Abstract
Objective
Knee osteoarthritis (OA) contributes significantly to disability in older individuals and racial/ethnic minorities are disproportionately affected. The present study aimed to characterize differences in clinical and experimental pain including pain inhibition among older African-Americans (AAs) and non-Hispanic whites (NHWs) with knee OA.
Methods
AAs and NHWs with knee OA (n=267) completed clinical and functional pain assessments including quantitative sensory testing (QST). We hypothesized that 1) AAs would display lower pain tolerance and higher heat, mechanical and cold pain ratings compared to NHWs; 2) AAs would display greater temporal summation compared to NHWs; 3) AAs would display reduced pain inhibition compared to NHWs; 4) AAs would demonstrate greater clinical pain and poorer function relative to NHWs; and 5) QST would significantly predict clinical pain within each race/ethnicity.
Results
AAs displayed increased pain sensitivity, temporal summation and reduced pain inhibition than NHWs. AAs reported greater clinical pain and poorer function than NHWs. Race/ethnic differences in clinical pain became non-significant when controlling for education and income, whereas differences in QST remained highly significant. Although pain inhibition predicted clinical pain in both groups, different QST measures were additionally predictive of clinical pain within groups.
Conclusion
Our study establishes race/ethnic differences in experimental and clinical pain and function in older individuals with knee OA. Our findings that different QST measures were associated with clinical pain within race/ethnic groups while reduced pain inhibition was important in all participants warrants further study evaluating common and group-specific pathophysiological mechanisms contributing to clinical pain in OA.
Osteoarthritis (OA) is a major source of years of life lost due to disability in the older population (1), and the knee is the most commonly affected joint. Approximately 1 in 2 people may develop symptomatic knee OA by 85 years of age (2). A disproportionate number of racial/ethnic minorities are affected by knee OA (3). Compared to non-Hispanic whites (NHWs), a greater proportion of African-Americans (AAs) report both radiographic knee OA and symptomatic knee OA (4). Several investigators have reported greater pain severity among AA compared to NHW individuals with knee OA (5-8), while others have reported no such race/ethnic differences in OA pain severity including knee OA (9). In addition to race/ethnic differences in OA-related clinical pain, several studies have reported significantly greater disability among AAs compared to whites with knee OA (10-12).
A complex web of factors contributes to these disparities in OA-related pain and disability ranging from differences in physiological pain processing to socioeconomic (9,13) (5,6,14-16) and healthcare-related factors (i.e., access to and preferences for treatment) (17,18). A substantial literature addressing race/ethnic group differences in experimentally-induced pain has demonstrated greater pain sensitivity among healthy AAs compared to NHWs across multiple stimulus modalities, including lower pain threshold and tolerance as well as significantly higher suprathreshold pain ratings among healthy AAs compared to NHWs (21-24). (19). In addition to differences in basal pain sensitivity, there is evidence supporting differences in endogenous pain modulatory systems (i.e., pain inhibition and facilitation). For example, NHWs showed more robust conditioned pain modulation (CPM) compared to AAs (20) suggesting reduced pain inhibitory function among healthy AAs. Also, AAs exhibited greater temporal summation of pain (i.e. application of repeated painful stimuli become progressively more painful) (25), which represents a transient form of central sensitization. Therefore, considerable evidence demonstrates race/ethnic group differences in pain perception as well as endogenous pain modulation among healthy AAs compared to healthy NHWs. Race/ethnicity comparisons of experimental pain sensitivity may elucidate differences in clinical pain, since experimental pain sensitivity is predictive of clinical pain (26-28). However, limited information is available regarding race/ethnic group differences in experimental pain responses among individuals with knee OA (8), which is particularly important given the public health impact of OA and its greater burden among AAs. Moreover, no investigator to date has determined whether experimental measures of basal pain sensitivity and endogenous pain modulation relate to clinical pain and disability in a race/ethnicity-dependent manner among persons with knee OA.
The aim of the present study was to characterize differences in experimental pain sensitivity, endogenous pain inhibition, clinical pain and pain-related disability among older AAs and NHWs with knee OA. We hypothesized that: 1) AAs would display lower pain tolerance and higher ratings of heat, mechanical and cold pain compared to NHWs; 2) AAs would display greater temporal summation, suggesting increased central pain sensitization among AAs compared to NHWs; 3) AAs would display lower CPM, suggesting reduced pain inhibition among AAs compared to NHWs; 4) AAs would demonstrate higher levels of self-reported clinical pain and disability and poorer performance on functional measures relative to NHWs; and 5) Experimental pain sensitivity would significantly predict clinical pain within each race/ethnic group. These hypotheses were also examined adjusting for important confounding demographic and OA variables.
Patients and Methods
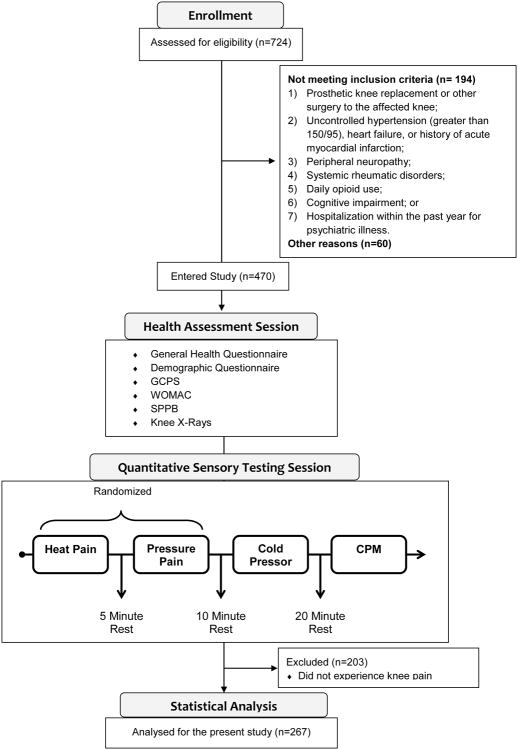
Individuals between 45 and 85 years of age who self-identified as African-American (AA) or non-Hispanic whites (NHW) were enrolled at the University of Florida (UF) and University of Alabama at Birmingham (UAB). Participants were recruited through community (e.g., posted fliers, radio and print media ads, word-of-mouth referrals) and clinic-based recruitment methods between January 2010 and October 2013. The study was approved by UF/UAB Institutional Review Boards. General study methodology is summarized in Figure 1.
Figure 1. Diagram detailing study methodology.
Participants presented with unilateral or bilateral symptomatic knee OA based upon the American College of Rheumatology clinical criteria (experience knee pain, stiffness (<30 minutes) and functional limitations), regardless of radiographic evidence. However, radiographs were performed in all participants in bilateral weight bearing, fixed flexion posteroanterior and lateral radiographic evaluation of the knee, as described elsewhere (29). Each knee joint was scored for Kellgren and Lawrence grade (0-4) (30). Participants were excluded if they had: 1) prosthetic knee replacement or other clinically significant surgery to the affected knee; 2) uncontrolled hypertension (greater than 150/95), heart failure, or history of acute myocardial infarction; 3) peripheral neuropathy; 4) systemic rheumatic disorders including rheumatoid arthritis, systemic lupus erythematosus, and fibromyalgia; 5) daily opioid use; 6) cognitive impairment; or 7) hospitalization within the preceding year for psychiatric illness.
General Study Procedures
After informed consent was obtained, participants attended a health assessment session (HAS) and a quantitative sensory testing (QST) session as previously described (8,31). Study participants completed questionnaires assessing general health and demographic information including participant's age, sex, weight, height, annual household income and highest level of education. During the HAS, a study physician or nurse practitioner completed a brief health history and physical examination including administration of the Short Physical Performance Battery (see below). To designate the participants' most painful knee as the index knee for QST procedures, the study physician or nurse practitioner performed joint palpation of the knees. The QST session took place within four weeks of the HAS. Individuals completed measures of clinical pain and function (detailed below) followed by thermal, mechanical, temporal summation, and conditioned pain modulation (CPM) assessments. The order of thermal and mechanical tests was counterbalanced, followed by cold pressor pain. Lastly, after a rest period, CPM was assessed. Recorded instructions were played prior to commencement of all procedures.
QST procedures
Thermal
Contact heat stimuli were delivered using a computer-controlled device (Medoc Pathway Pain & Sensory Evaluation System, Ramat Yishai, Israel). The thermode position was moved between trials to avoid sensitization and/or habituation of cutaneous receptors. Heat pain threshold and tolerance levels were assessed on both the index knee and ipsilateral ventral forearm using an ascending method of limits with a 16 × 16mm thermode. For each trial the probe would start at baseline temperature (32°C) and increase at a rate of 0.5°C/second until the participant responded by pressing a button. Participants were instructed to press the button when the sensation “first became painful”, and when they “no longer felt able to tolerate the pain”. The temperature was recorded for threshold and tolerance. The mean temperature from three trials was used for analysis.
Temporal summation of thermal pain was assessed by verbally rating the intensity of pain evoked by each of 5 brief, repetitive, suprathreshold heat pulses on a scale of 0 (no pain sensation) to 100 (the most intense pain sensation imaginable). Three target temperatures (44, 46, and 48°C) were delivered by a Contact Heat-Evoked Potential Stimulator (CHEPS) thermode for less than 1 second, with an approximately 2.5-second inter-pulse interval during which the temperature of the contactor returned to baseline (32°C). The procedure was terminated if the participant rated the thermal pain at 100. The average rating over the 5 trials, an index of overall sensitivity to suprathreshold heat pain, and the maximum increase in pain, a measure of temporal summation, were determined for each participant and used in the analyses. The latter was calculated by subtracting the first trial rating from the maximum rating provided at each temperature.
Mechanical
Pressure pain thresholds were evaluated at the medial and lateral aspects of the index knee as well as the ipsilateral quadriceps, trapezius and dorsal forearm. The order of testing sites was counterbalanced and randomized. For each site, a handheld Medoc digital pressure algometer (Algomed, MEDOC, Ramat Yishai, Israel) was applied at a constant rate of 30 kilopascals (kPa)/second. The participant was instructed to press a button when the sensation “first became painful,” and the pressure was recorded. An average pressure pain threshold was determined for each site from three trials. The maximum pressure for the knee sites was 600 pKa and 1000 pKa at the other sites. If participants did not report pain at the maximum pressure level, the procedure was terminated and a pressure of 600 or 1000 was assigned for that trial.
After pressure pain, we assessed sensitivity to punctate mechanical stimuli at the patella and back of the ipsilateral hand using a calibrated nylon monofilament delivering a target force of 300grams. Testing sites were randomized. Participants provided verbal pain ratings following a single contact and after 10 contacts at a rate of 1 contact/second. Ratings were made on a scale of 0 (no pain sensation) to 100 (the most intense pain sensation imaginable). The procedure was repeated and the ratings were summed for single and multiple contacts for analysis.
Cold Pressor Procedure
Following the thermal and mechanical procedures, cold sensitivity was probed with a modified procedure based on the cold pressor task (CPT). The procedure was three one-minute hand immersions in a cold-water bath (Thermo Scientific Refrigerated Bath) at 16°, 12°, and 8°C temperature. Participants placed their right hand (up to their wrist) into the water for up to 1 minute or until they wish to stop. During each immersion, participants were instructed to verbally indicate when the cold sensation “first became painful” (i.e., threshold). Participants also provided verbal ratings of pain intensity and unpleasantness at the end of the one-minute immersion. Verbal ratings of pain intensity and unpleasantness were on scale of 0 (no pain sensation/unpleasantness) to 100 (the most intense/unpleasantness pain sensation imaginable). If a participant withdrew the hand from the water bath before the one-minute period ended, the withdrawal time was recorded.
Conditioned Pain Modulation (CPM)
CPM was evaluated by determining the ability of a conditioning stimulus (i.e., cold-water immersion) to diminish the painfulness of a test stimulus (i.e., heat pain ratings). First, participants rated a series of 5 heat pain trials on the left ventral forearm to measure baseline sensitivity. Next, participants immersed their right hand into the cold-water bath for 1 minute. Then, participants removed their hand and rated a second series of heat pain trials. For both the conditioning and the test stimuli, temperatures were tailored to each participant to achieve a stimulus that produced a rating of 40-60 on the 0-100 scale.
Measures of Clinical Pain and Function
Graded Chronic Pain Scale (GCPS)
The GCPS evaluates global pain severity and pain-related interference over the past 6 months and consists of 7-items related to pain intensity and pain interference (i.e., loss of work days due to pain, interference in daily activities (32). With a 0-10 numerical rating scale, participants rated the intensity of their current knee pain and the worst and average pain during the past 6 months. These three items were averaged and multiplied by 10 to generate a “Characteristic Pain Intensity” score. Using the same scale, participants rated the degree that their knee pain interfered with daily activities (3 items) during the past 6 months, which were averaged and multiplied by 10 to generate a “Disability” score.
Western Ontario and McMaster Universities Index of Osteoarthritis (WOMAC)
The WOMAC (33) assesses symptoms of knee osteoarthritis in the past 48 hours. For this study, the 4-point Likert scale version was used. The WOMAC yields three subscales including pain during activities (5 items), stiffness during the day (2 items), and impairments in physical function (17 items) with higher scores indicating worse pain, stiffness, and impairments in physical function.
Short Physical Performance Battery (SPPB)
The SPPB (34) was used as an observed measure of functional limitations. The SPPB has been used as a reliable and valid performance-based measure of physical function in many various studies (35,36). Components include standing balance, 4-meter gait speed, and chair rising tasks. A single summary performance score is also calculated, ranging from 0 to 12 (lower scores = greater functional limitations) (34,37).
Statistical Methods
Data analysis was performed using version 22 of IBM SPSS software. Values reported are means and standard deviations unless otherwise stated. We used two separate approaches to assess race/ethnic differences: excluding and including confounding variables (i.e., covariates). Covariates were selected based on statistically significant pair-wise differences between race/ethnic groups. Race/ethnic differences were examined: 1) using Student's t-tests (i.e., unadjusted model), 2) using analysis of covariance (ANCOVA) with age, BMI and study site (i.e., UF or UAB) as covariates (i.e., partially adjusted model) and 3) using ANCOVA with income and education as additional covariates to model 2 above (i.e., fully adjusted model). Repeated measures during the mechanical temporal summation procedures were analyzed using repeated measure analysis of covariance (RM-ANCOVA) with a Greenhouse-Geisser correction if the sphericity assumption was not met. Statistical significance for all tests was set at 0.05.
Data Reduction of QST Variables
Due to the large number of QST variables obtained, they were subjected to a Principal Component Analyses (PCA) to reduce the number of statistical comparisons undertaken, and to minimize redundancy in the information reflected by the QST variables. PCA was performed with Oblique and Orthogonal rotations for variable reduction as previously described (38). Several PCA results were compared to determine agreement of primary loadings for individual variables. Components with eigenvalues greater than one were retained for interpretation. The variables entered in the PCA were: heat pain threshold and tolerance at the forearm and knee, pressure pain at all sites, cold pain threshold, cold pain intensity and unpleasantness ratings, punctate pain ratings at the hand and knee, heat temporal summation at the hand and knee and punctate temporal summation at the hand and knee. Once the factors were determined, pain index scores were computed from the individual measures above by computing z-scores for each pain measure and averaging the z-scores from measures that comprised the factors. These z-scores were entered in a hierarchical multiple regression to assess the ability of QST measures to predict clinical pain within each race/ethnic group after accounting for demographic and OA confounders.
Results
Two-hundred sixty-seven individuals (147 AA, 120 NHW) participated in the study. Significant race/ethnic differences emerged for age, BMI, study site, income, and education (Table 1, p's<0.001). Race/ethnic differences in QST and clinical pain/function measures were examined: 1) without covariates (i.e., unadjusted model), 2) with age, BMI and study site as covariates (i.e., partially adjusted model) and 3) age, BMI, study site, education and income as covariates (i.e., fully adjusted model). The results are displayed in Table 2 and summarized below.
Table 1. Basic characteristics of the OA participants by race/ethnic (n=267).
| NHW (n=120) | AA (n=147) | p-value | |
|---|---|---|---|
| Age (yrs), mean (SD) | 58.9 (8.3) | 55.1 (6.5) a | 0.004 |
| BMI, mean (SD) | 29.9 (6.9) | 32.6 (7.8) | 0.001 |
| Sex, % (n) | 0.991 | ||
| Female | 63.3 (76) | 63.3 (93) | |
| Male | 36.7 (44) | 36.7 (54) | |
| Income, % (n) | 0.001 | ||
| < $19,000 | 14.4 (17) | 54.5 (79) | |
| $20,000 - $29,000 | 20.3 (24) | 14.5 (21) | |
| $30,000 - $39,000 | 9.3 (11) | 13.8 (20) | |
| >$40,000 | 55.9 (66) | 17.2 (25) | |
| Education, % (n) | 0.001 | ||
| High school | 30.8 (37) | 59.2 (87) | |
| 2-year college degree | 21.7 (26) | 22.4 (33) | |
| 4-year college degree | 23.3 (28) | 13.6 (20) | |
| Graduate degree | 24.2 (29) | 4.8 (7) | |
| KL Scores | 0.634 | ||
| Grade 0 | 51.7 (62) | 49.7 (73) | |
| Grade 1 | 15.8 (19) | 15.0 (22) | |
| Grade 2 | 15.0 (18) | 10.9 (16) | |
| Grade 3 | 10.0 (12) | 15.0 (22) | |
| Grade 4 | 7.5 (9) | 9.5 (14) | |
| Test Site, % (n) | 0.030 | ||
| University of Florida | 75.0 (90) | 62.6 (92) | |
| University of Alabama at Birmingham | 25.0 (30) | 37.4 (55) |
BMI= Body mass index
Table 2. Means (SD) for the QST and clinical pain and function measures by race/ethnicity of our OA participants (n=267).
| NHW (n=120) | AA (n=147) | Unadjusted p-value | Partially Adjusted p-valuea | Fully Adjusted p-valueb | |
|---|---|---|---|---|---|
| Heat Pain | |||||
|
| |||||
| Threshold Forearm (°C) | 42.6 (2.8) | 40.9 (3.5) | 0.001 | 0.001 | 0.001 |
| Tolerance Forearm (°C) | 47.0 (2.2) | 45.0 (2.6) | 0.001 | 0.001 | 0.001 |
| Threshold Knee (°C) | 42.6 (2.9) | 41.3 (3.5) | 0.002 | 0.004 | 0.007 |
| Tolerance Knee (°C) | 47.0 (1.9) | 44.8 (3.1) | 0.001 | 0.001 | 0.001 |
| TS Forearm at 44° (Δ) | 9.5 (11.7) | 15.7 (17.8) | 0.009 | 0.013 | 0.005 |
| TS Knee at 44°(Δ) | 7.3 (10.6) | 10.8 (13.4) | 0.066 | 0.071 | 0.053 |
| TS Forearm at 46° (Δ) | 9.6 (11.8) | 17.1 (17.0) | 0.039 | 0.075 | 0.195 |
| TS Knee at 46°(Δ) | 11.0 (15.2) | 15.6 (15.4) | 0.025 | 0.031 | 0.078 |
| TS Forearm at 48° (Δ) | 11.2 (12.8) | 17.9 (18.9) | 0.001 | 0.004 | 0.010 |
| TS Knee at 48°(Δ) | 12.6 (15.8) | 17.5 (18.6) | 0.033 | 0.038 | 0.037 |
|
| |||||
| Mechanical Pain | |||||
|
| |||||
| PP Threshold Knee Med (KPa) | 327.7 (169.8) | 252.2 (149.3) | 0.001 | 0.002 | 0.011 |
| PP Threshold Knee Lat (KPa) | 355.7 (183.0) | 263.2 (155.1) | 0.001 | 0.001 | 0.032 |
| PP Threshold Quads (KPa) | 444.3 (234.0) | 387.0 (219.4) | 0.039 | 0.031 | 0.640 |
| PP Threshold Trapezius (KPa) | 291.6 (172.1) | 247.9 (170.8) | 0.019 | 0.013 | 0.305 |
| PP Threshold Forearm (KPa) | 264.7 (181.3) | 222.7 (148.7) | 0.035 | 0.034 | 0.332 |
| Punctate Pain Hand | 7.1 (13.3) | 15.4 (18.7) | 0.003 | 0.001 | 0.025 |
| Punctate Pain Knee | 10.4 (14.4) | 21.0 (23.4) | 0.001 | 0.001 | 0.022 |
| Punctate TS Hand (Δ) | 7.5 (12.1) | 23.0 (21.3) | 0.001 | 0.001 | 0.001 |
| Punctate TS Knee (Δ) | 14.4 (17.6) | 26.1 (19.1) | 0.001 | 0.001 | 0.001 |
|
| |||||
| Cold Pressor Procedure | |||||
|
| |||||
| Threshold at 16° | 34.2 (18.4) | 33.2 (18.1) | 0.673 | 0.379 | 0.168 |
| Intensity ratings 16° | 28.2 (27.2) | 37.7 (31.6) | 0.009 | 0.003 | 0.015 |
| Unpleasantness ratings 16° | 30.6 (27.7) | 41.4 (32.1) | 0.004 | 0.002 | 0.018 |
| Threshold at 12° | 19.7 (15.6) | 119.8 (15.0) | 0.960 | 0.886 | 0.656 |
| Intensity ratings 12° | 54.1 (31.9) | 64.3 (31.9) | 0.011 | 0.007 | 0.010 |
| Unpleasantness ratings 12° | 57.2 (30.9) | 69.2 (31.6) | 0.002 | 0.002 | 0.012 |
| Threshold at 8° | 12.9 (12.2) | 12.1 (10.5) | 0.557 | 0.412 | 0.414 |
| Intensity ratings 8° | 69.2 (29.0) | 75.7 (29.1) | 0.074 | 0.075 | 0.322 |
| Unpleasantness ratings 8° | 72.4 (28.1) | 78.3 (28.0) | 0.099 | 0.145 | 0.566 |
|
| |||||
| Clinical Pain and Function Measures | |||||
|
| |||||
| GCPS: Pain Intensity | 42.6 (19.2) | 57.6 (23.2) | 0.001 | 0.001 | 0.037 |
| GCPS: Disability | 37.1 (26.3) | 49.5 (29.9) | 0.001 | 0.003 | 0.496 |
| WOMAC: Pain | 6.1 (3.6) | 8.3 (4.8) | 0.001 | 0.001 | 0.278 |
| WOMAC: Stiffness | 3.2 (1.8) | 3.6 (2.1) | 0.062 | 0.225 | 0.771 |
| WOMAC: Function | 19.0 (12.5) | 27.1 (15.9) | 0.001 | 0.001 | 0.119 |
| SPPB: Total Score | 10.4 (1.7) | 9.3 (1.9) | 0.001 | 0.001 | 0.001 |
Partially adjusted for Age, BMI, and Study Site (University of Florida and University of Alabama at Birmingham) as covariates.
Fully adjusted for Age, BMI, Study Site (University of Florida and University of Alabama at Birmingham), Income (1=less than $19,000, 2=$20,000 to $29,000, 3= $30,000 to $39,000 and 4= greater than $40,000) and Highest Education (1=High school, Two-year college degree, Four–year college degree, Graduate degree) as covariates.
TS, temporal summation; PP, pressure pain
Race/ethnic Differences in Experimental Pain Sensitivity
Heat Pain
In unadjusted and adjusted analyses, AAs displayed significantly lower heat pain threshold and tolerance at forearm and knee compared to NHWs (p<0.01). Similarly, there were significant race/ethnic differences in temporal summation at the highest temperature (48°) even in the adjusted models. In all cases, the AA participants showed higher pain ratings and greater temporal summation than the NHW participants.
Mechanical Pain
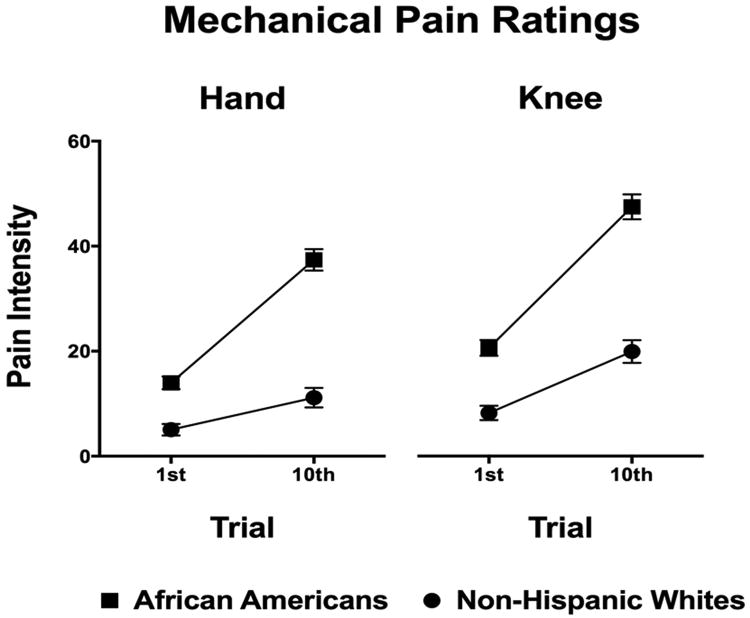
AA participants displayed significantly lower pressure pain thresholds in the unadjusted and partially adjusted models (p<0.05). After adding education and income (i.e., fully adjusted model) only pressure pain thresholds at the knee remained significantly different between race/ethnicities (p<0.05). Also, AAs showed significantly greater punctate pain ratings and punctate temporal summation than the NHW participants (p≤0.001, Table 2, Figure 2).
Figure 2. Mechanical temporal summation measures across the race groups.
Cold Pain
Cold pain threshold did not differ across groups at any temperature (p's>0.05). However, AAs provided higher ratings of pain intensity and unpleasantness at all temperatures (p's < 0.05), though this difference became non-significant in the fully adjusted model at 8 °C. Also, at all temperatures a greater proportion of AAs (59% at 8°C, 44% at 12°C, 12% at 16°C) compared to NHWs (28% at 8°C, 11% at 12°C, 2% at 16°C) withdrew their hand before one minute (p-values <0.01).
Conditioned Pain Modulation
Significant race/ethnic differences emerged in heat pain ratings from pre- to post-cold water immersion. AAs reported greater pain (54.6±24.2) at both time points than the NHWs (46.9±26.5, p=0.040). Also, the change in pain ratings of heat pain from pre- to post- cold water immersion differed by race/ethnicity (p=0.012). NHW participants' ratings did not significantly change (pre-46.9±26.1) to post-immersion (46.9±26.6) whereas the AA participants reported a significant increase in pain from pre- (52.6±24.4) to post-immersion (56.5±24.1).
Race/ethnic Differences in Measures of Clinical Pain and Function
AA participants reported significantly higher clinical pain and disability on the GCPS and WOMAC compared to NHWs in unadjusted and partially-adjusted models (p<0.01). However, race/ethnic group differences were non-significant for WOMAC measures and for GCPS disability scores when education and income were included as covariates, while group differences in GCPS pain intensity remained significant. SPPB scores were significantly lower among AAs compared to the NHWs in unadjusted and fully adjusted analyses (p<0.001).
Associations Among Measures of Clinical Pain, Function, and Experimental Pain
The PCA yielded six modality-specific components: 1) heat pain (threshold and tolerance at the forearm and knee), 2) pressure pain threshold at all sites, 3) cold pain (threshold, pain intensity and unpleasantness ratings), 4) punctate pain (pain ratings at the hand and knee), 5) punctate pain temporal summation (at the hand and knee) and 6) heat pain temporal summation (at the hand and knee) (Table 3). These pain index scores as well as CPM scores were subsequently included in the two regression models.
Table 3. Principal component analysis: Factor loadings and eigenvalues of experimental pain measures.
| Pain measures | Cold Pain | Pressure Pain | Heat TS | Heat Pain | Punctate Pain | Punctate TS |
|---|---|---|---|---|---|---|
| CP Intensity ratings 12°C | 0.880 | <0.100 | 0.145 | <0.100 | 0.158 | 0.136 |
| CP Unpleasantness ratings 12°C | 0.842 | <0.100 | 0.131 | <0.100 | 0.248 | 0.159 |
| CP Intensity ratings 8°C | 0.828 | -0.101 | <0.100 | -0.147 | 0.156 | 0.133 |
| CP Threshold at 12°C | -0.777 | 0.232 | <0.100 | 0.132 | 0.225 | <0.100 |
| CP Unpleasantness ratings 8°C | 0.774 | <0.100 | <0.100 | <0.100 | 0.244 | 0.141 |
| CP Intensity ratings 16°C | 0.759 | -0.116 | 0.110 | -0.104 | 0.195 | 0.158 |
| CP Threshold at 8°C | -0.729 | 0.233 | <0.100 | 0.170 | 0.238 | <0.100 |
| CP Unpleasantness ratings 16°C | 0.718 | <0.100 | <0.100 | -0.135 | 0.163 | 0.203 |
| CP Threshold at 16°C | -0.665 | 0.263 | <0.100 | <0.100 | 0.146 | <0.100 |
| PPTh Quadriceps (Kg) | -0.159 | 0.858 | <0.100 | 0.195 | <0.100 | <0.100 |
| PPTh Knee Lat (Kg) | -0.109 | 0.840 | <0.100 | 0.188 | -0.131 | -0.100 |
| PPTh Knee Med (Kg) | -0.139 | 0.834 | <0.100 | 0.216 | -0.134 | -0.121 |
| PPTh Trapezius (Kg) | -0.143 | 0.816 | <0.100 | 0.151 | <0.100 | <0.100 |
| PPTh Forearm (Kg) | -0.221 | 0.815 | <0.100 | 0.172 | <0.100 | <0.100 |
| HP TS Forearm at 46° (Δ) | <0.100 | <0.100 | 0.784 | -0.114 | <0.100 | <0.100 |
| HP TS Knee at 46°(Δ) | <0.100 | <0.100 | 0.758 | <0.100 | 0.205 | <0.100 |
| HP TS Knee at 44°(Δ) | <0.100 | <0.100 | 0.721 | <0.100 | <0.100 | 0.184 |
| HP TS Forearm at 48° (Δ) | <0.100 | -0.126 | 0.714 | -0.129 | <0.100 | -0.145 |
| HP TS Forearm at 44° (Δ) | 0.127 | <0.100 | 0.689 | -0.126 | <0.100 | <0.100 |
| HP TS Knee at 48°(Δ) | <0.100 | <0.100 | 0.679 | <0.100 | <0.100 | 0.112 |
| HP Tolerance Knee (°C) | <0.100 | 0.206 | -0.154 | 0.833 | -0.154 | -0.118 |
| HP Threshold Forearm (°C) | -0.157 | 0.268 | <0.100 | 0.790 | <0.100 | <0.100 |
| HP Tolerance Forearm (°C) | -0.217 | 0.213 | <0.100 | 0.789 | <0.100 | <0.100 |
| HP Threshold Knee (°C) | -0.112 | 0.221 | -0.222 | 0.748 | -0.227 | -0.170 |
| Punctate Pain Knee | 0.162 | -0.210 | <0.100 | -0.197 | 0.840 | <0.100 |
| Punctate Pain Hand | 0.205 | -0.220 | <0.100 | -0.193 | 0.821 | <0.100 |
| Punctate TS Hand (Δ) | 0.203 | -0.183 | 0.137 | -0.142 | <0.100 | 0.817 |
| Punctate TS Knee (Δ) | 0.225 | -0.141 | 0.148 | -0.128 | 0.137 | 0.817 |
|
| ||||||
| % of variance | 31.8 | 11.9 | 11.1 | 6.6 | 5.4 | 4.1 |
| Cumulative % variance | 31.8 | 43.7 | 54.9 | 61.5 | 66.8 | 70.9 |
HP, heat pain; TS, temporal summation; CP, cold pain; PPTh, pressure pain threshold
Two separate hierarchical multiple regressions were performed to assess the ability of experimental pain measures to predict clinical pain as measured by the WOMAC and GCPS (Table 4) stratified by race/ethnic group. Preliminary analyses were conducted to ensure no violation of the assumptions of normality, linearity, multicollinearity and homoscedasticity. Age, sex, BMI, study site, education, income and radiographic severity of OA were entered at Step 1 followed by the QST measures in Step 2. In the final model among the NHW individuals, increased punctate pain temporal summation significantly predicted greater WOMAC pain intensity. Among AA participants, increased pressure pain sensitivity and decreased CPM predicted greater WOMAC pain intensity. Among the NHW participants, increased punctate and heat pain temporal summation, cold pain sensitivity with decreased CPM predicted greater GCPS characteristic pain intensity. In the AA participants, increased punctate and pressure pain sensitivity, punctate pain temporal summation and decreased CPM significantly predicted greater GCPS characteristic pain intensity.
Table 4. Hierarchical multiple regressions predicting clinical pain stratified by race.
| DV: WOMAC | Non-Hispanic Whites | African Americans | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| β | t | p | R2 | ΔR2 | β | t | p | R2 | ΔR2 | |
| Step 1 | 0.21* | 0.21* | 0.24* | 0.24* | ||||||
| Age | -.126 | -1.41 | .160 | -.002 | -.02 | .982 | ||||
| Sex | -.106 | -1.21 | .227 | .063 | .76 | .448 | ||||
| BMI | .151 | 1.56 | .120 | .221 | 2.48 | .014 | ||||
| KL Score | .108 | 1.10 | .272 | .155 | 1.83 | .068 | ||||
| Study Site | -.020 | -.22 | .825 | -.283 | -3.22 | .002 | ||||
| Education | -.230 | -2.48 | .015 | -.178 | -1.83 | .068 | ||||
| Income | -.243 | -2.59 | .011 | -.079 | -.74 | .458 | ||||
| Step 2 | 0.35* | 0.14* | 0.35* | 0.11* | ||||||
| Cold Pain | .100 | .90 | .365 | -.074 | -.92 | .357 | ||||
| Punctate TS | .234 | 2.14 | .035 | .161 | 1.90 | .060 | ||||
| Pressure Pain | .070 | .60 | .547 | .240 | 2.67 | .009 | ||||
| Heat Pain TS | .131 | 1.40 | .164 | .015 | .18 | .851 | ||||
| Heat Pain | .024 | .22 | .820 | .064 | .74 | .461 | ||||
| Punctate Pain | .162 | 1.48 | .140 | .141 | 1.66 | .099 | ||||
| CPM | -.069 | -.78 | .435 | -.154 | -2.01 | .046 | ||||
|
| ||||||||||
| DV: GCPS | Non-Hispanic Whites | African Americans | ||||||||
|
|
|
|||||||||
| β | t | p | R2 | ΔR2 | β | t | p | R2 | ΔR2 | |
|
| ||||||||||
| Step 1 | 0.20* | 0.20* | 0.21* | 0.21* | ||||||
| Age | -.145 | -1.60 | .111 | -.111 | -1.31 | .190 | ||||
| Sex | -.048 | -.54 | .586 | -.009 | -.110 | .913 | ||||
| BMI | .141 | 1.44 | .153 | .209 | 2.31 | .023 | ||||
| KL Score | .087 | .87 | .382 | .196 | 2.26 | .025 | ||||
| Study Site | -.066 | -.72 | .473 | -.142 | -1.58 | .116 | ||||
| Education | -.255 | -2.71 | .008 | -.170 | -1.71 | .089 | ||||
| Income | -.184 | -1.94 | .055 | -.124 | -1.14 | .256 | ||||
| Step 2 | 0.42* | 0.22* | 0.39* | 0.18* | ||||||
| Cold Pain | .241 | 2.30 | .024 | -.037 | -.47 | .635 | ||||
| Punctate TS | .212 | 2.04 | .044 | .166 | 2.02 | .046 | ||||
| Pressure Pain | -.055 | -.50 | .616 | .201 | 2.31 | .022 | ||||
| Heat Pain TS | .196 | 2.21 | .029 | .025 | .31 | .752 | ||||
| Heat Pain | .008 | .08 | .934 | .112 | 1.34 | .181 | ||||
| Punctate Pain | .194 | 1.87 | .064 | .305 | 3.71 | .000 | ||||
| CPM | -.212 | -2.53 | .013 | -.228 | -3.09 | .002 | ||||
DV: dependent variable; BMI: Body mass index; KL: Kellgren: TS: temporal summation; CPM: Conditioned pain modulation
Discussion
The present study aimed to characterize differences in experimental pain sensitivity, conditioned pain modulation (CPM), clinical pain and pain-related disability among older African-Americans (AAs) and non-Hispanic whites (NHWs) with knee osteoarthritis (OA). First, AA participants displayed significantly greater sensitivity to all experimental pain modalities, including greater temporal summation compared to NHWs, suggesting greater central sensitization. Second, the CPM task revealed that concurrently applied cold pain did not produce any reductions in heat pain ratings in the NHW participants while it produced increased heat pain ratings in AAs, indicating that AAs displayed pain facilitation. Third, AAs reported higher levels of clinical pain, disability and poorer functional performance relative to NHW individuals, even after controlling for age, BMI and study site. Interestingly, group differences in most clinical variables became non-significant when additionally controlling for education and income, whereas race/ethnic differences in the QST measures were not impacted by education and income. Finally and notably, the association between QST measures and clinical pain differed across race/ethnic groups suggesting the possibility of distinct pathophysiological mechanisms.
Limited information is currently available regarding race/ethnic differences in experimental pain sensitivity among older adults with knee OA (8). To date, studies examining experimental pain responses across race/ethnic groups have primarily included healthy young participants, and these studies have generally not controlled for important confounders (21,24). Consistent with previous findings from healthy adults (19), race/ethnic differences were observed in experimental pain sensitivity across multiple stimulus modalities in our knee OA participants. In addition to increased basal pain sensitivity, AAs experienced greater temporal summation compared to NHWs suggesting increased central sensitization, a pain facilitatory mechanism. This was further supported by the CPM procedure. While the NHW participants did not exhibit significant pain inhibition- consistent with previous studies of knee OA (39) and non-clinical samples in this age range- (40), the AA participants displayed significant pain facilitation. Taken together, our findings extend previously reported race/ethnic differences in endogenous pain modulatory mechanisms in healthy adults (20,41) to individuals with knee OA.
The present investigation also determined race/ethnic group differences in measures of clinical pain, disability and function. Although most clinical measures significantly differed between race/ethnic groups after adjusting for age, BMI and study site, only GCPS characteristic pain intensity and SPPB remained statistically significant after also adjusting for education and income. Similarly, in a study of older adults attending a multidisciplinary pain center, neighborhood education and income mediated some, but not all associations between race/ethnicity and measures of pain and disability (42). Similar to our findings, Ang and colleagues (9) have reported no race differences in clinical pain after controlling for socioeconomic status (SES) using the WOMAC. It is possible that the differences in our measures account for some of the discrepancy since the WOMAC captures pain within the past 48 hours while the GCPS captures momentary, recent and pain within the past 6 months. On the other hand, health disparities researchers caution against the interpretation of analysis including SES as a covariate to model race differences (see 43, for a review). Some authors argue that race represents a social construct, and SES is a consequence of race, therefore, it is very difficult to disentangle the independent effects of these variables. These researchers suggest that studies with large sample sizes can be stratified by SES to better model the complex interactions between race and SES.
A major aim of the present study was to determine whether experimental pain sensitivity significantly predicted clinical pain within the race/ethnic groups, even after accounting for important demographic and OA-related confounders. Our results suggest that QST measures do indeed predict clinical pain, although which QST measures were predictive varied across race/ethnic groups. Specifically, pressure pain sensitivity was only predictive of clinical pain within our AA participants, while heat temporal summation and cold pain were only predictive of clinical pain within the NHW participants. Similar race/ethnic group differences in experimental pain responses (i.e., phenotypes) have been reported in healthy individuals (37) possibly reflecting underlying race/ethnic differences in the structure and function of the somatosensory system. This difference in predictors supports the idea that there are distinct pathophysiological mechanisms beyond cultural and socioeconomic factors contributing to differences in clinical pain between the race/ethnic groups. On the other hand, deficient pain inhibition significantly predicted pain for both race/ethnicities supporting the important role of deficient pain inhibition in chronic pain (44). Future research is needed to address these mechanistically-based differences and similarities among the race/ethnic groups.
The present investigation has several limitations worth mentioning. First, the lack of significant CPM effects in the present sample might be due to our methodology; although, previous studies have shown an absence of CPM in this age group and this patient population (39,40). Second, the cross-sectional nature of the study precludes conclusions regarding the direction of association between experimental and clinical pain measures. Third, the present sample was largely composed of community-dwelling adults with mild to moderate levels of knee pain and samples with different characteristics, such as those from clinical settings, may yield different results. Finally, large studies are needed to examine the complex relationships and interactions between education, income and race in pain disparities research.
The present study extends findings regarding race/ethnic differences in experimental pain sensitivity, clinical pain, disability and function among individuals with knee OA. The findings highlight the potential importance of SES, which should be examined further in future studies. Furthermore, our findings that different QST measures are associated with clinical pain in the two race/ethnic groups highlight the need for future research to evaluate the possibility that different underlying mechanisms contribute to clinical pain across race/ethnicity.
Acknowledgments
This research was supported by the following National Institutes of Health (NIH) grants: AG033906, AG039659 the UF Center for Clinical and Translational Science Grant Number UL1 TR000064 and the UAB Center for Clinical and Translational Science Grant Number UL1TR000165 from the National Center for Advancing Translational Sciences (NCATS) and National Center for Research Resources (NCRR).
Footnotes
Possible conflict of interest: R.B.F. is a stockholder in Algynomics.
References
- 1.Michaud CM, McKenna MT, Begg S, Tomijima N, Majmudar M, Bulzacchelli MT, Ebrahim S, Ezzati M, Salomon JA, Kreiser JG, Hogan M, Murray CJ. The burden of disease and injury in the United States 1996. Popul Health Metr. 2006;4:11. doi: 10.1186/1478-7954-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, Dragomir A, Kalsbeek WD, Luta G, Jordan JM. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008 Sep 15;59(9):1207–13. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen KD. Racial and ethnic disparities in osteoarthritis phenotypes. Curr Opin Rheumatol. 2010 Sep;22(5):528–32. doi: 10.1097/BOR.0b013e32833b1b6f. [DOI] [PubMed] [Google Scholar]
- 4.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. 2006 Nov;33(11):2271–9. Epub 2006 Oct 1. [PubMed] [Google Scholar]
- 5.Creamer P, Lethbridge-Cejku M, Hochberg MC. Determinants of pain severity in knee osteoarthritis: effect of demographic and psychosocial variables using 3 pain measures. J Rheumatol. 1999;26(8):1785–92. [PubMed] [Google Scholar]
- 6.Jones AC, Kwoh CK, Groeneveld PW, Mor M, Geng M, Ibrahim SA. Investigating racial differences in coping with chronic osteoarthritis pain. J Cross Cult Gerontol. 2008 Dec;23(4):339–47. doi: 10.1007/s10823-008-9071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen KD, Helmick CG, Schwartz TA, DeVellis RF, Renner JB, Jordan JM. Racial differences in self-reported pain and function among individuals with radiographic hip and knee osteoarthritis: the Johnston County Osteoarthritis Project. Osteoarthritis Cartilage. 2009 Sep;17(9):1132–6. doi: 10.1016/j.joca.2009.03.003. Epub 2009 Mar 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glover TL, Goodin BR, Horgas AL, Kindler LL, King CD, Sibille KT, Peloquin CA, Riley JL, 3rd, Staud R, Bradley LA, Fillingim RB. Vitamin D, race, and experimental pain sensitivity in older adults with knee osteoarthritis. Arthritis Rheum. 2012 Dec;64(12):3926–35. doi: 10.1002/art.37687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ang DC, Ibrahim SA, Burant CJ, Kwoh CK. Is there a difference in the perception of symptoms between african americans and whites with osteoarthritis? J Rheumatol. 2003;30(6):1305–10. [PubMed] [Google Scholar]
- 10.Foy CG, Penninx BW, Shumaker SA, Messier SP, Pahor M. Long-term exercise therapy resolves ethnic differences in baseline health status in older adults with knee osteoarthritis. J Am Geriatr Soc. 2005;53(9):1469–75. doi: 10.1111/j.1532-5415.2005.53459.x. [DOI] [PubMed] [Google Scholar]
- 11.Sowers M, Jannausch ML, Gross M, Karvonen-Gutierrez CA, Palmieri RM, Crutchfield M, Richards-McCullough K. Performance-based physical functioning in African-American and Caucasian women at midlife: considering body composition, quadriceps strength, and knee osteoarthritis. Am J Epidemiol. 2006;163(10):950–8. doi: 10.1093/aje/kwj109. [DOI] [PubMed] [Google Scholar]
- 12.Burns R, Graney MJ, Lummus AC, Nichols LO, Martindale-Adams J. Differences of self-reported osteoarthritis disability and race/ethnic. J Natl Med Assoc. 2007;99(9):1046–51. [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan JM, Luta G, Renner JB, Linder GF, Dragomir A, Hochberg MC, Fryer JG. Self-reported functional status in osteoarthritis of the knee in a rural southern community: the role of sociodemographic factors, obesity, and knee pain. Arthritis Care Res. 1996;9(4):273–8. doi: 10.1002/1529-0131(199608)9:4<273::aid-anr1790090412>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 14.Kington RS, Smith JP. Socioeconomic status and racial and ethnic differences in functional status associated with chronic diseases. Am J Public Health. 1997;87(5):805–10. doi: 10.2105/ajph.87.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green CR, Baker TA, Smith EM, Sato Y. The effect of race in older adults presenting for chronic pain management: a comparative study of black and white Americans. J Pain. 2003;4(2):82–90. doi: 10.1054/jpai.2003.8. [DOI] [PubMed] [Google Scholar]
- 16.Golightly YM, Dominick KL. Racial variations in self-reported osteoarthritis symptom severity among veterans. Aging Clin Exp Res. 2005;17(4):264–9. doi: 10.1007/BF03324608. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim SA, Siminoff LA, Burant CJ, Kwoh CK. Variation in perceptions of treatment and self-care practices in elderly with osteoarthritis: a comparison between African American and white patients. Arthritis Rheum. 2001 Aug;45(4):340–5. doi: 10.1002/1529-0131(200108)45:4<340::AID-ART346>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Dominick KL, Dudley TK, Grambow SC, Oddone EZ, Bosworth HB. Racial differences in health care utilization among patients with osteoarthritis. J Rheumatol. 2003;30(10):2201–6. [PubMed] [Google Scholar]
- 19.Rahim-Williams B, Riley JL, 3rd, Williams AK, Fillingim RB. A quantitative review of ethnic group differences in experimental pain response: do biology, psychology, and culture matter? Pain Med. 2012;13(4):522–40. doi: 10.1111/j.1526-4637.2012.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell CM, France CR, Robinson ME, Logan HL, Geffken GR, Fillingim RB. Ethnic differences in diffuse noxious inhibitory controls. J Pain. 2008;9(8):759–66. doi: 10.1016/j.jpain.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards RR, Fillingim RB. Ethnic differences in thermal pain responses. Psychosom Med. 1999;61:346–54. doi: 10.1097/00006842-199905000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Sheffield D, Biles PL, Orom H, Maxiner W, Sheps DS. Race and sex differences in cutaneous pain perception. Psychosom Med. 2000;62:517–23. doi: 10.1097/00006842-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Kim H, Neubert JK, Rowan JS, et al. Comparison of experimental and acute clinical pain responses in humans as pain phenotypes. J Pain. 2004;5:377–84. doi: 10.1016/j.jpain.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Campbell CM, Edwards RR, Fillingim RB. Ethnic differences in responses to multiple experimental pain stimuli. Pain. 2005;13:22–6. doi: 10.1016/j.pain.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Mechlin B, Heymen S, Edwards CL, Girdler SS. Ethnic differences in cardiovascular-somatosensory interactions and in the central processing of noxious stimuli. Psychophysiology. 2011 Jun;48(6):762–73. doi: 10.1111/j.1469-8986.2010.01140.x. Epub 2010 Oct 29. [DOI] [PubMed] [Google Scholar]
- 26.Fillingim RB, Maixner W, Kincaid S, Sigurdsson A, Harris MB. Pain sensitivity in patients with temporomandibular disorders: relationship to clinical and psychosocial factors. Clin J Pain. 1996 Dec;12(4):260–9. doi: 10.1097/00002508-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 27.D'Antono B, Ditto B, Rios N, Moskowitz DS. Risk for hypertension and diminished pain sensitivity in women: Autonomic and daily correlates. Int J of Psychophys. 1999;31:175–187. doi: 10.1016/s0167-8760(98)00057-9. [DOI] [PubMed] [Google Scholar]
- 28.Edwards RR, Doleys DM, Fillingim RB, Lowery D. Ethnic differences in pain tolerance: Clinical implications in a chronic pain population. Psychosom Med. 2001;63:316–323. doi: 10.1097/00006842-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Felson DT, Nevitt MC, Yang M, Clancy M, Niu J, Torner JC, Lewis CE, Aliabadi P, Sack B, McCulloch C, Zhang Y. A new approach yields high rates of radiographic progression in knee osteoarthritis. J Rheumatol. 2008;35(10):2047–54. [PMC free article] [PubMed] [Google Scholar]
- 30.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cruz-Almeida Y, King CD, Goodin BR, Sibille KT, Glover TL, Riley JL, Sotolongo A, Herbert MS, Schmidt J, Fessler BJ, Redden DT, Staud R, Bradley LA, Fillingim RB. Psychological profiles and pain characteristics of older adults with knee osteoarthritis. Arthritis Care Res (Hoboken) 2013 Jul 16; doi: 10.1002/acr.22070. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 33.Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS) Arthritis Care Res (Hoboken) 2011;63:S208–28. doi: 10.1002/acr.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 35.Vasunilashorn S, Coppin AK, Patel KV, Lauretani F, Ferrucci L, Bandinelli S, Guralnik JM. Use of the Short Physical Performance Battery Score to predict loss of ability to walk 400 meters: analysis from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2009;64(2):223–9. doi: 10.1093/gerona/gln022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wennie Huang WN, Perera S, VanSwearingen J, Studenski S. Performance measures predict onset of activity of daily living difficulty in community-dwelling older adults. Int J Geriatr Psychiatry. 2008;23(3):238–43. doi: 10.1111/j.1532-5415.2010.02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cruz-Almeida Y, Riley JL, 3rd, Fillingim RB. Experimental pain phenotype profiles in a racially and ethnically diverse sample of healthy adults. Pain Med. 2013 Nov;14(11):1708–18. doi: 10.1111/pme.12203. Epub 2013 Jul 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, Graven-Nielsen T. Sensitization in patients with painful knee osteoarthritis. Pain. 2010 Jun;149(3):573–81. doi: 10.1016/j.pain.2010.04.003. Epub 2010 Apr 24. [DOI] [PubMed] [Google Scholar]
- 40.Riley JL, 3rd, King CD, Wong F, Fillingim RB, Mauderli AP. Lack of endogenous modulation and reduced decay of prolonged heat pain in older adults. Pain. 2010 Jul;150(1):153–60. doi: 10.1016/j.pain.2010.04.020. Epub 2010 May 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mechlin MB, Maixner W, Light KC, Fisher JM, Girdler SS. African Americans show alterations in endogenous pain regulatory mechanisms and reduced pain tolerance to experimental pain procedures. Psychosom Med. 2005 Nov-Dec;67(6):948–56. doi: 10.1097/01.psy.0000188466.14546.68. [DOI] [PubMed] [Google Scholar]
- 42.Fuentes M, Hart-Johnson T, Green CR. The association among neighborhood socioeconomic status, race and chronic pain in black and white older adults. J Natl Med Assoc. 2007 Oct;99(10):1160–9. [PMC free article] [PubMed] [Google Scholar]
- 43.Shavers VL. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc. 2007 Sep;99(9):1013–23. [PMC free article] [PubMed] [Google Scholar]
- 44.Villanueva L. Diffuse Noxious Inhibitory Control (DNIC) as a tool for exploring dysfunction of endogenous pain modulatory systems. Pain. 2009 Jun;143(3):161–2. doi: 10.1016/j.pain.2009.03.003. Epub 2009 Mar 31. [DOI] [PubMed] [Google Scholar]