Abstract
Outcomes for patients with hematologic malignancies who experience overt relapse after allogeneic hematopoietic stem cell transplantation (HCT) are poor. There are limited data on outcomes of post-transplant minimal residual disease (MRD). In this single institution, retrospective cohort analysis of children with acute leukemia and myelodysplastic syndrome we document the pattern of relapse with a primary focus on outcomes of post-transplant MRD. Forty of 93 (43%) patients who underwent a first allogeneic HCT and who all had systematic pre and post-transplant MRD evaluations at +30, +60, +90, +180 days and at +1 and +2 years post-transplant, experienced relapse. The median time to relapse was 4.8 months post-transplant with a median survival of 4 months post-relapse. Despite frequent, systematic, routine post-HCT disease restaging evaluation, 31 patients (78%) presented with overt disease at the time of relapse. 7 patients with acute leukemia who had post-transplant MRD, presented at a median of 1 month post-transplant. Due to rapid disease progression or treatment-related mortality (TRM), there was no improvement in survival for those patients whose leukemia was detected in a state of MRD post-transplant. Our results suggest that early intervention strategies targeting post-transplant MRD for relapse prevention in acute leukemia may not be feasible.
Keywords: Relapse, Allogeneic hematopoietic cell transplantation, Pediatrics, Leukemia, Minimal Residual Disease
Introduction
Relapse is the primary cause of treatment failure for patients with hematologic malignancies who undergo allogeneic hematopoietic stem cell transplantation (HCT).(1) Once patients have relapsed after HCT, treatment options are limited and the outlook is in general poor.(2–7) One potential approach to improve post-transplant outcomes would be to utilize preemptive interventions for relapse prevention. Treatment of post-transplant minimal residual disease (MRD) (< 5% bone marrow blasts or positive cytogenetic or molecular markers of disease) to prevent overt relapse may be one such strategy.(8, 9)
The majority of studies evaluating post-transplant relapse in acute leukemia are based on patients who present with overt morphologic relapse or high disease burden, where outcomes are poor.(3, 4, 6) However, with frequent post-transplant surveillance and more sensitive measures of detection, disease recurrence could, in theory, be detected both earlier and at a state of lower disease burden that may be more amenable to treatment, potentially leading to improved outcomes.(10–12) Certainly, preemptive immunotherapy in the setting of mixed chimerism has shown promise in relapse prevention.(13–16) Additionally, treatment of MRD using donor lymphocyte infusion (DLI) in the setting of chronic myelogenous leukemia prior to hematologic relapse has lead to durable remissions.(17–19) Outcomes with DLI for treatment of acute leukemia, however, are quite variable.(20–22) There is limited data, in general, on the outcomes of post-transplant MRD specifically in the setting of acute leukemia.(21, 23–26)
In this study, we describe the presentation and management of children with hematologic malignancies who experience post-transplant relapse. With a focus on understanding the pattern of relapse, the goal was to determine if post-transplant MRD would be amenable to intervention for relapse prevention.
Methods
Patients and Inclusion Criteria
This was a single-institution, retrospective cohort study of pediatric patients (age less than or equal to 21 years) who relapsed after having undergone a first allogeneic HCT for a hematologic malignancy between January 1, 2003 and December 31, 2010 at The Johns Hopkins Hospital (JHH). This included all patients with a diagnosis of acute lymphoblastic leukemia (ALL), acute myelogenous leukemia (AML), myelodysplastic syndrome (MDS), mixed phenotypic acute leukemia (MPAL) and lymphoblastic lymphoma (LBL), irrespective of disease status, transplant conditioning, donor and stem cell source, HLA matching or other transplant-related variables. Patients with other types of leukemia, including blast crisis chronic myelogenous leukemia (CML), were excluded. For this analysis, one patient with LBL was analyzed with patients with ALL. This study was approved by the JHH Institutional Review Board.
Disease Monitoring, Surveillance, and Definitions
All patients had pre-transplant disease evaluation. Routine post-transplant surveillance occurred at 30, 60, 90, 180 days +/− 10 days and 1 year, and 2 years +/− 1 month post-transplant and then as clinically indicated. Evaluation was disease-specific and included evaluation of chimerism (peripheral blood and marrow), and flow cytometric, cytogenetic and molecular MRD studies (e.g., bcr/abl in Philadelphia chromosome positive ALL) from the bone marrow. In addition, lumbar punctures were routinely performed at above time points to assess CNS status in all patients.
The day of relapse after HCT was identified by the first day of laboratory confirmation of disease presence, inclusive of post-transplant MRD. In patients with ALL, MRD was assessed in our central reference lab using flow cytometric methods that have been previously described.(27) Following definitions published by Leung and colleagues (28) MRD was positive if the level was ≥ 0.01%. For AML, the sensitivity for routine flow cytometric analysis ranged from approximately 0.1% to 1% of cells depending upon the phenotype of the initial leukemia. Treatment related mortality (TRM) was defined as death unrelated to progressive disease and was inclusive of transplant-related mortality or death due to treatment of post-transplant relapse.
Statistical analysis
The primary endpoint was overall survival after post-transplant relapse. Overall survival was defined by the date of relapse until the date of death, censored at the last follow up date for patients who were alive at the time of this analysis. Probabilities of survival were evaluated using the Kaplan-Meier method. The cumulative incidence of relapse, adjusting for the competing risk of death from TRM was calculated using the method of Gooley.(29) T-test and Fisher’s exact test for numerical and categorical variables, respectively, were used to test for differences in patient characteristics between those who did and did not relapse. Analysis of variance was used to analyze the differences between the various presentations of post-transplant relapse, specifically by the time to relapse. The level of statistical significance was set at p<0.05. Statistical analyses were performed with Stata/IC software 12.0 (StataCorp LP, College Station, TX, USA)
Results
Patient and relapse characteristics
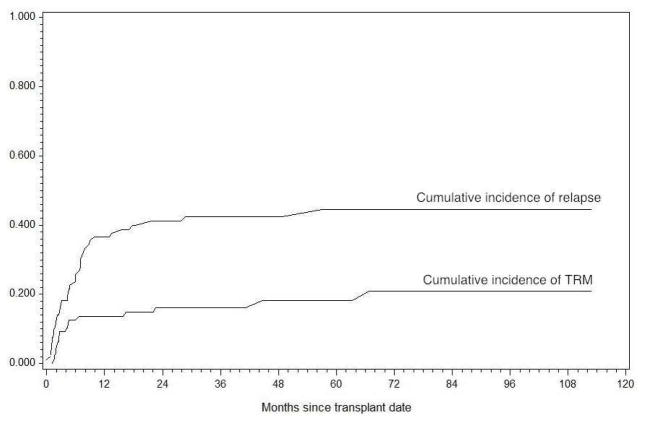
Forty of 93 pediatric patients (43%) who underwent a first allogeneic HCT for acute leukemia or MDS relapsed after HCT. Patient characteristics are shown in Table 1. This included 21 relapses amongst 57 patients (37%) with ALL or AML who were in a morphologic remission and underwent a myeloablative transplant. (Table 2) The cumulative incidence of post-HCT relapse, accounting for the competing risk of transplant-related mortality was 17%, 26%, 37% and 41% at 3, 6, 12 and 24 months respectively. (Figure 1) This included 41 patients with AML (18 relapsed), 34 with ALL (16 relapsed), 10 with MPAL (4 relapsed) and 8 with MDS (2 relapsed).
Table 1.
Characteristics of pediatric patients undergoing first allogeneic HCT for acute leukemia or MDS compared with the subset who relapsed after HCT
| Variable | All patients (n=93) | Patients who relapsed post-HCT (n=40) | Log-rank p-value for comparison of Relapse Free Survival (Relapse vs No relapse) | ||
|---|---|---|---|---|---|
| Median age at HCT, years (range) | 10 (0.6–21.2) | 9 (0.7–20.2) | NS | ||
| n | n (% of all patients) | ||||
| Gender | Male | 62 (67%) | 29 (47%) | NS | |
| Diagnosis | AML | 41 (44%) | 18 (44%) | NS | |
| ALL. | 34 (37%) | 16 (47%) | |||
| MPAL | 10 (11%) | 4 (40%) | |||
| MDS | 8 (9%) | 2 (25%) | |||
| Disease status at HCT, by disease | AML | Active Disease | 8 (9%) | 5 (63%)# | < 0.01** |
| MRD + CR | 13 (14%) | 6 (46%) | |||
| MRD Neg CR | 20 (22%) | 7 (35%) | |||
| ALL | Active Disease | 1 (8%) | 1 (100%) | ||
| MRD + CR | 13 (14%) | 8 (62%) | |||
| MRD Neg CR | 20 (22%) | 7 (35%) | |||
| MPAL | Active Disease | 1 (8%) | 1 (100%) | ||
| MRD + CR | 1 (8%) | 0 (0%) | |||
| MRD Neg CR | 8 (9%) | 3 (38%) | |||
| MDS | 8 (9%) | 2 (25%) | |||
| Performance Status at HCT | 80–100% | 84 (90%) | 33 (39%) | 0.02 | |
| 40–70% | 8 (9%) | 6 (75%) | |||
| Indication for HCT (For leukemia patients only, n=85) | Primary Induction Failure | 12 (13%) | 6 (50%) | NS | |
| High-Risk Disease* | 24 (26%) | 6 (25%) | |||
| Relapsed Disease | 25 (27%) | 10 (40%) | |||
| Multiple Indications | 24 (26%) | 16 (67%) | |||
| Remission Number (For leukemia patients only, n=85) | CR1 | 41 (44%) | 16 (39%) | NS | |
| CR2 | 27 (63%) | 12 (44%) | |||
| CR3+ | 7 (9%) | 4 (57%) | |||
| Refractory | 10 (11%) | 7 (70%)# | |||
| HCT Conditioning Regimen | Myeloablative | 79 (85%) | 30 (38%) | <0.01 | |
| Reduced Intensity | 14 (15%) | 10 (71%) | |||
| Stem Cell Source | Bone Marrow | 71 (76%) | 34 (48%) | NS | |
| Single Umbilical Cord | 18 (19%) | 6 (33%) | |||
| Double Umbilical Cord | 1 (1%) | 0 (0%) | |||
| Peripheral Blood | 3 (3%) | 0 (0%) | |||
| Donor Type | Matched sibling | 26 (28%) | 11 (42%) | NS | |
| Matched unrelated | 30 (32%) | 12 (40%) | |||
| Cord Blood | 19 (20%) | 6 (32%) | |||
| Haploidentical^ | 15 (16%) | 9 (60%) | |||
| Mismatched related/unrelated | 3 (3%) | 2 (67%) | |||
Abbreviations: ALL=acute lymphoblastic leukemia; AML = acute myelogenous leukemia; MPAL=mixed phenotypic acute leukemia; MDS=myelodysplastic syndrome. Active disease defined by > 5% blasts, including those with refractory disease; CR=complete remission, MRD=minimal residual disease which includes patients with levels > 0.01% by flow cytometry for ALL and > 0.1% for AML/MPAL, or detectable disease by cytogenetics.
High-risk determination was made by transplant physician using a constellation of multiple assessments which included cytogenetics (e.g., monosomy 7, hypodiploid < 43 chromosomes, FLT3/ITD), end-induction MRD positivity and/or phenotype (MPAL) and in conjunction with standard accepted criteria for transplant indications.
p-value is in comparison of relapse free survival for those with active disease to those in a CR.
Many patients who received the initial haploidentical transplants were on an institutional non-myeloablative protocol which also included patients with active disease at the time of transplant.
2 patients with refractory disease prior to HCT died from early TRM before day 100. 1 patient with refractory AML remains a long-term survivor post-HCT without relapse
Table 2.
Relapse Rate and Time to Relapse for Patients with ALL and AML in a Morphologic Remission at HCT who Underwent a Myeloablative Preparative Regimen, by pre-HCT MRD status
| Disease | Pre-HCT MRD Status | Total (n) | Number of Patients Who had Experienced Relapse at or Prior to the Planned Restaging Evaluation Time Points | Total Relapse (n) | Crude Relapse Rate | Median Time to Relapse (Days) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 30 | Day 60 | Day 90 | Day 180 | Day 365 | Day 365+ | ||||||
| ALL | Negative | 20 | 1 | 0 | 1 | 2 | 1 | 2 | 7 | 35% | 182 |
| + | 11 | 0 | 3* | 0 | 1 | 3 | 0 | 7 | 64% | 132 | |
| AML | Negative | 15 | 0 | 0 | 0 | 0 | 2 | 1 | 3 | 20% | 238 |
| + | 11 | 0 | 0 | 0 | 2 | 1 | 1 | 4 | 36% | 224 | |
MRD considered positive when < 5% and ≥ 0.01% in ALL and ≥ 0.1% in AML.
2 subjects presented with post-HCT MRD.
Figure 1. Cumulative Incidence of Relapse and Transplant Related Mortality (TRM).
Cumulative incidence of relapse and TRM were analyzed at competing risks starting at the date of HCT for 93 consecutive patients who underwent a first allogeneic transplant for acute leukemia or MDS
At the time of relapse, the majority (n=31, 78%) presented with morphologic (> 5% disease) relapse. Twenty-two patients (56%) had clinical signs and symptoms consistent with relapse, including presentation with peripheral blasts, extramedullary disease, cytopenias prompting disease evaluation and/or other symptoms concerning for disease recurrence (e.g., pain). Specifically 3 patients had leukemia cutis or chloromatous masses and 1 presented with a testicular mass that prompted further evaluation. Eight (21%) were asymptomatic and relapse was discovered at pre-specified times of routine disease evaluation, including 2 patients who were found to have isolated CNS relapse. Nine patients (23%) presented with post-transplant MRD that was detected on routine surveillance. This included 7 patients with a diagnosis of leukemia and 2 with MDS. Details regarding the presentation of relapse were not available for one subject who presented with confirmed morphologic relapse.
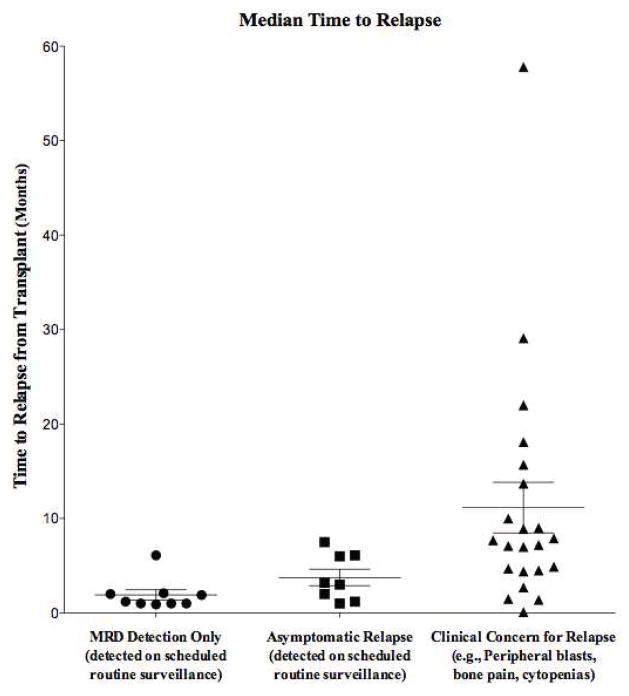
The median time to relapse for all patients was 4.8 months (range, 0.1 –57 months) post-transplant, with a statistically significant difference in the time to relapse by presentation: MRD positive relapse presented at a median of 1 month post-transplant (n=9); patients with evidence of disease detected by routine surveillance presented at a median of 3 months post-transplant (n=8); and those with overt relapse presented at a median time of: 7.5 months post-transplant (n=22) (p < 0.001) (Figure 2) After exclusion of those with refractory disease, the median time to relapse for patients with AML and ALL was 4.5 months (range, 1–15.8 months; n=12) and 6 months (range, 1–29 months; n=14) respectively.
Figure 2. Median time to relapse, by relapse presentation.
Routine marrow evaluation was performed for all pediatrics patients post-transplant at approximately days: + 30, + 60, + 90, + 180, and + 1 and +2 years post-transplant. Analysis of variance was used to analyze the differences in the time to relapse by the various presentations of post-transplant relapse. Middle line of box-plot indicates the median with whiskers indicating top and bottom quarter percentile. (p<0.001)
Management of relapse
Decisions regarding the treatment of relapse varied and were based on the timing of relapse, the patient’s condition and physician and patient/family preference. Six patients received supportive care, including hospice, palliative or complementary medicine only; 3 received withdrawal of immunosuppression in response to MRD detection; 24 patients received cytotoxic and/or radiation therapy; and 13 received DLI (with or without prior chemotherapy). Eleven patients were able to proceed to a second allogeneic HCT after attaining remission.
Overall survival after post-transplant relapse and non-relapse mortality
Overall survival (OS) at 6 months and 1, 2 and 5 years post relapse was 30%, 17.5%, 15% and 11% respectively. Median survival after relapse was 4 months (range 0.1–33 months). Five of 40 (12.5%) patients are currently alive with a median follow-up of 39 months, including 2 patients who continue to be treated for active disease. One survivor had MDS and presented with MRD alone. The remaining 4 presented with overt disease and included 3 patients with ALL and 1 with MPAL.
Death post-relapse was due to a variety of causes. The majority died with progressive disease (n=28). Zero of 18 patients with AML survived after post-transplant relapse. Survival did not appear to differ by therapeutic approach to relapse with the exception of those who underwent a second HCT. The three-year overall survival probability among the 11 patients who underwent a second transplant was 27% (95% CI 6.5–54%) compared to 5.4% (95% CI 0–20%,) for those who did not (p=0.02). The patients who proceeded to a second transplant more often had a later relapse (median time to relapse 8 months (range, 1–29 months)) than those who did not undergo a second transplant (median time to relapse, 3.8 months (range, 1–58 months)). Eight patients died from TRM related to second transplant, which included 3 patients who developed grade IV GVHD. Three remain long-term survivors following second transplant.
Outcomes of post-transplant MRD
All patients who presented with post-transplant MRD were discovered on routine planned surveillance. These patients (n=9) presented at a median of 1-month post-transplant (range, 1–6 months), with 8 of 9 patients having some evidence of pre-transplant disease. Amongst the 7 patients with leukemia, 5 had very rapid progression of disease to overt relapse at a median of 21 days (range, 13–24 days) from first detection of MRD, despite intervention in response to MRD, including early withdrawal of immunosuppression (n=3) and donor lymphocyte infusion (DLI) (n=2) (Table 3). All patients received disease-directed therapy, with the exception of one patient who died from early TRM at day 118 post-transplant with rising levels of MRD at the time of death. All patients receiving disease directed therapy died from TRM with the exception of one patient with MDS who presented with MRD by evidence of cytogenetic relapse at day +180 post-transplant and received DLI prior to any further disease progression. This patient remains a long-term survivor. Survival of patients who presented with MRD post-transplant was no better than those who presented with frank relapse, despite pre-emptive intervention for treatment of MRD.
Table 3.
Outcomes of Patients with Leukemia and Post-Transplant Minimal Residual Disease
| Patient # | Disease | Pre-HCT Disease Status |
Transplant Conditioning |
Donor | Days from HCT to detection of Post- HCT MRD |
% MRD1 | Mode of detection |
Days from HCT to overt relapse |
Days from HCT to first intervention |
Intervention | Survival after HCT (Days) |
Cause of Death | Disease status at last evaluation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | ALL | + BCR-ABL 0.01% by RT-PCR only, flow negative | MA (Cy/TBI) | MSD | 60 | 0.01 | Flow cytometric and cytogenetic, + BCR-ABL PCR | 84 | 90 | Chemo | 115 | Multi-system organ failure | PD |
| 6 | 0.29% MRD by flow | MA (Cy/TBI) | MSD | 56 | 0.01 | Flow cytometric | N/A | 70 | DLI | 83 | CMV PNA, ARDS | None performed prior to death | |
| 76 | +BCR-ABL 0.03% by RT-PCR only, flow negative | NMA | Haploidentical | 35 | BCR-ABL detection by RT-PCR, non-quantifiable | Cytogenetic | Increasing copies of BCR-ABL without overt relapse | No treatment initiated due to ongoing toxicities post HCT | N/A | 153 | Adenovirus, pulmonary hemorrhage, fungal infection | CR | |
| 46 | AML | MRD Negative CR | NMA | Haploidentical | 30 | 0.08 | Cytogenetic | 51 | 30* | WIS→ 2nd HCT | 247 | Multi-system organ failure, GVHD, Sepsis | CR |
| 49 | 1% by flow | NMA | Haploidentical | 29 | 3 | Flow cytometric | 50 | 52 | Chemo + DLI | 81 | Sepsis | PD | |
| 63 | Refractory Disease | MA (Bu/Cy) | MUD | 64 | 1 | Flow cytometric | 77 | 69* | WIS → Chemo | 138 | Sepsis | PD | |
| 65 | Refractory Disease, CNS negative | MA (Bu/Cy) | MUD | 28 | 2 to 3 | Flow cytometric | 45 | 29* | WIS→ Chemo | 150 | VOD, GVHD | PD |
Indicates the level of disease detected at the first evidence for minimal residual disease. Specifically, for those patients who presented at day 56, 60 and 64 prior disease restaging at 1 month post-transplant was negative.
Indicates that therapy was initiated prior to overt relapse.
HCT=hematopoietic cell transplantation; MRD=minimal residual disease; WIS=withdrawal of immunosuppression; DLI=donor lymphocyte infusion; CMV=cytomegalovirus; PNA=pneumonia; ARDS=acute respiratory distress syndrome; GVHD=graft-versus host disease; CR=complete remission; PD=progressive disease; ALL=acute lymphoblastic leukemia; AML=acute myelogenous leukemia; Chemo=chemotherapy; MA=myeloablative; Cy=cyclophosphamide; TBI=Total body irradiation; Bu=busulfan; NMA=non-myeloablative; MSD=matched sibling donor; MUD=matched unrelated donor
Discussion
Despite the hypothesis that treatment of post-transplant MRD may represent a window of opportunity for relapse prevention, and thereby improve post-transplant outcomes, our findings do not support this as an optimal strategy for relapse prevention in patients with acute leukemia. First, despite the frequent monitoring, one critical observation is that the majority of patients (78%) who experienced relapse had already progressed to morphologic relapse by the time of disease detection. Second, amongst those whose disease was detected in a state of MRD, disease progression was often rapid, detected early post-transplant or identified at a time when ongoing toxicities compromised the efficacy of therapeutic interventions.
With the ability to monitor for much lower degrees of disease burden using techniques that greatly increase the sensitivity of disease detection,(11) post-transplant MRD-monitoring was performed at frequent, accepted standard intervals at our center. Despite this rigorous and multimodal routine post-transplant evaluation, inclusive of complete data from all disease evaluation time points with disease assessment by flow cytometric (with ALL MRD analysis performed at our central reference lab), molecular and chimerism studies, it was unexpected that the vast majority of patients would still present with morphologic relapse. This was especially notable in the patients with ALL and AML who were in a morphologic remission at the time of transplant and underwent a myeloablative transplant. In this subgroup, only 2 of 21 relapsed patients presented with post-transplant MRD prior to morphologic relapse.
One possibility for this finding may be related to the timing of disease recurrence in relation to the timing of disease evaluation. In our study, the median time to relapse was at 4.8 months post-transplant, right between the day 90 (3 month) and 6 month scheduled evaluation. This suggests that adding an interval evaluation between 3 and 6 months may be useful in earlier detection of MRD prior to morphologic relapse. Similarly, in a recent study by Zhao et al (26) which evaluated post-HCT MRD in ALL, they also noted MRD was not detected prior to hematologic relapse in the patients who relapsed between 3 and 6 months, suggesting the need for additional evaluation during this period. At our center, we continue to do frequent MRD surveillance, and often add an additional MRD evaluation between the 3–6 month period and around 9 months post-transplant for patients with high-risk disease.
Additionally, the sensitivity of disease detection methods may also be an important consideration. Balduzzi et al(21) in a recent study evaluating pre and post-HCT MRD in ALL using real-time quantitative polymerase chain reaction methods demonstrated that intervention upon low-level post-transplant MRD (< 1 × 10−4) could prevent overt relapse. However, in their study, all those with higher level MRD (≥ 1 × 10−3) or those who experienced a one-log increase in MRD, (albeit still at low levels), all ultimately experienced overt relapse despite pre-emptive interventions. In our study, 4 patients with AML relapse had higher-level MRD and those with ALL progressed rapidly. This suggests that a more sensitive method of disease detection in the post-transplant setting, where there may be prognostic implications of very low levels of disease, should be incorporated. This strategy, however, may not be useful for predicting extramedullary relapse, which may not be reliably detected with bone marrow monitoring. (21, 26, 30) Six patients in our study presented with extramedullary relapse as the first manifestation of disease recurrence without prior marrow disease involvement.
Even with more frequent monitoring and more sensitive measures of disease detection, it is uncertain if these measures would improve outcomes for the majority with post-transplant relapse given the ability to treat only very low levels of disease and the potential for rapid disease progression. In our study, the median time for disease progression from the first post-transplant MRD detection to overt relapse was rapid (median time, 21 days, in our cohort), with other studies reporting time to overt relapse within 1–3 months after detection of MRD.(26) Immunotherapeutic approaches to induce a graft-versus-leukemia effect with early withdrawal of immunosuppression or DLI may be beneficial and most effective in patients with early relapse and low burden disease, but may require weeks to take effect and efficacy is limited in the setting of rapid disease progression or higher burden disease. (21, 31, 32) Additionally, this may not be an option in those with pre-existing GVHD.(13, 33–37) Disappointingly, in our study, similar to other reports, use of DLI, even pre-emptively, was not associated with long-term survival in patients with acute leukemia. (5, 21, 32, 38) Other treatment options for MRD, especially in the early post-transplant setting, are limited due to ongoing transplant-related co-morbidities. Cytoreductive therapy is generally poorly tolerated; accordingly all patients who received chemotherapy to treat post-transplant MRD died from treatment-related toxicity.
In light of the limited ability to treat post-transplant MRD, our study provides further support of the need for improved pre-transplant risk stratification for identification of those at highest risk of relapse in whom early interventions, such as early withdrawal of immunosuppression or DLI, for relapse prevention would be indicated. (21, 25, 39) Because of the important prognostic value of pre-transplant MRD status on post-transplant outcomes, specifically in ALL,(40) pre-transplant MRD reduction is another strategy that may lead to improved post-transplant outcomes. (21) Since this population has relatively chemotherapy refractory disease, we now consider referring patients with pre-transplant MRD for novel immunotherapeutic clinical trials for MRD reduction prior to transplant (e.g., chimeric antigen receptor therapy, immunotoxin therapy), in an attempt to improve post-transplant outcomes, an approach that needs further evaluation.
While pre-transplant MRD positivity is the most predictive factor of post-transplant relapse(40–42), we do not believe that this should preclude proceeding to transplant. Certainly, improved pre-emptive interventions may reduce relapse risk in those with pre-transplant MRD. In our study, amongst those who underwent a myeloablative conditioning and were in a morphologic remission, 10 of 35 (28.5%) who were MRD negative and 11 of 22 (50%) who were MRD positive experienced relapse. (Table 2) Consistent with other studies, (28) patients with pre-transplant MRD had a higher rate of relapse, but many were able to experience disease-free survival, with a lesser prognostic value of pre-transplant MRD in AML than in ALL.
The main limitation of our study is in the retrospective design incorporating a heterogeneous patient population, including higher-risk patients with refractory disease and/or those who have undergone a nonmyeloablative/reduced intensity conditioning. However, as our study demonstrated, even for those who were in remission and underwent a myeloablative transplant, findings were similar. Additionally, the limited sensitivity of our AML flow cytometric MRD may have missed very low levels of MRD prior to overt relapse, biasing more of the AML patients to present at a state of higher disease burden, when intervention was less effective. Certainly ongoing development focused on optimizing evaluation of AML MRD should be implemented to improve upon the ability of disease detection to attempt early preemptive intervention for relapse prevention.(42–44)
In conclusion, our results illustrate the challenges in treating post-transplant MRD for relapse prevention in patients with acute leukemia. Primarily, most patients who relapse may already be in a state of overt relapse at the time of disease detection. Additionally, for those whose relapse is detected at the stage of MRD, our results do not demonstrate a survival advantage. Whether more frequent or more sensitive measures of disease evaluations in the early post-transplant period, to potentially identify an even lower degree of MRD or detect disease prior to overt relapse, would lead to improved outcomes is one possibility that needs to be further explored—but post-transplant intervention may still be limited by the early timing of relapse and/or rapid disease progression. Given the poor outcomes once post-transplant disease is detected, improved pre-transplant risk-stratification and shifting the focus to relapse prevention is needed to improve post-transplant outcomes.
Acknowledgments
We would like to thank our patients and their families as well as the clinical care teams. Partial funding support for A.R.C from NIH P30 CA006973.
Footnotes
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pasquini M, Wang Z. Current use and outcome of hepatopoietic stem cell transplantation: CIBMTR Summary Slides, 2011. 2011 [Google Scholar]
- 2.Thanarajasingam G, Kim HT, Cutler C, et al. Outcome and prognostic factors for patients who relapse after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:1713–1718. doi: 10.1016/j.bbmt.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spyridonidis A, Labopin M, Schmid C, et al. Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the Acute Leukemia Working Party of EBMT. Leukemia. 2012;26:1211–1217. doi: 10.1038/leu.2011.351. [DOI] [PubMed] [Google Scholar]
- 4.Schmid C, Labopin M, Nagler A, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012;119:1599–1606. doi: 10.1182/blood-2011-08-375840. [DOI] [PubMed] [Google Scholar]
- 5.Nishikawa T, Inagaki J, Nagatoshi Y, et al. The second therapeutic trial for children with hematological malignancies who relapsed after their first allogeneic SCT: long-term outcomes. Pediatr Transplant. 2012;16:722–728. doi: 10.1111/j.1399-3046.2012.01737.x. [DOI] [PubMed] [Google Scholar]
- 6.Bajwa R, Schechter T, Soni S, et al. Outcome of children who experience disease relapse following allogeneic hematopoietic SCT for hematologic malignancies. Bone Marrow Transplant. 2013;48:661–665. doi: 10.1038/bmt.2012.209. [DOI] [PubMed] [Google Scholar]
- 7.Poon LM, Hamdi A, Saliba R, et al. Outcomes of adults with acute lymphoblastic leukemia relapsing after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:1059–1064. doi: 10.1016/j.bbmt.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pulsipher MA, Langholz B, Wall DA, et al. The Relationship of Acute Gvhd and Pre- and Post-Transplant Flow-MRD to the Incidence and Timing of Relapse in Children Undergoing Allogeneic Transplantation for High Risk ALL: Defining a Target Population and Window for Immunological Intervention to Prevent Relapse. ASH Annual Meeting Abstracts. 2012;120:470. [Google Scholar]
- 9.de Lima M, Porter DL, Battiwalla M, et al. Proceedings from the National Cancer Institute’s Second International Workshop on the Biology, Prevention, and Treatment of Relapse after Hematopoietic Stem Cell Transplantation: Part III. Prevention and Treatment of Relapse after Allogeneic Transplantation. Biol Blood Marrow Transplant. 2013 doi: 10.1016/j.bbmt.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alyea EP, DeAngelo DJ, Moldrem J, et al. NCI First International Workshop on The Biology, Prevention and Treatment of Relapse after Allogeneic Hematopoietic Cell Transplantation: report from the committee on prevention of relapse following allogeneic cell transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2010;16:1037–1069. doi: 10.1016/j.bbmt.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroger N, Bacher U, Bader P, et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: report from the Committee on Disease-Specific Methods and Strategies for Monitoring Relapse following Allogeneic Stem Cell Transplantation. Part I: Methods, acute leukemias, and myelodysplastic syndromes. Biol Blood Marrow Transplant. 2010;16:1187–1211. doi: 10.1016/j.bbmt.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porter DL, Alyea EP, Antin JH, et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2010;16:1467–1503. doi: 10.1016/j.bbmt.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rettinger E, Willasch AM, Kreyenberg H, et al. Preemptive immunotherapy in childhood acute myeloid leukemia for patients showing evidence of mixed chimerism after allogeneic stem cell transplantation. Blood. 2011;118:5681–5688. doi: 10.1182/blood-2011-04-348805. [DOI] [PubMed] [Google Scholar]
- 14.Bader P, Niemeyer C, Willasch A, et al. Children with myelodysplastic syndrome (MDS) and increasing mixed chimaerism after allogeneic stem cell transplantation have a poor outcome which can be improved by pre-emptive immunotherapy. Br J Haematol. 2005;128:649–658. doi: 10.1111/j.1365-2141.2004.05354.x. [DOI] [PubMed] [Google Scholar]
- 15.Bader P, Kreyenberg H, Hoelle W, et al. Increasing mixed chimerism defines a high-risk group of childhood acute myelogenous leukemia patients after allogeneic stem cell transplantation where pre-emptive immunotherapy may be effective. Bone Marrow Transplant. 2004;33:815–821. doi: 10.1038/sj.bmt.1704444. [DOI] [PubMed] [Google Scholar]
- 16.Bader P, Kreyenberg H, Hoelle W, et al. Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: possible role for pre-emptive immunotherapy? J Clin Oncol. 2004;22:1696–1705. doi: 10.1200/JCO.2004.05.198. [DOI] [PubMed] [Google Scholar]
- 17.van Rhee F, Lin F, Cullis JO, et al. Relapse of chronic myeloid leukemia after allogeneic bone marrow transplant: the case for giving donor leukocyte transfusions before the onset of hematologic relapse. Blood. 1994;83:3377–3383. [PubMed] [Google Scholar]
- 18.Gilleece MH, Dazzi F. Donor lymphocyte infusions for patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukaemia. Leuk Lymphoma. 2003;44:23–28. doi: 10.1080/1042819021000050061. [DOI] [PubMed] [Google Scholar]
- 19.Huang XJ, Xu LP, Liu KY, et al. Individualized intervention guided by BCR-ABL transcript levels after HLA-identical sibling donor transplantation improves HSCT outcomes for patients with chronic myeloid leukemia. Biol Blood Marrow Transplant. 2011;17:649–656. doi: 10.1016/j.bbmt.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Tan Y, Du K, Luo Y, et al. Superiority of preemptive donor lymphocyte infusion based on minimal residual disease in acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Transfusion. 2013 doi: 10.1111/trf.12524. [DOI] [PubMed] [Google Scholar]
- 21.Balduzzi A, Di Maio L, Silvestri D, et al. Minimal residual disease before and after transplantation for childhood acute lymphoblastic leukaemia: is there any room for intervention? Br J Haematol. 2014;164:396–408. doi: 10.1111/bjh.12639. [DOI] [PubMed] [Google Scholar]
- 22.Yan CH, Liu DH, Liu KY, et al. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood. 2012;119:3256–3262. doi: 10.1182/blood-2011-09-380386. [DOI] [PubMed] [Google Scholar]
- 23.Diez-Campelo M, Perez-Simon JA, Perez J, et al. Minimal residual disease monitoring after allogeneic transplantation may help to individualize post-transplant therapeutic strategies in acute myeloid malignancies. Am J Hematol. 2009;84:149–152. doi: 10.1002/ajh.21340. [DOI] [PubMed] [Google Scholar]
- 24.Spinelli O, Peruta B, Tosi M, et al. Clearance of minimal residual disease after allogeneic stem cell transplantation and the prediction of the clinical outcome of adult patients with high-risk acute lymphoblastic leukemia. Haematologica. 2007;92:612–618. doi: 10.3324/haematol.10965. [DOI] [PubMed] [Google Scholar]
- 25.Lankester AC, Bierings MB, van Wering ER, et al. Preemptive alloimmune intervention in high-risk pediatric acute lymphoblastic leukemia patients guided by minimal residual disease level before stem cell transplantation. Leukemia. 2010;24:1462–1469. doi: 10.1038/leu.2010.133. [DOI] [PubMed] [Google Scholar]
- 26.Zhao XS, Liu YR, Zhu HH, et al. Monitoring MRD with flow cytometry: an effective method to predict relapse for ALL patients after allogeneic hematopoietic stem cell transplantation. Ann Hematol. 2012;91:183–192. doi: 10.1007/s00277-011-1285-1. [DOI] [PubMed] [Google Scholar]
- 27.Borowitz MJ, Pullen DJ, Shuster JJ, et al. Minimal residual disease detection in childhood precursor-B-cell acute lymphoblastic leukemia: relation to other risk factors. A Children’s Oncology Group study. Leukemia. 2003;17:1566–1572. doi: 10.1038/sj.leu.2403001. [DOI] [PubMed] [Google Scholar]
- 28.Leung W, Pui CH, Coustan-Smith E, et al. Detectable minimal residual disease before hematopoietic cell transplantation is prognostic but does not preclude cure for children with very-high-risk leukemia. Blood. 2012;120:468–472. doi: 10.1182/blood-2012-02-409813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 30.Harris AC, Kitko CL, Couriel DR, et al. Extramedullary relapse of acute myeloid leukemia following allogeneic hematopoietic stem cell transplantation: incidence, risk factors and outcomes. Haematologica. 2013;98:179–184. doi: 10.3324/haematol.2012.073189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins RH, Jr, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15:433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 32.Collins RH, Jr, Goldstein S, Giralt S, et al. Donor leukocyte infusions in acute lymphocytic leukemia. Bone Marrow Transplant. 2000;26:511–516. doi: 10.1038/sj.bmt.1702555. [DOI] [PubMed] [Google Scholar]
- 33.Horn B, Soni S, Khan S, et al. Feasibility study of preemptive withdrawal of immunosuppression based on chimerism testing in children undergoing myeloablative allogeneic transplantation for hematologic malignancies. Bone Marrow Transplant. 2009;43:469–476. doi: 10.1038/bmt.2008.339. [DOI] [PubMed] [Google Scholar]
- 34.Ma X, Wu D, Sun A, et al. The value of monitoring minimal residual disease in the patients with donor lymphocyte infusion as intervention of relapsed/refractory acute lymphoblastic leukemia after allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2010;85:141–142. doi: 10.1002/ajh.21573. [DOI] [PubMed] [Google Scholar]
- 35.Pulsipher MA, Bader P, Klingebiel T, Cooper LJ. Allogeneic transplantation for pediatric acute lymphoblastic leukemia: the emerging role of peritransplantation minimal residual disease/chimerism monitoring and novel chemotherapeutic, molecular, and immune approaches aimed at preventing relapse. Biol Blood Marrow Transplant. 2009;15:62–71. doi: 10.1016/j.bbmt.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Bader P, Klingebiel T, Schaudt A, et al. Prevention of relapse in pediatric patients with acute leukemias and MDS after allogeneic SCT by early immunotherapy initiated on the basis of increasing mixed chimerism: a single center experience of 12 children. Leukemia. 1999;13:2079–2086. doi: 10.1038/sj.leu.2401581. [DOI] [PubMed] [Google Scholar]
- 37.Roddie C, Peggs KS. Donor lymphocyte infusion following allogeneic hematopoietic stem cell transplantation. Expert Opin Biol Ther. 2011;11:473–487. doi: 10.1517/14712598.2011.554811. [DOI] [PubMed] [Google Scholar]
- 38.Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- 39.Lutz C, Massenkeil G, Nagy M, et al. A pilot study of prophylactic donor lymphocyte infusions to prevent relapse in adult acute lymphoblastic leukemias after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:805–812. doi: 10.1038/sj.bmt.1705981. [DOI] [PubMed] [Google Scholar]
- 40.Bader P, Hancock J, Kreyenberg H, et al. Minimal residual disease (MRD) status prior to allogeneic stem cell transplantation is a powerful predictor for post-transplant outcome in children with ALL. Leukemia. 2002;16:1668–1672. doi: 10.1038/sj.leu.2402552. [DOI] [PubMed] [Google Scholar]
- 41.Anthias C, Dignan FL, Morilla R, et al. Pre-transplant MRD predicts outcome following reduced-intensity and myeloablative allogeneic hemopoietic SCT in AML. Bone Marrow Transplant. 2014 doi: 10.1038/bmt.2014.9. [DOI] [PubMed] [Google Scholar]
- 42.Walter RB, Buckley SA, Pagel JM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood. 2013;122:1813–1821. doi: 10.1182/blood-2013-06-506725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin JA, O’Brien MA, Hills RK, Daly SB, Wheatley K, Burnett AK. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: results of the United Kingdom MRC AML-15 trial. Blood. 2012;120:2826–2835. doi: 10.1182/blood-2012-06-435669. [DOI] [PubMed] [Google Scholar]
- 44.Walter RB, Gooley TA, Wood BL, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 2011;29:1190–1197. doi: 10.1200/JCO.2010.31.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]