Abstract
A recent report from our group has described that upon engulfment of pathogens, a subset of phagosomes is formed to preserve antigens for prolonged presentation on MHC class II molecules. The distinctive feature of these particular vesicles is their coating with LC3/Atg8, a key component of the autophagy machinery. Here we discuss the possible outcomes of LC3-associated phagocytosis and its implications in the context of immunity.
Keywords: Atg8/LC3, macroautophagy, phagocytosis, phagosomal maturation, MHC class II
Macroautophagy is a catalytic process meant to degrade intracellular components by directing them to lysosomes. This evolutionarily conserved pathway was initially described to be responsible for the recycling of nutrients during starvation. Apart from its impact on cell survival, macroautophagy is also a relevant process in both innate and adaptive immunity, because it can facilitate detection and antigen presentation of pathogens, as well as promote microbial digestion. The macroautophagy core machinery consists of a set of ATG proteins. Among these, LC3 is at the core of the macroautophagic process. It is the only essential macroautophagy protein that can be found in the autophagosome after its completion, and therefore is the marker par excellence to experimentally monitor the fate of these vesicles.
However, researchers have provided evidence for an alternative LC3-dependent process, first described in 2007 and termed LC3-associated phagocytosis (LAP). LAP recruits LC3 molecules to phagosome membranes after receptor engagement. Several receptors can mediate LC3 recruitment, including TLR1/2, TLR2/6, TLR4, FCRL (Fc receptor-like), CLEC7A/Dectin-1 and also recognition of apoptotic, necrotic, or entotic cells. But why is LC3 being diverted from its traditional location, associated with phagophores and autophagosomes, to phagosomes? How often and under which conditions does it occur? The existing literature, including our recent report, suggests that the outcome of LAP differs depending on its cellular context. Importantly, the majority of studies describing LAP used murine cell lines or mouse models. In our study we used human phagocytes instead. The frequency of LC3-positive phagosomes seems to differ between species, with between 40% and 80% of the total phagosomes in mouse but only 5% to 10% in human cells being LC3-associated. Furthermore, the maintenance kinetics of LC3-positive phagosomes depends on their species origin. In murine cells they have been reported to be fairly short-lived, with LAP peaking at 30 min to 1 h after phagocytosis and significantly decreasing 2 h after the initial stimulus. However, in human cells we observed that LC3-positive phagosomes can persist for more than 4 h after their formation. We suggest that these dissimilarities might account for different functions of this subset of phagosomes.
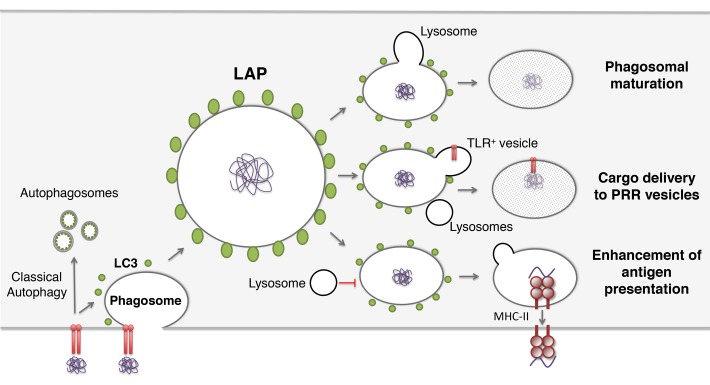
So far 3 main functions have been attributed to LAP (Fig. 1). One line of evidence supports the idea that LAP can be promoting the fusion of lysosomes with phagosomes and, therefore, favoring phagosomal maturation. Second, LAP might deliver phagosome cargo to pathogen recognition receptors. LAP has also been seen as a facilitator of peptide presentation on MHC class II molecules.
Figure 1. Possible outcomes for LC3-associated phagocytosis (LAP). LC3 recruitment to phagosomes can increase fusion with lysosomes and consequently promote phagosomal maturation. Alternatively, cargo contained in LC3-positive phagosomes might be delivered to pathogen recognition receptors (PRRs). In addition, LAP can facilitate antigen presentation, by preserving phagosomal cargo for longer periods of time.
LAP as a Pathway to Enhance Pathogen Degradation within Phagosomes
One of the proposed functions for LAP is the enhanced killing of engulfed pathogens. This notion was supported by comparing autophagy-deficient and -proficient cells for clearance of bacteria and yeast. In the absence of membrane-bound LC3, intraphagosomal pathogens obtain a survival advantage compared with control cells. Later on, it was observed that degradation of engulfed dead cells or entotic cells is also compromised in Atg5, Atg7, Becn1, and Pik3c3/Vps34 knockdown or knockout cells. Altogether, this led to the conclusion that LC3 recruitment to phagosomes is required for phagosome maturation. Some of these studies monitored the recruitment of lysosomal dyes or membrane proteins (LAMP1) to phagosomes, but did not distinguish LC3-positive vs. -negative phagosomes in these fusion events. Moreover the fate of phagosomal membrane-coupled LC3 was not characterized in detail.
LAP as a Pathway to Delay Hydrolytic Capacity of Phagosomes
Our lab studied the kinetics of the LAP vesicles in human antigen-presenting cells. In general, LC3-positive phagosomes are found to be long-lived structures with delayed recruitment of lysosomes. In the face of these observations we suggested that LC3-associated phagosomes are a special compartment for prolonged antigen storage. Alkalinization of the phagosomal lumen had been previously reported to result in slow degradation of internalized antigens. Indeed, dendritic cells (DCs), which are more potent antigen-presenting cells than macrophages, contain fewer proteolytic phagosome compartments. The mechanisms described to sustain antigen presentation involve recruitment of the NADPH oxidase CYBB/NOX2, and SEC22B-dependent recruitment of ER proteins. In addition, LAP seems to be required for IFN-α secretion in plasmacytoid DCs. Activation of inflammatory cytokine production results from TLR9 trafficking into specialized IRF7 compartments. LAP is necessary to generate IRF7-signaling vesicles in response to DNA-containing immune complexes and later on is required to attract TLR9+ phagosomes to those vesicles. This evidence supports the hypothesis that LAP can stabilize cargo for delayed processing and recognition, rather than immediate delivery for degradation. Finally, in line with our report, a recent publication also suggested that MHC class II presentation is facilitated by LAP. LC3B-deficient murine DCs were pulsed with yeast expressing ovalbumin and co-cultured with ovalbumin-specific CD4+ T cells. They present this model antigen less efficiently than wild-type DCs. In addition, LC3 and MHC class II colocalize in vesicles containing whole yeast or β-glucan particles, suggesting that LAP could be implicated in potentiating processing of fungal-derived antigens.
Furthermore, during herpes simplex virus infection in vivo, macroautophagy-deficient animals exhibit impaired CD4+ T cell responses. The authors suggested LAP function in the regulation of phagosome maturation as the underlying mechanism. Indeed, despite the fact that atg5−/− DCs present normal endocytic and phagocytic capacity, they fail to process viral antigen for MHC class II presentation. Although lysosomal pH does not seem to be altered, cathepsin proteases are less efficiently recruited to phagosomes in Atg5-deficient DCs, but it remains unclear if LC3-associated vs. LC3-negative phagosomes differ in cathepsin accumulation. Thus, LAP seems to contribute to antigen processing for MHC class II presentation, but only a subset of phagosomes within each cell is regulated by this pathway, and LC3 association changes the fate of the affected phagosomes depending on the cellular context. In human phagocytes, LAP stabilizes substrates for delayed processing toward MHC class II presentation to CD4+ T cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.