Abstract
The large conductance, calcium-activated BK-α/β4 potassium channel, localized to the intercalated cells of the distal nephron, mediates potassium secretion during high potassium, alkaline diets. Here we determine whether BK-α/β4-mediated potassium transport is dependent on epithelial sodium channel (ENaC)-mediated sodium reabsorption. We maximized sodium-potassium exchange in the distal nephron by feeding mice a low sodium, high potassium diet. Wild type and BK-β4 knockout mice were maintained on low sodium, high potassium, alkaline diet or a low sodium, high potassium, acidic diet for 7–10 days. Wild type mice maintained potassium homeostasis on the alkaline but not acid diet. BK-β4 knockout mice could not maintain potassium homeostasis on either diet. During the last 12 hours of diet, wild type mice on either a regular, alkaline or an acid diet, or knockout mice on an alkaline diet were administered amiloride (an ENaC inhibitor). Amiloride enhanced sodium excretion in all wild type and knockout groups to similar values; however, amiloride diminished potassium excretion by 59% in wild type but only by 33% in knockout mice on an alkaline diet. Similarly, amiloride decreased the transtubular potassium gradient by 68% in wild type but only by 42% in knockout mice on an alkaline diet. Amiloride treatment equally enhanced sodium excretion and diminished potassium secretion in knockout mice on an alkaline diet and wild type mice on an acid diet. Thus, the enhanced effect of amiloride on potassium secretion in wild type compared to knockout mice on the alkaline diet, clarify a BK- α/β4-mediated potassium secretory pathway in intercalated cells driven by ENaC-mediated sodium reabsorption linked to bicarbonate secretion.
Keywords: BK-α/β4, ENaC, K secretion, Na reabsorption, intercalated cell, TTKG, math modeling
Introduction
The prevailing mechanism for K secretion in the distal nephron involves a cation exchange process with epithelial Na channels (ENaC) on the apical membrane of principal cells mediating Na uptake in series with the basolateral Na-K-ATPase, which actively extrudes Na and transports K into the cell. Potassium secretion can be enhanced by aldosterone, which increases the basolateral Na-K-ATPase [1], apical ENaC [2;3], and apical renal outer medullary K channel (ROMK) [4] in principal cells. By this mechanism, the maximum K secreted to Na reabsorbed ratio is 0.67, which is the ratio dictated by the 2 K per 3 Na exchange of the Na-K-ATPase. However, the Yanomami of South America ingest a diet of very low Na and high K content, indicating a mammalian mechanism that enhances the K secreted per Na reabsorbed ratio to a value greater than 0.67. It should be noted that the urine of the Yanomami is very alkaline, as shown by their very low urinary [Cl] [5]. We hypothesized that the alkalinity of their diet was critical for their ability to eliminate K with very low Na intake [6].
The mechanism for a high ratio of K secretion to Na reabsorption may involve secreting K independently of ENaC-mediated Na reabsorption [7]. Supporting this notion, an isolated perfused cortical collecting duct (CCD) study showed that large conductance, Ca-activated K channels (BK) mediated luminal K exit. The Na-K-ATPase delivered the K into the cell but Na entered via the basolateral Na-H exchanger [8] instead of ENaC. This mechanism for Na-independent K secretion would require enhanced Na-K-ATPase in the basolateral membrane. However, the majority of BK channels reside in acid/base transporting intercalated cells, which contain minimal Na-K-ATPase [9;10].
In intercalated cells, the pore-forming BK-α is associated with the BK-β4 subunit [11]. Unlike wild type, BK-β4 knock-out mice (KO) do not maintain K homeostasis when consuming a high K alkaline diet [6]. The high K diet causes an increase in total cellular expression of BK-α, via aldosterone [12]. However, the BK-α only appears in the apical membrane, where it can mediate K secretion, in the presence of the BK-β4 subunit. The BK-β4 expression is enhanced when the urine is alkaline.
If the Yanomami have adapted to low Na intake and are secreting K independently of Na reabsorption then we would predict that mice on a low Na, high K alkaline diet would maintain K balance after treating with amiloride, which completely blocks ENaC-mediated Na reabsorption and K secretion in isolated CCDs from rabbits on a regular diet [13]. However, we found that mice use the BK-α/β4 of intercalated cells to mediate K secretion by a mechanism that is mostly dependent on ENaC-mediated Na reabsorption in principal cells. We propose a new model for K secretion that involves recently discovered intercalated cell transporters and a Na recycling mechanism involving active pumping by principal cells and passive leak by intercalated cells.
Results
For all experiments of this study, the tables show the electrolyte, osmolality, urinary volume and hematocrit values read directly from the analytical instruments. The figures show comparisons of results calculated from these values.
Role of alkalinity in BK-α/β4-mediated K secretion with low Na, high K diet
We previously showed that WT on an alkaline high K diet maintained K balance whereas WT on an acidic high K diet exhibited increased plasma [K] and diminished urinary K output [6]. The following experiments determined the importance of alkaline urine for handling a low Na, high K diet. In these experiments, we compared K handling between WT and KO on a low Na, high K alkaline diet (WT-Alk and KO-Alk, respectively) and between WT and KO on a low Na, high K, acid diet (WT-Acid and KO-Acid, respectively) for 7 to 10 days.
As shown in table 1, the urine pH was similarly alkaline in WT-Alk and KO-Alk and was similarly acidic for WT-Acid and KO-Acid. The plasma [K] (mM) was significantly elevated in KO-Alk, compared with WT-Alk; however, the plasma [K] of WT-Acid was elevated to a value not significantly different from the value for KO-Acid.
Table 1.
Comparison of various measurements (KW = kidney weight; V = urine volume; U[K] = urine [K]; U[Na] = urine [Na]; Uosm = urine osmolality; POsm = plasma osmolalilty; Hct. = hematocrit; UpH = urine pH) between wild type (WT) and BK-β4 knockouts (KO) on diets containing regular chow (control), low Na, high K, alkaline (Alk) and low Na, high K, acidic (Acid).
| KW | N | V | N | food | N | water | N | U[K] | N | U[Na] | N | P[K] | N | P[Na] | N | Uosm | N | Posm | N | Hct. | N | UpH | N | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| units | mgm | ml/day | gm/d | ml/day | mM | mM | mM | mM | mOsm/L | mOsm/L | % | |||||||||||||
| WT-control | 319 | 14 | 1.16 | 14 | 2.58 | 8 | 4.5 | 13 | 263.2 | 14 | 176.1 | 12 | 4.35 | 14 | 147.9 | 12 | 2090 | 13 | 306 | 10 | 44.3 | 10 | 6.29 | 10 |
| ±SEM | 14 | 0.09 | 0.29 | 0.5 | 23.4 | 17.6 | 0.017 | 1.4 | 201 | 3 | 0.6 | 0.13 | ||||||||||||
| WT-Alk | 305 | 13 | 5.61 | 13 | 3.99 | 13 | 12.2 | 13 | 634.7 | 13 | 7.2 | 13 | 5.23 | 13 | 145.5 | 9 | 1616 | 13 | 295 | 8 | 45.6 | 9 | 8.85 | 8 |
| ±SEM | 8 | 0.38 | 0.19 | 0.7 | 37.2 | 0.6 | 0.16 | 2.5 | 128 | 5 | 0.9 | 0.3 | ||||||||||||
| WT-Acid | 245 | 6 | 5 | 6 | 2.37 | 6 | 9.5 | 6 | 448.2 | 6 | 6.4 | 6 | 9.3 | 6 | 140.2 | 6 | 1380 | 6 | 287 | 6 | 43.2 | 6 | 5.89 | 6 |
| ±SEM | 10 | 0.59 | 0.12 | 0.6 | 17 | 0.3 | 0.48 | 1.9 | 68 | 2 | 1.7 | 0.14 | ||||||||||||
| KO-control | 293 | 16 | 1.25 | 16 | 2.83 | 12 | 5.6 | 15 | 239.4 | 16 | 122.9 | 14 | 4.09 | 16 | 149.4 | 16 | 2204 | 16 | 298 | 6 | 44.7 | 16 | 6.18 | 13 |
| ±SEM | 11 | 0.15 | 0.27 | 0.6 | 30.5 | 11.8 | 0.09 | 1.3 | 252 | 3 | 0.7 | 0.13 | ||||||||||||
| KO-Alk | 299 | 12 | 5.15 | 9 | 3.45 | 12 | 10.3 | 12 | 525.6 | 12 | 8.3 | 9 | 7.61 | 10 | 143.2 | 5 | 1311 | 12 | 284 | 5 | 44.1 | 9 | 8.85 | 5 |
| ±SEM | 11 | 0.38 | 0.41 | 0.8 | 22.6 | 1 | 0.2 | 1.2 | 61 | 6 | 0.7 | 0.08 | ||||||||||||
| KO-Acid | 223 | 4 | 3.45 | 4 | 2.53 | 4 | 8.3 | 4 | 413.3 | 4 | 5.6 | 4 | 8.49 | 4 | 135.1 | 4 | 1330 | 4 | 288 | 4 | 44.3 | 4 | 5.6 | 4 |
| ±SEM | 6 | 0.4 | 0 | 0.3 | 47.2 | 1 | 0.33 | 0.7 | 98 | 3 | 0.5 | 0.02 | ||||||||||||
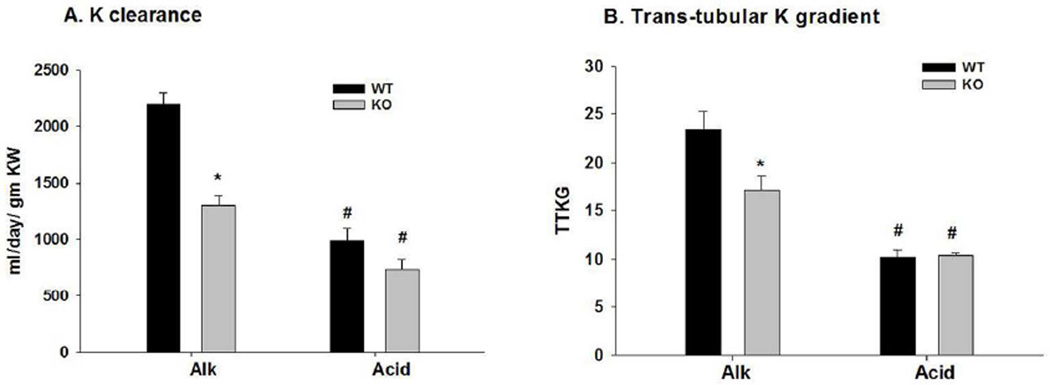
As shown in figure 1A, the K clearance (ml/day/gm kidney weight) for KO -Alk was significantly lower, with a value of 1297.9 ± 85.2 (n = 9), compared with a value of 2192.3 ± 112.8 (n = 13) for WT-Alk. The K clearance was depressed significantly in WT-Acid to 986.1 ± 108.8 (n = 6), a value not significantly different from KO-acid (738.4 ± 85.4; n = 4).
Figure 1.
Bar plots illustrating (A) K clearance and (B) trans-tubular K gradient (TTKG) for WT and KO on Alk and Acid diets. Clearance and TTKG were calculated from values of table 1. *P < 0.01 vs WT; #P < 0.05 vs Alk.
The trans-tubular K gradient (TTKG) was determined as a measure o K secretion in the connecting tubule (CNT) and initial CCD, before water extraction (see Supplement 2). As shown in figure 1B, the TTKG of KO-Alk was 17.2 ± 1.4 (n = 5), a value significantly less than the value of 23.4 ± 1.9 (n = 8) for WT-Alk; however, for KO-Acid, the TTKG value of 10.4 ± 0.2 (n = 4) was not different from the value of 10.2 ± 0.7 (n = 6) for WT-Acid. These results indicate that K homeostasis of mice on a low Na, high K diet is dependent on BK-α/β4-mediated K secretion in concert with urinary alkalinization.
Thiazide and Amiloride-sensitive Na and K transport in the ASDN
Experiments were designed to determine the Na dependency of BK-α/β4-mediated K secretion in mice on the low Na, high K, alkaline diet. For WT-Alk and KO-Alk, a bolus of vehicle, hydrochlorothiazide (HCTZ) or amiloride was given to determine the amount of Na-Cl co-transporter (NCC) mediated Na reabsorption vs. ENaC-dependent Na reabsorption/K secretion in each genotype. HCTZ (50 mg/Kg) was given for 12 hours to determine whether WT or KO was preferentially reabsorbing Na via NCC, rather than ENaC, in the aldosterone-sensitive distal nephron (ASDN). It was shown previously that doses of thiazides ≥ 2 mg/kg elicited a maximal natriuretic response in mice [14]. The High Performance Liquid Chromatography measurements of amiloride in the plasma and the calculated values of the distal lumen are shown in figure 1S.
We found that vehicle treatment caused a discouragement of drinking a high volume of water during the last 12 hours, thereby causing a reduction in urinary flow and a small increase in plasma [K]. These results show the importance of maintaining a high urinary flow to maintain K balance. Nevertheless, the thiazide and amiloride data provide information regarding the relative values of amiloride-sensitive Na reabsorption and K secretion with respect to vehicle in these mice.
As shown in table 2 and figure 2, HCTZ treatment increased Na excretion only in WT and KO on control diets. As indicated by the decrease in plasma [Na] and the increase in hematocrit, amiloride, but not HCTZ, caused Na and volume depletion and an elevation of plasma [K] in WT and KO on either a control or Alk diet.
Table 2.
Comparison of various measurements (symbols are same as in table 1) between WT and β4KO on control, Alk, or Acid diets and treated with vehicle (veh), hydrochlorothiazide (HCTZ), or amiloride (amil).
| KW | V | U[K] | U[Na] | P[K] | P[Na] | Uosm | Posm | Hct. | UpH | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| units | mgm | N | ml/day | N | mM | N | mM | N | mM | N | mM | N | mOsm/L | N | mOsm/L | N | % | N | N | |
| WT-veh | 275 | 4 | 1.3 | 4 | 298 | 4 | 62.3 | 4 | 4.32 | 4 | 158.1 | 4 | 2180 | 4 | 277.5 | 4 | 40.8 | 4 | 6.17 | 4 |
| ±SEM | 10 | 0.24 | 82.3 | 5 | 0.15 | 0.8 | 410 | 2.5 | 1.5 | 0.09 | ||||||||||
| WT-HCTZ | 273 | 8 | 1.66 | 7 | 282 | 7 | 137.9 | 7 | 4.19 | 8 | 154.9 | 7 | 2141 | 7 | 297.5 | 8 | 42.6 | 3 | 6.24 | 7 |
| ±SEM | 11 | 0.27 | 66.9 | 35.5 | 0.11 | 1.7 | 440 | 4.9 | 1.9 | 0.19 | ||||||||||
| WT-amil | 282 | 4 | 2.4 | 4 | 110 | 4 | 244.5 | 4 | 5.81 | 4 | 140.3 | 4 | 1670 | 4 | 282.5 | 4 | 46.7 | 4 | 6.3 | 4 |
| ±SEM | 8 | 0.35 | 39 | 20.9 | 0.22 | 1.6 | 385 | 2.5 | 1.9 | 0.11 | ||||||||||
| WT-Alk-veh | 276 | 9 | 2 | 9 | 716 | 9 | 4.44 | 9 | 5.62 | 9 | 147.4 | 7 | 2339 | 9 | 291.1 | 9 | 40.4 | 9 | 7.31 | 9 |
| ±SEM | 8 | 0.34 | 93.4 | 1.01 | 0.23 | 1.6 | 376 | 3.1 | 1.1 | 0.32 | ||||||||||
| WT-Alk-HCTZ | 274 | 4 | 2.15 | 4 | 487 | 4 | 3.55 | 4 | 4.89 | 4 | 146.9 | 4 | 1647 | 4 | 277.5 | 4 | 42.9 | 4 | 7.18 | 4 |
| ±SEM | 10 | 0.35 | 75.5 | 0.76 | 0.21 | 2.1 | 145 | 2.5 | 1.6 | 0.51 | ||||||||||
| WT-Alk-amil | 256 | 8 | 1.78 | 8 | 282 | 6 | 184.2 | 8 | 7.2 | 8 | 135.7 | 8 | 1657 | 8 | 280 | 8 | 49.2 | 7 | 6 | 8 |
| ±SEM | 1 | 0.18 | 37 | 17.5 | 0.05 | 0.8 | 201 | 2.7 | 1 | 0.25 | ||||||||||
| KO-Alk-veh | 248 | 7 | 1,61 | 7 | 373 | 7 | 4.79 | 7 | 5.67 | 7 | 150 | 6 | 1769 | 7 | 284 | 7 | 39 | 7 | 6.32 | 7 |
| ±SEM | 14 | 0.24 | 94 | 0.95 | 0.28 | 2 | 280 | 3.7 | 0.8 | 0.25 | ||||||||||
| KO-Alk-HCTZ | 244 | 5 | 1.8 | 4 | 253 | 4 | 4.01 | 4 | 5.04 | 5 | 148.3 | 4 | 1225 | 4 | 302 | 5 | 38.3 | 3 | 6.34 | 4 |
| ±SEM | 8 | 0.11 | 35 | 0.64 | 0.37 | 1.1 | 118 | 4 | 1.9 | 0.08 | ||||||||||
| KO-Alk-amil | 225 | 5 | 0.64 | 5 | 528 | 5 | 448 | 5 | 7.55 | 5 | 132.5 | 5 | 3137 | 5 | 290 | 5 | 47.2 | 4 | 6.25 | 5 |
| ±SEM | 3 | 0.02 | 63.6 | 24.9 | 0.63 | 1 | 409 | 3.2 | 1.2 | 0.14 |
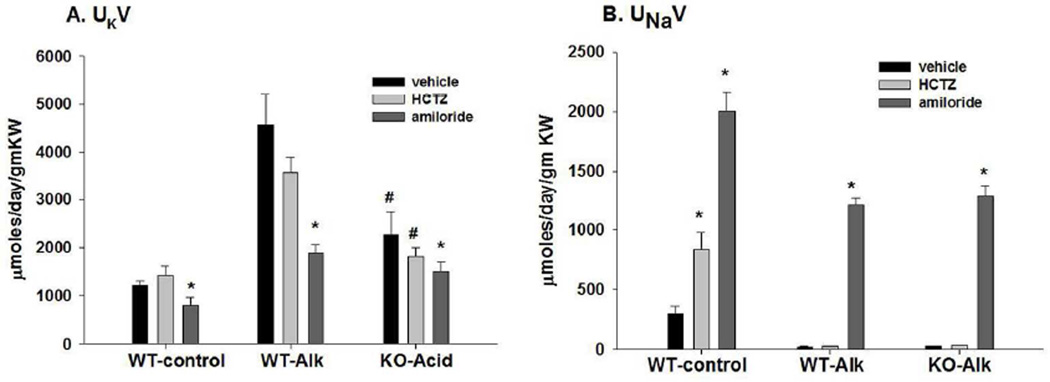
Figure 2.
Summary bar plots illustrating the effects of hydrochlorothiazide (HCTZ) and amiloride vs vehicle on (A) K excretion and (B) Na excretion for WT on a control diet (WT-control), WT-Alk, and KO-Alk. *P < 0.05 vs vehicle. #P < 0.05 vs. WT-Alk.
Figure 2A reveals that amiloride treatment decreased urinary K excretion (UKV; µmoles/day/gm kidney weight) in WT-control from 1206.5 ± 107.1 in vehicle (n = 4) to 807.3 ± 145.8 (n = 4), and in WT-Alk from 4787.5 ± 609.8 in vehicle (n = 10) to 1890.2 ± 173.6 (n = 8), and in KO-Alk from 2137.0 ± 365.2 in vehicle (n = 6) to 1504.3 ± 202.4 (n = 5). As shown in figure 2B, HCTZ treatment increased urinary Na excretion (UNaV; µmoles/day/gm kidney weight) in WT-control from a value of 297.4 ± 66.8 in vehicle (n = 4) to 836.5 ± 142.2 (n = 6) with HCTZ and to 2005.5 ± 157.8 (n = 4) with amiloride. In WT-Alk, Na excretion increased from a vehicle value of 26.2 ± 3.2 (n = 10) to 1208.0 ± 56.8 (n = 8) with amiloride and in KO from 19.3 ± 1.4 (n = 7) in vehicle to 1278.1 ± 103.1 (n = 5) with amiloride.
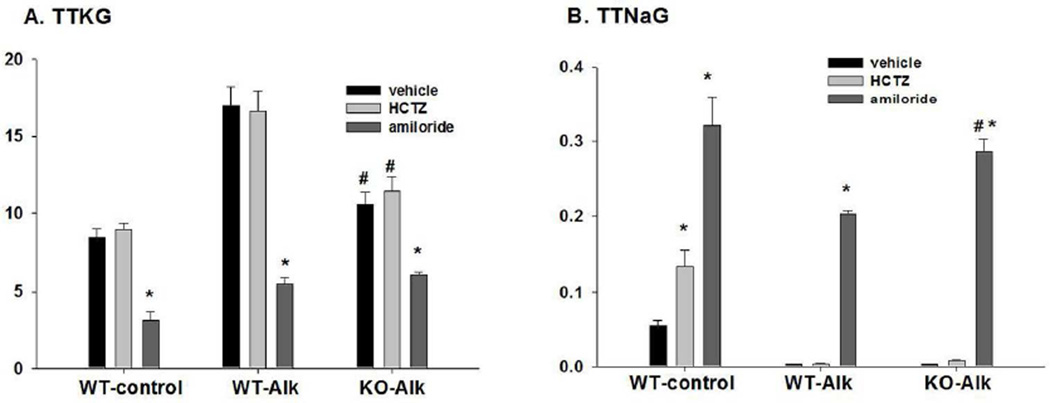
As shown in figure 3A, amiloride treatment significantly decreased TTKG in WT-control from 8.4 ± 0.6 (vehicle; n = 4) to 3.1 ± 0.6 (n = 4), in WT-Alk from 17.0 ± 1.3 (vehicle; n = 10) to 5.5 ± 0.4 (n = 8), and in KO-Alk from 9.7 ± 0.9 (n = 5) to 6.1 ± 0.1 (n = 5), a value not significantly different from that of amiloride-treated WT-Alk. The trans-tubular Na gradient (TTNaG) was determined as Na reabsorption in the CNT and initial CCD and shown in figure 3B. In WT-control, the TTNaG increased from 0.054 ± 0.007 (n=4) in vehicle to 0.133 ± 0.022 (n = 7) with HCTZ, and to 0.320 ± 040 (n = 4) with amiloride treatment. The TTNaG of WT-Alk increased from 0.0041 ± 0.0003 (n = 7) in vehicle to 0.286 ± 0.017 (n = 5) with amiloride. The TTNaG of KO-Alk increased from 0.0039 ± 0.0003 (n = 7) in vehicle to 0.203 ± 0.004 (n=6) with amiloride. That there was an undetectable effect of HCTZ on Na and water balance in WT-Alk and KO-Alk is consistent with a high percentage of Na bypassing NCC and reabsorbing via ENaC in exchange for secreted K. These results show that despite increased amiloride-sensitive Na reabsorption in KO, the amiloride-sensitive K secretion is reduced.
Figure 3.
Summary bar plots illustrating the effects of HTZ and amiloride vs vehicle on (A) TTKG and (B) trans-tubular Na gradient (TTNaG) for WT-control, WT-Alk, and KO-Alk. *P < 0.05 vs vehicle. #P < 0.05 vs. WT-Alk.
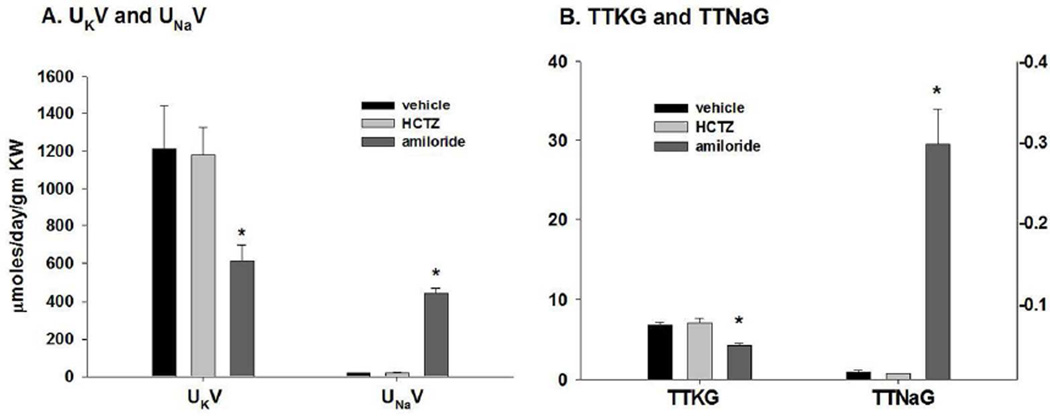
We gave amiloride to determine whether the defect in K secretion in WT-Acid resulted from reduced ENaC-mediated Na reabsorption or decreased K secretion coupled with Na reabsorption. When WT-Acid were given amiloride, the UKV (figure 4A) decreased from 1211.8 ± 230.3 (vehicle; n = 5) to 615.2 ± 83.6 (n = 3) and the UNaV increased from 21.9 ± 2.3 (n = 5) in vehicle to 441.1 ± 30.9 (n = 3) with amiloride. As shown in figure 4B, amiloride decreased the TTKG from 6.8 ± 0.4 (n=5) to 4.3 ± 0.2 (n=3) and increased the TTNaG from 0.0100 ± 0.0015 (n=5) to 0.33 ± 0.02 (n=3). These results show that the amiloride-sensitive Na reabsorption in WT-Acid is similar to WT-Alk; however the amiloride-sensitive K secretion is considerably attenuated as was the KO-Alk.
Figure 4.
Summary bar plots illustrating effects of HCTZ and amiloride on (A) rates of K and Na excretion and (B) TTKG (left y-axis) and TTNaG (right y-axis) for WT-Acid. *P < 0.05 vs vehicle.
Discussion
The KO exhibit defective K secretion when consuming a high K, alkaline diet [6], indicating that BK-α/β4 mediates K secretion in these conditions. We fed mice low Na, high K diet to maximize Na-K exchange and determine the Na dependency of K secretion by BK-α/β4 in the aldosterone-sensitive distal nephron. HCTZ was used to determine whether decreased K secretion in KO resulted from enhanced NCC-mediated Na reabsorption and less delivery of Na to ENaC. Amiloride treatment enabled the determination of ENaC-dependent Na reabsorption and K secretion by WT and KO.
TTKG
In the current experiments, cortical K handling has been inferred from the TTKG computed from the final urine. The TTKG, used frequently in previous studies [6;15–17], was used in this study as a determination of K secretion in the CNT and initial CCD. The accuracy of this approach may be open to question, especially in view of the likelihood of K reabsorption along the collecting ducts (CD). Traditionally, the formula for TTKG is derived by assuming that the only CD flux is that of water, while luminal solutes are neither reabsorbed nor secreted. As shown in Supplement 2, this problem is reconsidered from the perspective of a 2-solute system (nominally KCl and urea), in which there may be fluxes of both. The resulting formula for the isotonic (cortical) [K] is the standard TTKG, plus an upward perturbation when there is K+ reabsorption, and a downward perturbation when there is urea reabsorption. In the presence of both K and urea reabsorption, these terms can cancel, preserving the accuracy of the standard term.
The model calculations of Supplement 2, Table S1 also allow examination of the accuracy of the TTKG. In the second to last column of the table, the cortical K+ concentration is estimated from the final urine using the standard TTKG; the right-most column is determined by going back into the luminal concentration profiles from the model and selecting the K concentration at the point that the lumen is isotonic (300 mM). Overall, agreement of the TTKG estimate with the model value is at the 10% level, and supports the approach of the current study.
Effects of HCTZ
HCTZ treatment caused an expected Na diuresis in mice on a regular diet. However, HCTZ did not cause a Na diuresis in either WT or KO on Alk or Acid. These results show that all Na in mice on Alk or KO was reabsorbed by ENaC, and not NCC in the distal nephron. That HCTZ was ineffective regardless of acid/base shows that the high plasma [K] or its mediator, aldosterone, and not the anion in the distal lumen, was responsible for the increased ENaC- vs. NCC-mediated Na reabsorption. This result is consistent with a previous study showing a diminished natriuretic response to thiazides after high K feeding [18].
A high K diet induces the WNK4-SPAK pathway [19] and signals the dephosphorylation of NCC resulting in a shift of distal Na reabsorption from NCC to ENaC in order to maximize Na-K exchange [20]. However, our mathematical modeling (see Supplement 2) using previously determined Na reabsorptive permeabilities, revealed that the NCC-mediated Na reabsorption was not necessarily turned off but was merely undetectable compared with the overwhelmingly enhanced ENaC-mediated Na reabsorption.
Na dependency of BK-α/β4-mediated K secretion
Our results demonstrated that neither WT nor β4KO can adapt to the low Na, high K diet with an ENaC-independent, Na reabsorbing mechanism that can maintain K, Na and fluid balance. Our findings differ from two studies that demonstrated Na-independent K secretion. Using isolated perfused rabbit CCD, it was shown that BK was involved in a mechanism to secrete K independently from Na reabsorption [8]. Another study used amiloride to demonstrate Na-independent K secretion in rats [7]. However, for mice adapted to a low Na, high K diet for 7–10 days in the present study, amiloride treatment reduced K secretion, elevated plasma [K], decreased volume and reduced plasma [Na]. The plasma amiloride concentration was at least 1.4 µM during the course of 12 hrs, and estimated in the CNT lumen at 22 to 60 µM (figure S1). With an inhibitory constant (Ki) of 100 nM [21;22], amiloride will have inhibited ENaC by more than 99% for the 12 hour duration. Amiloride will not have substantially inhibited the proximal tubule Na-H exchanger, with a Ki >100 µM [23]. Moreover, inhibition of the Na-H exchanger would result in increased urinary pH, which was not observed.
It is unlikely that we did not observe Na-independent K secretion because the flow was not great enough in the amiloride-treated mice. A high luminal volume maintains a low luminal K concentration, thereby preventing a high gradient for K reabsorption [24;25]. Moreover, high flow activates BK but cannot account for a lumen negative potential that drives K secretion, independent of Na reabsorption. Our results are consistent with previous studies revealing tight coupling of ENaC-mediated Na reabsorption for K secretion despite high distal flow [26–28].
Mechanism of enhanced K secretion in WT-Alk
Amiloride treatment revealed that the defect of KO-Alk was the inability to couple K secretion with ENaC-mediated Na reabsorption to the magnitude of WT-Alk. The amount of ENaC-sensitive K secretion and Na reabsorption ratio (Ks/Nar) can be estimated by the changes in TTKG and TTNaG after amiloride treatment, and assuming that the distal flow does not change substantially. With this assumption, the Ks/Nar in WT-Alk is greater than 1.5 and the Ks/Nar in KO-Alk is less than 0.5. This value for KO-Alk is near the Ks/Nar of 0.40 in rabbits on a control diet and the Ks/Nar of 0.57 when rabbits were treated with deoxycorticosterone acetate (DOCA; synthetic mineralocorticoid) in isolated rabbit CCD [29]. In another study of isolated CCDs from DOCA-treated rabbits, the Ks/Nar was 0.76, which exceeded the pump ratio of 0.67 [28].
The DOCA-treated condition is different than adapting mice to the Alk diet because the animal on Alk will enhance K secretion with minimal Na delivery while alkalinizing the urine. That aldosterone promotes HCO3 secretion via pendrin [30] is consistent with neutral exchange of anions and electrical coupling of Na absorption with K secretion. Moreover, enhanced pendrin-mediated HCO3 secretion is associated with increased ENaC-mediated Na reabsorption [31], which would result in KHCO3 secretion driven by Na-K-ATPase in the principal cells (PC) and H-ATPase in the intercalated cells (IC). However, if Ks/Nar is greater than one then the mechanism must involve Na recycling.
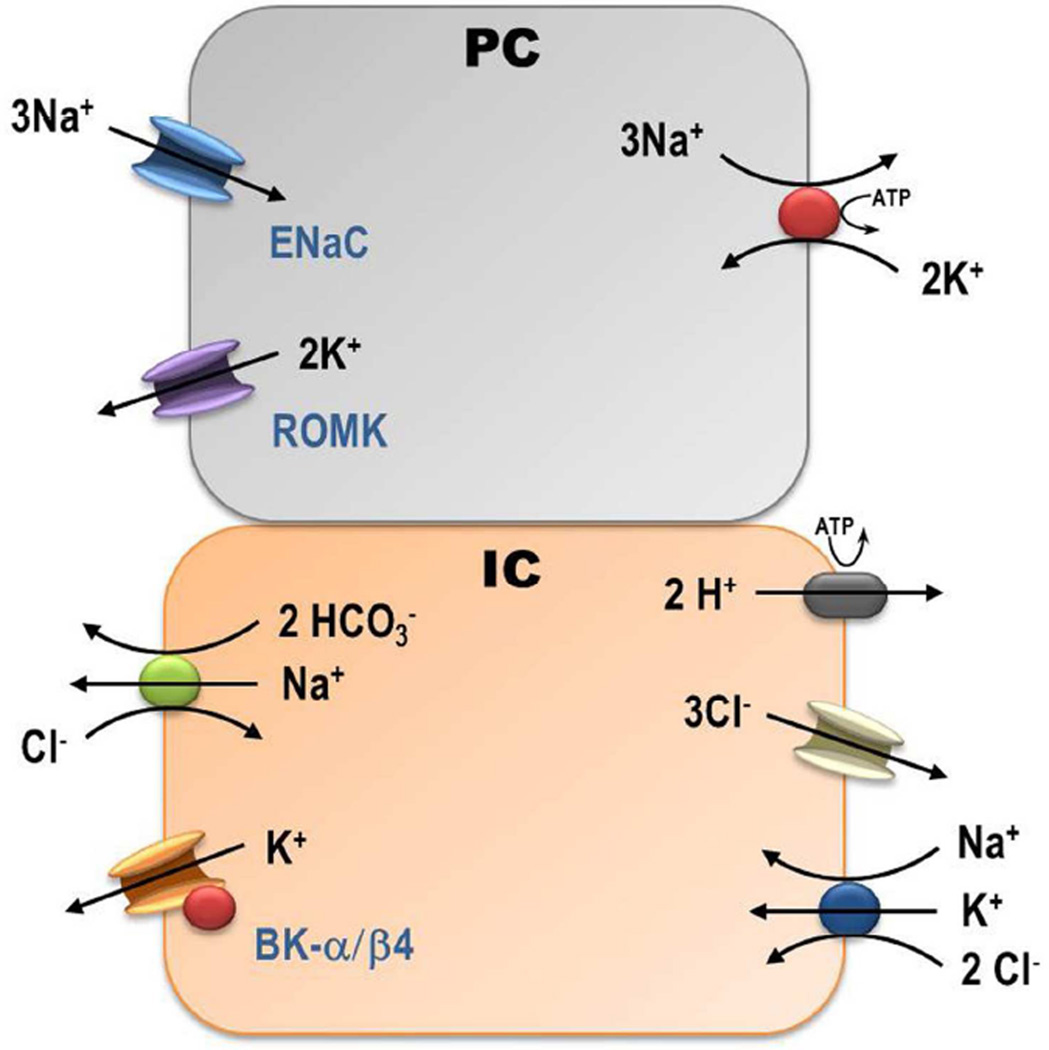
The mechanism for a very high Ks/Nar in WT-Alk may involve two novel transporters in the IC. As depicted in the cell model of figure 5, the secretory Na-K-2Cl co-transporter 1 (NKCC1) was revealed in the basolateral membrane [32] and the Na dependent Cl-HCO3 exchanger (NDCBE) in the apical membrane [33;34] of IC. With a luminal [Na] of 1 mM, a cellular [Na] of at least 15 mM, minimal Na-K-ATPase in IC, and a drive for HCO3 secretion, the chemical gradients favor Na secretion/recycling. The basolateral NKCC1 would transport K into the cell from approximately 5 mM (plasma) to 120 mM (intracellular) utilizing the chemical gradients favoring Na and Cl cell entry. The basolateral H-ATPase, which increases with a high K, alkaline diet [6], supports the cell electro-negative gradient [35] for Cl recycling via the basolateral Cl channel [36]. In isolated cortical collecting ducts, there is considerable passive leak of Na from bath to lumen [28;29]. Reducing luminal [Na] below 15 mM results in a reversal of Na transport across the renal distal tubule [25]. After amiloride treatment, the Na concentration rises to 36 mM and the Na transports into the cell with HCO3 recycling via pendrin and NDCBE as previously described [33;34]. This could explain the failure to alkalinize the urine when amiloride is given to Alk mice (see table 2). One caveat to this model is the notion that NKCC1 is in the basolateral membrane of β-IC as well as α-IC, where they were first described [37]. Because the alkaline diet causes an increase in β-IC, compared with α-IC, as indicated by an increase in pendrin expression [12], we speculate that recycling of Na would occur in the β-IC.
Figure 5.
Illustration of interaction between defined transporters of the cortical collecting duct that can explain how the Na-K-ATPAse of the principal cells (PC) can drive BK-α/β4-mediated K secretion in the intercalated cells (IC). Secretion of HCO3 in exchange for Cl via pendrin raises the luminal [HCO3] as the luminal Cl is forced through the IC instead of through the paracellular pathway, where it would short-circuit the Vte. The greater resistance of the tight junction pathway augments the electronegative lumen potential and driving force for K secretion. The large plasma to lumen chemical gradient for Na and the generation of intracellular HCO3 create a driving force for BK-mediated K secretion and NaHCO3 secretion with Na recycling to generate a higher ratio of amiloride sensitive K secretion per Na reabsorption.
Conclusion
We conclude that the BK-α/β4, localized in intercalated cells of the connecting tubules and cortical collecting duct, mediates K secretion by an ENaC-mediated, Na-dependent pathway. The BK-α/β4 pathway accounts for a very high ratio of K secreted per Na reabsorbed when mice are on a low Na, high K, alkaline diet, similar to the diets of the Yanomami of South America.
Methods
Animal Studies
Wild type (WT; C57Bl/6, Charles River, Wilmington, MA) and BK-β4 knockout mice (generously provided by R. Brenner) were maintained in accordance with the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center. For all experiments, 12–20 week-old mice had full access to water. Mice were fed either regular mouse chow (control; 0.6% K, 0.32% Na), or a special diet (Harlan Teklad, Madison, WI) for 7–10 days before sacrifice. Special diets were as follows: low Na, high K with alkaline anions (#TD.07278; Alk; 5.0% K with 5% of equal carbonate/citrate/Cl and 0.01% Na), or low Na, high K with Cl as the counter anion (#TD.09075; Acid; 5.0% K, 5.0% Cl, 0.01% Na). Twelve hours before sacrifice, a subset of mice were treated with an intraperitoneal (IP) bolus of vehicle, hydrochlorothiazde (HCTZ; 50 mg/kg), at a concentration of 10 mg/ml, or amiloride (5 mg/kg), at a concentration of 1 mg/ml. HCTZ and amiloride were dissolved in poly-ethylene glycol.
After treatment, urine was collected in metabolic cages (Nalgene) as previously described [10]. The urinary Na and K concentrations were analyzed by flame photometer (Jenway Clinical PFP7) as previously described [38] and for pH and osmolality using a Model 215 pH meter (Denver Instruments) and Model 3250 osmometer (Advanced Instruments), respectively. At sacrificing, we extracted blood from the carotid artery, measured hematocrit and centrifuged for measurement of plasma [K], [Na], and osmolality. The trans-tubular Na and K gradients were calculated using the formulas: urine [Na] X plasma [Osm]/ urine [Osm] X plasma [Na], and urine [K] X plasma [Osm] / urine [Osm] X plasma [K], respectively.
Supplementary Material
Table 3.
Comparison of various measurements (symbols are same as in table 1) of WT mice on Acid diet and treated with veh, HCTZ, or amil.
| KW | V | U[K] | U[Na] | P[K] | P[Na] | Uosm | Posm | Hct. | UpH | |
|---|---|---|---|---|---|---|---|---|---|---|
| units | mgm | ml/day | mM | mM | mM | mM | mOsm/L | mOsm/L | % | |
| Acid-veh | 246 | 1.28 | 227.6 | 4.4 | 11.1 | 150.5 | 934 | 306 | 55.9 | 5.4 |
| ±SEM (n=5) | 5 | 0.19 | 9.5 | 0.6 | 0.74 | 2.3 | 29 | 6.8 | 2.8 | 0.05 |
| Acid-HCTZ | 248 | 1.67 | 185.3 | 3.2 | 9.64 | 141.7 | 823 | 303.3 | 50.9 | 5.6 |
| ±SEM (n=3) | 10 | 0.33 | 26 | 0.4 | 0.1 | 2.2 | 103 | 12 | 1.5 | 0.1 |
| Acid-amil | 246 | 0.93 | 174.4 | 124.3 | 11.54 | 131.7 | 1107 | 310 | 55.3 | 5.8 |
| ±SEM (n=3) | 26 | 0.24 | 36.3 | 18.2 | 0.68 | 0.8 | 320 | 11.3 | 3.7 | 0.1 |
Acknowledgments
This project was funded by National Institutes of Diabetes and Digestive and Kidney Diseases Grants R01-DK-29857 (AMW) and RO1 DK071014 and RO1 DK92474 (SCS), and a fellowship (#11PRE7530018) from the American Heart Association MWA Affiliate (RJC). We appreciate the helpful comments of Dr. Gerhard Giebisch.
Footnotes
Disclosure
None
Supplementary information is available at Kidney International’s website.
Reference List
- 1.Garg LC, Knepper MA, Burg MB. Mineralocorticoid effects on Na-K-ATPase in individual nephron segments. Am.J.Physiol. 1981;240:F536–F544. doi: 10.1152/ajprenal.1981.240.6.F536. [DOI] [PubMed] [Google Scholar]
- 2.Masilamani S, Kim GH, Mitchell C, et al. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J.Clin.Invest. 1999;104:R19–R23. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbry P, Hofman P. Molecular biology of Na+ absorption. Am.J.Physiol. 1997;273:G571–G585. doi: 10.1152/ajpgi.1997.273.3.G571. [DOI] [PubMed] [Google Scholar]
- 4.Yoo D, Kim BY, Campo C, et al. Cell surface expression of the ROMK (Kir 1.1) channel is regulated by the aldosterone-induced kinase, SGK-1, and protein kinase A. J.Biol.Chem. 2003;278:23066–23075. doi: 10.1074/jbc.M212301200. [DOI] [PubMed] [Google Scholar]
- 5.Oliver WJ, Cohen EL, Neel JV. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a "no-salt" culture. Circulation. 1975;52:146–151. doi: 10.1161/01.cir.52.1.146. [DOI] [PubMed] [Google Scholar]
- 6.Cornelius RJ, Wen D, Hatcher LI, Sansom SC. Bicarbonate promotes BK-alpha/beta4-mediated K excretion in the renal distal nephron. Am.J.Physiol Renal Physiol. 2012;303:F1563–F1571. doi: 10.1152/ajprenal.00490.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frindt G, Palmer LG. K+ secretion in the rat kidney: Na+ channel-dependent and -independent mechanisms. Am.J.Physiol Renal Physiol. 2009;297:F389–F396. doi: 10.1152/ajprenal.90528.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muto S, Tsuruoka S, Miyata Y, et al. Basolateral Na+/H+ exchange maintains potassium secretion during diminished sodium transport in the rabbit cortical collecting duct. Kidney Int. 2009;75:25–30. doi: 10.1038/ki.2008.447. [DOI] [PubMed] [Google Scholar]
- 9.Sabolic I, Herak-Kramberger CM, Breton S, Brown D. Na/K-ATPase in intercalated cells along the rat nephron revealed by antigen retrieval. J Am Soc.Nephrol. 1999;10:913–922. doi: 10.1681/ASN.V105913. [DOI] [PubMed] [Google Scholar]
- 10.Holtzclaw JD, Grimm PR, Sansom SC. Intercalated cell BK-alpha/beta4 channels modulate sodium and potassium handling during potassium adaptation. J.Am.Soc.Nephrol. 2010;21:634–645. doi: 10.1681/ASN.2009080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimm PR, Foutz RM, Brenner R, Sansom SC. Identification and localization of BK-beta subunits in the distal nephron of the mouse kidney. Am.J.Physiol Renal Physiol. 2007;293:F350–F359. doi: 10.1152/ajprenal.00018.2007. [DOI] [PubMed] [Google Scholar]
- 12.Wen D, Cornelius RJ, Yuan Y, Sansom SC. Regulation of BK-alpha expression in the distal nephron by aldosterone and urine pH. Am.J.Physiol Renal Physiol. 2013;305:F463–F476. doi: 10.1152/ajprenal.00171.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Neil RG, Boulpaep EL. Effect of amiloride on the apical cell membrane cation channels of a sodium-absorbing, potassium-secreting renal epithelium. J.Membr.Biol. 1979;50:365–387. doi: 10.1007/BF01868898. [DOI] [PubMed] [Google Scholar]
- 14.Foy JM, Furman BL. Effect of diuretics on mouse blood sugar following single dose administration. Br.J.Pharmacol. 1971;42:287–297. doi: 10.1111/j.1476-5381.1971.tb07110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasuvattakul S, Quaggin SE, Scheich AM, et al. Kaliuretic response to aldosterone: influence of the content of potassium in the diet. Am.J.Kidney Dis. 1993;21:152–160. doi: 10.1016/s0272-6386(12)81086-9. [DOI] [PubMed] [Google Scholar]
- 16.Gil-Ruiz MA, Alcaraz AJ, Maranon RJ, et al. Electrolyte disturbances in acute pyelonephritis. Pediatr.Nephrol. 2012;27:429–433. doi: 10.1007/s00467-011-2020-9. [DOI] [PubMed] [Google Scholar]
- 17.Mc GC, Horan J, Jones D, et al. A study of tubular potassium secretory capacity in older patients with hyperkalaemia. J.Nutr.Health Aging. 2008;12:152–155. doi: 10.1007/BF02982569. [DOI] [PubMed] [Google Scholar]
- 18.Shirley DG, Skinner J, Walter SJ. The influence of dietary potassium on the renal tubular effect of hydrochlorothiazide in the rat. Br.J.Pharmacol. 1987;91:693–699. doi: 10.1111/j.1476-5381.1987.tb11264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Reilly M, Marshall E, Macgillivray T, et al. Dietary electrolyte-driven responses in the renal WNK kinase pathway in vivo. J.Am.Soc.Nephrol. 2006;17:2402–2413. doi: 10.1681/ASN.2005111197. [DOI] [PubMed] [Google Scholar]
- 20.Sorensen MV, Grossmann S, Roesinger M, et al. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int. 2013;83:811–824. doi: 10.1038/ki.2013.14. [DOI] [PubMed] [Google Scholar]
- 21.Schild L, Schneeberger E, Gautschi I, Firsov D. Identification of amino acid residues in the alpha, beta, and gamma subunits of the epithelial sodium channel (ENaC) involved in amiloride block and ion permeation. J.Gen.Physiol. 1997;109:15–26. doi: 10.1085/jgp.109.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer LG, Frindt G. Amiloride-sensitive Na channels from the apical membrane of the rat cortical collecting tubule. Proc.Natl.Acad.Sci.U.S.A. 1986;83:2767–2770. doi: 10.1073/pnas.83.8.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masereel B, Pochet L, Laeckmann D. An overview of inhibitors of Na(+)/H(+) exchanger. Eur.J.Med.Chem. 2003;38:547–554. doi: 10.1016/s0223-5234(03)00100-4. [DOI] [PubMed] [Google Scholar]
- 24.Good DW, Wright FS. Luminal influences on potassium secretion: transepithelial voltage. Am J Physiol. 1980;239:F289–F298. doi: 10.1152/ajprenal.1980.239.3.F289. [DOI] [PubMed] [Google Scholar]
- 25.Good DW, Wright FS. Luminal influences on potassium secretion: sodium concentration and fluid flow rate. Am J Physiol. 1979;236:F192–F205. doi: 10.1152/ajprenal.1979.236.2.F192. [DOI] [PubMed] [Google Scholar]
- 26.Engbretson BG, Stoner LC. Flow-dependent potassium secretion by rabbit cortical collecting tubule in vitro. Am J Physiol. 1987;253:F896–F903. doi: 10.1152/ajprenal.1987.253.5.F896. [DOI] [PubMed] [Google Scholar]
- 27.Grantham JJ, Kurg MB, Obloff J. The nature of transtubular Na and K transport in isolated rabbit renal collecting tubules. J.Clin.Invest. 1970;49:1815–1826. doi: 10.1172/JCI106399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stokes JB. Potassium secretion by cortical collecting tubule: relation to sodium absorption, luminal sodium concentration, and transepithelial voltage. Am.J.Physiol. 1981;241:F395–F402. doi: 10.1152/ajprenal.1981.241.4.F395. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz GJ, Burg MB. Mineralocorticoid effects on cation transport by cortical collecting tubules in vitro. Am.J.Physiol. 1978;235:F576–F585. doi: 10.1152/ajprenal.1978.235.6.F576. [DOI] [PubMed] [Google Scholar]
- 30.Verlander JW, Hassell KA, Royaux IE, et al. Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension. 2003;42:356–362. doi: 10.1161/01.HYP.0000088321.67254.B7. [DOI] [PubMed] [Google Scholar]
- 31.Pech V, Pham TD, Hong S, et al. Pendrin modulates ENaC function by changing luminal HCO3- J.Am.Soc.Nephrol. 2010;21:1928–1941. doi: 10.1681/ASN.2009121257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu W, Schreck C, Coleman RA, et al. Role of NKCC in BK channel-mediated net K+ secretion in the CCD. Am.J.Physiol Renal Physiol. 2011;301:F1088–F1097. doi: 10.1152/ajprenal.00347.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leviel F, Hubner CA, Houillier P, et al. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J.Clin.Invest. 2010;120:1627–1635. doi: 10.1172/JCI40145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eladari D, Chambrey R, Peti-Peterdi J. A new look at electrolyte transport in the distal tubule. Annu.Rev.Physiol. 2012;74:325–349. doi: 10.1146/annurev-physiol-020911-153225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chambrey R, Kurth I, Peti-Peterdi J, et al. Renal intercalated cells are rather energized by a proton than a sodium pump. Proc.Natl.Acad.Sci.U.S.A. 2013;110:7928–7933. doi: 10.1073/pnas.1221496110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmer LG, Frindt G. Cl- channels of the distal nephron. Am.J.Physiol Renal Physiol. 2006;291:F1157–F1168. doi: 10.1152/ajprenal.00496.2005. [DOI] [PubMed] [Google Scholar]
- 37.Ginns SM, Knepper MA, Ecelbarger CA, et al. Immunolocalization of the secretory isoform of Na-K-Cl cotransporter in rat renal intercalated cells. J.Am.Soc.Nephrol. 1996;7:2533–2542. doi: 10.1681/ASN.V7122533. [DOI] [PubMed] [Google Scholar]
- 38.Grimm PR, Irsik DL, Settles DC, et al. Hypertension of Kcnmb1−/− is linked to deficient K secretion and aldosteronism. Proc.Natl.Acad.Sci.U.S.A. 2009;106:11800–11805. doi: 10.1073/pnas.0904635106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.