Abstract
Chronic obstructive pulmonary disease (COPD) involves aberrant airway inflammatory responses to cigarette smoke (CS) associated with respiratory epithelial cell cilia shortening and impaired mucociliary clearance (MCC). The underlying cellular and molecular mechanisms for CS-associated cilia shortening have remained incompletely understood. We have previously demonstrated increased autophagy in the lungs of COPD patients; however, whether or not this process is selective for specific autophagic targets in the lung was not elucidated. Based on observations that increased morphological and biochemical indicators of autophagy correlate with cilia shortening in our models, we posited that autophagy might regulate cilia length in response to CS in the lung. We demonstrate that CS-induced cilia shortening occurs through an autophagy-dependent mechanism mediated by the deacetylase HDAC6 (histone deacetylase 6). Autophagy-impaired (Becn1+/−, map1lc3b−/−, or Hdac6-/Y) mice resist CS-induced cilia shortening. Furthermore, cilia components are identified as autophagic substrates during CS exposure. Assessment of airway cilia function using a 3D MCC assay demonstrates that Becn1+/−, map1lc3b−/−, and Hdac6-/Y mice or mice injected with the HDAC6 inhibitor tubastatin A are protected from CS-associated mucociliary dysfunction. We concluded that an autophagy-dependent pathway regulates cilia length during CS exposure, which identifies new pathways and targets in COPD.
Keywords: cilia, autophagy, lung, COPD, HDAC6
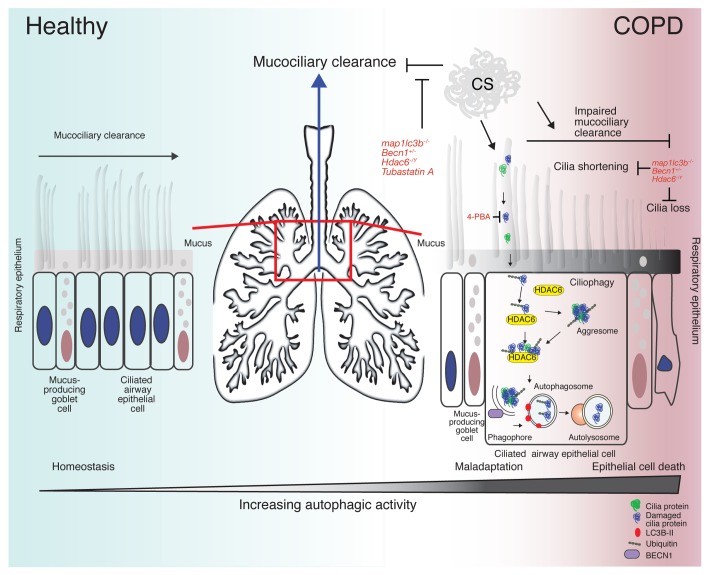
As the 4th leading cause of death in the USA, COPD remains a complex, debilitating lung disease that encompasses a variety of pathological conditions ranging from airway inflammation (chronic bronchitis) to the destruction of peripheral lung tissue (emphysema). The pathogenesis of COPD remains poorly understood, but involves aberrant cellular and inflammatory responses of the lung to cigarette smoke, resulting in the disruption of airway epithelial cell function. Such disruption has been attributed to a reduction in epithelial cell cilia length and airway epithelial cell death, followed by re-epithelialization by goblet cells, resulting in excess mucus production leading to impaired mucociliary clearance (Fig. 1). Impaired airway clearance prevents the elimination of particles and pathogens trapped in mucus from the airways and may promote susceptibility to respiratory infections that exacerbate COPD.
Figure 1. Ciliophagy and COPD. The mucociliary escalator covers most of the bronchi, bronchioles, and nose. It is composed of mucus-producing goblet cells and the ciliated epithelium (left). The cilia are constantly beating, pushing mucus up and out into the throat (center). Cigarette smoke induces oxidative stress, which leads to cilia protein damage, misfolding, ubiquitination, and the formation of intracellular protein aggregates (right). HDAC6 recognizes ubiquitinated protein aggregates and delivers them to the autophagosome. Cilia proteins are subsequently delivered to the lysosome for degradation or recycling. In cases of chronic oxidative stress, cilia proteins are degraded resulting in the shortening of airway cilia contributing to impaired mucociliary clearance (right). Genetic deletion of LC3B (map1lc3b), BECN1 (Becn1) or HDAC6 (Hdac6) or inhibiting HDAC6 (tubastatin A) or protein misfolding (4-PBA) alleviates CS-induced impairment of MCC. Severe oxidative stress eventually leads to programmed epithelial cell death, a process that may involve excessive autophagy.
Ciliated cells of the respiratory tract have large numbers of motile cilia each nucleated by a basal body, from which extends the ciliary axoneme. As cultured epithelial or primary cell lines cannot form motile cilia, we developed a physiologically relevant model of differentiated mouse tracheal epithelial cells (MTECs) grown at the air-liquid interface and exposed them to mainstream cigarette smoke. In this model and in vivo, increased morphological and biochemical indicators of autophagy correlate with cilia shortening. Furthermore, short-term exposure to CS in vitro, or subchronic exposure in vivo, results in increases in the autophagic processing of substrate proteins (increased flux) in MTECs, and in lung tissue, respectively.
Mice with autophagy-deficiency (Becn1+/− and map1lc3b−/−) resist cilia shortening caused by CS exposure in vitro and in vivo. These observations are consistent with previous studies, where knockout of Atg7 in the airways disrupts cilia formation, hyperactivation of mechanistic target of rapamycin (MTOR) results in abnormally long cilia, and rapamycin shortens embryonic cilia. Similarly, we identified cilia components as substrates for autophagic pathway processing during CS exposure. Cilia components colocalize with autophagosomes, and cilia proteins are enriched in autophagosome/lysosomal fractions basally and upon CS exposure in vitro and in vivo, suggesting that cilia proteins may be sequestered within autophagosomes in response to CS exposure.
Evaluation of the expression levels of proteins known to regulate primary cilia length in human bronchial epithelial cells subjected to CS treatment identified HDAC6, a protein that regulates primary cilia resorption and the autophagy pathway through autophagosome-lysosome fusion. Hdac6-/Y MTECs and mice, which display an autophagy-deficient phenotype, are protected against CS-induced cilia shortening. HDAC6 is also upregulated and hypomethylated at the epigenetic level in human COPD patients. HDAC6 has dual functionality as a ubiquitin-binding protein and as a regulator of protein acetylation. We identified the ubiquitin-binding activity and not the deacetylase activity of HDAC6 as critical for its function in the transport of CS-associated ubiquitinated proteins to aggresomes facilitating autophagy. HDAC6 expression was increased by oxidative and proteostatic stress but counter-regulated by NFE2L2/Nrf2 and acetylated by SIRT1/Sirtuin 1.
Ciliated airway epithelial cells are of crucial importance in airway physiology and pathophysiology. Measurement of MCC in humans represents an important clinical tool to assess airway function in lung diseases like COPD or cystic fibrosis. To test the hypothesis that autophagy plays a maladaptive role in cilia homeostasis we implemented a physiological model of airway MCC. We observed that genetic deletion of the autophagy mediators BECN1 and LC3B, or genetic and chemical inhibition of HDAC6 protect mice from MCC disruption upon CS exposure. Treatment of mice with the chemical chaperone 4-PBA also improves MCC following CS exposure, which suggests that the preservation of proteins required for ciliated cell function might reduce autophagic substrates and enhance MCC. Consistently, COPD severity is associated with a general increase in protein aggregates in human lung COPD tissues.
The effect of CS on mucociliary clearance is reversible and not associated with increased apoptosis, suggesting that epithelial cell death is a minor contributor to airway dysfunction in our in vivo model. However, the accumulation of CS-denatured protein that exceeds cellular degradative capacity into aggresomes might represent a harmful intermediate state prior to cell death. While we show that the activation of the proposed ciliophagy pathway leads to cilia shortening, we also demonstrate that excessive activation of autophagy might ultimately lead to ciliated cell loss and ciliated cell death. These findings are consistent with our previous observations in which epithelial cells from LC3B knockout (map1lc3b−/−) mice are protected from CS-induced apoptosis, and these mice are resistant to CS-induced emphysema development.
Currently, few advances have been made to alleviate MCC disruption and bronchitis associated with the pathogenesis of COPD. Our study demonstrates that HDAC6 and other regulators of the autophagic pathway adversely impact cilia length, phenotypic responses and functional airway outcomes during CS exposure, in a process we term “ciliophagy” (Fig. 1). Our findings suggest potential new therapeutic targets for improving airway function during chronic lung diseases such as COPD through the maintenance of epithelial cell proteostasis and modulation of the autophagic pathway.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by NIH grants, R01-HL60234, R01-HL55330, R01-HL079904, P01-HL70807, HL007118 and P01-HL105339 as well as a FAMRI clinical innovator award, an American Lung Association award (09PRE2250120), and salary support from the Lovelace Respiratory Research Institute.