Abstract
Mesenchymal stem cells (MSC) have great therapeutic potential for the repair of nonhealing bone defects due to their proliferative capacity, multilineage potential, trophic factor secretion, and lack of immunogenicity. However, a major barrier to the translation of cell-based therapies into clinical practice is ensuring their survival and function upon implantation into the defect site. We hypothesized that forming MSC into more physiologic 3-dimensional spheroids, rather than employing dissociated cells from 2-dimensional monolayer culture, would enhance their survival when exposed to a harsh microenvironment while maintaining their osteogenic potential. MSC spheroids were formed using the hanging drop method with increasing cell numbers. Compared to larger spheroids, the smallest spheroids which contained 15,000 cells exhibited increased metabolic activity, reduced apoptosis, and the most uniform distribution of proliferating cells. Spheroids were then entrapped in fibrin gels and cultured in serum-free media and 1% oxygen. Compared to identical numbers of dissociated MSC in fibrin gels, spheroids exhibited significantly reduced apoptosis and secreted up to 100-fold more VEGF. We also observed that fibrin gels containing spheroids and those containing an equivalent number of dissociated cells exhibited similar expression levels of early and late markers of osteogenic differentiation. These data demonstrate that MSC spheroids exhibit greater resistance to apoptosis and enhanced proangiogenic potential, while maintaining similar osteogenic potential to dissociated MSC entrapped in a clinically relevant biomaterial, supporting the use of MSC spheroids in cell-based approaches to bone repair.
Keywords: Spheroid, fibrin, mesenchymal stromal cell, cell survival, osteogenic potential
INTRODUCTION
Bone has a remarkable capacity to heal and remodel following trauma or insult. However, native bone repair processes are insufficient for approximately 10% of patients whose bone defects either extend beyond a critical size or are associated with impaired native healing secondary to age or disease. Clinically, these defects can manifest through nonunion fractures, deep-seated infections that require debridement, tumor resection or irradiation, inherited bone disorders, necrosis secondary to compromised blood supply, and failed hip and knee replacements. In the United States alone, there are 1.6 million surgical procedures (at a cost of $5 billion) performed annually that require some type of bone substitute (O’Keefe and Mao, 2011). The gold standard for bone substitutes remains the autologous bone graft (Laurencin, et al., 2006), whereby healthy bone tissue is removed from a remote site, such as the iliac crest of the pelvic bone, and used to fill the bone defect within the same patient. Although these autologous bone grafts minimize the risk of rejection and retain inherent osteogenic and osteoinductive properties, this approach is fraught with limitations including limited tissue availability and tissue morbidity at the collection site. All of these factors serve as the driving force for developing engineered bone tissue that can be used in clinical practice.
Unlike materials-based strategies that are dependent upon the availability of responsive and functional host cells, cell-based therapies represent an exciting approach to promote tissue repair by delivering functional cells to the defect site. Mesenchymal stem cells (MSC) are under widespread investigation due to their osteogenic and pro-angiogenic potential (Caplan and Dennis, 2006, Decaris, et al., 2012, Hoch, et al., 2012). These benefits, however, are often lost with successive passaging and expansion of these cells ex vivo, which relies on culturing them as a nonphysiologic monolayer (Siddappa, et al., 2007). Furthermore, although a cell’s surrounding milieu may be readily controlled in vitro, cells are often transplanted in vivo into a harsh ischemic environment lacking a microvascular network (Kneser, et al., 2006). This leads to sudden oxygen and nutrient deprivation, inducing massive cell death (Potier, et al., 2007). Thus, it is imperative to develop improved methods to enhance cell survival and maximize cell function for cell-based therapies in bone tissue engineering.
A number of strategies are under investigation to effectively prime MSC prior to their implantation to improve in vivo survival and function. While the culture of MSC in the presence of various exogenous growth factors enhances survival upon transplantation (Herrmann, et al., 2010, Pasha, et al., 2008), this approach is limited by an insufficient knowledge of the appropriate dosage and duration of factor exposure (Mehta, et al., 2012), as well as high costs associated with prolonged use of recombinant proteins. Preconditioning in low oxygen has similar pro-survival effects, yet this approach can inhibit differentiation and stunt proliferation (Holzwarth, et al., 2010). Furthermore, these strategies seek to manipulate the behavior of cells grown in monolayer culture, a nonphysiologic condition. MSC expanded in nonphysiologic monolayer culture rapidly undergo apoptosis following transplantation (Zhang, et al., 2001). However, the implantation of multiple cellular populations as interconnected sheets exhibits reduced apoptosis and prolonged survival compared to equivalent numbers of dissociated cells (Shimizu, et al., 2002, Yang, et al., 2005). This distinct improvement in survival is potentially due to the retention of critical cell-cell contacts established during culture, which are enzymatically severed with standard trypsinization methods. However, due to diffusive limitations, thick, viable, 3D tissues such as bone cannot be created with this cell-sheet technology (Yang, Yamato, Kohno, Nishimoto, Sekine, Fukai and Okano, 2005).
In order to increase cell survival and efficacy of stem cell therapy, our lab and others have demonstrated that MSC exhibit increased overall function when formed into spheroids – multicellular aggregates formed through promoting cell-cell interactions. Recent data reveal that MSC spheroids survive better in ischemic conditions compared to dissociated cells expanded in monolayer culture (Bhang, et al., 2012a). This is likely because spheroids avoid the need for cell detachment from the ECM, allowing them to preserve their native environment and provide essential signals for cell survival (Wang, et al., 2009). Additionally, spheroid formation also greatly enhances the pro-angiogenic potential of MSC, causing up-regulation of a wide array of growth factors including vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), and angiogenin (Bhang, Lee, Lee, La, Yang, Cho and Kim, 2012a, Lee, et al., 2012, Wang, Chen, Hwang, Lin, Huang, Lee, Chang and Sung, 2009). However, these studies were performed with undifferentiated MSC, and osteogenic induction of MSC before transplantation is commonly employed to enhance the potential contribution of transplanted cells toward bone formation. The impact of spheroid formation using osteogenically-induced MSC on cell survival, trophic factor secretion, and osteogenesis is unknown.
We hypothesized that MSC formed into 3-dimensional spheroids and suspended in fibrin hydrogels would exhibit increased survival and function when exposed to a harsh microenvironment compared to dissociated MSC. To explore this hypothesis, we examined the osteogenic, pro-angiogenic, and apoptotic resistance of MSC spheroids compared to cells expanded in monolayer culture. We examined the response of MSC spheroids to an experimentally-controlled ischemic microenvironment when entrapped in fibrin gels as a model of their behavior upon implantation into tissue defects.
MATERIALS AND METHODS
Cell Culture
Human bone marrow-derived mesenchymal stem cells (MSC) were purchased from Lonza (Lonza, Walkersville, MD) and were listed as CD105, CD166, CD29, and CD44 positive, as well as CD14, CD34 and CD45 negative, as tested by flow cytometry. Cells were used without further characterization, and during expansion MSC were seeded at approximately 4,000 cells/cm2. Cells were cultured in αMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (JR Scientific, Woodland, CA), and 100 units/mL of penicillin and 100 μg/mL of streptomycin (Mediatech, Manassas, VA) under standard culture conditions (37°C, 21% O2, 5% CO2) until use at passage 5.
Spheroid Formation and Characterization
Spheroidal aggregates of MSC were formed using the hanging drop technique (Del Duca, et al., 2004) with 15,000; 30,000; or 60,000 cells per 25 μL droplet. Identical numbers of MSC were plated as adherent monolayers in 12-well plates as controls. After allowing spheroids to aggregate for 48 hours, spheroid diameter was quantified via bright field microscopy (n=12 per seeding density) and analyzed with ImageJ (NIH, Bethesda, MD). The 25-μL media droplets containing spheroids were refreshed every two days by careful aspiration of 14 μL from and addition of 20 μL to each droplet to account for evaporative loss.
Assessment of Cellular Viability, Metabolism, and Proliferation
After allowing spheroids to aggregate for 48 hours, MSC were lysed in passive lysis buffer (Promega, Madison, WI) and apoptosis was quantitatively measured by analyzing 100 μL of lysate per sample using a Caspase-Glo 3/7 assay (Promega) (Binder, et al., 2013, Davis, et al., 2011). Luminescence was detected on a microplate reader and normalized to DNA content, which was determined from the same lysate using the Quant-iT PicoGreen DNA Assay Kit (Invitrogen). In order to measure cellular metabolic activity, the media was refreshed on adherent monolayers, and the spheroids were collected and placed in 12-well plates with 1 mL of αMEM. 100 μL of Alamar Blue (Invitrogen) was added to both spheroid and monolayer wells and allowed to incubate with the cells for 3 hours, after which fluorescence was detected on a microplate reader and normalized to total DNA content from the same sample.
The distribution of proliferating cells within intact spheroids and corresponding adherent monolayer cultures was determined using a Click-iT™ EdU imaging kit (Invitrogen) according to the manufacturer’s protocols. Spheroids were then fixed in 3% glutaraldehyde (Fisher Scientific, Pittsburgh, PA), counterstained with Hoechst, and imaged using a Nikon Eclipse TE2000U microscope (Melville, NY) and SpotRT digital camera (Diagnostic Instruments, Sterling Heights, MI). Additional sections were stained with Toluidine Blue (Sigma Aldrich, St. Louis, MO) to visualize cellular distribution.
Entrapment of Spheroids into Fibrin Gels
Fibrin gels were prepared as we previously described (Davis, Miller, Case and Leach, 2011, Murphy and Leach, 2012) by combining 20 mg/mL fibrinogen (Calbiochem, Gibbstown, NJ), 2.3% (w/v) NaCl (Sigma Aldrich), 2.5 U/mL thrombin (Calbiochem), 20 mM CaCl2 (Sigma Aldrich), and 250 KIU/mL aprotinin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), all in PBS. Spheroids containing 15,000 cells/sphere were formed over 48 hours and suspended in fibrin gels at 2×106 cells/mL of gel (e.g., 4 spheroids per 30 μL gel), cast into custom PDMS molds, and allowed to gel for 1 hour (Murphy and Leach, 2012). An equal number of dissociated cells was suspended in gels as control groups. Gels were then transferred into individual wells of 24-well plates and the media was refreshed every 3 days.
Spheroid Response to Impaired Microenvironment
Fibrin gels containing spheroids or dissociated cells were allowed to equilibrate in αMEM under standard culture conditions for 24 hours, after which their media was exchanged for serum-free αMEM and they were moved to 1% oxygen (serum-deprived/hypoxic conditions, SD/H conditions) using oxygen-controlled HERAcell 150i incubators (Thermo Scientific, Pittsburgh, PA). These conditions are sufficient to induce apoptosis in MSC (Binder, Genetos and Leach, 2013). Gels were collected in passive lysis buffer (Promega) at 0, 2, and 5 days following the change in culture environment, and cellular proliferation and apoptosis were quantified as described above. The media was refreshed 24 hours prior to collection, and the concentration of VEGF in the media was determined using a human VEGF ELISA kit according to the manufacturer’s protocol (R&D Systems, Minneapolis, MN).
Assessment of Osteogenic Potential
To determine if osteogenic induction was required prior to spheroid formation, MSC were cultured as adherent monolayers for 10 days in either αMEM or osteogenic media under full serum and normoxic conditions (OM; α-MEM supplemented with 10 mM β-glycerophosphate, 50 μg/mL ascorbate-2-phosphate, and 100 nM dexamethasone, all from Sigma). Cells were trypsinized and formed into 15,000-cell spheroids using either α-MEM or OM, and osteogenic potential was assessed 7 days after spheroid formation by quantifying intracellular alkaline phosphatase (ALP) activity via PNPP assay and calcium deposition using the o-cresolphthalein assay (Davis, et al., 2013b, Decaris, Binder, Soicher, Bhat and Leach, 2012).
To assess the osteogenic potential of spheroids within fibrin gels, MSC in monolayer culture were osteogenically induced in OM for 10 days prior to spheroid formation (15,000 cells/spheroid). Spheroids were formed over 48 hours and then suspended in fibrin gels at 2×106 cells/mL of gel as described above, and an equal number of dissociated cells were suspended in gels as control groups. Gels were then cultured in OM for up to 21 days. The osteogenic potential of the entrapped MSC was determined by quantifying ALP activity and calcium deposition at 0, 7, 14, and 21 days following gel fabrication (Davis, et al., 2013a).
Statistical Analysis
Data are presented as mean ± standard deviation. Statistical analysis was performed using two-way ANOVA with Bonferroni correction for multiple comparisons in Prism 6 software (GraphPad); p values less than 0.05 were considered statistically significant.
RESULTS
Spheroid Formation and Characterization
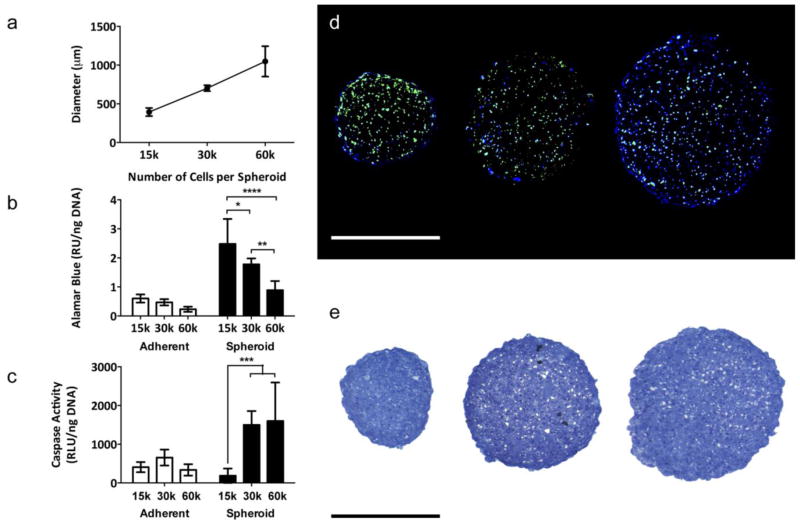
The hanging drop technique enabled MSC to coalesce into a single spheroid per droplet, and spheroids were visible to the naked eye within 48 hours. Spheroid diameter increased linearly with increasing cell number, ranging from 394 ± 53 μm for the 15,000-cell spheroid to 1049 ± 196 μm for the 60,000-cell spheroid (Fig. 1a).
Fig. 1.
MSC spheroid diameter, metabolic activity, viability, and proliferation can be controlled by cell number. (a) Spheroid diameter increases linearly with increasing cell number (n=12). (b) The smaller 15,000-cell spheroids exhibited increased metabolic activity, as measured by Alamar Blue, than the larger 30,000-cell and 60,000-cell spheroids (*p<0.05 vs. 30,000-cell spheroid, ****p<0.0001 vs. 60,000-cell spheroid; n=6). (c) The smaller 15,000-cell spheroids exhibited reduced apoptosis, as measured by caspase 3/7 activity, than the larger 30,000-cell and 60,000-cell spheroids (***p<0.001 vs. 30,000-cell and 60,000-cell spheroids; n=6). (d) EdU (green) and Hoechst (blue) staining revealed the cells in the 15,000-cell spheroid (left) are more proliferative than those in the larger 30,000-cell (middle) and 60,000-cell spheroids (right). Scale bar represents 500 μm. (e) Toluidine Blue staining indicated the 15,000-cell spheroids are more compact than the larger 30,000-cell and 60,000-cell spheroids. Scale bar represents 500 μm.
Metabolic Activity, Viability, and Proliferation of MSC Spheroids is Dependent on Size
The smallest spheroids formed of 15,000 cells exhibited increased metabolic activity as measured by Alamar Blue (Fig. 1b) and reduced caspase 3/7 activity, indicative of less apoptosis, compared to the larger 30,000-cell and 60,000-cell spheroids (p<0.001). The 30,000- and 60,000-cell spheroids exhibited higher caspase activity than their adherent counterparts (p<0.05 and p<0.01, respectively); however, caspase activity within 15,000-cell spheroids was comparable to that of its adherent counterpart (Fig. 1c). The 15,000-cell spheroids also had the most uniform distribution of proliferating cells, as indicated by sections stained for EdU and Hoechst (Fig. 1d). Spheroids formed from 30,000 and 60,000-cells exhibited large voids and uneven morphology compared to the more compact 15,000-cell spheroids (Fig. 1e). The 15,000-cell spheroid exhibited the greatest viability amongst the three sizes evaluated; thus, 15,000-cell spheroids were used for all subsequent studies.
MSC Spheroids Entrapped in Fibrin Gels Resist Apoptosis and Secrete More VEGF
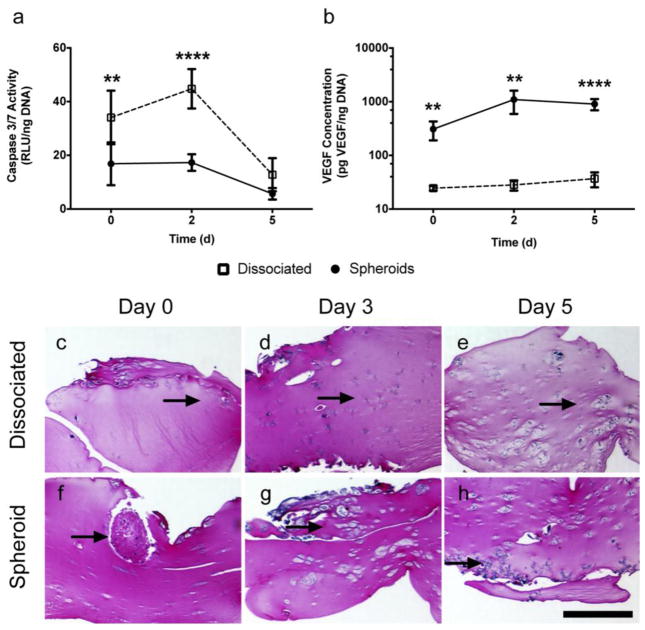
When cultured in serum-free media and 1% oxygen (SD/H conditions), entrapped spheroids exhibited significantly reduced caspase activity, indicative of less apoptosis, compared to dissociated MSC (Fig. 2a). In both groups, normalized caspase activity decreased over 5 days, likely due to loss of more vulnerable cells upon changing culture conditions and the persistence of more robust cells in culture. Additionally, MSC spheroids secreted up to 100 times the amount of VEGF compared to an equal number of dissociated MSC (Fig. 2b). Proliferation, as measured by DNA content, did not differ between the entrapped spheroids and dissociated cells, and these trends were observed in all serum-oxygen tension combinations (Supplementary Fig. 1). Sections of fibrin gels stained with H&E indicated that the cells slowly migrated out of the spheroids into the surrounding gel, and after 5 days in culture, both groups appeared morphologically similar (Fig. 2e, 2h).
Fig. 2.
MSC spheroids entrapped in a fibrin gel and cultured in SD/H conditions exhibit enhanced survival and VEGF secretion compared to dissociated MSC. (a) Caspase 3/7 activity in MSC spheroids compared to dissociated cells (**p<0.01 vs. spheroids at day 0, ****p<0.0001 vs. spheroids, n=4). (b) VEGF secretion in spheroids compared to dissociated cells (**p<0.01 vs. spheroids, ****p<0.0001 vs. spheroids, n=4). (c–h) H&E staining of cells entrapped in fibrin gels over time. Arrows denote cells; scale bar represents 500 μm.
Osteogenic Potential of MSC Spheroids is Dependent Upon Timing of Induction
We examined the contribution of the timing of exposure to osteogenic media on spheroid formation and osteogenic potential. When cells were cultured in αMEM for 10 days prior to OM exposure, MSC spheroids exhibited significantly lower ALP activity, an early marker of osteogenesis, compared to adherent monolayers. However, if both spheroid and adherent monolayers were exposed to osteogenic media throughout the experiment, ALP activity was not statistically different between the two groups (Fig. 3a). Calcium content, a late stage marker of osteogenesis, significantly increased in both MSC spheroids and adherent monolayers as the duration of culture in OM was increased (Fig. 3b). For all media combinations, the total normalized calcium content was higher in spheroids compared to adherent monolayers. Absolute DNA content was greater for adherent monolayers than MSC spheroids, with no statistical differences found between media groups (Supplementary Fig. 2).
Fig. 3.
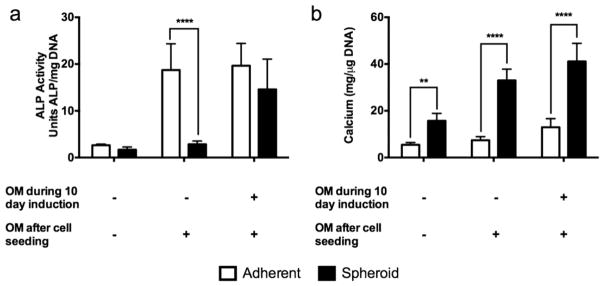
Osteogenic potential of MSC spheroids and adherent monolayers is influenced by timing of osteogenic induction. Osteogenic potential was assessed 7 days after spheroid formation. (a) When cells were cultured in αMEM prior to spheroid formation in OM (- OM during 10 day induction), ALP activity was significantly lower in spheroidal culture compared to adherent monolayers. However, ALP activity was not statistically different between the two groups if both spheroid and adherent monolayers received osteogenic media throughout the experiment (****p<0.0001 vs. spheroids, n=4). (b) Calcium content significantly increased in both MSC spheroids and adherent monolayers as the duration of culture in OM increased (**p<0.01 vs. spheroids, ****p<0.0001 vs. spheroids, n=4).
Spheroid Osteogenic Potential in Fibrin Gels is Comparable to Dissociated MSC
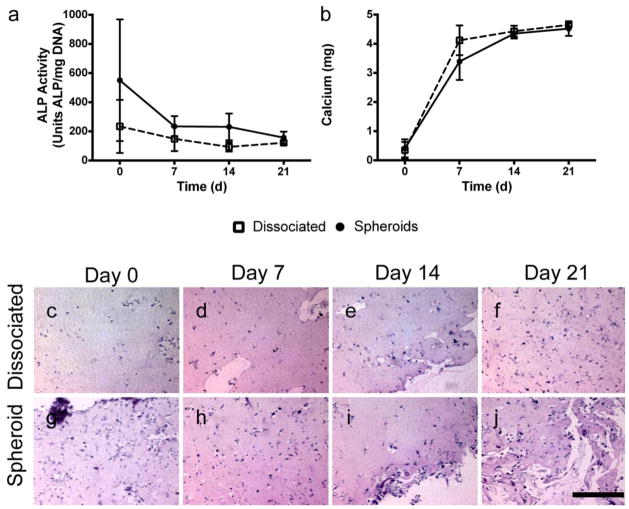
In light of data presented above, we osteogenically induced MSC for 10 days prior to forming 15,000-cell spheroids to examine the osteogenic potential of MSC entrapped in fibrin gels over time. We did not detect any differences in ALP activity (Fig. 4a) or calcium secretion (Fig. 4b) for spheroids entrapped in fibrin gels over the 21-day period compared to an equivalent number of dissociated cells. Cells persisted in the gels throughout the 21-day period, and by 7 days, the cells had migrated out of the spheroids and were morphologically similar to the gels containing dissociated cells (Fig. 4c).
Fig. 4.
MSC spheroids entrapped in fibrin gels exhibit comparable osteogenic potential to dissociated MSC. (a) ALP activity was statistically indistinguishable between spheroids and dissociated cells entrapped in fibrin gels at all time points (n=3). (b) Total calcium content was statistically indistinguishable between MSC spheroids and dissociated cells entrapped in fibrin gels at all time points (n=3). (c–j) H&E staining of cells entrapped in fibrin gels over time. Scale bar represents 200 μm.
DISCUSSION
In this study, we examined the viability, pro-angiogenic, and osteogenic potential of MSC spheroids compared to MSC maintained as traditional adherent monolayers and/or dissociated cells. We report that MSC spheroids formed with 15,000 cells exhibit greater resistance to apoptosis and increased secretion of VEGF, a potent trophic factor that enhances collateral vessel formation, under normoxic, hypoxic, full serum, and serum-free conditions. Furthermore, spheroids are as responsive to osteogenic induction as their monolayer or dissociated cell counterparts, exhibiting similar osteogenic potential when cultured in fibrin gels – a promising vehicle for cell delivery. These data provide strong evidence that formation of MSC into spheroids has the potential to facilitate the clinical translation of MSC-based therapies for the repair of nonhealing bone defects.
Spheroids can be formed using numerous techniques including agarose-coated wells (Friedrich, et al., 2009, Laschke, et al., 2013), siliconized spinner flasks (Frith, et al., 2010, Kunz-Schughart, et al., 1998, Mueller-Klieser, 1997), methylcellulose-containing media (Correa de Sampaio, et al., 2012, Wang, Chen, Hwang, Lin, Huang, Lee, Chang and Sung, 2009), and specialized plates (Fey and Wrzesinski, 2012, Lai and Kisaalita, 2012). We employed the hanging drop technique (Bartosh, et al., 2010) due to its simplicity, reliability, and cost-effectiveness. Varying sizes of spheroids have been investigated, ranging from 50 μm (Wenger, et al., 2004) to 1,500 μm (Bartosh, Ylostalo, Mohammadipoor, Bazhanov, Coble, Claypool, Lee, Choi and Prockop, 2010). The size of these 3-dimensional multicellular aggregates must be considered carefully due to limitations in the diffusive length of nutrient transport (Curcio, et al., 2007), which renders cells in the core of spheroids with radii greater than 200 μm vulnerable to hypoxia and cell death. The presence of a hypoxic core within the spheroid may preprogram the cells to promote survival (Korff and Augustin, 1998) and enhance trophic factor secretion (Shweiki, et al., 1995). Thus, there may be additional advantages conferred when utilizing a spheroid whose radius is near the nutrient transport limitation. However, the link between a hypoxic core and cell activity has not been firmly demonstrated. We chose to examine the range of 15,000 – 60,000 cells per spheroid, as others have observed MSC spheroids within this range to exhibit radii on the order of 200 μm (Bartosh, et al., 2010, Bhang, et al. 2012b). In our study, spheroids containing approximately 15,000 cells had a mean radius of approximately 200 μm and were subsequently the most viable – as measured by caspase 3/7 activity, metabolic activity, and proliferation – compared to spheroids of larger sizes. We did not examine smaller spheroid sizes, which would require fewer cells, in order to maintain a radius near the limitation of diffusive nutrient transport. Furthermore, cell-based therapies require a substantial number of cells to be delivered in order to drive tissue formation, and larger spheroids facilitate the transplantation of fewer aggregates.
These studies confirm that spheroidal conformation drastically increases the production of VEGF, a potent mediator of angiogenesis. Bone formation is dependent upon angiogenesis, and many studies report that accelerated angiogenesis enhances bone formation (He, et al., 2012, He, et al., 2010, Kaigler, et al., 2006, Leu, et al., 2009). However, the pro-angiogenic potential of MSC is inversely related to their degree of osteogenic differentiation (Hoch, Binder, Genetos and Leach, 2012). Spheroidal formation of MSC may address this shortcoming, as MSC spheroids entrapped in a fibrin gel secreted 100-fold more VEGF than an equal number of dissociated cells while exhibiting similar osteogenic potential. Although these changes were observed under serum-free and hypoxic conditions lacking osteogenic cues, the magnitude of VEGF increase provides support for the use of MSC spheroids to promote mineral deposition, as they can simultaneously establish a vascular network.
In contrast to MSC expanded in monolayer which must be trypsinized from tissue culture plastic, spheroidal culture preserves cell-cell contacts and native extracellular matrix thought to provide the survival advantage we and others have observed (Wang, Chen, Hwang, Lin, Huang, Lee, Chang and Sung, 2009). This survival advantage, however, cannot come at the expense of osteogenic potential. Baraniak and McDevitt demonstrated that MSC spheroids retain their tri-lineage potential (Baraniak and McDevitt, 2012). Our study builds upon these findings by demonstrating that upon incorporation into a clinically-relevant biomaterial, MSC spheroids are as osteogenic as dissociated cells. However, our results demonstrate that if MSC are not osteogenically-induced prior to spheroid formation, the spheroids appear to lack osteogenic potential. This phenomenon may stem from the fact that MSC grown as adherent monolayers are receiving both soluble and mechanical osteogenic cues, while spheroids in hanging drops rely solely on soluble cues. Tissue culture plastic possesses a Young’s Modulus of approximately 3 GPa, and it has been well demonstrated that MSC grown on stiff substrates exhibit increased osteogenic potential (Engler, et al., 2006, Titushkin and Cho, 2007, Winer, et al., 2009). Thus, incorporating the cells into a relevant biomaterial allows for a more equal comparison in terms of osteogenic potential. Similar to cells grown as monolayers, MSC spheroids respond to soluble osteogenic cues with increased ALP activity and mineral deposition indicative of osteogenic differentiation. Cell density within the fibrin gel may also contribute to our detected differences. At a consistent seeding density (2×106 cells/mL), there are differences in cell-cell contact and communication between MSC spheroids and dissociated cells. The effect of seeding density on spheroid function in fibrin hydrogels merits further investigation. This study provides strong evidence that spheroid formation does not inhibit the responsiveness of MSC in spheroidal form to osteogenic cues, thereby harnessing the survival advantage of spheroids without sacrificing their ability to directly participate in bone formation.
While others have transplanted spheroids in vivo without a biomaterial carrier (Bhang, et al., 2011, Bhang, et al., 2012b), primarily for promoting neovascularization of ischemic tissue, the delivery of cells within a biomaterial can promote cell persistence at the defect site and provide cues to guide cellular differentiation. Fibrin gels are particularly promising as a cell delivery vehicle, as endogenously, fibrin serves as a scaffold for tissue repair and regeneration and a provisional matrix for the invasion of regenerative cells. Furthermore, fibrin gels can be delivered in a minimally-invasive approach or molded to specific shapes and are tunable to achieve a particular gelation rate, compressive stiffness, and degradation rate (Barsotti, et al., 2011, Davis, Miller, Case and Leach, 2011, Janmey, et al., 2009, Shaikh, et al., 2008). In agreement with similar studies, we observed that MSC entrapped in fibrin gels were capable of proliferation, osteogenic differentiation, and secretion of pro-angiogenic factors (Davis, Miller, Case and Leach, 2011). When cultured in fibrin gels, entrapped MSC migrated out of the spheroid and into the surrounding biomaterial. These findings are in agreement with others that have shown MSC migrate out of the spheroid when plated on adherent culture surfaces (Bartosh, Ylostalo, Mohammadipoor, Bazhanov, Coble, Claypool, Lee, Choi and Prockop, 2010). Thus, the maintenance of MSC as spheroids is not required for continued performance, indicating that spheroidal conformation can be used as a tool to precondition the entrapped MSC. Additionally, the material properties of the fibrin gel should be considered when choosing a delivery vehicle for MSC spheroids, as cell migration through the gel may play a key role in their enhanced survival and osteogenic potential.
CONCLUSION
Our results demonstrate that MSC formed into 3-dimensional spheroids, compared to those derived from traditional 2-dimensional monolayer culture, maintain osteogenic potential and exhibit improved survival when exposed to a harsh microenvironment. MSC formed as spheroids secrete significantly more VEGF and exhibit greater resistance to apoptosis compared to dissociated MSC, suggesting that they would promote angiogenesis and be more apt to survive than dissociated cells upon transplantation. Additionally, formation of MSC into spheroids did not inhibit their responsiveness to osteogenic induction. Collectively, our findings demonstrate that spheroidal culture of MSC has the potential to facilitate the clinical translation of MSC-based therapies for the repair of slow- and non-healing bone defects.
Supplementary Material
Acknowledgments
This project was supported by NIH Grant 1R03DE021704-01 to JKL; SF was supported by the Clinical and Translational Science Center (CTSC) T32 Pre-Doctoral Clinical Research Training Program.
Footnotes
DISCLOSURE STATEMENT
The authors indicate no potential conflicts of interest.
References
- Baraniak PR, McDevitt TC. Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential. Cell Tissue Res. 2012;347:701–711. doi: 10.1007/s00441-011-1215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsotti MC, Magera A, Armani C, Chiellini F, Felice F, Dinucci D, Piras AM, Minnocci A, Solaro R, Soldani G, Balbarini A, Di Stefano R. Fibrin acts as biomimetic niche inducing both differentiation and stem cell marker expression of early human endothelial progenitor cells. Cell Prolif. 2011;44:33–48. doi: 10.1111/j.1365-2184.2010.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosh TJ, Ylostalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, Lee RH, Choi H, Prockop DJ. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010;107:13724–13729. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhang SH, Cho SW, La WG, Lee TJ, Yang HS, Sun AY, Baek SH, Rhie JW, Kim BS. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. 2011;32:2734–2747. doi: 10.1016/j.biomaterials.2010.12.035. [DOI] [PubMed] [Google Scholar]
- Bhang SH, Lee S, Lee TJ, La WG, Yang HS, Cho SW, Kim BS. Three-dimensional cell grafting enhances the angiogenic efficacy of human umbilical vein endothelial cells. Tissue Eng Part A. 2012a;18:310–319. doi: 10.1089/ten.tea.2011.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhang SH, Lee S, Shin JY, Lee TJ, Kim BS. Transplantation of cord blood mesenchymal stem cells as spheroids enhances vascularization. Tissue Eng Part A. 2012b;18:2138–2147. doi: 10.1089/ten.tea.2011.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BY, Genetos DC, Leach JK. Lysophosphatidic acid protects human mesenchymal stromal cells from differentiation-dependent vulnerability to apoptosis. Tissue Eng Part A. 2013 doi: 10.1089/ten.tea.2013.0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Correa de Sampaio P, Auslaender D, Krubasik D, Failla AV, Skepper JN, Murphy G, English WR. A heterogeneous in vitro three dimensional model of tumour-stroma interactions regulating sprouting angiogenesis. PLoS One. 2012;7:e30753. doi: 10.1371/journal.pone.0030753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio E, Salerno S, Barbieri G, De Bartolo L, Drioli E, Bader A. Mass transfer and metabolic reactions in hepatocyte spheroids cultured in rotating wall gas-permeable membrane system. Biomaterials. 2007;28:5487–5497. doi: 10.1016/j.biomaterials.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Davis HE, Binder BY, Schaecher P, Yakoobinsky DD, Bhat A, Leach JK. Enhancing Osteoconductivity of Fibrin Gels with Apatite-Coated Polymer Microspheres. Tissue Eng Part A. 2013a doi: 10.1089/ten.tea.2012.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis HE, Binder BY, Schaecher P, Yakoobinsky DD, Bhat A, Leach JK. Enhancing osteoconductivity of fibrin gels with apatite-coated polymer microspheres. Tissue Eng Part A. 2013b;19:1773–1782. doi: 10.1089/ten.tea.2012.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis HE, Miller SL, Case EM, Leach JK. Supplementation of fibrin gels with sodium chloride enhances physical properties and ensuing osteogenic response. Acta Biomater. 2011;7:691–699. doi: 10.1016/j.actbio.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Decaris ML, Binder BY, Soicher MA, Bhat A, Leach JK. Cell-derived matrix coatings for polymeric scaffolds. Tissue Eng Part A. 2012;18:2148–2157. doi: 10.1089/ten.TEA.2011.0677. [DOI] [PubMed] [Google Scholar]
- Del Duca D, Werbowetski T, Del Maestro RF. Spheroid preparation from hanging drops: characterization of a model of brain tumor invasion. J Neurooncol. 2004;67:295–303. doi: 10.1023/b:neon.0000024220.07063.70. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Fey SJ, Wrzesinski K. Determination of drug toxicity using 3D spheroids constructed from an immortal human hepatocyte cell line. Toxicol Sci. 2012;127:403–411. doi: 10.1093/toxsci/kfs122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA. Spheroid-based drug screen: considerations and practical approach. Nat Protoc. 2009;4:309–324. doi: 10.1038/nprot.2008.226. [DOI] [PubMed] [Google Scholar]
- Frith JE, Thomson B, Genever PG. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods. 2010;16:735–749. doi: 10.1089/ten.TEC.2009.0432. [DOI] [PubMed] [Google Scholar]
- He J, Decaris ML, Leach JK. Bioceramic-mediated trophic factor secretion by mesenchymal stem cells enhances in vitro endothelial cell persistence and in vivo angiogenesis. Tissue Eng Part A. 2012;18:1520–1528. doi: 10.1089/ten.tea.2011.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Genetos DC, Leach JK. Osteogenesis and trophic factor secretion are influenced by the composition of hydroxyapatite/poly(lactide-co-glycolide) composite scaffolds. Tissue Eng Part A. 2010;16:127–137. doi: 10.1089/ten.tea.2009.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann JL, Wang Y, Abarbanell AM, Weil BR, Tan J, Meldrum DR. Preconditioning mesenchymal stem cells with transforming growth factor-alpha improves mesenchymal stem cell-mediated cardioprotection. Shock. 2010;33:24–30. doi: 10.1097/SHK.0b013e3181b7d137. [DOI] [PubMed] [Google Scholar]
- Hoch AI, Binder BY, Genetos DC, Leach JK. Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PLoS One. 2012;7:e35579. doi: 10.1371/journal.pone.0035579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzwarth C, Vaegler M, Gieseke F, Pfister SM, Handgretinger R, Kerst G, Muller I. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol. 2010;11:11. doi: 10.1186/1471-2121-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey PA, Winer JP, Weisel JW. Fibrin gels and their clinical and bioengineering applications. J R Soc Interface. 2009;6:1–10. doi: 10.1098/rsif.2008.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaigler D, Wang Z, Horger K, Mooney DJ, Krebsbach PH. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J Bone Miner Res. 2006;21:735–744. doi: 10.1359/jbmr.060120. [DOI] [PubMed] [Google Scholar]
- Kneser U, Schaefer DJ, Polykandriotis E, Horch RE. Tissue engineering of bone: the reconstructive surgeon’s point of view. J Cell Mol Med. 2006;10:7–19. doi: 10.1111/j.1582-4934.2006.tb00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korff T, Augustin HG. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol. 1998;143:1341–1352. doi: 10.1083/jcb.143.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz-Schughart LA, Kreutz M, Knuechel R. Multicellular spheroids: a three-dimensional in vitro culture system to study tumour biology. Int J Exp Pathol. 1998;79:1–23. doi: 10.1046/j.1365-2613.1998.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Kisaalita WS. Performance evaluation of 3D polystyrene 96-well plates with human neural stem cells in a calcium assay. J Lab Autom. 2012;17:284–292. doi: 10.1177/2211068212442503. [DOI] [PubMed] [Google Scholar]
- Laschke MW, Schank TE, Scheuer C, Kleer S, Schuler S, Metzger W, Eglin D, Alini M, Menger MD. Three-dimensional spheroids of adipose-derived mesenchymal stem cells are potent initiators of blood vessel formation in porous polyurethane scaffolds. Acta Biomater. 2013;9:6876–6884. doi: 10.1016/j.actbio.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Laurencin C, Khan Y, El-Amin SF. Bone graft substitutes. Expert Rev Med Devices. 2006;3:49–57. doi: 10.1586/17434440.3.1.49. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Park SJ, Kang SK, Kim GH, Kang HJ, Lee SW, Jeon HB, Kim HS. Spherical bullet formation via E-cadherin promotes therapeutic potency of mesenchymal stem cells derived from human umbilical cord blood for myocardial infarction. Mol Ther. 2012;20:1424–1433. doi: 10.1038/mt.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu A, Stieger SM, Dayton P, Ferrara KW, Leach JK. Angiogenic response to bioactive glass promotes bone healing in an irradiated calvarial defect. Tissue Eng Part A. 2009;15:877–885. doi: 10.1089/ten.tea.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta M, Schmidt-Bleek K, Duda GN, Mooney DJ. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Adv Drug Deliv Rev. 2012;64:1257–1276. doi: 10.1016/j.addr.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Klieser W. Three-dimensional cell cultures: from molecular mechanisms to clinical applications. Am J Physiol. 1997;273:C1109–1123. doi: 10.1152/ajpcell.1997.273.4.C1109. [DOI] [PubMed] [Google Scholar]
- Murphy KC, Leach JK. A reproducible, high throughput method for fabricating fibrin gels. BMC Res Notes. 2012;5:423. doi: 10.1186/1756-0500-5-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe RJ, Mao J. Bone tissue engineering and regeneration: from discovery to the clinic--an overview. Tissue Eng Part B Rev. 2011;17:389–392. doi: 10.1089/ten.teb.2011.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008;77:134–142. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- Potier E, Ferreira E, Meunier A, Sedel L, Logeart-Avramoglou D, Petite H. Prolonged hypoxia concomitant with serum deprivation induces massive human mesenchymal stem cell death. Tissue Eng. 2007;13:1325–1331. doi: 10.1089/ten.2006.0325. [DOI] [PubMed] [Google Scholar]
- Shaikh FM, Callanan A, Kavanagh EG, Burke PE, Grace PA, McGloughlin TM. Fibrin: a natural biodegradable scaffold in vascular tissue engineering. Cells Tissues Organs. 2008;188:333–346. doi: 10.1159/000139772. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, Abe K, Kikuchi A, Umezu M, Okano T. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res. 2002;90:e40. doi: 10.1161/hh0302.105722. [DOI] [PubMed] [Google Scholar]
- Shweiki D, Neeman M, Itin A, Keshet E. Induction of vascular endothelial growth factor expression by hypoxia and by glucose deficiency in multicell spheroids: implications for tumor angiogenesis. Proc Natl Acad Sci U S A. 1995;92:768–772. doi: 10.1073/pnas.92.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddappa R, Licht R, van Blitterswijk C, de Boer J. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J Orthop Res. 2007;25:1029–1041. doi: 10.1002/jor.20402. [DOI] [PubMed] [Google Scholar]
- Titushkin I, Cho M. Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophys J. 2007;93:3693–3702. doi: 10.1529/biophysj.107.107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Chen CH, Hwang SM, Lin WW, Huang CH, Lee WY, Chang Y, Sung HW. Spherically symmetric mesenchymal stromal cell bodies inherent with endogenous extracellular matrices for cellular cardiomyoplasty. Stem Cells. 2009;27:724–732. doi: 10.1634/stemcells.2008-0944. [DOI] [PubMed] [Google Scholar]
- Wenger A, Stahl A, Weber H, Finkenzeller G, Augustin HG, Stark GB, Kneser U. Modulation of in vitro angiogenesis in a three-dimensional spheroidal coculture model for bone tissue engineering. Tissue Eng. 2004;10:1536–1547. doi: 10.1089/ten.2004.10.1536. [DOI] [PubMed] [Google Scholar]
- Winer JP, Janmey PA, McCormick ME, Funaki M. Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng Part A. 2009;15:147–154. doi: 10.1089/ten.tea.2007.0388. [DOI] [PubMed] [Google Scholar]
- Yang J, Yamato M, Kohno C, Nishimoto A, Sekine H, Fukai F, Okano T. Cell sheet engineering: recreating tissues without biodegradable scaffolds. Biomaterials. 2005;26:6415–6422. doi: 10.1016/j.biomaterials.2005.04.061. [DOI] [PubMed] [Google Scholar]
- Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.