Abstract
Background
Glycogen synthase kinase-3 (GSK-3) plays an important role in human cancer. The aim of this study is to evaluate the clinicopathological significance of expression of GSK-3α/β and pGSK-3α/βTyr279/216 in patients with epithelial ovarian cancer and to investigate whether GSK-3 inhibition can influence cell viability and tumor growth of ovarian cancer.
Methods
Immunohistochemistry was used to examine expression of GSK-3α/β and pGSK-3α/βTyr279/216 in 71 human epithelial ovarian cancer tissues and correlations between protein expression, and clinicopathological factors were analyzed. Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay following exposure of ovarian carcinoma cells to pharmacological inhibitors of GSK-3 or GSK-3 small interfering RNA. In vivo validation of tumor growth inhibition was performed with xenograft mice.
Results
The expression levels of GSK-3α/β and pGSK-3α/βTyr279/216 in ovarian cancers were significantly higher than those in benign tumors. High expression of GSK-3α/β was more likely to be found in patients with advanced International Federation of Gynecology and Obstetrics (FIGO) stages and high serum cancer antigen 125. Higher expression of pGSK-3α/βTyr279/216 was associated with advanced FIGO stages, residual tumor mass, high serum cancer antigen 125, and poor chemoresponse. Worse overall survival was revealed by Kaplan–Meier survival curves in patients with high expression of GSK-3α/β or pGSK-3α/βTyr279/216. Multivariate analysis indicated that FIGO stage, GSK-3α/β expression, and pGSK-3α/βTyr279/216 expression were independent prognostic factors for overall survival. GSK-3 inhibition by lithium chloride, 4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione (TDZD-8), or GSK-3 small interfering RNA can decrease viability of SKOV3 and SKOV3-TR30 ovarian cancer cells. Additionally, lithium chloride-treated SKOV3 xenograft mice had a significant reduction in tumor growth compared with control-treated animals.
Conclusion
Our findings suggest that overexpression and aberrant activation of GSK-3 may contribute to progression and poor prognosis in ovarian cancer. Inhibition of GSK-3 may be a potential therapy for ovarian cancer.
Keywords: ovarian carcinoma, immunohistochemistry, lithium chloride, TDZD-8
Introduction
Despite the skillful surgical techniques and improvements in combined chemotherapy, ovarian cancer remains the leading cause of death in gynecological malignancies with 5-year survival rates of 44%.1 The main reason for poor prognosis of ovarian cancer is that about 70% of the cases are still diagnosed in advanced stages. The majority of advanced patients, including those who achieve a complete response to first-line chemotherapy, will ultimately relapse and do not successfully respond to further treatments.2 There is clearly an urgent need to develop new classes of treatment modalities, represented by molecular target-directed therapies.
Glycogen synthase kinase-3 (GSK-3) is a multifunctional serine (Ser)/threonine kinase known to play a pivotal role in the regulation of metabolism, embryonic development, cell differentiation, and apoptosis.3,4 Two isoforms of GSK-3, GSK-3α and GSK-3β, have been identified with high homology and similar but not identical biochemical functions. The actions of GSK-3 are often regulated by phosphorylation. In opposition to inhibitory regulation by phosphorylation of Ser9 in GSK-3β and Ser21 in GSK-3α, GSK-3 activity is enhanced by phosphorylation of tyrosine (Tyr)216 in GSK-3β and Tyr279 in GSK-3α.4 Surprisingly, most of the research so far has primarily focused on GSK-3β only. The dysregulation of GSK-3β has been implicated in tumorigenesis and cancer progression.5 It remains controversial whether GSK-3β is a “tumor promoter” or “tumor suppressor”.5,6
Our interest in GSK-3 stemmed from our previous study in which we found that enhanced expression of GSK-3 is associated with acquired resistance to paclitaxel in ovarian carcinoma cells.7 Previous studies have investigated the effect of GSK-3β inhibition in ovarian cancer cells.8–11 However, the clinicopathological and biological significance of GSK-3 in ovarian cancer remains inconclusive.
In the present study, we extended the previous study to evaluate the expression status of GSK-3α/β and its actively phosphorylated form, pGSK-3α/βTyr279/216, in ovarian cancer tissues using immunohistochemical analysis.7 The associations between clinicopathological factors and expression of GSK-3α/β and pGSK-3α/βTyr279/216 were evaluated. In addition, we provide experimental evidence that GSK-3 inhibition by pharmacological agents or RNA interference can decrease viability of ovarian carcinoma cells and slow tumor growth in vivo.
Materials and methods
Clinical data and tissue samples
This study included 71 patients with epithelial ovarian cancer (EOC) who underwent surgical resection followed by paclitaxel/platinum combined chemotherapy in Women’s Hospital, School of Medicine, Zhejiang University, in the period 2003 to 2005. The paraffin-embedded specimens were retrieved from the archives of the Department of Pathology of our hospital. Meanwhile, we also collected 20 samples of benign ovarian tumor (ten serous and ten mucinous cystadenoma) as controls. The use of all tissues has been approved by the Ethics Committee of Women’s Hospital, School of Medicine, Zhejiang University (No 20100009). Because the use of redundant paraffin-embedded specimens did not bring any damage to patients, the Ethics Committee waived the need for consent from the patients. All patients with EOC were followed-up two to four times annually until December 2010. The following data were collected: age, date of diagnosis, date of relapse, date of last follow-up, date and cause of death, serum cancer antigen 125 (CA125) values, International Federation of Gynecology and Obstetrics (FIGO) stage, histologic type and grade, residual tumor mass, and response to chemotherapy. Chemotherapeutic response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST).12 Patients achieving complete response or partial response to treatment were considered as responders, and patients achieving progressive disease or stable disease were considered as nonresponders.
Immunohistochemistry
Expression of GSK-3α/β and pGSK-3α/βTyr279/216 in tissues was examined immunohistochemically by the avidin–biotin– peroxidase complex method following microwave antigen retrieval of paraffin sections, using a mouse monoclonal antibody to human GSK-3α/β (diluted 1:30; Santa Cruz Biotechnology Inc., Dallas, TX, USA) and a rabbit polyclonal antibody to human pGSK-3α/βTyr279/216 (diluted 1:80; Santa Cruz Biotechnology), respectively. The expression levels of GSK-3α/β and pGSK-3α/βTyr279/216 were determined semi-quantitatively according to the described method.13 Briefly, the percentage of positive cells was rated as follows: 0 points, 0% to 25%; 1 point, 26% to 50%; 2 points, 51% to 75%; 3 points, >75%. The staining intensity was rated as follows: 0 points, no staining; 1 point, weak intensity; 2 points, moderate intensity; 3 points, strong intensity. Points for expression intensity and percentage of positive cells were added (total score of 0–6). Thus, the score 0 was taken as negative (−); 1 to 2 as weak positive (+); 3 to 4 as moderate positive (++); and 5 to 6 as strong positive (+++).
Cell culture and reagents
Human ovarian carcinoma cell line SKOV3 was obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in McCoy’s 5A medium (Gibco®; Thermo Fisher Scientific, Waltham, MA, USA), supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C and 5% CO2. Paclitaxel-resistant cell subline, SKOV3-TR30, was developed by exposing parental SKOV3 cells to increased concentration of paclitaxel as described previously.14 Two GSK-3 inhibitors, lithium chloride (LiCl) and 4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione (TDZD-8), were purchased from Sigma-Aldrich (St Louis, MO, USA).
Cell viability assay
The effect of LiCl and TDZD-8 on cell viability was determined by 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazo-lium bromide (MTT) assay as described previously.14 Briefly, 2,000 cells per well were seeded in 96-well plates, incubated in a CO2 incubator overnight at 37°C, and then treated with different doses of LiCl (0–80 mM) and TDZD-8 (0–20 μM) in triplicate for indicated times. A volume of 10 μL of MTT (5 mg/mL) was added to the cells and incubated at 37°C for 3 hours. The MTT solution was removed and 200 μL of dimethyl sulfoxide was added to each well to dissolve the blue formazan crystals. The optical density was measured at 490 nm wavelength using a Universal microplate reader Elx800 (BIO-TEK, Winooski, VT, USA). The absorbance values were normalized by assigning the value of the control line in the medium without drug to 1.0 and the value of the no-cell control to 0. Mean values were calculated from three independent experiments.
RNA interference
Small interfering RNA (siRNA) specific to human GSK3α (GSK-3α siRNA [h] sc-29339) and GSK3β (GSK-3β siRNA [h] sc-35527) were purchased from Santa Cruz Biotechnology. Unrelated control siRNA (sc-37007; Santa Cruz Biotechnology) was also used. Transfection was carried out using DharmaFECT siRNA transfection reagents (Thermo Fisher Scientific) according to the manufacturer’s recommendations. Briefly, 24 hours after plating, cells were incubated with the siRNA/DharmaFECT complexes at the appropriate concentration (70 nM for GSK3α, 50 nM for GSK3β) in antibiotic- and serum-free medium for 6 hours at 37°C. After this incubation period, transfection medium was replaced with fresh medium containing FBS. The effect of siRNA on protein expression was determined by Western immunoblotting, and the relative cell viability was determined by MTT assay 72 hours after transfection.
Western blotting
Cells were harvested and lysed in modified protein lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% sodium dodecyl sulfate [SDS], 1% NP-40, 0.02% sodium azide) added with 1% proteinase inhibitor cocktail (Sigma-Aldrich). The protein concentration was measured by the Bradford method.15 Equal amounts of sample lysates were separated by SDS-PAGE and electrophoretically transferred onto a nitrocellulose membrane. The membrane was blocked with 5% nonfat dried milk in TBST buffer (20 mM Tri-HCl, pH 7.4, 150 mM NaCl, and 0.1% Tween-20) and incubated overnight at 4°C with the antibodies against GSK-3α/β (1:500 mouse monoclonal antibody) and β-actin (1:1,000 mouse monoclonal antibody; Santa Cruz Biotechnology), respectively. The membrane was washed with TBST buffer and incubated with appropriate secondary antibodies. The protein bands were visualized using the enhanced Pierce chemiluminescence kits (Thermo Fisher Scientific). Signal data was normalized for β-actin bands, and a mean value was calculated from three independent experiments.
Nude mice and xenografts
Eight female BALB/c-nude mice, aged 4–5 weeks, were purchased from Shanghai Laboratory Animal Center (Shanghai, People’s Republic of China) and housed within a dedicated specific pathogen-free facility at the Laboratory Animal Center of Zhejiang University. To develop the tumor xenografts, 5×106 SKOV3 cells (in 0.2 mL PBS) were injected subcutaneously near the axillary fossa of nude mice. Once tumors were palpable, mice were randomly divided into two groups (four mice per group) and treated with various regimens: normal saline control or LiCl. LiCl (340 mg/kg, intraperitoneally) or normal saline were injected every 2 days for a total of seven treatments (day 1, 3, 5, 7, 9, 11, 13). Length (l) and width (w) of tumors were measured with a vernier caliper once a week, and the tumor volumes were calculated using the formula,
| (1) |
At the end of the study (7 weeks after drug treatment), animals were sacrificed according to the protocol developed by the Guidance of the Animal Center, and tumors were excised and weighed.
Statistical analysis
The data were compiled with the software package SPSS, version 16.0 (Chicago, IL, USA). Mann–Whitney U tests were used to assess the correlation between clinicopathological parameters and expressions of GSK-3α/β and pGSK-3α/βTyr279/216. Univariate survival analysis was performed by the Kaplan–Meier method (log-rank test). The multivariate survival analysis was performed according to the Cox regression model. Differences in data among treatment groups in experiments in vitro and in vivo were analyzed using one-way analysis of variance (ANOVA). All tests were two-tailed, and P<0.05 was considered to be significant.
Results
Increased expression of GSK-3α/β and pGSK-3α/βTyr279/216 in ovarian cancer tissues
Using immunohistochemistry, we investigated the expression of GSK-3α/β and pGSK-3α/βTyr279/216 in specimens of EOC and benign tumors. Figures 1 and 2 show the representative features of immunohistochemical staining of GSK-3α/β and pGSK-3α/βTyr279/216, respectively. The expression levels of GSK-3α/β and pGSK-3α/βTyr279/216 in EOC were significantly higher than those in benign tumors (P<0.001) (Tables 1 and 2). Moreover, a significant correlation between expression of GSK-3α/β and pGSK-3α/βTyr279/216 was observed (r=0.278, P=0.019). Thus, it is likely that overexpression and aberrant activation of GSK-3 is a pathologic characteristic of clinical ovarian cancer.
Figure 1.
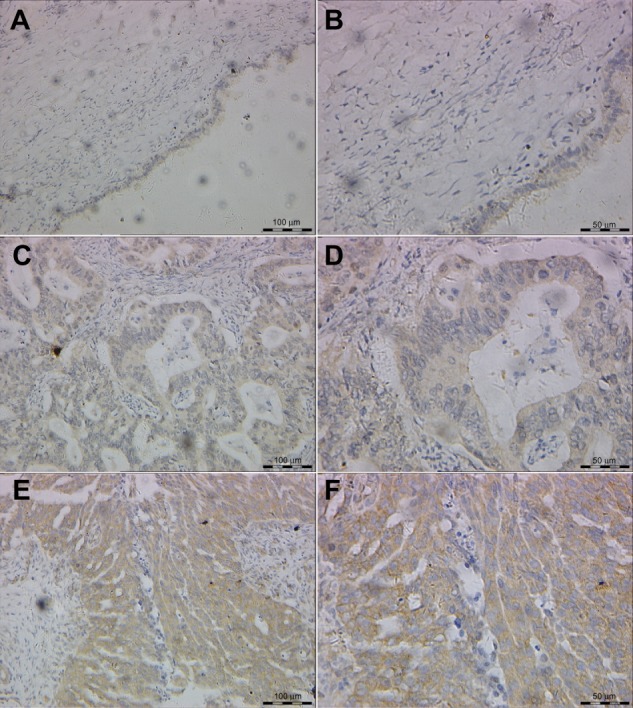
Representative features of immunohistochemical staining of GSK-3α/β.
Notes: Little staining of GSK-3α/β in benign epithelial ovarian tumor (200×) (A) and (400×) (B); EOC case with low expression of GSK-3α/β (200×) (C) and (400×) (D); EOC case with high expression of GSK-3α/β (200×) (E) and (400×) (F).
Abbreviations: EOC, epithelial ovarian cancer; GSK, glycogen synthase kinase.
Figure 2.
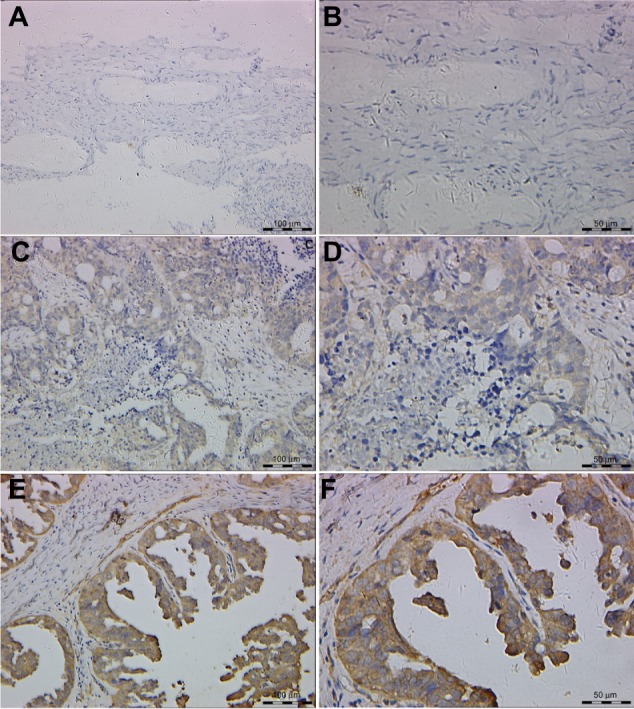
Representative features of immunohistochemical staining of pGSK-3α/βTyr279/216.
Notes: Little staining of pGSK-3α/βTyr279/216 in benign epithelial ovarian tumor (200×) (A) and (400×) (B); EOC case with low expression of pGSK-3α/βTyr279/216 (200×) (C) and (400×) (D); EOC case with high expression of pGSK-3α/βTyr279/216 (200×) (E) and (400×) (F).
Abbreviations: EOC, epithelial ovarian cancer; GSK, glycogen synthase kinase.
Table 1.
Expression of GSK-3α/β in epithelial ovarian cancer and benign ovarian tumor
| Parameters | GSK-3α/β immunostaining
|
z | P-value | |||
|---|---|---|---|---|---|---|
| − | + | ++ | +++ | |||
| Benign or malignant | −5.956 | <0.001 | ||||
| Benign tumor (n=20) | 18 | 2 | 0 | 0 | ||
| EOC (n=71) | 8 | 21 | 19 | 23 | ||
| Age of EOC patient (years) | −0.222 | 0.824 | ||||
| ≤50 (n=38) | 3 | 13 | 11 | 11 | ||
| >50 (n=33) | 5 | 8 | 8 | 12 | ||
| Stage of EOC | −3.328 | 0.001 | ||||
| FIGO I + II (n=27) | 5 | 11 | 9 | 2 | ||
| FIGO III + IV (n=44) | 3 | 10 | 10 | 21 | ||
| Residual tumor of EOC | −1.406 | 0.160 | ||||
| ≤1 cm (n=53) | 8 | 15 | 15 | 15 | ||
| >1 cm (n=18) | 0 | 6 | 4 | 8 | ||
| Histological type of EOC | −0.078 | 0.938 | ||||
| Serous (n=39) | 3 | 14 | 9 | 13 | ||
| Other (n=32) | 5 | 7 | 10 | 10 | ||
| Histological grade of EOC | −1.552 | 0.121 | ||||
| Grade 1 (n=11) | 1 | 5 | 5 | 0 | ||
| Grade 2 + 3 (n=60) | 7 | 16 | 14 | 23 | ||
| Serum CA125 of EOC patient (U/mL) | −2.053 | 0.040 | ||||
| ≤500 (n=47) | 8 | 15 | 11 | 13 | ||
| >500 (n=24) | 0 | 6 | 8 | 10 | ||
| Chemotherapy response of EOC | −1.234 | 0.217 | ||||
| Response (n=55) | 6 | 19 | 14 | 16 | ||
| Nonresponse (n=16) | 2 | 2 | 5 | 7 | ||
Abbreviations: CA125, cancer antigen 125; EOC, epithelial ovarian cancer; FIGO, International Federation of Gynecology and Obstetrics; GSK, glycogen synthase kinase.
Table 2.
Expression of pGSK-3α/βTyr279/216 in epithelial ovarian cancer and benign ovarian tumor
| Parameters | pGSK-3α/βTyr279/216 immunostaining
|
z | P-value | |||
|---|---|---|---|---|---|---|
| − | + | ++ | +++ | |||
| Benign or malignant | −4.759 | <0.001 | ||||
| Benign tumor (n=20) | 16 | 2 | 1 | 1 | ||
| EOC (n=71) | 11 | 22 | 14 | 24 | ||
| Age of EOC patient (years) | −0.685 | 0.508 | ||||
| ≤50 (n=38) | 8 | 10 | 8 | 12 | ||
| >50 (n=33) | 3 | 12 | 6 | 12 | ||
| Stage of EOC | −2.815 | 0.004 | ||||
| FIGO I + II (n=27) | 5 | 13 | 6 | 3 | ||
| FIGO III + IV (n=44) | 6 | 9 | 8 | 21 | ||
| Residual tumor of EOC | −2.005 | 0.044 | ||||
| ≤1 cm (n=53) | 10 | 18 | 10 | 15 | ||
| >1 cm (n=18) | 1 | 4 | 4 | 9 | ||
| Histological type of EOC | −1.518 | 0.132 | ||||
| Serous (n=39) | 5 | 9 | 10 | 15 | ||
| Other (n=32) | 6 | 13 | 4 | 9 | ||
| Histological grade of EOC | −0.298 | 0.791 | ||||
| Grade 1 (n=11) | 1 | 5 | 2 | 3 | ||
| Grade 2 + 3 (n=60) | 10 | 17 | 12 | 21 | ||
| Serum CA125 of EOC patient (U/mL) | −2.920 | 0.003 | ||||
| ≤500 (n=47) | 10 | 17 | 9 | 11 | ||
| >500 (n=24) | 1 | 5 | 5 | 13 | ||
| Chemotherapy response of EOC | −3.292 | 0.001 | ||||
| Response (n=55) | 10 | 20 | 13 | 12 | ||
| Nonresponse (n=16) | 1 | 2 | 1 | 12 | ||
Abbreviations: CA125, cancer antigen 125; EOC, epithelial ovarian cancer; FIGO, International Federation of Gynecology and Obstetrics; GSK, glycogen synthase kinase.
In patients with EOC, the clinicopathologic and prognostic significance of expression levels of GSK-3α/β and pGSK-3α/βTyr279/216 were also analyzed. High expression of GSK-3α/β was more likely to be found in EOC patients with advanced FIGO stages (P=0.001) and high serum CA125 (P=0.040) (Table 1). Higher expression of pGSK-3α/βTyr279/216 was more prevalent in tissues from patients with advanced FIGO stages (P=0.004), residual tumor mass (P=0.044), high serum CA125 (P=0.003), and poor chemoresponse (P=0.001) (Table 2). Until the final follow-up time, the median follow-up time of the total 71 patients was 59 months (range 5–87). Worse overall survival was revealed by Kaplan– Meier survival curves in patients with high expression of GSK-3α/β (P=0.004) or pGSK-3α/βTyr279/216 (P=0.005) (Figure 3). Multivariate analysis indicated that FIGO stage (hazard ratio [HR] =11.1, P=0.02), GSK-3α/β expression (HR =3.1, P=0.045), and pGSK-3α/βTyr279/216 expression (HR =2.8, P=0.041) were independent prognostic factors for overall survival.
Figure 3.
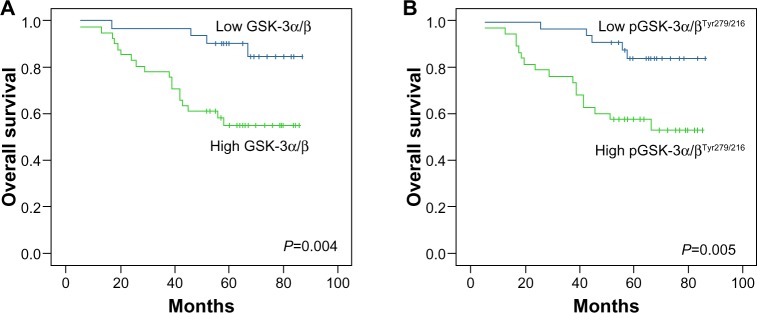
Kaplan–Meier survival analysis among 71 patients with epithelial ovarian cancer according to expression of GSK-3α/β and pGSK-3α/βTyr279/216.
Notes: Patients with high expression (++ − +++) of GSK-3α/β (A) or pGSK-3α/βTyr279/216 (B) had a significantly inferior overall survival time than those with low expression (P=0.004, P=0.005, respectively).
Abbreviation: GSK, glycogen synthase kinase.
Inhibition of GSK-3 decreases viability of ovarian carcinoma cells
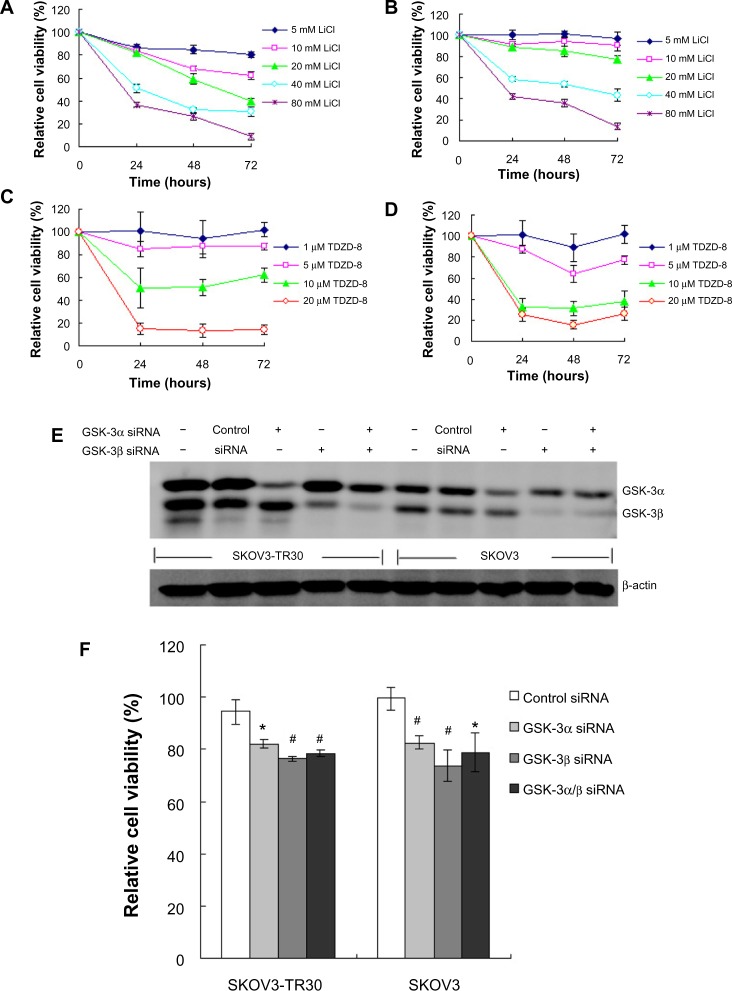
The immunohistochemical results in our study indicate that GSK-3 has a putative pathologic role in ovarian carcinoma. To determine whether active GSK-3 is essential for survival of ovarian carcinoma cells, we tested the effect of two GSK-3 inhibitors, LiCl and TDZD-8, in SKOV3 and SKOV3-TR30, a paclitaxel-resistant cell subline derived from SKOV3. We found that both LiCl and TDZD-8 can decrease cell viability of SKOV3 and SKOV3-TR30 (Figure 4A–D).
Figure 4.
Effect of GSK-3 inhibition on cell viability.
Notes: LiCl (A and B) and TDZD-8 (C and D) reduces viability of ovarian carcinoma cells; SKOV3 (A and C) and SKOV3-TR30 (B and D) cells were treated with various concentrations of LiCl or TDZD-8, and cell viability was determined using the MTT assay at 24, 48, and 72 hours; Data represent the mean of three different experiments with triplicate wells; To determine GSK-3α/β protein levels after siRNA treatment, protein extracts were obtained using a lysis buffer containing protease inhibitors cocktail 72 hours posttransfection and analyzed by Western blot (E); A reduction of ≥70% in the expression levels of the corresponding GSK-3 isoforms was observed when compared to untransfected or scrambled siRNA transfected controls. GSK-3 silencing decreases viability of ovarian carcinoma cells (F); Cell viability was determined by MTT assay 72 hours after transfection with GSK-3α siRNA (70 nM), GSK-3β siRNA (50 nM), or both in SKOV3 and SKOV3-TR30 cells; Data represent the mean of three different experiments with triplicate wells. *P<0.05, #P<0.01 compared with control siRNA.
Abbreviations: GSK, glycogen synthase kinase; LiCl, lithium chloride; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; siRNA, small interfering RNA; TDZD-8, 4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione.
To determine whether the reduction of cell viability by pharmacological inhibition of GSK-3 was specific to GSK-3 isoforms, we depleted the expression of GSK-3α or GSK-3β in SKOV3 and SKOV3-TR30 cells using RNA interference. Following a 3-day exposure to GSK-3-specific siRNAs, immunoblotting showed ≥70% reduction in expression levels of the corresponding GSK-3 isoforms when compared to untransfected or scrambled siRNA transfected controls (Figure 4E). We found that depletion of either GSK-3α or β isoforms can significantly decrease cell viability of ovarian cancer (Figure 4F). These results indicate that both GSK-3 isoforms may contribute to regulate cell viability of ovarian carcinoma.
Effect of LiCl on tumor growth in vivo
To determine whether GSK-3 inhibition affects ovarian tumor growth in vivo, we used a xenograft model of ovarian cancer generated by SKOV3 cells. As shown in Figure 5A, tumor growth was slowed in the LiCl group as measured by calculated tumor volume. The differences between the control and LiCl-treated tumor volumes at days 21, 28, and 35 were statistically significant. By the end of the study, the differences of tumor volumes between control and treatment group were statistically insignificant (Figure 5A and B). However, tumor growth was significantly reduced in the LiCl group as measured by tumor weight when tumors were excised at the end of the study (Figure 5C).
Figure 5.
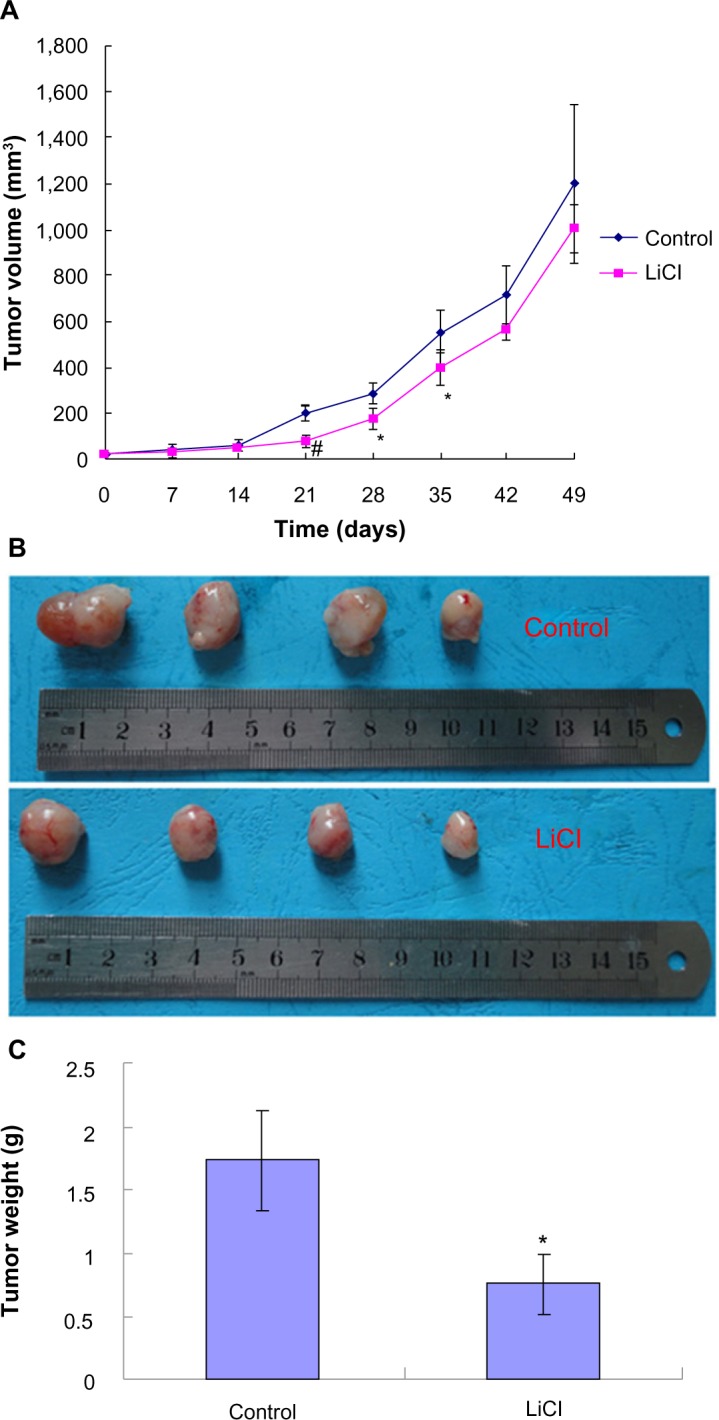
In vivo validation of tumor growth inhibition by LiCl treatment.
Notes: After palpable tumors generated by SKOV3 cells developed, ip injections of LiCl (340 mg/kg) were done every 2 days for a total of seven treatments, the tumors were measured, and the tumor volume was calculated once a week (A); The growth of the LiCl-treated tumors was slowed compared with the control; Data are means ± SD (n=4); *P<0.05, #P<0.01 versus control; Tumor specimens dissected from the nude mice xenografted with SKOV3 cells at the end of the study (B); Comparison of tumor weight between LiCl group and control (C); Data are means ± SD (n=4); *P,0.05 versus control.
Abbreviations: ip, intraperitoneal; LiCl, lithium chloride; SD, standard deviation.
Discussion
In view of the known biological importance of GSK-3 in human cancers and the unknown clinicopathological significance of GSK-3 expression in ovarian carcinoma, we used immunohistochemistry to assess the expression status of GSK-3α/β and its actively phosphorylated form, pGSK-3α/βTyr279/216, in a series of 71 EOC. In this study, we performed immunohistochemical analysis using two antibodies which can simultaneously recognize GSK-3α and GSK-3β and the activated form of GSK-3α and GSK-3β, respectively. We found that the expression levels of GSK-3α/β and pGSK-3α/βTyr279/216 in EOC were significantly higher than those in benign ovarian tumors. High expressions (++ − +++) of GSK-3α/β were observed in 59.2% (42/71) of the EOC samples, which correlated with advanced FIGO stage and high serum CA125. High expressions (++− +++) of pGSK-3α/βTyr279/216 were observed in 53.5% (38/71) of EOC, which associated with advanced FIGO stage, postoperative residual tumor mass, and high serum CA125. These findings reflect that expression of GSK-3α/β and pGSK-3α/βTyr279/216 seem to increase with tumor growth and invasiveness, and the aberrant activation of GSK-3α/β may play a role in the progression of EOC. Consistently, Do et al found that pGSK-3α/βSer9/21 immunostaining in benign neoplasias was significantly higher than in ovarian carcinomas.16 Loss of pGSK-3α/βSer9/21 immunoreactivity indirectly indicates upregulation of GSK-3 activity, despite having not detected pGSK-3α/βTyr279/216 immunostaining.
EOC is mainly treated by paclitaxel/platinum combined chemotherapy, and this study has evaluated the expression of GSK-3α/β or pGSK-3α/βTyr279/216 as a marker of chemotherapeutic responsiveness in such patients. In our study, 77.5% (55/71) of EOC patients showed a response to the combined chemotherapy, and no correlation was observed between GSK-3α/β expression and chemotherapeutic responsiveness. However, we found that expressions of pGSK-3α/βTyr279/216 were clearly lower in responders than those in nonresponders. The response rate (30/33) in patients with low expression (− − +) of pGSK-3α/βTyr279/216 was increased significantly. These data strongly suggest that the expression level of activated GSK-3 in ovarian cancer is inversely associated with responsiveness of paclitaxel/platinum chemotherapy. High expression of pGSK-3α/βTyr279/216 in cancer tissue may be a potential predictor for primary resistance to the first-line chemotherapy. Our results are inconsistent with the report by Cai et al,9 who demonstrated that GSK-3β inhibition may confer resistance to cisplatin in the A2780 ovarian carcinoma cell line in vitro. However, their results came from only one cell model, without verification of clinical samples. It cannot be excluded that GSK-3 may play a different role in different ovarian cancer cells.
There have been a number of conflicting reports concerning the prognostic values of GSK-3β in human cancers.17–23 In the present study, we found that high expressions of GSK-3α/β and pGSK-3α/βTyr279/216 were significantly associated with shortened overall survival in patients with EOC. It gave us a clue that overexpression and aberrant activation of GSK-3 might be potential independent predictors for poor prognosis in ovarian cancer. Our results agreed with previous results shown in lung cancer18 and bladder cancer.22 Nevertheless, the current results do not agree with those in tongue cancer,17 breast cancer,19,20 gastric cancer,21 and lymphoma.23 Previous studies indicated that the biological significance of GSK-3 activation or inactivation appears to be cell-type specific. In cancers of the pancreas,24 colon,25 ovary,8 and osteosarcoma,26 GSK-3β activity is believed to promote cell survival or tumor growth. Additionally, cell proliferation and survival were attenuated by inhibiting the activity of GSK-3β in myeloma,27 thyroid cancer,28 leukemia,29 glioma,30,31 renal cancer,32 gastrointestinal cancer,33 and bladder cancer22 cells. Moreover, GSK-3 inhibition may enhance the antitumor effect of TNF-related apoptosis-inducing ligand (TRAIL) in pancreatic cancer cells or sorafenib in renal cell carcinoma.34,35 Inconsistently, GSK-3β seems to be a “tumor suppressor” or “proapoptotic protein” in other cancer cells, such as hepatoma36,37 and breast cancer38 cells. Considering the biologic complexity of GSK-3 in various normal cellular functions and the pathogenesis of various cancers, we believe that this discrepancy is not surprising.
Recently, Cao et al8 and Hilliard et al10 showed GSK-3 as a potential therapeutic target in ovarian cancer. Nevertheless, the biological significance of GSK-3 in paclitaxel-resistant ovarian cancer cells has not been evaluated. Our study revealed that pharmacological inhibitors of GSK-3 can reduce viability of SKOV3 and SKOV3-TR30 cells, a paclitaxel-resistant cell subline derived from SKOV3. We also found that genetic depletion of either GSK-3α or β isoforms can significantly decrease viability of paclitaxel-sensitive and -resistant ovarian carcinoma cells. Although most of the previous studies have not focused on the significance of GSK-3α, a role for GSK-3α in acute myeloid leukemia has been demonstrated.39 Our results indicate that both GSK-3 isoforms may contribute to regulate cell viability in ovarian carcinoma.
To validate the in vitro findings of growth inhibition in an in vivo situation, nude mice were implanted with SKOV3 cells. The dose of LiCl was chosen based on previous reports evaluating subcutaneously grafted thyroid cancers.28 Our study showed that treatment of LiCl in mice bearing tumor xenograft could significantly slow tumor growth compared with the saline control. This finding was in agreement with a previous study, although Cao et al mixed LiCl and tumor cells together before subcutaneous injection.8 The safety of lithium ions has been established as a result of its use in the treatment of psychotic diseases for over 60 years. Thus, the possibility that LiCl could be a viable treatment for ovarian cancer is exciting.
In summary, we have shown that overexpression and aberrant activation of GSK-3 may contribute to progression of ovarian cancer and have a potential role as independent prognostic factors in EOC patients, and that inhibition of GSK-3 may be a potential therapy for ovarian cancer. Further studies are warranted to confirm and expand these findings, including screening more reasonable administration schedules of GSK-3 inhibitor/chemotherapy combination.
Acknowledgments
This work was supported by the Natural Science Foundation of Zhejiang Province, People’s Republic of China (LY12H16023) and the National Natural Science Foundation of China (30901591).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Guarneri V, Piacentini F, Barbieri E, Conte PF. Achievements and unmet needs in the management of advanced ovarian cancer. Gynecol Oncol. 2010;117(2):152–158. doi: 10.1016/j.ygyno.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 3.Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A. Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol. 2009;156(6):885–898. doi: 10.1111/j.1476-5381.2008.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29(2):95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Luo J. Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009;273(2):194–200. doi: 10.1016/j.canlet.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel S, Woodgett J. Glycogen synthase kinase-3 and cancer: good cop, bad cop? Cancer Cell. 2008;14(5):351–353. doi: 10.1016/j.ccr.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu Y, Hu D, Qiu J, Xie X, Ye F, Lu WG. Overexpression of glycogen synthase kinase-3 in ovarian carcinoma cells with acquired paclitaxel resistance. Int J Gynecol Cancer. 2011;21(3):439–444. doi: 10.1097/IGC.0b013e31820d7366. [DOI] [PubMed] [Google Scholar]
- 8.Cao Q, Lu X, Feng YJ. Glycogen synthase kinase-3beta positively regulates the proliferation of human ovarian cancer cells. Cell Res. 2006;16(7):671–677. doi: 10.1038/sj.cr.7310078. [DOI] [PubMed] [Google Scholar]
- 9.Cai G, Wang J, Xin X, Ke Z, Luo J. Phosphorylation of glycogen synthase kinase-3 beta at serine 9 confers cisplatin resistance in ovarian cancer cells. Int J Oncol. 2007;31(3):657–662. [PubMed] [Google Scholar]
- 10.Hilliard TS, Gaisina IN, Muehlbauer AG, Gaisin AM, Gallier F, Burdette JE. Glycogen synthase kinase 3β inhibitors induce apoptosis in ovarian cancer cells and inhibit in-vivo tumor growth. Anticancer Drugs. 2011;22(10):978–985. doi: 10.1097/CAD.0b013e32834ac8fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novetsky AP, Thompson DM, Zighelboim I, et al. Lithium chloride and inhibition of glycogen synthase kinase 3β as a potential therapy for serous ovarian cancer. Int J Gynecol Cancer. 2013;23(2):361–366. doi: 10.1097/IGC.0b013e31827cfecb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Xie X, Lu WG, Ye DF, Chen HZ, Fu YF. The value of curettage in diagnosis of endometrial hyperplasia. Gynecol Oncol. 2002;84(1):135–139. doi: 10.1006/gyno.2001.6476. [DOI] [PubMed] [Google Scholar]
- 14.Fu Y, Ye D, Chen H, Lu W, Ye F, Xie X. Weakened spindle checkpoint with reduced BubR1 expression in paclitaxel-resistant ovarian carcinoma cell line SKOV3-TR30. Gynecol Oncol. 2007;105(1):66–73. doi: 10.1016/j.ygyno.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 15.Kruger NJ. The Bradford method for protein quantitation. Methods Mol Biol. 1994;32:9–15. doi: 10.1385/0-89603-268-X:9. [DOI] [PubMed] [Google Scholar]
- 16.Do TV, Kubba LA, Antenos M, Rademaker AW, Sturgis CD, Woodruff TK. The role of activin A and Akt/GSK signaling in ovarian tumor biology. Endocrinology. 2008;149(8):3809–3816. doi: 10.1210/en.2007-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goto H, Kawano K, Kobayashi I, Sakai H, Yanagisawa S. Expression of cyclin D1 and GSK-3beta and their predictive value of prognosis in squamous cell carcinomas of the tongue. Oral Oncol. 2002;38(6):549–556. doi: 10.1016/s1368-8375(01)00121-x. [DOI] [PubMed] [Google Scholar]
- 18.Zheng H, Saito H, Masuda S, Yang X, Takano Y. Phosphorylated GSK3-beta-ser9 and EGFR are good prognostic factors for lung carcinomas. Anticancer Res. 2007;27(5B):3561–3569. [PubMed] [Google Scholar]
- 19.Ding Q, He X, Xia W, et al. Myeloid cell leukemia-1 inversely correlates with glycogen synthase kinase-3beta activity and associates with poor prognosis in human breast cancer. Cancer Res. 2007;67(10):4564–4571. doi: 10.1158/0008-5472.CAN-06-1788. [DOI] [PubMed] [Google Scholar]
- 20.Armanious H, Deschenes J, Gelebart P, Ghosh S, Mackey J, Lai R. Clinical and biological significance of GSK-3β inactivation in breast cancer-an immunohistochemical study. Hum Pathol. 2010;41(12):1657–1663. doi: 10.1016/j.humpath.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Cho YJ, Kim JH, Yoon J, et al. Constitutive activation of glycogen synthase kinase-3beta correlates with better prognosis and cyclin- dependent kinase inhibitors in human gastric cancer. BMC Gastroenterol. 2010;10:91. doi: 10.1186/1471-230X-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naito S, Bilim V, Yuuki K, et al. Glycogen synthase kinase-3beta: a prognostic marker and a potential therapeutic target in human bladder cancer. Clin Cancer Res. 2010;16(21):5124–5132. doi: 10.1158/1078-0432.CCR-10-0275. [DOI] [PubMed] [Google Scholar]
- 23.Chung R, Peters AC, Armanious H, Anand M, Gelebart P, Lai R. Biological and clinical significance of GSK-3beta in mantle cell lymphoma – an immunohistochemical study. Int J Clin Exp Pathol. 2010;3(3):244–253. [PMC free article] [PubMed] [Google Scholar]
- 24.Ougolkov AV, Fernandez-Zapico ME, Savoy DN, Urrutia RA, Billadeau DD. Glycogen synthase kinase-3beta participates in nuclear factor kappaB-mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res. 2005;65(6):2076–2081. doi: 10.1158/0008-5472.CAN-04-3642. [DOI] [PubMed] [Google Scholar]
- 25.Shakoori A, Ougolkov A, Yu ZW, et al. Deregulated GSK3beta activity in colorectal cancer: its association with tumor cell survival and proliferation. Biochem Biophys Res Commun. 2005;334(4):1365–1373. doi: 10.1016/j.bbrc.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 26.Tang QL, Xie XB, Wang J, et al. Glycogen synthase kinase-3β, NF-κB signaling, and tumorigenesis of human osteosarcoma. J Natl Cancer Inst. 2012;104(10):749–763. doi: 10.1093/jnci/djs210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.G-Amlak M, Uddin S, Mahmud D, et al. Regulation of myeloma cell growth through Akt/Gsk3/forkhead signaling pathway. Biochem Biophys Res Commun. 2002;297(4):760–764. doi: 10.1016/s0006-291x(02)02278-7. [DOI] [PubMed] [Google Scholar]
- 28.Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, Chen H. Inactivation of glycogen synthase kinase-3beta, a downstream target of the raf-1 pathway, is associated with growth suppression in medullary thyroid cancer cells. Mol Cancer Ther. 2007;6(3):1151–1158. doi: 10.1158/1535-7163.MCT-06-0665. [DOI] [PubMed] [Google Scholar]
- 29.Ougolkov AV, Bone ND, Fernandez-Zapico ME, Kay NE, Billadeau DD. Inhibition of glycogen synthase kinase-3 activity leads to epigenetic silencing of nuclear factor kappaB target genes and induction of apoptosis in chronic lymphocytic leukemia B cells. Blood. 2007;110(2):735–742. doi: 10.1182/blood-2006-12-060947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotliarova S, Pastorino S, Kovell LC, et al. Glycogen synthase kinase-3 inhibition induces glioma cell death through c-MYC, nuclear factor- kappaB, and glucose regulation. Cancer Res. 2008;68(16):6643–6651. doi: 10.1158/0008-5472.CAN-08-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aguilar-Morante D, Morales-Garcia JA, Sanz-SanCristobal M, Garcia-Cabezas MA, Santos A, Perez-Castillo A. Inhibition of glioblastoma growth by the thiadiazolidinone compound TDZD-8. PLoS One. 2010;5(11):e13879. doi: 10.1371/journal.pone.0013879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bilim V, Ougolkov A, Yuuki K, et al. Glycogen synthase kinase-3: a new therapeutic target in renal cell carcinoma. Br J Cancer. 2009;101(12):2005–2014. doi: 10.1038/sj.bjc.6605437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mai W, Kawakami K, Shakoori A, et al. Deregulated GSK3{beta} sustains gastrointestinal cancer cells survival by modulating human telomerase reverse transcriptase and telomerase. Clin Cancer Res. 2009;15(22):6810–6819. doi: 10.1158/1078-0432.CCR-09-0973. [DOI] [PubMed] [Google Scholar]
- 34.Mamaghani S, Simpson CD, Cao PM, et al. Glycogen synthase kinase-3 inhibition sensitizes pancreatic cancer cells to TRAIL-induced apoptosis. PLoS One. 2012;7(7):e41102. doi: 10.1371/journal.pone.0041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawazoe H, Bilim VN, Ugolkov AV, et al. GSK-3 inhibition in vitro and in vivo enhances antitumor effect of sorafenib in renal cell carcinoma (RCC) Biochem Biophys Res Commun. 2012;423(3):490–495. doi: 10.1016/j.bbrc.2012.05.147. [DOI] [PubMed] [Google Scholar]
- 36.Beurel E, Kornprobst M, Blivet-Van Eggelpoël MJ, et al. GSK-3beta inhibition by lithium confers resistance to chemotherapy-induced apoptosis through the repression of CD95 (Fas/APO-1) expression. Exp Cell Res. 2004;300(2):354–364. doi: 10.1016/j.yexcr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Beurel E, Kornprobst M, Blivet-Van Eggelpoël MJ, Cadoret A, Capeau J, Desbois-Mouthon C. GSK-3beta reactivation with LY294002 sensitizes hepatoma cells to chemotherapy-induced apoptosis. Int J Oncol. 2005;27(1):215–222. [PubMed] [Google Scholar]
- 38.Alao JP, Stavropoulou AV, Lam EW, Coombes RC. Role of glycogen synthase kinase 3 beta (GSK3beta) in mediating the cytotoxic effects of the histone deacetylase inhibitor trichostatin A (TSA) in MCF-7 breast cancer cells. Mol Cancer. 2006;5:40. doi: 10.1186/1476-4598-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banerji V, Frumm SM, Ross KN, et al. The intersection of genetic and chemical genomic screens identifies GSK-3α as a target in human acute myeloid leukemia. J Clin Invest. 2012;122(3):935–947. doi: 10.1172/JCI46465. [DOI] [PMC free article] [PubMed] [Google Scholar]