Abstract
Remarkably little has been written on the biology of essential tremor (ET), despite its high prevalence. The olivary model, first proposed in the 1970s, is the traditional disease model for ET; however, the model is problematic for a number of reasons. Recently, intensive tissue-based studies have identified a series of structural changes in the brains of most ET cases, and nearly all of the observed changes are located in the cerebellar cortex. These studies suggest that Purkinje cells are central to the pathogenesis of ET and may thus provide a focus for the development of novel therapeutic strategies. Arising from these studies, a new model of ET proposes that the population of Purkinje cells represents the site of the initial molecular/cellular events leading to ET. Furthermore, a number of secondary changes/remodeling observed in the molecular and granular layers (i.e., in the Purkinje cell “neighborhood”) are likely to be of additional mechanistic importance. On a physiological level, the presence of remodeling indicates the likely formation of aberrant synapses and the creation of new/abnormal cortical circuits in ET. Specific efforts need to be devoted to understanding the cascade of biochemical and cellular events occurring in the Purkinje cell layer in ET and its neuron neighborhood, as well as the physiological effects of secondary remodeling/rewiring that are likely to be occurring in this brain region in ET.
Keywords: Essential tremor, Cerebellum, Biology, Pathophysiology, Purkinje, Neurodegeneration
Introduction
Essential tremor (ET) is one of the most common neurological diseases [1–3], yet little is understood about its basic biological underpinnings. The traditional pathophysiological model has been the olivary model, which posits that pacemaking neurons in the inferior olivary nucleus, firing in a coupled and rhythmic manner, produce tremor. In this model, the cerebellar cortex, through its receipt of this abnormal olivary output, is involved in a passive manner. However, recent studies cast considerable doubt on the validity of this model and suggest that ET may be a primary disorder of the cerebellar cortex. These studies suggest that a primary problem of the Purkinje neurons in ET leads to a secondary remodeling/ rewiring within the cerebellar cortex, with changes in adjacent neuronal populations (e.g., basket cells), and that the formation of the resultant aberrant cerebellar circuitry is probably central to the pathogenesis of ET. In this manner, both the Purkinje neuron and its local “neuron neighborhood” are of central importance in terms of disease pathophysiology. The purpose of this review is to present the recent data and to broaden the discussion of disease mechanisms and biology in ET, particularly as these relate to the Purkinje cell, its neuronal environment, and the possible presence/role of cerebellar cortical rewiring in this disease.
ET—a Review of the Clinical Entity
ET is among the most commonly encountered movement disorders. The prevalence is approximately 4 % among persons aged 40 and older [4, 5], and increasingly higher through advancing age, with the prevalence estimated in some studies to reach 20 % or higher in the oldest old [1, 6–8]. The defining clinical feature of ET is an 8–12-Hz kinetic tremor of the arms (i.e., tremor occurring during volitional movement); postural and/or kinetic tremors of cranial structures (i.e., neck, jaw, and/or voice) also often occur [9, 10]. Aside from kinetic tremor, ET patients may eventually exhibit a range of other forms of arm tremor, particularly as the disease advances; these include postural tremor [11, 12], rest tremor [13], and intention tremor [14, 15], with the latter being one of several telling indications that the disorder involves an abnormal cerebellar output. In numerous studies, a mild gait ataxia is reported in ET [16–24]; in some patients, this problem has been reported to be considerable [25]. Aside from these motor features, research in recent years has focused on a variety of cognitive and psychiatric features that seem to be linked with the disorder, some of which could be the direct result of the underlying problem in the cerebellum. Thus, a cerebellar disorder, via a cerebellar–cortical syndrome, could contribute to cognitive impairment (esp. deficits in executive function) observed in patients with ET [26, 27]. The psychiatric features linked with ET include a personality profile [28–30] and an associated mood disturbance [31–33], and it is possible that several of these features could be primary rather than secondary. The expansion of the clinical picture to include a wider range of motor and non-motor features [34], along with evidence from postmortem studies of pathological heterogeneity [35, 36], has prompted the question whether “essential tremor” is actually a family of diseases, perhaps better referred to as “the essential tremors,” rather than a single clinical–pathological entity [37, 38].
Localizing the Problem in ET—Clinical and Imaging Evidence of Cerebellar System Involvement
Both clinical and neuroimaging evidence strongly implicate the presence of a pervasive underlying abnormality of cerebellar function in ET. In addition to the more typical kinetic tremor of ET, an intention (i.e., “cerebellar”) tremor occurs in the arms in approximately 60 % of ET patients [14], and in 10 % of ET patients, such intention tremor also involves the head/neck [15]. Postural and kinetic tremor, typical of ET, may also occur in patients with disorders of cerebellar degeneration, including multiple system atrophy [39], certain spinocerebellar ataxias (SCAs) [40–42], and ataxia telangiec-tasia [43], and thus, such tremors are likely to have, in part, a cerebellar basis. Abnormalities in tandem gait and balance have been described repeatedly in ET patients, and the presence of a mild ataxia is well-accepted [16–24]. In some patients, the gait problem is more marked [25]. Furthermore, oculomotor deficits indicative of cerebellar dysfunction [44, 45] are reported in ET, as well as impairments in both temporal variability in voluntary movement and predictions in timing of movement, each under cerebellar control [46–50]. Unilateral cerebellar stroke has been observed to abruptly terminate ipsilateral arm tremor in patients with ET [51], and cerebellar outflow (dentato-rubrothalamic) pathways are the target of deep brain stimulation, which is a highly effective surgical treatment for ET [52]. A growing array of neuroimaging studies now indicates the presence not only of functional and metabolic abnormalities in the ET cerebellum, but of structural abnormalities in both the cerebellar gray and white matter. These studies include functional magnetic resonance imaging studies, positron emission tomography studies, [1H] magnetic resonance spectroscopic imaging studies, diffusion tensor imaging studies, and voxel-based morphometry studies [53–64]. Finally, animal studies provide some corroborative evidence. Tremor is not common in animals, yet a sea otter was reported with a late-life tremor that resembled ET clinically; interestingly, at necropsy, the main pathological finding was in the cerebellum, where there was extensive vacuolation of Purkinje cells [65].
The Traditional Disease Model in ET: Reviewing the Olivary Model
As noted above, the traditional model of ET, the olivary model, was first proposed in the early 1970s [66, 67]. The olivary model is based on three primary observations. Firstly, the β-carboline alkaloids (e.g., harmaline, harmine, harmane, and others) are a class of chemicals that are highly neurotoxic, and their administration to a broad range of laboratory species produces an action tremor, the hallmark feature of ET [68–71]. These toxins, by producing an excessive climbing fiber-derived glutamate discharge, result in Purkinje cell destruction [72]. Second, a variety of neurons in the central nervous system have pacemaking properties, that is, they are capable of firing in a coordinated and rhythmical fashion. Among the neurons with pacemaking properties are the climbing fibers in the inferior olivary nucleus [73–75]. Third, early studies on the pathology of ETconcluded that there was no ET pathology [36]. These studies, however, were based on small numbers of ET cases, a limited examination of the cerebellum, and an absence of control brains for comparison [36, 66].
The olivary model is based on sound primary neurophysiological observations, and it is one that has been with us for quite some time, yet as reviewed in detail elsewhere [66], there are major problems with this as an ET model. First, there is no empirical evidence that the hypothesized process is occurring in the human disease ET. In other words, the model is purely conjectural [66]. Second, pacemakers exist in numerous locations in the central nervous system, including the locus ceruleus [76], dorsal raphe nucleus [77], thalamus [78], and even in the cerebellum (Purkinje cells) itself [79] (Table 1). Thus, olivary pacemakers are not unique, and there has been no attempt to explain why these rather than the numerous other pacemakers are posited to be pathomechanistically relevant in ET [66]. Third, the harmaline model is an animal toxin model of tremor and not a model of the human disease, ET, which occurs in nature. Action tremor is a nonspecific neurological sign and is not the equivalent of the human disease ET. Also, harmaline-exposed animals develop an acute, total body tremor of high frequency that resolves after a few hours. Hence, it is a model of acute tremor rather than chronic tremor [66]. Finally, positron emission tomography studies, which began to emerge in the 1990s, did not demonstrate involvement of the inferior olivary nucleus in ET, nor did later postmortem studies reveal structural changes in that nucleus [56, 80]. In summary, the olivary model of ET suffers from a number of critical problems [66]. Its relevance to ET has been called into question.
Table 1.
Pacemakers in the central nervous system
Recent Postmortem Findings and the Cerebellar Degenerative Model of ET
Leaving aside the problems with the olivary model, recent evidence from detailed neuropathological studies reveal that the inferior olivary nucleus appears normal in ET [80]; furthermore, these studies report extensive changes localized to the cerebellar cortex [36, 81, 82]. With the emergence of these studies, there is accumulating evidence that the cerebellum is involved in the pathogenesis of ET and, furthermore, that ET may be a primary disorder of the cerebellar cortex [83]. Thus, there has been a shift in the focus of recent studies to more careful appraisal of the cerebellar cortex in this disease.
As noted above, postmortem studies over the past 5–10 years have revealed a number of structural changes in the cerebellum in most ETcases compared to age-matched control brains [66]. These studies have systematically identified changes in the following five cerebellar compartments: (1) Purkinje cell dendrites (i.e., an increase in number of Purkinje cell dendritic swellings) [84], (2) Purkinje cell bodies (i.e., reductions in Purkinje cell counts in some studies [81] and Purkinje cell linear density in others [85, 86] as well as heterotopic displacement of the Purkinje cell soma) [87], (3) Purkinje cell axons (i.e., a broad range of changes in axonal shape, including increased numbers of thickened axonal profiles and torpedoes [rounded swellings], and changes in axonal connectivity including increased numbers of axonal recurrent collaterals, axonal branching, and terminal axonal sprouting) [81, 82], (4) basket cell axonal processes (i.e., hypertrophy of perisomal processes [“hairy baskets”] [88] and elongated Leucine-rich repeat and Ig domain (Lingo)-1-labeled pinceau processes) [89], and (5) possible gamma-aminobutyric acid (GABA) receptor changes in the dentate nucleus [90]. At the Essential Tremor Centralized Brain Repository, we have also performed a systematic postmortem study of the other brain regions that form loop connections with the cerebellum (i.e., the thalamus, inferior olivary nucleus, red nucleus, and motor cortex). Significant pathologic changes are not evident in these brain regions in our studies or in those of others [35, 80, 91], reinforcing the notion that the cerebellum is the focal point of interest in the pathogenesis of ET. As can be seen, most of the above-listed changes are restricted to a specific region of the cerebellum, that is, the cerebellar cortex, although a study by a group in Canada reported that there are GABA receptor changes in the deep cerebellar nuclei in ET as well [90]. The structural changes described to date likely represent a mixture of regenerative/ compensatory responses to injury as well as degenerative changes [66], as will be discussed further below.
There is some debate over the presence and extent of Purkinje cell loss in ET [92, 93], and this debate will not be re-reviewed here, although as can be seen from the data above, Purkinje cell loss is only one of many features of the cerebellar derangement observed in ET. Moreover, the data suggest that Purkinje cell loss is likely to be a downstream, terminal event in the long chain of pathogenesis, and that the underlying molecular abnormality is one that only in some circumstances overwhelms the Purkinje cell economy, resulting in Purkinje cell death. This would also be in keeping with current knowledge of Purkinje cell biology and the hardiness of its response to injury/stress in the context of various lesions, stressors, and disease states [94–98].
Although it is still quite controversial, there is growing support for the notion that ET itself is a neurodegenerative disease [37, 60, 99–103]. Indeed, the notion that ETcould be a degenerative condition is not new; this was suggested more than 50 years ago [104]. The degenerative model has a number of potential problems, which have been discussed and addressed in detail elsewhere [66]. A major one, worth reviewing, is that one would expect that the longer the duration of symptoms, the more pronounced the morphological changes in the brain. Although some of the postmortem morphological changes in the ET brain clearly correlate with disease duration [82], others do not. While on the surface this is concerning, there are a number of plausible explanations. In general, cerebellar response to injury leads to changes that are likely to be partly regenerative/compensatory while others are regressive/degenerative [66, 94]. Thus, as has been observed in Purkinje cells in various settings [97], some axonal changes likely represent abortive regenerative attempts that are later followed by degeneration. Thus, at any one time, the observed morphological changes in a given brain region likely represent a complex mélange of regenerative, aborted regenerative, and degenerative events [66]. This mixed array of ambi-directional events is likely to make simple linear models of morphological counts by disease duration unrevealing. Furthermore, models of Purkinje cell count by disease duration do not assess loss of Purkinje cells per se [66]. The number of Purkinje cells that individuals have at baseline is known to vary considerably, and simple counts of Purkinje cells do not take this change from baseline into consideration; therefore, models of Purkinje cell count by disease duration may be equally unrevealing [66].
One may ask whether it is conceivable that a disease that is neurodegenerative could have such a long disease course, in some cases, over many decades? There are several other examples of this; indeed, some forms of SCA progress remarkably slowly. Thus, patients with SCA15 have a very slow progression (e.g., patients may have few objective signs even after 10 years and may remain symptomatic for 59 years) [105], patients with SCA 20 progress remarkably slowly (e.g., only becoming wheelchair dependent after 40 years of symptoms) [106], and patients with SCA28 are reported to have only mild clinical symptomatology even 25 years after symptom onset [107].
A Remodeling of the Cerebellar Cortex in ET?
Some of the structural changes that have been observed in the cerebellar cortex in ET suggest a remodeling of neurons and neuronal processes. Below, we will discuss these, beginning with the changes that have been observed in the Purkinje cell axonal compartment and then discussing more widespread changes.
First, torpedoes (Fig. 1), which are abnormal swellings of the proximal portion of the Purkinje cell axon, have been observed to be approximately sevenfold more common in ET brains compared with brains from age-matched controls [36, 81], with detailed assessments showing that a single Purkinje cell axon in ET may contain several such torpedoes [108]. Torpedoes contain a massive accumulation of abnormally phosphorylated and disorganized neurofilaments and other disrupted organelles [83, 109, 110]. Neurofilament mis-accumulations and resulting axonal swellings, in general [111, 112], are thought to inhibit both anterograde and retrograde axonal transport, ultimately leading to cell strangulation [102–115]. This strangulation, in general, leads to the selective degeneration and, in some instances, death of neurons [116–120]. Of interest is that the reduction in numbers of Purkinje cells is greatest in ET brains that contain the most torpedoes [81]. It is also likely this axonal strangulation leads to regenerative axonal changes as well, with attempts at axonal remodeling. Thus, as will be discussed below in ET, the extent of recurrent collateral formation is directly proportional to the number of torpedoes [82]. Moreover, in ET cases, thickened axonal profiles, axonal recurrent collaterals, and branched axons were observed to be three- to fivefold more frequent on the axons of Purkinje cells with torpedoes versus axons of Purkinje cells without torpedoes [82].
Fig. 1.
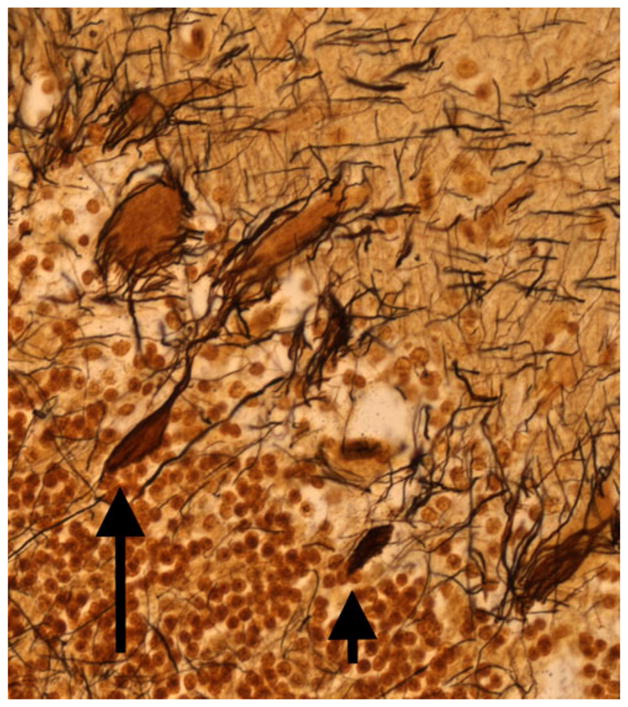
Bielschowsky-stained cerebellar cortical section of an ET case showing a torpedo (long arrow) adjacent to a Purkinje cell. A second torpedo is also seen (short arrow). ×40 magnification
Probably related to torpedo formation is the thickening of axonal profiles (Fig. 2), which has been observed to occur to a greater degree in ET cases than controls [82]. These lesions appear as more linear and elongated in contrast to the ovoid expansions of the Purkinje cell axon that characterize the torpedo [82]. In one study of ET, a thickening was defined as an axon that was at least double the width of other apparently normal axons [82]. Mechanistically, the relation of such thickenings to torpedoes is unclear, but they probably represent either a similar event or perhaps an even earlier, local axonal event prior to the formation of a full, ovoid, out- pouching or torpedo. In ET, Purkinje cells with torpedoes were 5.3 times more likely to also have a thickened axonal profile in comparison to Purkinje cells without torpedoes [82].
Fig. 2.
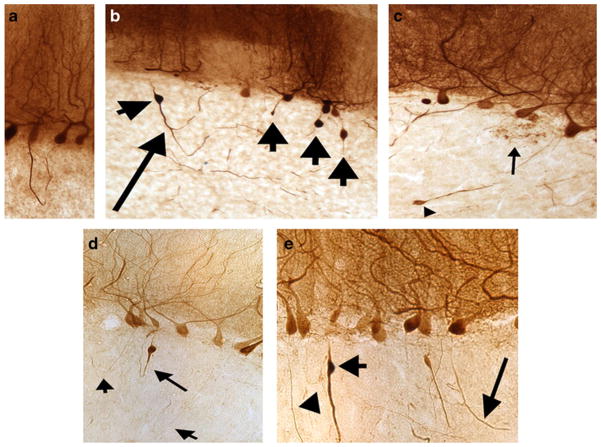
Calbindin-stained cerebellar cortical sections of ET cases (×10 magnification), showing a a thickened axonal profile that forms a recurrent collateral; the distal portion of the axon divides into several branches, b multiple torpedoes (short arrows) and a recurrent collateral with two collateral branches (long arrow), and c Increased terminal axonal sprouting (arrow) as well as a small torpedo (arrowhead) are shown d Three recurrent collaterals (arrows). The longer arrow indicates an axon with both a torpedo and a recurrent collateral. e a thickened axon with a torpedo (short arrow). An axonal of normal caliber is also shown (arrowhead). An arciform axon is seen as well (long arrow)
More extensive changes have also been observed in Purkinje cell axons in ET [82]. The observed changes are highly correlated with one another [82]. Before reviewing these changes, it is important to step back and point out that the general/broad response of Purkinje cells to stress/disease involves a range of axonal changes [95]. In this manner, fetal Purkinje cells in organotypic cultures are able to regenerate their axons on mature cerebellar slices, and the regrowing axons invade all cerebellar regions of the apposed mature slices, including white matter [98]. We know that injured Purkinje cells, in contrast to most neurons in the central nervous system, often still survive despite marked injury [95, 121, 122], exhibiting a vigorous inclination towards sprouting and other changes both along the intracortical segment and the distal stump [94, 96–98]. These reparative axonal changes may represent the neuron’s attempt to access trophic factors by establishing additional connections with Purkinje cells or other granule layer neurons [82]. However, it is possible that the disease-challenged Purkinje cells in ET, in addition to expressing these changes themselves, are also provoking this response in nearby intact neurons/axons in an attempt to compensate. For example, it is known more generally that deafferented Purkinje cells influence intact, nearby axons, probably by releasing diffusible factors, thereby provoking their outgrowth and synaptogenesis [97]. The strong correlation seen among the various Purkinje cell axon morphological changes within a brain in ET (i.e., ET cases with an abundance of one type of change also have an abundance of the other types of changes), as well as their greater tendency to occur in cells with torpedoes, indicates that they are part of a related biological process [82]; i.e., the presence of this related series of axonal changes supports the notion that the maintenance of Purkinje cell function is challenged in ET [82]. On a physiological level, this axonal remodeling indicates the formation of aberrant synapses and the creation of new/abnormal cortical circuits in ET [97].
More specifically, these morphological changes include (1) an increased number of arciform axons (i.e., axons that gradually curve back towards the Purkinje cell layer rather than continuing downward to the deep cerebellar nuclei—i.e., axons that are seemingly heading in a cortical direction rather than a corticofugal direction) (Fig. 2), (2) the formation of axon recurrent collaterals (i.e., axons or axon branches that make at least a 90° turn back towards the Purkinje cell layer) (Fig. 2), (3) increased axonal branching (i.e., the presence of axons with at least one branch point and as many as multiple bifurcations) (Fig. 2), and (4) increased terminal axonal sprouting (i.e., the presence of a frayed terminal axonal region juxtaposed within or near to the Purkinje cell layer) (Fig. 2). These structural abnormalities indicate a likely increase in aberrant Purkinje–Purkinje interactions and/or aberrant Purkinje cell interactions with other neurons.
Aside from the above-described changes in the axonal compartment, a number of other discrete structural changes have been observed in the cerebellar cortex in ET, and these changes suggest an in situ rewiring and remodeling. Some of these changes are likely the reactive result of Purkinje cell loss in ET [87], although this sequence of events remains to be definitively established. First, there seems to be an increase in the number of heterotopic Purkinje cells in ET (Fig. 3). Heterotopic Purkinje cells are Purkinje cells whose cell body is mislocalized in the molecular rather than Purkinje cell layer. In a study of ET cases versus controls, the median number of such mislocalized cells was three times higher in ET cases [87]. Furthermore, the number of these cells was inversely related to the number of Purkinje cells (i.e., ET cases with fewer Purkinje cells had higher heterotopic Purkinje cell counts) [87]. The mechanism underlying Purkinje cell heterotopia is not clear. The cerebellar molecular layer is occupied by Purkinje cell dendrites, climbing fibers, parallel fibers, interneurons, Bergmann glia, and glia [87]. Each of these structures is dynamically regulated. Purkinje cell dendrites regress during neurodegeneration, and with the death of a Purkinje cell, the immense Purkinje cell dendritic arbor disappears completely [87]. Such a situation could result in the remodeling of the molecular layer, leading to defective Purkinje cell body localization (i.e., a dislocation of Purkinje cells) [83]. The fact that the number of heterotopic Purkinje cells in ET correlates inversely with the number of Purkinje cells supports such a sequence of events [87].
Fig. 3.
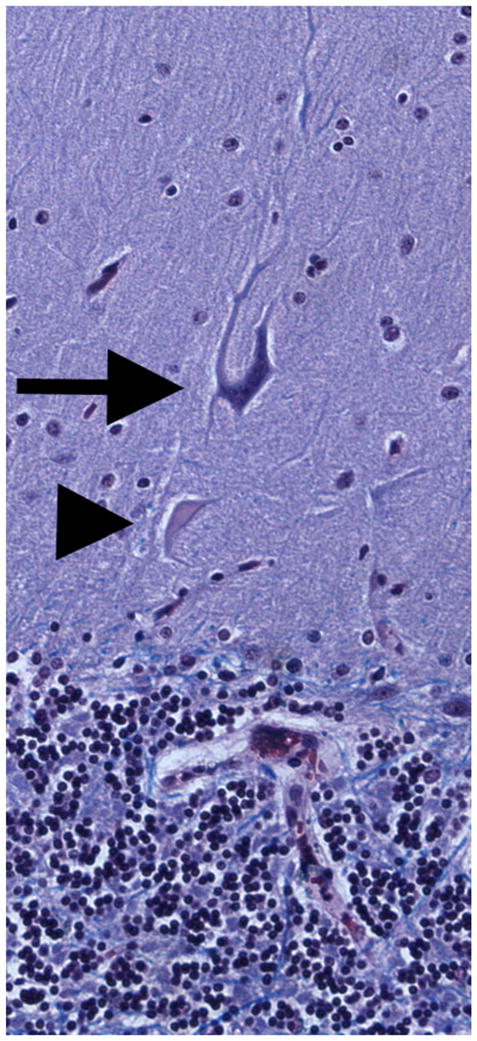
Luxol fast blue hematoxylin and eosin-stained cerebellar cortical section of an ET case showing a heterotopic Purkinje cell (long arrow). The Purkinje cell is oriented abnormally and a dendritic swelling is shown closer to the granular layer (i.e., beneath the cell, arrowhead). ×20 magnification
Second, changes have been observed in the basket cell population in ET, and these indicate that structural changes are not restricted to Purkinje cells, but rather, other neurons within the Purkinje cell functional network seem to be involved in disease pathogenesis. Thus, Erickson-Davis et al. recently observed an unusual dense and tangled (what was termed “hairy”) appearance of the basket cell axonal plexus surrounding Purkinje cell soma in Bielschowsky preparations of cerebellar cortical sections in ET cases [88] (Fig. 4). Basket cells are GABA-ergic inhibitory interneurons in the molecular layer. These cells send out axonal collaterals to form a pericellular basket around Purkinje cell perikarya [88]. The observed hypertrophic changes were inversely correlated with the number of Purkinje cells (i.e., greater Purkinje cell loss was associated with more basket cell changes) [88]. In humans, up to 50 axon collaterals from neighboring basket cells descend from the molecular layer and then combine to form a complex basket structure (i.e., constituting the basket cells’ entire axonal output) around the Purkinje cell body [88, 123]. The mechanism by which the hypertrophy of the basket cell axonal plexus occurs is unknown. One possible explanation is that the increased profiles observed in ET represent an accumulation of converging basket cell processes recruited from neighboring Purkinje cells that have been damaged or died [88]. Although there has been little investigation of such a phenomenon in the cerebellum, selective preservation and reorganization of basket cell axonal processes has been demonstrated in basket cells in the CA1 and CA3 regions of the hippocampus [88, 124, 125]. These cells form baskets around hippocampal pyramidal cells and thus function as local circuit inhibitory GABA-ergic interneurons, analogous to the relationship between cerebellar basket cells and Purkinje cells. These disease-resistant hippocampal basket cells undergo extensive reorganization in the setting of pyramidal cell death [86, 126]. Thus, in a similar fashion, the hypertrophied basket cell processes observed in ET might be converging on and reorganizing around remaining Purkinje cells.
Fig. 4.
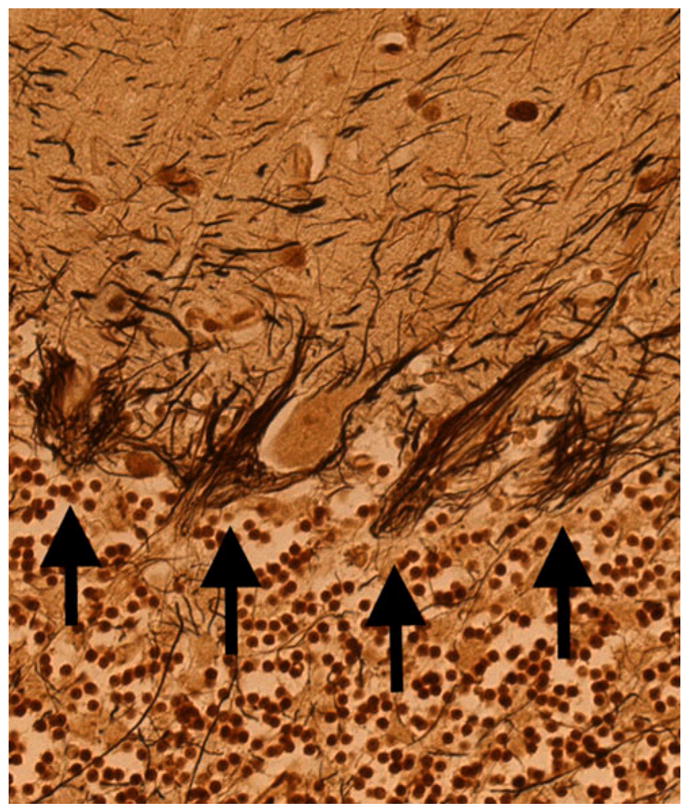
Bielschowsky-stained cerebellar cortical section of an ET case shows a dense and tangled (what was termed “hairy”) appearance of the basket cell axonal plexus surrounding Purkinje cell soma. Four baskets are marked by arrows. ×20 magnification
That there is a disturbance in the region of the basket cell pinceau in ET is further evident in recent work demonstrating an additional abnormality in the pinceau. A Lingo-1 sequence variant has been associated with ET in several genetic studies [127–130]. The Lingo-1 protein is a negative regulator of axonal regeneration and neurite outgrowth [127]. Kuo et al. determined that the Lingo-1 protein expression level was significantly increased in the cerebellar cortex in ET cases versus controls and that Lingo-1 was enriched in the basket cell pinceau [89]. These Lingo-1 positive pinceau were abnormally elongated in ET cases compared to controls [89] (Fig. 5).
Fig. 5.
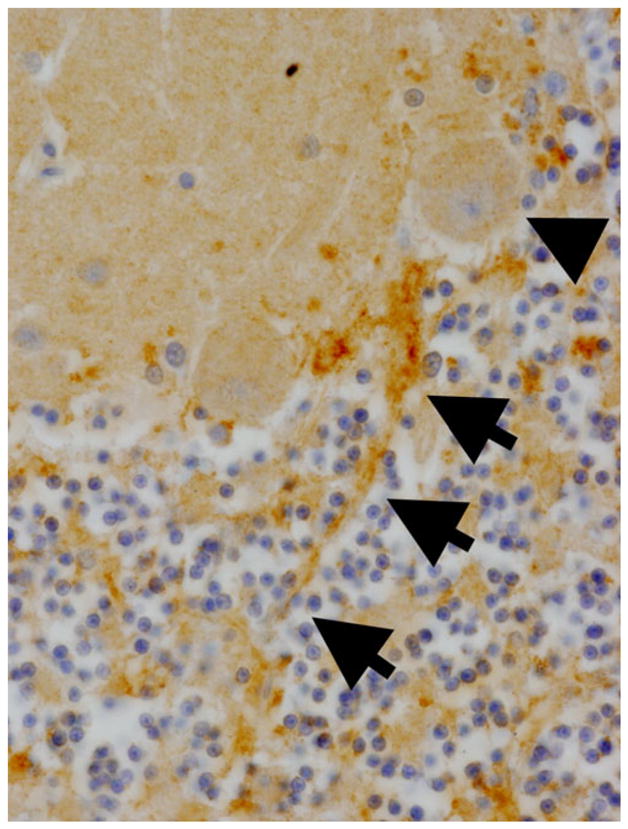
Lingo-1 immunostaining showing a Purkinje cell body (arrowhead) and an elongated pinceau (tracked with three arrows). ×20 magnification
The disease-associated decline in the Purkinje cell population is likely to lead to downstream effects as well. For example, a recent study reported a postmortem decrease in GABA(A) and GABA(B) receptors in the cerebellar dentate nucleus in individuals with ET compared to controls [90]. Further work is needed to explore these downstream effects.
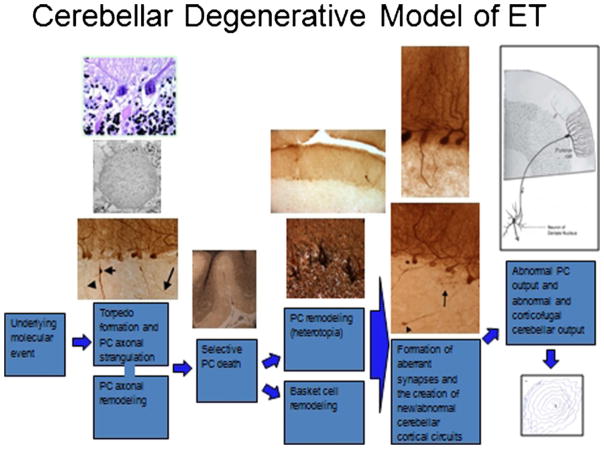
From the above studies, it would seem that Purkinje cells are central to the pathogenesis of ET [83]. In this model (Fig. 6), an as yet undefined underlying molecular abnormality leads to Purkinje cells stress/injury. A broad range of regenerative/responsive and degenerative changes are observed in these neurons. In some instances, these neurons are eventually failing to survive, resulting in a modest depletion of the Purkinje cell population. That is, the degenerative process progresses to the death of the Purkinje cell in some areas. Yet, as has been observed more broadly in cerebellar biology, Purkinje cell loss is likely to only be a later pathomechanistic event [97]. Loss of the Purkinje cell likely results in further remodeling changes in the surrounding (e.g., basket cell) neuronal populations. Further work is needed to understand the precise spatiotemporal sequence of these changes.
Fig. 6.
In the proposed model, an underlying molecular abnormality, as yet undefined, results in Purkinje cell stress and injury. A cascade of attempted regenerative and degenerative and changes occur, resulting both in anatomical remodeling and physiological changes (rewiring)
As noted above, the remodeling of Purkinje cell axons likely leads to a rewiring. On a physiological level, this indicates the likely formation of aberrant synapses, and the creation of new/abnormal cortical circuits in ET [97]. The increased number of recurrent collaterals and terminal sprouts, in particular, likely establishes synaptic contacts and produces abnormal feedback loops [97]. Thus, abnormalities in local intracerebellar circuits are likely important features of the pathogenesis of ET [83]. Additional circuit changes in the Purkinje cell-connected populations (basket cells, neurons in the deep cerebellar nuclei) are likely to be of mechanistic importance as well.
While some of the observed remodeling/rewiring may represent a garden variety/nonspecific cerebellar response to injury, it is likely that the particular cascade of observed events, from beginning to end, is distinct in ET. There is already preliminary evidence that this is the case. Thus, Purkinje cell loss is less marked in ET than in many other disorders of cerebellar degeneration, indicating that the disease pathophysiologies do not proceed along identical pathways [81]. Heterotopia, observed to increase in ET, was not observed to be increased in patients with progressive supranuclear palsy [87]. Finally, the types of Lingo-1 pinceau-related changes observed in ET were not identical from those seen in patients with SCA [89]. Animal studies are also revealing in this regard; in various mutant mice strains whose Purkinje cells degenerate, each mutation is characterized by a set of distinctive morphological abnormalities affecting Purkinje cells, and one observes in these strains a variable onset time and progression rate of neuronal death, indicating that the genetic defects responsible for Purkinje cell degeneration in the various strains act through different mechanisms that operate at different life stages [94]. Cerebellar pathobiology is not unitary.
The pace and timing of these events is also important to consider, particularly as ET is a slowly progressive disorder [131]. The interval between lesion creation and the initiation of axonal sprouting differs greatly between different cell populations in the central nervous system. The process is relatively rapid in the spinal cord, whereas for Purkinje cells, the process is known to be both delayed and protracted [97]. Indeed, Purkinje cells are unusual in the sense that they are the only known neuronal population in which late and protracted terminal sprouting occurs [97]. In Purkinje cells, the process of sprouting requires as long as 3 months for its initiation, and it becomes extremely active only 12–18 months after a lesion [97]. The remodeling process in ET is likely to be a slow one and not likely to manifest en bloc across the entire cerebellum at the same time point.
Conclusions
In conclusion, Purkinje cells are appearing in recent studies to be central to the pathogenesis of ET and, thus, may provide a focus for the development of novel therapeutic strategies [66, 83]. A new model of ET proposes that the population of Purkinje cells represents the site of the initial molecular/ cellular events leading to ET and that specific efforts need to be devoted to understanding the cascade of biochemical and cellular events occurring in the Purkinje cell layer of the cerebellar cortex in ET [66, 83]. It is equally important to realize that the Purkinje cells do not exist in anatomical or physiological isolation and that a number of changes in the Purkinje cell neighborhood are likely to be mechanistically important. The observed remodeling of the Purkinje and other neuronal populations in this brain region could be of vital importance in terms of disease mechanisms. Hence, we must begin to think more broadly in terms of local circuitry changes in the Purkinje cell neighborhood in ET.
Acknowledgments
The National Institutes of Health R01NS042859 is acknowledged for the support.
Footnotes
Conflict of Interest There are no conflicts of interest or competing financial interests.
References
- 1.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25(5):534–41. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 2.Dogu O, Sevim S, Camdeviren H, et al. Prevalence of essential tremor: door-to-door neurologic exams in Mersin Province, Turkey. Neurology. 2003;61(12):1804–6. doi: 10.1212/01.wnl.0000099075.19951.8c. [DOI] [PubMed] [Google Scholar]
- 3.Benito-León J, Bermejo-Pareja F, Morales JM, Vega S, Molina JA. Prevalence of essential tremor in three elderly populations of central Spain. Mov Disord. 2003;18(4):389–94. doi: 10.1002/mds.10376. [DOI] [PubMed] [Google Scholar]
- 4.Louis ED, Ford B, Frucht S, Barnes LF, X-Tang M, Ottman R. Risk of tremor and impairment from tremor in relatives of patients with essential tremor: a community-based family study. Ann Neurol. 2001;49(6):761–9. doi: 10.1002/ana.1022. [DOI] [PubMed] [Google Scholar]
- 5.Louis ED, Ottman R, Hauser WA. How common is the most common adult movement disorder? Estimates of the prevalence of essential tremor throughout the world. Mov Disord. 1998;13(1):5–10. doi: 10.1002/mds.870130105. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman A, Imke S, Brewer M, et al. High prevalence of tremor in a retirement community. Neurology. 1994;44:A213. [Google Scholar]
- 7.Elble RJ. Tremor in ostensibly normal elderly people. Mov Disord. 1998;13(3):457–64. doi: 10.1002/mds.870130314. [DOI] [PubMed] [Google Scholar]
- 8.Louis ED, Thawani SP, Andrews HF. Prevalence of essential tremor in a multiethnic, community-based study in northern Manhattan, New York, N.Y. Neuroepidemiology. 2009;32(3):208–14. doi: 10.1159/000195691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louis ED. Clinical practice. Essential tremor. N Engl J Med. 2001;345(12):887–91. doi: 10.1056/NEJMcp010928. [DOI] [PubMed] [Google Scholar]
- 10.Louis ED. Essential tremor. Lancet Neurol. 2005;4(2):100–10. doi: 10.1016/S1474-4422(05)00991-9. [DOI] [PubMed] [Google Scholar]
- 11.Brennan KC, Jurewicz EC, Ford B, Pullman SL, Louis ED. Is essential tremor predominantly a kinetic or a postural tremor? A clinical and electrophysiological study. Mov Disord. 2002;17(2):313–6. doi: 10.1002/mds.10003. [DOI] [PubMed] [Google Scholar]
- 12.Louis ED. The primary type of tremor in essential tremor is kinetic rather than postural: cross-sectional observation of tremor phenomenology in 369 cases. Eur J Neurol. 2013;20(4):725–7. doi: 10.1111/j.1468-1331.2012.03855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen O, Pullman S, Jurewicz E, Watner D, Louis ED. Rest tremor in patients with essential tremor: prevalence, clinical correlates, and electrophysiologic characteristics. Arch Neurol. 2003;60(3):405–10. doi: 10.1001/archneur.60.3.405. [DOI] [PubMed] [Google Scholar]
- 14.Louis ED, Frucht SJ, Rios E. Intention tremor in essential tremor: prevalence and association with disease duration. Mov Disord. 2009;24(4):626–7. doi: 10.1002/mds.22370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leegwater-Kim J, Louis ED, Pullman SL, et al. Intention tremor of the head in patients with essential tremor. Mov Disord. 2006;21(11):2001–5. doi: 10.1002/mds.21079. [DOI] [PubMed] [Google Scholar]
- 16.Kronenbuerger M, Konczak J, Ziegler W, et al. Balance and motor speech impairment in essential tremor. Cerebellum. 2009;8(3):389–98. doi: 10.1007/s12311-009-0111-y. [DOI] [PubMed] [Google Scholar]
- 17.Earhart GM, Clark BR, Tabbal SD, Perlmutter JS. Gait and balance in essential tremor: variable effects of bilateral thalamic stimulation. Mov Disord. 2009;24(3):386–91. doi: 10.1002/mds.22356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parisi SL, Héroux ME, Culham EG, Norman KE. Functional mobility and postural control in essential tremor. Arch Phys Med Rehabil. 2006;87(10):1357–64. doi: 10.1016/j.apmr.2006.07.255. [DOI] [PubMed] [Google Scholar]
- 19.Stolze H, Petersen G, Raethjen J, Wenzelburger R, Deuschl G. The gait disorder of advanced essential tremor. Brain. 2001;124(Pt 11):2278–86. doi: 10.1093/brain/124.11.2278. [DOI] [PubMed] [Google Scholar]
- 20.Hubble JP, Busenbark KL, Pahwa R, Lyons K, Koller WC. Clinical expression of essential tremor: effects of gender and age. Mov Disord. 1997;12(6):969–72. doi: 10.1002/mds.870120620. [DOI] [PubMed] [Google Scholar]
- 21.Hoskovcová M, Ulmanová O, Sprdlík O, et al. Disorders of balance and gait in essential tremor are associated with midline tremor and age. Cerebellum. 2013;12(1):27–34. doi: 10.1007/s12311-012-0384-4. [DOI] [PubMed] [Google Scholar]
- 22.Louis ED, Rios E, Rao AK. Tandem gait performance in essential tremor: clinical correlates and association with midline tremors. Mov Disord. 2010;25(11):1633–8. doi: 10.1002/mds.23144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao AK, Uddin J, Gillman A, Louis ED. Cognitive motor interference during dual-task gait in essential tremor. Gait Posture. 2013;38(3):403–9. doi: 10.1016/j.gaitpost.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singer C, Sanchez-Ramos J, Weiner WJ. Gait abnormality in essential tremor. Mov Disord. 1994;9(2):193–6. doi: 10.1002/mds.870090212. [DOI] [PubMed] [Google Scholar]
- 25.Louis ED, Galecki M, Rao AK. Four essential tremor cases with moderately impaired gait: how impaired can gait be in this disease? Tremor Other Hyperkinet Mov (N Y) 2013 Nov 4;3 doi: 10.7916/D8QV3K7G. pii: tre-03-200-4597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tröster AI, Woods SP, Fields JA, et al. Neuropsychological deficits in essential tremor: an expression of cerebello-thalamocortical pathophysiology? Eur J Neurol. 2002;9(2):143–51. doi: 10.1046/j.1468-1331.2002.00341.x. [DOI] [PubMed] [Google Scholar]
- 27.Benito-León J, Louis ED, Bermejo-Pareja F. Population-based case–control study of cognitive function in essential tremor. Neurology. 2006;66(1):69–74. doi: 10.1212/01.wnl.0000192393.05850.ec. [DOI] [PubMed] [Google Scholar]
- 28.Thenganatt MA, Louis ED. Personality profile in essential tremor: a case–control study. Parkinsonism Relat Disord. 2012;18(9):1042–4. doi: 10.1016/j.parkreldis.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chatterjee A, Jurewicz EC, Applegate LM, Louis ED. Personality in essential tremor: further evidence of non-motor manifestations of the disease. J Neurol Neurosurg Psychiatry. 2004;75(7):958–61. doi: 10.1136/jnnp.2004.037176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan EK, Fook-Chong S, Lum SY, et al. Non-motor manifestations in essential tremor: use of a validated instrument to evaluate a wide spectrum of symptoms. Parkinsonism Relat Disord. 2005;11(6):375–80. doi: 10.1016/j.parkreldis.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Louis ED, Benito-León J, Bermejo-Pareja F. Self-reported depression and anti-depressant medication use in essential tremor: cross-sectional and prospective analyses in a population-based study. Eur J Neurol. 2007;14(10):1138–46. doi: 10.1111/j.1468-1331.2007.01923.x. [DOI] [PubMed] [Google Scholar]
- 32.Miller KM, Okun MS, Fernandez HF, Jacobson CE, 4th, Rodriguez RL, Bowers D. Depression symptoms in movement disorders: comparing Parkinson’s disease, dystonia, and essential tremor. Mov Disord. 2007;22(5):666–72. doi: 10.1002/mds.21376. [DOI] [PubMed] [Google Scholar]
- 33.Lorenz D, Poremba C, Papengut F, Schreiber S, Deuschl G. The psychosocial burden of essential tremor in an outpatient- and a community-based cohort. Eur J Neurol. 2011;18(7):972–9. doi: 10.1111/j.1468-1331.2010.03295.x. [DOI] [PubMed] [Google Scholar]
- 34.Benito-León J. Essential tremor: from a monosymptomatic disorder to a more complex entity. Neuroepidemiology. 2008;31(3):191–2. doi: 10.1159/000154933. [DOI] [PubMed] [Google Scholar]
- 35.Shill HA, Adler CH, Sabbagh MN, et al. Pathologic findings in prospectively ascertained essential tremor subjects. Neurology. 2008;70(16 Pt 2):1452–5. doi: 10.1212/01.wnl.0000310425.76205.02. [DOI] [PubMed] [Google Scholar]
- 36.Louis ED. Essential tremor: evolving clinicopathological concepts in an era of intensive post-mortem enquiry. Lancet Neurol. 2010;9(6):613–22. doi: 10.1016/S1474-4422(10)70090-9. [DOI] [PubMed] [Google Scholar]
- 37.Louis ED. Essential tremors: a family of neurodegenerative disorders? Arch Neurol. 2009;66(10):1202–8. doi: 10.1001/archneurol.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Louis ED. “Essential tremor” or “the essential tremors”: is this one disease or a family of diseases? Neuroepidemiology. 2013;42(2):81–9. doi: 10.1159/000356351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaindlstorfer C, Granata R, Wenning GK. Tremor in multiple system atrophy—a review. Tremor Other Hyperkinet Mov (N Y) 2013 Sep 3;3 doi: 10.7916/D8NV9GZ9. pii: tre-03-165-4252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perlman SL. Spinocerebellar degenerations. Handb Clin Neurol. 2011;100:113–40. doi: 10.1016/B978-0-444-52014-2.00006-9. [DOI] [PubMed] [Google Scholar]
- 41.Gardner RJ, Knight MA, Hara K, Tsuji S, Forrest SM, Storey E. Spinocerebellar ataxia type 15. Cerebellum. 2005;4(1):47–50. doi: 10.1080/14734220410019029. [DOI] [PubMed] [Google Scholar]
- 42.Holmes SE, O’Hearn E, Margolis RL. Why is SCA12 different from other SCAs? Cytogenet Genome Res. 2003;100(1–4):189–97. doi: 10.1159/000072854. [DOI] [PubMed] [Google Scholar]
- 43.Shaikh AG, Zee DS, Mandir AS, Lederman HM, Crawford TO. Disorders of upper limb movements in ataxia-telangiectasia. PLoS One. 2013;8(6):e67042. doi: 10.1371/journal.pone.0067042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gitchel GT, Wetzel PA, Baron MS. Slowed saccades and increased square wave jerks in essential tremor. Tremor Other Hyperkinet Mov (N Y) 2013 Sep 3;3 doi: 10.7916/D8251GXN. pii: tre-03-178-4116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Helmchen C, Hagenow A, Miesner J, et al. Eye movement abnormalities in essential tremor may indicate cerebellar dysfunction. Brain. 2003;126(Pt 6):1319–32. doi: 10.1093/brain/awg132. [DOI] [PubMed] [Google Scholar]
- 46.Bares M, Lungu OV, Husárová I, Gescheidt T. Predictive motor timing performance dissociates between early diseases of the cerebellum and Parkinson’s disease. Cerebellum. 2010;9(1):124–35. doi: 10.1007/s12311-009-0133-5. [DOI] [PubMed] [Google Scholar]
- 47.Trillenberg P, Führer J, Sprenger A, et al. Eye-hand coordination in essential tremor. Mov Disord. 2006;21(3):373–9. doi: 10.1002/mds.20729. [DOI] [PubMed] [Google Scholar]
- 48.Avanzino L, Bove M, Tacchino A, et al. Cerebellar involvement in timing accuracy of rhythmic finger movements in essential tremor. Eur J Neurosci. 2009;30(10):1971–9. doi: 10.1111/j.1460-9568.2009.06984.x. [DOI] [PubMed] [Google Scholar]
- 49.Manto M, Bower JM, Conforto AB, et al. Consensus paper: roles of the cerebellum in motor control—the diversity of ideas on cerebellar involvement in movement. Cerebellum. 2012;11(2):457–87. doi: 10.1007/s12311-011-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bareš M, Husárová I, Lungu OV. Essential tremor, the cerebellum, and motor timing: towards integrating them into one complex entity. Tremor Other Hyperkinet Mov (N Y) 2012;2 pii: tre-02-93-653-1. Epub 2012 Sep 12. [PMC free article] [PubMed] [Google Scholar]
- 51.Dupuis MJ, Delwaide PJ, Boucquey D, Gonsette RE. Homolateral disappearance of essential tremor after cerebellar stroke. Mov Disord. 1989;4(2):183–7. doi: 10.1002/mds.870040210. [DOI] [PubMed] [Google Scholar]
- 52.Schuurman PR, Bosch DA, Bossuyt PM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. 2000;342(17):461–8. doi: 10.1056/NEJM200002173420703. [DOI] [PubMed] [Google Scholar]
- 53.Bucher SF, Seelos KC, Dodel RC, Reiser M, Oertel WH. Activation mapping in essential tremor with functional magnetic resonance imaging. Ann Neurol. 1997;41(1):32–40. doi: 10.1002/ana.410410108. [DOI] [PubMed] [Google Scholar]
- 54.Colebatch JG, Findley LJ, Frackowiak RS, Marsden CD, Brooks DJ. Preliminary report: activation of the cerebellum in essential tremor. Lancet. 1990;336(8722):1028–30. doi: 10.1016/0140-6736(90)92489-5. [DOI] [PubMed] [Google Scholar]
- 55.Jenkins IH, Bain PG, Colebatch JG, et al. A positron emission tomography study of essential tremor: evidence for overactivity of cerebellar connections. Ann Neurol. 1993;34(1):82–90. doi: 10.1002/ana.410340115. [DOI] [PubMed] [Google Scholar]
- 56.Wills AJ, Jenkins IH, Thompson PD, Findley LJ, Brooks DJ. Red nuclear and cerebellar but no olivary activation associated with essential tremor: a positron emission tomographic study. Ann Neurol. 1994;36(4):636–42. doi: 10.1002/ana.410360413. [DOI] [PubMed] [Google Scholar]
- 57.Louis ED, Shungu DC, Chan S, Mao X, Jurewicz EC, Watner D. Metabolic abnormality in the cerebellum in patients with essential tremor: a proton magnetic resonance spectroscopic imaging study. Neurosci Lett. 2002;333(1):17–20. doi: 10.1016/s0304-3940(02)00966-7. [DOI] [PubMed] [Google Scholar]
- 58.Pagan FL, Butman JA, Dambrosia JM, Hallett M. Evaluation of essential tremor with multi-voxel magnetic resonance spectroscopy. Neurology. 2003;6(8):1344–7. doi: 10.1212/01.wnl.0000065885.15875.0d. [DOI] [PubMed] [Google Scholar]
- 59.Klein JC, Lorenz B, Kang JS, et al. Diffusion tensor imaging of white matter involvement in essential tremor. Hum Brain Mapp. 2011;32(6):896–904. doi: 10.1002/hbm.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nicoletti G, Manners D, Novellino F, et al. Diffusion tensor MRI changes in cerebellar structures of patients with familial essential tremor. Neurology. 2010;74(12):988–94. doi: 10.1212/WNL.0b013e3181d5a460. [DOI] [PubMed] [Google Scholar]
- 61.Shin DH, Han BS, Kim HS, Lee PH. Diffusion tensor imaging in patients with essential tremor. AJNR Am J Neuroradiol. 2008;29(1):151–3. doi: 10.3174/ajnr.A0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quattrone A, Cerasa A, Messina D, et al. Essential head tremor is associated with cerebellar vermis atrophy: a volumetric and voxel-based morphometry MR imaging study. AJNR Am J Neuroradiol. 2008;29(9):1692–7. doi: 10.3174/ajnr.A1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benito-León J, Alvarez-Linera J, Hernández-Tamames JA, Alonso-Navarro H, Jiménez-Jiménez FJ, Louis ED. Brain structural changes in essential tremor: voxel-based morphometry at 3-Telsa. J Neurol Sci. 2009;287(1–2):138–42. doi: 10.1016/j.jns.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 64.Cerasa A, Messina D, Nicoletti G, et al. Cerebellar atrophy in essential tremor using an automated segmentation method. AJNR Am J Neuroradiol. 2009;30(6):1240–3. doi: 10.3174/ajnr.A1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Louis ED, Murray MJ, Miller MA, Pullman SL, Vonsattel JP. Late-life action tremor in a southern sea otter (enhydris lutris nereis) Mov Disord. 2004;19(2):222–6. doi: 10.1002/mds.10645. [DOI] [PubMed] [Google Scholar]
- 66.Louis ED. Re-thinking the biology of essential tremor: from models to morphology. Parkinsonism Related Disord. 2013;20:S88–93. doi: 10.1016/S1353-8020(13)70023-3. [DOI] [PubMed] [Google Scholar]
- 67.Llinás R, Volkind RA. The olivo-cerebellar system: functional properties as revealed by harmaline-induced tremor. Exp Brain Res. 1973;18:69–87. doi: 10.1007/BF00236557. [DOI] [PubMed] [Google Scholar]
- 68.Zetler G, Singbartl G, Schlosser L. Cerebral pharmacokinetics of tremor-producing harmala and iboga alkaloids. Pharmacology. 1972;7:237–48. doi: 10.1159/000136294. [DOI] [PubMed] [Google Scholar]
- 69.Martin FC, Le Thu A, Handforth A. Harmaline-induced tremor as a potential preclinical screening method for essential tremor medications. Mov Disord. 2005;20(3):298–305. doi: 10.1002/mds.20331. [DOI] [PubMed] [Google Scholar]
- 70.Robertson HA. Harmaline-induced tremor: the benzodiazepine receptor as a site of action. Eur J Pharmacol. 1980;67(1):129–32. doi: 10.1016/0014-2999(80)90020-5. [DOI] [PubMed] [Google Scholar]
- 71.Handforth A, Krahl SE. Suppression of harmaline-induced tremor in rats by vagus nerve stimulation. Mov Disord. 2001;16(1):84–8. doi: 10.1002/1531-8257(200101)16:1<84::aid-mds1010>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 72.O’Hearn E, Molliver ME. The olivocerebellar projection mediates ibogaine-induced degeneration of Purkinje cells: a model of indirect, trans-synaptic excitotoxicity. J Neurosci. 1997;17(22):8828–41. doi: 10.1523/JNEUROSCI.17-22-08828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O’Hearn E, Molliver ME. Administration of a non-NMDA antagonist, GYKI 52466, increases excitotoxic Purkinje cell degeneration caused by ibogaine. Neuroscience. 2004;127(2):373–83. doi: 10.1016/j.neuroscience.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 74.Elble RJ. Animal models of action tremor. Mov Disord. 1998;13 (Suppl 3):35–9. doi: 10.1002/mds.870131306. [DOI] [PubMed] [Google Scholar]
- 75.Handforth A. Harmaline tremor: underlying mechanisms in a potential animal model of essential tremor. Tremor Other Hyperkinet Mov (N Y) 2012;2 doi: 10.7916/D8TD9W2P. pii: 02-92-769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Oliveira RB, Howlett MC, Gravina FS, et al. Pacemaker currents in mouse locus coeruleus neurons. Neuroscience. 2010;170(1):166–77. doi: 10.1016/j.neuroscience.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 77.Penington NJ, Tuckwell HC. Properties of I(A) in a neuron of the dorsal raphe nucleus. Brain Res. 2012;1449:60–8. doi: 10.1016/j.brainres.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 78.Jahnsen H, Llinás R. Ionic basis for the electroresponsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984;349:227–47. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hansen ST, Meera P, Otis TS, Pulst SM. Changes in Purkinje cell firing and gene expression precede behavioral pathology in a mouse model of SCA2. Hum Mol Genet. 2013;22(2):271–83. doi: 10.1093/hmg/dds427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Louis ED, Babij R, Cortés E, Vonsattel JP, Faust PL. The inferior olivary nucleus: a postmortem study of essential tremor cases versus controls. Mov Disord. 2013;28(6):779–86. doi: 10.1002/mds.25400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130(Pt 12):3297–307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 82.Babij R, Lee M, Cortés E, Vonsattel JP, Faust PL, Louis ED. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain. 2013;136(Pt 10):3051–61. doi: 10.1093/brain/awt238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grimaldi G, Manto M. Is essential tremor a Purkinjopathy? The role of the cerebellar cortex in its pathogenesis. Mov Disord. 2013 doi: 10.1002/mds.25645. [DOI] [PubMed] [Google Scholar]
- 84.Yu M, Ma K, Faust PL, et al. Increased number of Purkinje cell dendritic swellings in essential tremor. Eur J Neurol. 2012;19(4):625–30. doi: 10.1111/j.1468-1331.2011.03598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Axelrad JE, Louis ED, Honig LS, et al. Reduced Purkinje cell number in essential tremor: a postmortem study. Arch Neurol. 2008;65(1):101–7. doi: 10.1001/archneurol.2007.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Louis ED, Babij R, Lee M, Cortés E, Vonsattel JP. Quantification of cerebellar hemispheric purkinje cell linear density: 32 ET cases versus 16 control. Mov Disord. 2013 doi: 10.1002/mds.25629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuo SH, Erickson-Davis C, Gillman A, Faust PL, Vonsattel JP, Louis ED. Increased number of heterotopic Purkinje cells in essential tremor. J Neurol Neurosurg Psychiatry. 2011;82(9):1038–40. doi: 10.1136/jnnp.2010.213330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Erickson-Davis CR, Faust PL, Vonsattel JP, Gupta S, Honig LS, Louis ED. “Hairy baskets” associated with degenerative Purkinje cell changes in essential tremor. J Neuropathol Exp Neurol. 2010;69(3):262–71. doi: 10.1097/NEN.0b013e3181d1ad04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuo SH, Tang G, Louis ED, et al. Lingo-1 expression is increased in essential tremor cerebellum and is present in the basket cell pinceau. Acta Neuropathol. 2013;125(6):879–89. doi: 10.1007/s00401-013-1108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paris-Robidas S, Brochu E, Sintes M, et al. Defective dentate nucleus GABA receptors in essential tremor. Brain. 2012;135(Pt 1):105–16. doi: 10.1093/brain/awr301. [DOI] [PubMed] [Google Scholar]
- 91.Rajput A, Robinson CA, Rajput AH. Essential tremor course and disability: a clinicopathologic study of 20 cases. Neurology. 2004;62(6):932–6. doi: 10.1212/01.wnl.0000115145.18830.1a. [DOI] [PubMed] [Google Scholar]
- 92.Rajput AH, Robinson CA, Rajput ML, Robinson SL, Rajput A. Essential tremor is not dependent upon cerebellar Purkinje cell loss. Parkinsonism Relat Disord. 2012;18(5):626–8. doi: 10.1016/j.parkreldis.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 93.Louis ED, Faust PL, Vonsattel JP. Purkinje cell loss is a characteristic of essential tremor: towards a more mature understanding of pathogenesis. Parkinsonism Relat Disord. 2012;18(8):1003–4. doi: 10.1016/j.parkreldis.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 94.Rossi F, Jankovski A, Sotelo C. Target neuron controls the integrity of afferent axon phenotype: a study on the Purkinje cell-climbing fiber system in cerebellar mutant mice. J Neurosci. 1995;15(3 Pt 1):2040–56. doi: 10.1523/JNEUROSCI.15-03-02040.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rossi F, Jankovski A, Sotelo C. Differential regenerative response of Purkinje cell and inferior olivary axons confronted with embryonic grafts: environmental cues versus intrinsic neuronal determinants. J Comp Neurol. 1995;359(4):663–77. doi: 10.1002/cne.903590412. [DOI] [PubMed] [Google Scholar]
- 96.Chan-Palay V. The recurrent collaterals of Purkinje cell axons: a correlated study of the rat’s cerebellar cortex with electron microscopy and the Golgi method. Z Anat Entwicklungsgesch. 1971;134(2):200–34. doi: 10.1007/BF00519300. [DOI] [PubMed] [Google Scholar]
- 97.Dusart I, Morel MP, Wehrlé R, Sotelo C. Late axonal sprouting of injured Purkinje cells and its temporal correlation with permissive changes in the glial scar. J Comp Neurol. 1999;408(3):399–418. [PubMed] [Google Scholar]
- 98.Carulli D, Buffo A, Strata P. Reparative mechanisms in the cerebellar cortex. Prog Neurobiol. 2004;72(6):373–98. doi: 10.1016/j.pneurobio.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 99.Bermejo-Pareja F. Essential tremor—a neurodegenerative disorder associated with cognitive defects? Nat Rev Neurol. 2011;7(5):273–82. doi: 10.1038/nrneurol.2011.44. [DOI] [PubMed] [Google Scholar]
- 100.Benito-León J. Essential tremor: one of the most common neurodegenerative diseases? Neuroepidemiology. 2011;36(2):77–8. doi: 10.1159/000323572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.LaRoia H, Louis ED. Association between essential tremor and other neurodegenerative diseases: what is the epidemiological evidence? Neuroepidemiology. 2011;37(1):1–10. doi: 10.1159/000328866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deuschl G, Elble R. Essential tremor—neurodegenerative or nondegenerative disease towards a working definition of ET. Mov Disord. 2009;24(14):2033–41. doi: 10.1002/mds.22755. [DOI] [PubMed] [Google Scholar]
- 103.Bonuccelli U. Essential tremor is a neurodegenerative disease. J Neural Transm. 2012;119(11):1383–7. doi: 10.1007/s00702-012-0878-8. [DOI] [PubMed] [Google Scholar]
- 104.Critchley M, Greenfield JC. Olivo-ponto-cerebellar atrophy. Brain. 1948;71:343–64. [PubMed] [Google Scholar]
- 105.Mckinlay Gardner RJ, Knight MA, Hara K, Tsuji S, Forrest SM, Storey E. Spinocerebellar ataxia type 15. Cerebellum. 2005;4:47–50. doi: 10.1080/14734220410019029. [DOI] [PubMed] [Google Scholar]
- 106.Storey E, Knight MA, Forrest SM, Mckinlay Gardner RJ. Spinocerebellar ataxia type 20. Cerebellum. 2005;4:55–7. doi: 10.1080/14734220410019048. [DOI] [PubMed] [Google Scholar]
- 107.Edener U, Wollner J, Hehr U, et al. Early onset and slow progression of SCA28, a rare dominant ataxia in a large four-generation family with a novel AFG3L2 mutation. Eur J Human Gen. 2010;18:965–8. doi: 10.1038/ejhg.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Louis ED. Essential tremor and other forms of kinetic tremor. In: Grimaldi G, Manto M, editors. Mechanisms and emerging therapies in tremor disorders. New York: Springer; 2013. pp. 167–201. [Google Scholar]
- 109.Louis ED, Yi H, Erickson-Davis C, Vonsattel JP, Faust PL. Structural study of Purkinje cell axonal torpedoes in essential tremor. Neurosci Lett. 2009;450(3):287–91. doi: 10.1016/j.neulet.2008.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Louis ED, Ma K, Babij R, et al. Neurofilament protein levels: quantitative analysis in essential tremor cerebellar cortex. Neurosci Lett. 2012;518(1):49–54. doi: 10.1016/j.neulet.2012.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu Z, Cork LC, Griffin JW, Cleveland DW. Increased expression of neurofilament subunit NF-L produces morphological alterations that resemble the pathology of human motor neuron disease. Cell. 1993;73(1):23–33. doi: 10.1016/0092-8674(93)90157-l. [DOI] [PubMed] [Google Scholar]
- 112.Liem RK, Leung CL. Neuronal intermediate filament overexpression and neurodegeneration in transgenic mice. Exp Neurol. 2003;184(1):3–8. doi: 10.1016/s0014-4886(03)00291-7. [DOI] [PubMed] [Google Scholar]
- 113.Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001;2(11):806–19. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- 114.Robertson J, Kriz J, Nguyen MD, Julien JP. Pathways to motor neuron degeneration in transgenic mouse models. Biochimie. 2002;84(11):1151–60. doi: 10.1016/s0300-9084(02)00025-1. [DOI] [PubMed] [Google Scholar]
- 115.Beaulieu JM, Nguyen MD, Julien JP. Late onset of motor neurons in mice overexpressing wild-type peripherin. J Cell Biol. 1999;147(3):531–44. doi: 10.1083/jcb.147.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mentis GZ, Díaz E, Moran LB, Navarrete R. Early alterations in the electrophysiological properties of rat spinal motoneurones following neonatal axotomy. J Physiol. 2007;582(Pt 3):1141–61. doi: 10.1113/jphysiol.2007.133488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ma WY, Vacca-Galloway LL. Reduced branching and length of dendrites detected in cervical spinal cord motoneurons of Wobbler mouse, a model for inherited motoneuron disease. J Comp Neurol. 1991;311(2):210–22. doi: 10.1002/cne.903110204. [DOI] [PubMed] [Google Scholar]
- 118.March PA, Thrall MA, Brown DE, Mitchell TW, Lowenthal AC, Walkley SU. GABAergic neuroaxonal dystrophy and other cytopathological alterations in feline Niemann-Pick disease type C. Acta Neuropathol. 1997;94(2):164–72. doi: 10.1007/s004010050689. [DOI] [PubMed] [Google Scholar]
- 119.Sasaki S, Iwata M. Dendritic synapses of anterior horn neurons in amyotrophic lateral sclerosis: an ultrastructural study. Acta Neuropathol. 1996;91(3):278–83. doi: 10.1007/s004010050426. [DOI] [PubMed] [Google Scholar]
- 120.Rossi F, Borsello T, Strata P. Exposure to kainic acid mimics the effects of axotomy in cerebellar Purkinje cells of the adult rat. Eur J Neurosci. 1994;6(3):392–402. doi: 10.1111/j.1460-9568.1994.tb00282.x. [DOI] [PubMed] [Google Scholar]
- 121.Bravin M, Savio T, Strata P, Rossi F. Olivocerebellar axon regeneration and target reinnervation following dissociated Schwann cell grafts in surgically injured cerebella of adult rats. Eur J Neurosci. 1997;9(12):2634–49. doi: 10.1111/j.1460-9568.1997.tb01693.x. [DOI] [PubMed] [Google Scholar]
- 122.Dusart I, Sotelo C. Lack of Purkinje cell loss in adult rat cerebellum following protracted axotomy: degenerative changes and regenerative attempts of the severed axons. J Comp Neurol. 1994;347(2):211–32. doi: 10.1002/cne.903470206. [DOI] [PubMed] [Google Scholar]
- 123.Leclerc N, Gravel C, Plioplys A, Hawkes R. Basket cell development in the normal and hypothyroid rat cerebellar cortex studied with a monoclonal anti-neurofilament antibody. Can J Biochem Cell Biol. 1985;63(6):564–76. doi: 10.1139/o85-075. [DOI] [PubMed] [Google Scholar]
- 124.Buckmaster PS, Jongen-Rêlo AL. Highly specific neuron loss preserves lateral inhibitory circuits in the dentate gyrus of kainate-induced epileptic rats. J Neurosci. 1999;19(21):9519–29. doi: 10.1523/JNEUROSCI.19-21-09519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495(2):387–95. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- 126.Arellano JI, Muñoz A, Ballesteros-Yáñez I, Sola RG, DeFelipe J. Histopathology and reorganization of chandelier cells in the human epileptic sclerotic hippocampus. Brain. 2004;127(Pt 1):45–64. doi: 10.1093/brain/awh004. [DOI] [PubMed] [Google Scholar]
- 127.Stefansson H, Steinberg S, Petursson H, et al. Variant in the sequence of the LINGO1 gene confers risk of essential tremor. Nat Genet. 2009;41(3):277–9. doi: 10.1038/ng.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vilariño-Güell C, Wider C, Ross OA, et al. LINGO1 and LINGO2 variants are associated with essential tremor and Parkinson disease. Neurogenetics. 2010;11(4):401–8. doi: 10.1007/s10048-010-0241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Clark LN, Park N, Kisselev S, Rios E, Lee JH, Louis ED. Replication of the LINGO1 gene association with essential tremor in a North American population. Eur J Hum Genet. 2010;18(7):838–43. doi: 10.1038/ejhg.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Deng H, Gu S, Jankovic J. LINGO1 variants in essential tremor and Parkinson’s disease. Acta Neurol Scand. 2012;125(1):1–7. doi: 10.1111/j.1600-0404.2011.01516.x. [DOI] [PubMed] [Google Scholar]
- 131.Louis ED, Agnew A, Gillman A, Gerbin M, Viner AS. Estimating annual rate of decline: prospective, longitudinal data on arm tremor severity in two groups of essential tremor cases. J Neurol Neurosurg Psychiatry. 2011;82(7):761–5. doi: 10.1136/jnnp.2010.229740. [DOI] [PMC free article] [PubMed] [Google Scholar]