99mTc-nanocolloid albumin (Nanocoll®; Nycomed Amersham Sorin SRL, VC, Italy).76 In that study, the authors compared data from the results of Phase III trials of 99mTctilmanocept with historical data from 99mTc-nanocolloid-albumin-based protocols. It was postulated that the localization rate relative to the study population was 99.99% for 99mTc-tilmanocept compared with 95.91% for 99mTc-nanocolloid albumin (P<0.0001). In addition, the localization of SLN was significantly superior for 99mTc-tilmanocept compared with 99mTc-nanocolloid albumin.76
A small Phase III trial involving 20 patients with oral cavity squamous cell carcinoma was recently reported. In that study, 99mTc-tilmanocept combined with single-photon emission computed tomography/computed tomography achieved a sensitivity of 100% in 12 node-positive patients.77
Based on the results of these trials, 99mTc-tilmanocept was approved for SNNS by the United States Food and Drug Administration in March 2013.
Conclusion
Because of its small diameter, 99mTc-tilmanocept is cleared from the injection site much quicker than other radioisotopes or blue dye. The mannose residues of 99mTc-tilmanocept bind to mannose receptors (CD206) expressed on reticuloendothelial cells in lymph nodes. Therefore, the tracer remains in the SLN without migrating to the second echelon lymph nodes. The use of 99mTc-tilmanocept in patients with early cancer might avoid unnecessary resection of additional second echelon nodes and, thus, avoid the morbidity associated with this procedure. However, the comparative Phase III trials of breast cancer and malignant melanoma demonstrated that this approach was only suitable for early breast cancer.74,75 Only 18% (27/148) of patients with breast cancer and 22% (34/154) of patients with melanoma presented with nodal involvement. The rate of nodal involvement in that study was low compared with the rate in other breast cancer trials (18% versus 26%–34%).9,11,57,78 Therefore, it is still unclear whether these data can be applied to a higher-risk population with higher rates of nodal involvement where the likelihood of false-negative results decreases when second echelon lymph nodes are removed together with the SLN.57 Since the clinical implementation of the data from the American College of Surgeons Oncology Group Z0011 trial, surgeons now prefer to resect more than one node in patients with nodal involvement in order to achieve more oncological safety when omitting full axillary clearance.30,31,79 When SLNB is performed after neoadjuvant chemotherapy, especially in patients where lymph node involvement is found initially, a larger number of sentinel nodes should be resected to decrease the false-negative rate.26
The clinical value of 99mTc-tilmanocept in SNNS will be more clearly demonstrated in comparative studies using other 99mTc radiotracers than in studies using blue dye, which was used in the previous multicenter Phase III trials.74,75 The results of such studies might support the use of 99mTc-tilmanocept instead of other radioisotopes used in combination with blue dye or used alone.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen SL, Iddings DM, Scheri RP, Bilchik AJ. Lymphatic mapping and sentinel node analysis: current concepts and applications. CA Cancer J Clin. 2006;56(5):292–309. doi: 10.3322/canjclin.56.5.292. [DOI] [PubMed] [Google Scholar]
- 2.Cabanas RM. An approach for the treatment of penile carcinoma. Cancer. 1977;39(2):456–466. doi: 10.1002/1097-0142(197702)39:2<456::aid-cncr2820390214>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127(4):392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 4.Krag DN1, Weaver DL, Alex JC, Fairbank JT. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol. 1993;2(6):335–339. doi: 10.1016/0960-7404(93)90064-6. [DOI] [PubMed] [Google Scholar]
- 5.Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Ann Surg. 1994;220(3):391–398. doi: 10.1097/00000658-199409000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albertini JJ, Lyman GH, Cox C, et al. Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. JAMA. 1996;276:1818–1822. [PubMed] [Google Scholar]
- 7.Pijpers R, Meijer S, Hoekstra OS, et al. Impact of lymphoscintigraphy on sentinel node identification with technetium-99mcolloidal albumin in breast cancer. J Nucl Med. 1997;38:366–368. [PubMed] [Google Scholar]
- 8.Veronesi U, Paganelli G, Galimberti V, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph nodes. Lancet. 1997;349:1864–1867. doi: 10.1016/S0140-6736(97)01004-0. [DOI] [PubMed] [Google Scholar]
- 9.Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer – a multicenter validation study. N Engl J Med. 1998;339(14):941–946. doi: 10.1056/NEJM199810013391401. [DOI] [PubMed] [Google Scholar]
- 10.McMasters KM, Tuttle TM, Carlson DJ, et al. Sentinel lymph node biopsy for breast cancer: a suitable alternative to routine axillary dissection in multi-institutional practice when optimal technique is used. J Clin Oncol. 2000;18(13):2560–2566. doi: 10.1200/JCO.2000.18.13.2560. [DOI] [PubMed] [Google Scholar]
- 11.Noguchi M, Motomura K, Imoto S, et al. A multicenter validation study of sentinel lymph node biopsy by the Japanese Breast Cancer Society. Breast Cancer Res Treat. 2000;63(1):31–40. doi: 10.1023/a:1006428105579. [DOI] [PubMed] [Google Scholar]
- 12.Turner RR, Ollila DW, Krasne DL, Giuliano AE. Histopathologic validation of the sentinel lymph node hypothesis for breast carcinoma. Ann Surg. 1997;226(3):271–276. doi: 10.1097/00000658-199709000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th ed. New York: Springer-Verlag; 2002. [Google Scholar]
- 14.Goldhirsch A, Glick JH, Gelber RD, Coates AS, Senn HJ. Meeting highlights: International Consensus Panel on the Treatment of Primary Breast Cancer. Seventh International Conference on Adjuvant Therapy of Primary Breast Cancer. J Clin Oncol. 2001;19(18):3817–3827. doi: 10.1200/JCO.2001.19.18.3817. [DOI] [PubMed] [Google Scholar]
- 15.Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23(30):7703–7720. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11(10):927–933. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashikaga T, Krag DN, Land SR, et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. 2010;102(2):111–118. doi: 10.1002/jso.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98(9):599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 19.Moody LC1, Wen X, McKnight T, Chao C. Indications for sentinel lymph node biopsy in multifocal and multicentric breast cancer. Surgery. 2012;152(3):389–396. doi: 10.1016/j.surg.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Schrenk P, Hochreiner G, Fridrik M, Wayand W. Sentinel node biopsy performed before preoperative chemotherapy for axillary lymph node staging in breast cancer. Breast J. 2003;9(4):282–287. doi: 10.1046/j.1524-4741.2003.09406.x. [DOI] [PubMed] [Google Scholar]
- 21.Schrenk P, Tausch C, Wölfl S, Bogner S, Fridrik M, Wayand W. Sentinel node mapping performed before preoperative chemotherapy may avoid axillary dissection in breast cancer patients with negative or micrometastatic sentinel nodes. Am J Surg. 2008;196(2):176–183. doi: 10.1016/j.amjsurg.2007.08.068. [DOI] [PubMed] [Google Scholar]
- 22.Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–618. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 23.Mamounas EP, Brown A, Anderson S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2005;23(12):2694–2702. doi: 10.1200/JCO.2005.05.188. [DOI] [PubMed] [Google Scholar]
- 24.Tausch C, Steger GG, Haid A, et al. Sentinel node biopsy after primary chemotherapy in breast cancer: a note of caution from results of ABCSG-14. Breast J. 2011;17(3):230–238. doi: 10.1111/j.1524-4741.2011.01073.x. [DOI] [PubMed] [Google Scholar]
- 25.Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(14):1455–1461. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen JJ, Wu J. Management strategy of early-stage breast cancer patients with a positive sentinel lymph node: with or without axillary lymph node dissection. Crit Rev Oncol Hematol. 2011;79(3):293–301. doi: 10.1016/j.critrevonc.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27(18):2946–2953. doi: 10.1200/JCO.2008.19.5750. [DOI] [PubMed] [Google Scholar]
- 28.Yi M, Giordano SH, Meric-Bernstam F, Mittendorf EA, et al. Trends in and outcomes from sentinel lymph node biopsy (SLNB) alone vs SLNB with axillary lymph node dissection for node-positive breast cancer patients: experience from the SEER database. Ann Surg Oncol. 2010;17(Suppl 3):343–351. doi: 10.1245/s10434-010-1253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milgrom S, Cody H, Tan L, et al. Characteristics and outcomes of sentinel node-positive breast cancer patients after total mastectomy without axillary-specific treatment. Ann Surg Oncol. 2012;19(12):3762–3770. doi: 10.1245/s10434-012-2386-3. [DOI] [PubMed] [Google Scholar]
- 30.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252(3):426–432. doi: 10.1097/SLA.0b013e3181f08f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galimberti V, Cole BF, Zurrida S, et al. International Breast Cancer Study Group Trial 23-01 investigators. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14(4):297–305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong SL, Balch CM, Hurley P, et al. Sentinel lymph node biopsy for melanoma: American Society of Clinical Oncology and Society of Surgical Oncology joint clinical practice guideline. Ann Surg Oncol. 2012;19:3313–3324. doi: 10.1245/s10434-012-2475-3. [DOI] [PubMed] [Google Scholar]
- 34.Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370(7):599–609. doi: 10.1056/NEJMoa1310460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balch CM, Gershenwald JE. Clinical value of the sentinel-node biopsy in primary cutaneous melanoma. N Engl J Med. 2014;370(7):663–664. doi: 10.1056/NEJMe1313690. [DOI] [PubMed] [Google Scholar]
- 36.Matthey-Giè M, Boubaker A, Letovanec I, Demartines N, Matter M. Sentinel lymph node biopsy in nonmelanoma skin cancer patients. J Skin Cancer. 2013;2013:267474. doi: 10.1155/2013/267474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Zaag ES, Kooij N, van de Vijver MJ, Bemelman WA, Peters HM, Buskens CJ. Diagnosing occult tumour cells and their predictive value in sentinel nodes of histologically negative patients with colorectal cancer. Eur J Surg Oncol. 2010;36:350–357. doi: 10.1016/j.ejso.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Saha S, Johnston G, Korant A, et al. Aberrant drainage of sentinel lymph nodes in colon cancer and its impact on staging and extent of operation. Am J Surg. 2013;205:302–305. doi: 10.1016/j.amjsurg.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 39.van der Zaag ES, Bouma WH, Tanis PJ, Ubbink DT, Bemelman WA, Buskens CJ. Systematic review of sentinel lymph node mapping procedure in colorectal cancer. Ann Surg Oncol. 2012;19:3449–3459. doi: 10.1245/s10434-012-2417-0. [DOI] [PubMed] [Google Scholar]
- 40.Bembenek AE, Rosenberg R, Wagler E, et al. Sentinel lymph node biopsy in colon cancer: a prospective multicenter trial. Ann Surg. 2007;245:858–863. doi: 10.1097/01.sla.0000250428.46656.7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Retter SM, Herrmann G, Schiedeck TH. Clinical value of sentinel node mapping in carcinoma of the colon. Colorectal Dis. 2011;13:855–859. doi: 10.1111/j.1463-1318.2010.02293.x. [DOI] [PubMed] [Google Scholar]
- 42.Resch A, Langner C. Lymph node staging in colorectal cancer: old controversies and recent advances. World J Gastroenterol. 2013;19(46):8515–8526. doi: 10.3748/wjg.v19.i46.8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mistrangelo DM, Bellò M, Cassoni P, et al. Value of staging squamous cell carcinoma of the anal margin and canal using the sentinel lymph node procedure: an update of the series and a review of the literature. Br J Cancer. 2013;108(3):527–532. doi: 10.1038/bjc.2012.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Filip B1, Scarpa M, Cavallin F, Alfieri R, Cagol M, Castoro C. Minimally invasive surgery for esophageal cancer: a review on sentinel node concept. Surg Endosc. 2014;28(4):1238–1249. doi: 10.1007/s00464-013-3314-8. [DOI] [PubMed] [Google Scholar]
- 45.Takeuchi H, Kawakubo H, Takeda F, Omori T, Kitagawa Y. Sentinel node navigation surgery in early-stage esophageal cancer. Ann Thorac Cardiovasc Surg. 2012;18(4):306–313. doi: 10.5761/atcs.ra.12.01951. [DOI] [PubMed] [Google Scholar]
- 46.Can MF, Yagci G, Cetiner S. Systematic review of studies investigating sentinel node navigation surgery and lymphatic mapping for gastric cancer. J Laparoendosc Adv Surg Tech A. 2013;23(8):651–662. doi: 10.1089/lap.2012.0311. [DOI] [PubMed] [Google Scholar]
- 47.Miyashiro I, Hiratsuka M, Sasako M, et al. Gastric Cancer Surgical Study Group (GCSSG) in the Japan Clinical Oncology Group (JCOG). High false-negative proportion of intraoperative histological examination as a serious problem for clinical application of sentinel node biopsy for early gastric cancer: final results of the Japan Clinical Oncology Group multicenter trial JCOG0302. Gastric Cancer. 2014;17(2):316–323. doi: 10.1007/s10120-013-0285-3. [DOI] [PubMed] [Google Scholar]
- 48.Palla VV, Karaolanis G, Moris D, Antsaklis A. Sentinel lymph node biopsy in uterine cervical cancer patients: ready for clinical use? A review of the literature. ISRN Surg. 2014;2014:841618. doi: 10.1155/2014/841618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hassanzade M, Attaran M, Treglia G, Yousef Z, Sadeghi R. Lymphatic mapping and sentinel node biopsy in squamous cell carcinoma of the vulva: systematic review and meta-analysis of the literature. Gynecol Oncol. 2013;130(1):237–245. doi: 10.1016/j.ygyno.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 50.Levenback CF, Ali S, Coleman RL, et al. Lymphatic mapping and sentinel lymph node biopsy in women with squamous cell carcinoma of the vulva: a gynecologic oncology group study. J Clin Oncol. 2012;30(31):3786–3791. doi: 10.1200/JCO.2011.41.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadeghi R1, Gholami H, Zakavi SR, Kakhki VR, Tabasi KT, Horenblas S. Accuracy of sentinel lymph node biopsy for inguinal lymph node staging of penile squamous cell carcinoma: systematic review and meta-analysis of the literature. J Urol. 2012;187(1):25–31. doi: 10.1016/j.juro.2011.09.058. [DOI] [PubMed] [Google Scholar]
- 52.Yamauchi K, Kogashiwa Y, Nakamura T, Moro Y, Nagafuji H, Kohno N. Diagnostic evaluation of sentinel lymph node biopsy in early head and neck squamous cell carcinoma: A meta-analysis. Head Neck. 2013 Oct 25; doi: 10.1002/hed.23526. Epub. [DOI] [PubMed] [Google Scholar]
- 53.Gilmore DM, Khullar OV, Colson YL. Developing intrathoracic sentinel lymph node mapping with near-infrared fluorescent imaging in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2012;144(3):S80–S84. doi: 10.1016/j.jtcvs.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 54.Winter A, Kneib T, Henke RP, Wawroschek F. Sentinel lymph node dissection in more than 1200 prostate cancer cases: rate and prediction of lymph node involvement depending on preoperative tumor characteristics. Int J Urol. 2014;21(1):58–63. doi: 10.1111/iju.12184. [DOI] [PubMed] [Google Scholar]
- 55.Goyal A, Newcombe RG, Chhabra A, Mansel RE, ALMANAC Trialists Group Factors affecting failed localisation and false-negative rates of sentinel node biopsy in breast cancer – results of the ALMANAC validation phase. Breast Cancer Res Treat. 2006;99(2):203–208. doi: 10.1007/s10549-006-9192-1. [DOI] [PubMed] [Google Scholar]
- 56.Veronesi U, Paganelli G, Viale G, et al. Sentinel lymph node biopsy and axillary dissection in breast cancer: results in a large series. J Natl Cancer Inst. 1999;91(4):368–373. doi: 10.1093/jnci/91.4.368. [DOI] [PubMed] [Google Scholar]
- 57.Martin RC, 2nd, Edwards MJ, Wong SL, et al. Practical guidelines for optimal gamma probe detection of sentinel lymph nodes in breast cancer: results of a multi-institutional study. For the University of Louisville Breast Cancer Study Group. Surgery. 2000;128(2):139–144. doi: 10.1067/msy.2000.108064. [DOI] [PubMed] [Google Scholar]
- 58.Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8(10):881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 59.Chagpar AB, Martin RC, Scoggins CR, et al. Factors predicting failure to identify a sentinel lymph node in breast cancer. Surgery. 2005;138(1):56–63. doi: 10.1016/j.surg.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 60.Rodier JF, Velten M, Wilt M, et al. Prospective multicentric randomized study comparing periareolar and peritumoral injection of radiotracer and blue dye for the detection of sentinel lymph node in breast sparing procedures: FRANSENODE trial. J Clin Oncol. 2007;25(24):3664–3669. doi: 10.1200/JCO.2006.08.4228. [DOI] [PubMed] [Google Scholar]
- 61.Motomura K, Inaji H, Komoike Y, Kasugai T, Noguchi S, Koyama H. Sentinel node biopsy guided by indocyanine green dye in breast cancer patients. Jpn J Clin Oncol. 1999;29(12):604–607. doi: 10.1093/jjco/29.12.604. [DOI] [PubMed] [Google Scholar]
- 62.Jung SY, Kim SK, Kim SW, et al. Comparison of sentinel lymph node biopsy guided by the multimodal method of indocyanine green fluorescence, radioisotope, and blue dye versus the radioisotope method in breast cancer: a randomized controlled trial. Ann Surg Oncol. 2014;21(4):1254–1259. doi: 10.1245/s10434-013-3437-0. [DOI] [PubMed] [Google Scholar]
- 63.Verbeek FP, Troyan SL, Mieog JS, et al. Near-infrared fluorescence sentinel lymph node mapping in breast cancer: a multicenter experience. Breast Cancer Res Treat. 2014;143(2):333–342. doi: 10.1007/s10549-013-2802-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ballardini B, Santoro L, Sangalli C, et al. The ndocyanine green method is equivalent to the 99mTc-labeled radiotracer method for identifying the sentinel node in breast cancer: a concordance and validation study. Eur J Surg Oncol. 2013;39(12):1332–1336. doi: 10.1016/j.ejso.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 65.Sugie T, Sawada T, Tagaya N, et al. Comparison of the indocyanine green fluorescence and blue dye methods in detection of sentinel lymph nodes in early-stage breast cancer. Ann Surg Oncol. 2013;20(7):2213–2218. doi: 10.1245/s10434-013-2890-0. [DOI] [PubMed] [Google Scholar]
- 66.Douek M, Klaase J, Monypenny I, et al. Sentinel node biopsy using a magnetic tracer versus standard technique: the SentiMAG multicentre trial. Ann Surg Oncol. 2014;21(4):1237–1245. doi: 10.1245/s10434-013-3379-6. [DOI] [PubMed] [Google Scholar]
- 67.Wallace AM, Hoh CK, Vera DR, Darrah D, Schulteis G. Lymphoseek: a molecular radiopharmaceutical for sentinel node detection. Ann Surg Oncol. 2003;10:531–538. doi: 10.1245/aso.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 68.Leong SP, Kim J, Ross MI, et al. A phase 2 study of (99m) Tc-tilmanocept in the detection of sentinel lymph nodes in melanoma and breast cancer. Ann Surg Oncol. 2011;18:961–969. doi: 10.1245/s10434-010-1524-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wallace AM, Hoh CK, Ellner SJ, Darrah DD, Schulteis G, Vera DR. Lymphoseek: a molecular imaging agent for melanoma sentinel lymph node mapping. Ann Surg Oncol. 2007;14:913–921. doi: 10.1245/s10434-006-9099-4. [DOI] [PubMed] [Google Scholar]
- 70.Wallace AM, Hoh CK, Darrah DD, Schulteis G, Vera DR. Sentinel lymph node mapping of breast cancer via intradermal administration of Lymphoseek. Nucl Med Biol. 2007;34:849–853. doi: 10.1016/j.nucmedbio.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vera DR, Wallace AM, Hoh CK, Mattrey RF. A synthetic macromolecule for sentinel node detection: (99m) Tc-DTPA-mannosyl-dextran. J Nucl Med. 2001;42:951–959. [PubMed] [Google Scholar]
- 72.Méndez J, Wallace AM, Hoh CK, Vera DR. Detection of gastric and colonic sentinel nodes through endoscopic administration of 99mTc-DTPA-mannosyl-dextran in pigs. J Nucl Med. 2003;44(10):1677–1681. [PubMed] [Google Scholar]
- 73.Ellner SJ, Hoh CK, Vera DR, Darrah DD, Schulteis G, Wallace AM. Dose-dependent biodistribution of [(99m)Tc]DTPA-mannosyl-dextran for breast cancer sentinel lymph node mapping. Nucl Med Biol. 2003;30(8):805–810. doi: 10.1016/j.nucmedbio.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 74.Wallace AM, Han LK, Povoski SP, et al. Comparative evaluation of [(99m)tc]tilmanocept for sentinel lymph node mapping in breast cancer patients: results of two phase 3 trials. Ann Surg Oncol. 2013;20(8):2590–2599. doi: 10.1245/s10434-013-2887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sondak VK, King DW, Zager JS, et al. Combined analysis of phase III trials evaluating [99mTc]tilmanocept and vital blue dye for identification of sentinel lymph nodes in clinically node-negative cutaneous melanoma. Ann Surg Oncol. 2013;20(2):680–688. doi: 10.1245/s10434-012-2612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tokin CA, Cope FO, Metz WL, et al. The efficacy of Tilmanocept in sentinel lymph mode mapping and identification in breast cancer patients: a comparative review and meta-analysis of the 99mTc-labeled nanocolloid human serum albumin standard of care. Clin Exp Metastasis. 2012;29(7):681–686. doi: 10.1007/s10585-012-9497-x. [DOI] [PubMed] [Google Scholar]
- 77.Marcinow AM, Hall N, Byrum E, Teknos TN, Old MO, Agrawal A. Use of a novel receptor-targeted (CD206) radiotracer, 99mTc-tilmanocept, and SPECT/CT for sentinel lymph node detection in oral cavity squamous cell carcinoma: initial institutional report in an ongoing phase 3 study. JAMA Otolaryngol Head Neck Surg. 2013;139(9):895–902. doi: 10.1001/jamaoto.2013.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349(6):546–553. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 79.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes – dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22(8):1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]


