Abstract
Objectives
Obesity is a major risk factor for the development of osteoarthritis (OA) that is associated with a state of low-grade inflammation, and increased circulating adipokines and free fatty acids (FFA). The aim of this study was to analyze effects of saturated (palmitate) and monounsaturated (oleate) free fatty acids (FFA) on articular chondrocytes and cartilage.
Methods
Human articular chondrocytes and fibroblast-like synoviocytes obtained from young healthy donors, and OA chondrocytes from patients undergoing total knee replacement were treated with palmitate or oleate alone or with interleukin 1-β (IL-1β). Cell viability, caspase activation, and gene expression of proinflammatory factors, extracellular matrix proteins, and extracellular proteases were studied. In addition, chondrocyte viability, interleukin-6 (IL-6) production and matrix damage were assessed in bovine and human articular cartilage explants cultured with FFA with or without IL-1β.
Results
Palmitate, but not oleate, induced caspase activation and cell death in IL-1β-stimulated normal chondrocytes, and upregulated il6 and cox2 expression in chondrocytes and fibroblast-like synoviocytes through toll-like receptor-4 signaling. In cartilage explants, palmitate induced chondrocyte death, IL-6 release and extracellular matrix degradation. Palmitate synergized with IL-1β in stimulating proapoptotic and proinflammatory cellular responses. Pharmacological inhibition of caspases or TLR-4 signaling reduced palmitate and IL-1β-induced cartilage damage.
Conclusions
Palmitate acts as a pro-inflammatory and catabolic factor that, in synergy with IL-1β, induces chondrocyte apoptosis and articular cartilage breakdown. Collectively, our data suggest that elevated levels of saturated FFA often found in obesity may contribute to OA pathogenesis.
Keywords: Osteoarthritis, cartilage, fatty acids
INTRODUCTION
Osteoarthritis (OA) is the most prevalent form of arthritis and a leading cause of pain and disability (1, 2). Previously considered a non-inflammatory, “wear and tear” disease of articular cartilage, OA has proven to be more complex, consisting of various clinical subtypes, including post-traumatic, aging related, genetic and metabolic OA (3). Metabolic OA is characterized by the presence of obesity and chronic, low-grade inflammation, and has an earlier onset and a faster progression than other subtypes (4). In spite of being preventable through lifestyle modifications, the prevalence of both obesity and metabolic OA has dramatically increased during the last decade (5).
The molecular mechanisms underlying metabolic OA have not been fully elucidated. In weight-bearing joints, altered biomechanics are a major contributor (6, 7). However, recent epidemiologic data have shown an association between obesity and hand OA that cannot be explained by increased joint loading (8, 9). Further, an increased risk of knee OA in obese patients with metabolic syndrome (MetS) as compared to obese patients without MetS has been reported (10). These findings suggest that systemic factors are involved, and have led to the discovery of pro-inflammatory cytokines and adipokines produced in adipose tissue as central contributors of metabolic OA (9, 11). Adipokines, such as leptin, resistin, adiponectin, visfatin and chemerin, positively correlate with changes in body mass index and, in addition to other cytokines such as interleukin-1β (IL-1 β) and IL-6, account for the systemic, low-grade inflammatory state present in obesity (12, 13). However, the role of adipokines in OA remains to be conclusively elucidated. In the setting of experimental OA, the general body of evidence supports pro-inflammatory and catabolic actions of adipokines in joint tissues (4, 9), while a few studies show no association (14) or a protective effect (4, 15). In the clinical setting, leptin and chemerin concentrations are increased in synovial fluid of OA patients, correlating with disease severity (16, 17), and circulating levels of adiponectin are positively associated with hand (18) and knee OA (19). However, a lack of association between adipokines and OA has also been reported (20, 21).
Additional factors and mechanisms are likely to mediate the obesity-inflammation-OA relationship. In fact, MetS and obesity are associated with other metabolic alterations that could also contribute to OA pathogenesis, such as disorders in glucose and lipid metabolism. In this context, saturated free fatty acids (FFA) are of particular interest, since their circulating levels are elevated in obesity, they cause apoptosis, insulin resistance and inflammation in peripheral tissues, and they play a central role in other chronic diseases related to MetS such as diabetes and atherosclerosis (12, 13, 22, 23). However, little is know about the relationship between saturated FFA and OA pathophysiology. Accumulation of palmitate and oleate, the most abundant saturated and monounsaturated FA in humans, has been described in OA cartilage (24, 25). Here, we examined the individual effects of palmitate and oleate on articular chondrocytes and cartilage.
MATERIALS AND METHODS
Human articular chondrocytes and fibroblast-like synoviocytes
Normal human articular cartilage and synovium were harvested at the time of autopsy from the knees of donors (mean ± SD age 22±3 years) without a history of joint disease and with macroscopically normal cartilage surfaces. OA cartilage was obtained from patients (mean ± SD age 56±9 years) with OA who were undergoing total knee arthroplasty. Tissue collection was approved by the Scripps Human Subjects Committee. Chondrocytes were isolated and cultured as described previously (26). Fibroblast-like synoviocytes were isolated from synovial membranes after collagenase (Sigma-Aldrich) digestion for 3-hours at 37°C. After isolation, cells were plated at a density of 104 cells/cm2 in Dulbecco's modified Eagle's medium (DMEM; Corning) containing 10% calf serum (CS) and incubated at 37°C in 5% CO2. Second-passage cells were used in all the experiments.
For experiments, low-endotoxin, fatty-acid-free bovine serum albumin (Sigma-Aldrich) was diluted in serum-free DMEM (1%) and used as vehicle. Palmitate and oleate (Sigma-Aldrich) solutions were prepared in serum free DMEM as described previously (27). Human recombinant IL-1β (PrepoTech) was used at 10 pg/ml or 1 ng/ml. Pan-caspase inhibitor Z-VAD-FMK (Calbiochem) was used at 100 μM. Toll-like receptor-4 signaling inhibitor CLI-095 (InvivoGen) was used at 1 μg/ml. In the experiments involving inhibitors, they were added at the same time as fatty acids or IL-1 and no pretreatment period was carried out.
Cell viability and caspase 3/7 activity assays
Chondrocytes were plated in 96-well plates and treated for the time periods indicated. Cell viability was assessed using CellTiter-Glo® Luminescent Cell Viability Assay (Promega) and caspase 3/7 activation was measured using Caspase-Glo® 3/7 Assay (Promega) following manufacturer's instructions.
RNA isolation and real-time reverse transcription–polymerase chain reaction
Total RNA was isolated from treated cells using RNAeasy kits (Qiagen). Quantitative polymerase chain reaction (qPCR) was performed using a LightCycler 480 (Roche Diagnostics) and TaqMan Gene Expression Assay probes for interleukin-6 (il6), cyclooxygenase-2 (cox2), inducible-nitric oxide synthase (nos2), collagenase-3 (mmp-13), a disintegrin and metalloproteinase with thrombospondin motifs-4 (adamts4), aggrecan (acan), α1 helix of collagen type-2 (col2a1), and GAPDH (gapdh), according to the manufacturer's instructions (Applied Biosystems).
Enzyme-linked immunosorbent assay (ELISA)
Secreted interleukin-6 (IL-6) concentrations in conditioned media from the monolayer chondrocyte cultures or human cartilage explants were determined by a 2-site, sandwich-type ELISA using microplate wells coated with a monoclonal capture antibody (R&D Systems: MAB206), and an alkaline phosphatase–labeled polyclonal detection antibody (R&D Systems: BAF206). The detection limit was 1 μg/ml and the intraassay and interassay variations were 4.8% and 7.3%, respectively.
Human cartilage explants
Full-thickness articular cartilage explants without subchondral bone were obtained from the knees of two donors (ages 47 and 51 years) with no previous joint pathology, and cultured for 24 hours at 37C in 5% CO2 in DMEM supplemented with 10% CS and antibiotics. Explants (n=10-12 per experimental condition) were treated with palmitate (0.5 mM), IL-1β (1 ng/ml) or a combination of both. After 72-hours, media was collected for glycosaminoglycan (GAG) and IL-6 quantification.
Bovine cartilage explants
Full-thickness articular cartilage explants without subchondral bone were harvested from adult bovine knees and cultured for 48 hours at 37C in 5% CO2 in DMEM supplemented with 10% CS and antibiotics. Explants (n=6 per experimental condition in each experiment) were simultaneously treated with palmitate (0.5 mM), oleate (0.5 mM), IL-1β (1 ng/ml) and Z-VAD-FMK (100 μM). After 72-hours, media was collected for GAG quantification, and explants were processed for live/dead cell assay and immunohistochemistry analysis as described below.
GAG assay
GAG level in supernatants of explant cultures was quantified as described elsewhere (28). GAG concentration was normalized to explant weight, and the results expressed as micrograms of GAG per milligram of cartilage.
Live/dead cell assay
Live and dead chondrocytes were simultaneously viewed using confocal microscopy as described previously (28). Three explants per experimental condition were analyzed. To quantify live/dead chondrocytes, two digital images per explant were obtained and cells were manually counted in five fields per image. Percentage of live cells was calculated by dividing the number of live chondrocytes by the total number of cells.
Immunohistochemistry
Explants were fixed in 10% zinc-buffered formalin (Z-Fix; Anatech) for 24-hours and embedded in paraffin. Paraffin-embedded samples were deparaffinized in the xylene substitute Pro-Par Clearant (Anatech) and rehydrated in graded ethanol and water. After washing with TBS, sections were blocked with 2.5% horse serum for 1 hour at room temperature and then incubated with rabbit anti-cleaved poly (ADP-ribose) polymerase (PARP) polyclonal antibody (Promega; dilution 1:50) overnight at 4°C. After washing with TBS, sections were incubated with ImmPRESS-AP Anti-Rabbit IgG Polymer Detection Reagent (Vector Laboratories) for 30 min following manufacturer's instructions, counterstained with hematoxylin (Thermo Scientific), dehydrated and mounted. Positive and total cells were counted in two different sections per explant, and results are reported as percentage of PARP positive cells.
Statistical analysis
Data are given as mean ± SEM. Differences between groups were assessed by ANOVA. P values <0.05 were considered significant.
RESULTS
Palmitate causes apoptotic cell death in human articular chondrocytes treated with IL-1β
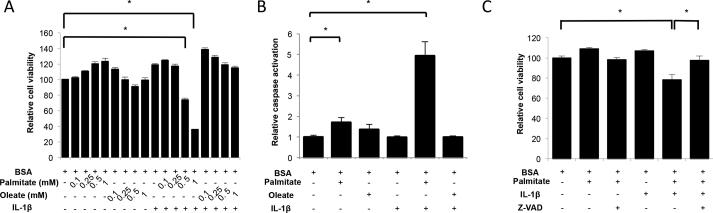
First, we evaluated the impact of saturated and monounsaturated FFAs on chondrocyte viability. Chondrocytes from normal human knee cartilage were treated with increasing concentrations of palmitate or oleate alone or in combination with IL-1β for 72 hours. As shown in Figure 1A, treatment with FFA did not affect cell viability, even at doses as high as 1 mM. IL-1β alone also had no detectable effect. However, a significant (p<0.001) cell death induction was seen when cells were treated with 0.5 mM or 1 mM palmitate and IL-1β. This effect was not observed with oleate and IL-1β co-incubation. In subsequent experiments, we used FFA at a concentration of 0.5 mM since it is in the high physiological range and it has been extensively used in experimental studies (29).
Figure 1.
Free fatty acids, cell viability and caspase activation in human articular chondrocytes. (A) Cell viability in normal human articular chondrocytes treated with different concentrations of palmitate or oleate alone or with IL-1β (1 ng/ml) for 72 hours. (B) Caspase 3/7 activation in normal human articular chondrocytes treated with palmitate (0.5 mM), oleate (0.5 mM), or IL-1β (1 ng/ml) as indicated for 72 hours. (C) Cell viability in normal human articular chondrocytes treated with palmitate (0.5 mM), IL-1β (1 ng/ml) or Z-VAD-FMK (100 μM) for 72 hours. Values are expressed as mean ± SEM. *, p<0.05.
To test if the decrease in chondrocyte viability was associated with apoptosis, caspase 3 and 7 activities were quantified using a luminescence assay. Figure 1B shows a significant (p<0.001) increase in caspase 3/7 activities upon treatment with palmitate and IL-1β. Oleate and IL-1β alone or in combination did not induce caspase activity. The pan-caspase inhibitor Z-VAD-FMK prevented the palmitate and IL-1β-induced decrease in chondrocyte viability (Figure 1C), further supporting the notion that the combination of palmitate and IL-1β causes chondrocyte death via apoptosis induction.
Palmitate synergizes with IL-1β to increase IL-6 and COX2 expression in human articular chondrocytes and fibroblast-like synoviocytes
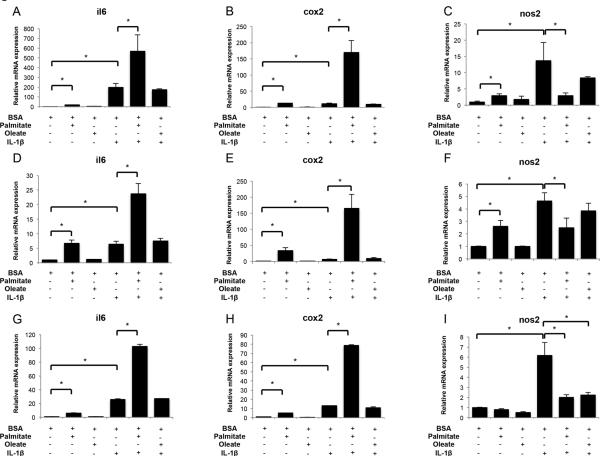
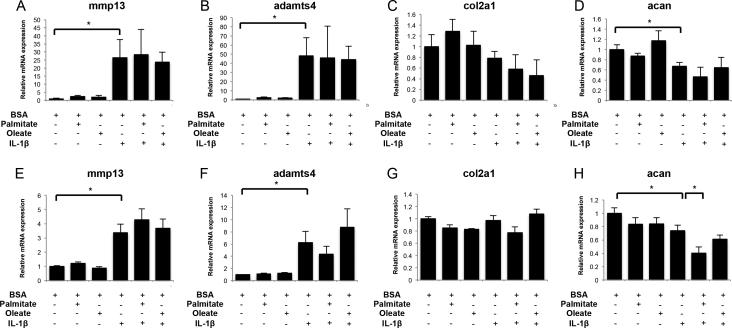
Next, we analyzed FFA effects on gene expression of catabolic and anabolic factors in human articular chondrocytes. Expression of il6, cox2, nos2, mmp13, adamts4, acan and col2a1 was assessed in normal and OA human chondrocytes treated with palmitate or oleate, with or without IL-1β. In normal chondrocytes, palmitate, but not oleate, increased il6, cox2 and nos2 mRNA (Figures 2A-C), as well as IL-6 secretion (mean ± SD; BSA: 1.3 ± 1.1 ng/ml; palmitate: 2.5 ± 0.4 ng/ml; IL-1β: 32.4 ± 1.8 ng/ml; palmitate+IL-1β: 43.4 ± 4.1 ng/ml; BSA vs palmitate p=0.049; IL-1β vs palmitate+IL-1β p=0.0036). When cells were incubated with both palmitate and IL-1β, the expression of il6 and cox2 was synergistically increased (p<0.05) (Figures 2A,B). This synergy was not observed for nos2, since co-treatment with palmitate prevented IL-1β-induced nos2 upregulation (Figure 2C). OA chondrocytes exhibited similar il6, cox2 and nos2 gene expression patterns as normal chondrocytes (Figures 2D-F). Incubation with FFAs did not modify MMP13, ADAMTS4, and collagen type 2 gene expression regardless of the presence of IL-1β in normal or OA articular chondrocytes (Figures 3A-C,G-E). However, whereas acan levels were unchanged upon FFA treatment in normal chondrocytes, palmitate significantly (p<0.05) downregulated acan expression in OA chondrocytes treated with IL-1β (Figures 3D,H).
Figure 2.
Free fatty acids and proinflammatory mediators expression in human articular chondrocytes and fibroblast-like synoviocytes. Il6, cox2 and nos2 mRNA levels assessed by qPCR in normal human articular chondrocytes (A-C), osteoarthritic articular chondrocytes (D-F), and fibroblast-like synoviocytes (G-I) treated with palmitate (0.5 mM), oleate (0.5 mM), or IL-1β (10 pg/ml) as indicated for 24 hours. Values are expressed as mean ± SEM. *, p<0.05.
Figure 3.
Expression of extracellular matrix (ECM) proteins and proteases in human articular chondrocytes treated with palmitate, oleate and IL-1β. Mmp13, adamts4, col2a1 and acan mRNA levels assessed by qPCR in normal human articular chondrocytes (A-D) and osteoarthritic articular chondrocytes (E-H) treated with palmitate (0.5 mM), oleate (0.5 mM), and IL-1β (10 pg/ml) as indicated for 24 hours. Values are expressed as mean ± SEM. *, p<0.05.
In addition, we analyzed il6, cox2 and nos2 expression in human fibroblast-like synoviocytes treated with palmitate or oleate, alone or in combination with IL-1β. Palmitate, but not oleate, significantly (p<0.01) increased il6 and cox2 expression and co-treatment with IL-1β further enhanced this effect (Figures 2G-H). FFA alone did not modify nos2 expression and significantly (p<0.01) decreased IL-1β-induced nos2 upregulation (Figure 2I).
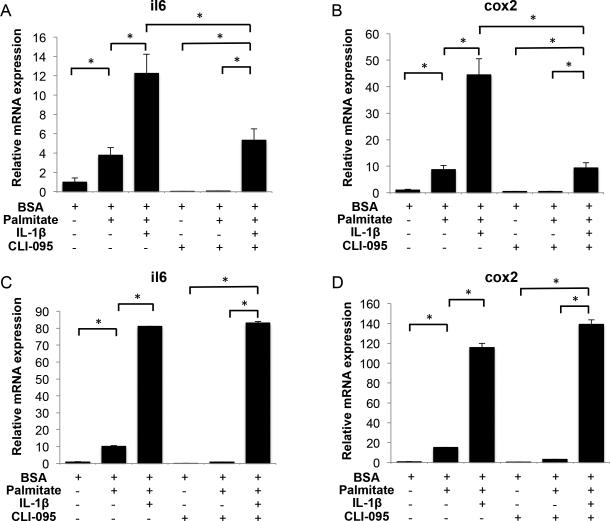
To determine whether palmitate effects are receptor-mediated, we tested CLI-095, a pharmacological inhibitor of TLR-4 signaling (30), which completely blocked il6 and cox2 expression induced by palmitate, but not by IL-1β, in normal articular chondrocytes and fibroblast-like synoviocytes (Figures 4A-D).
Figure 4.
Effects of TLR-4 signaling inhibition in human articular chondrocytes and fibroblast-like synoviocytes treated with palmitate and IL-1β. (A) Il6 and (B) cox2 gene expression assessed by qPCR in normal human chondrocytes treated with palmitate (0.5 mM), IL-1β (10 pg/ml) or CLI-095 (1 μg/ml) as indicated for 24 hours. (C) Il6 and (D) cox2 gene expression assessed by qPCR in fibroblast-like synoviocytes s treated with palmitate (0.5 mM), IL-1β (10 pg/ml) or CLI-095 (1 μg/ml) as indicated for 24 hours. Values are expressed as mean ± SEM. *, p<0.05.
Palmitate induces chondrocyte death and extracellular matrix damage in bovine cartilage explants
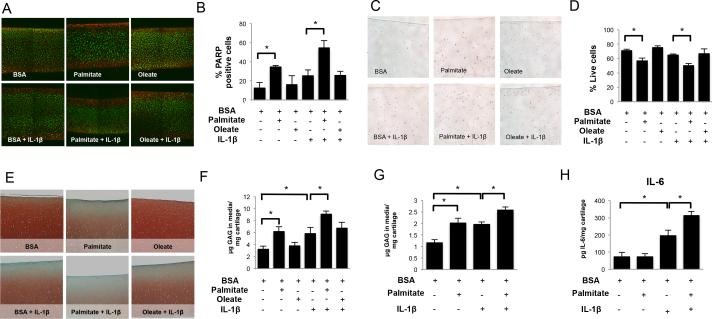
To evaluate long-term palmitate effects on articular cartilage integrity, bovine cartilage explants were treated with palmitate or oleate, alone or combined with IL- 1β. Palmitate, but not oleate, significantly (p<0.05) increased cell death in cartilage explants as evidenced by a decrease in cell viability and an increase in cleaved-PARP staining, particularly in the cartilage surface (Figures 5A-D). This effect was synergistically enhanced by IL-1β. ECM breakdown was assessed by Safranin-O staining and analysis of GAGs levels in the media. Palmitate, but not oleate, significantly (p<0.05) decreased Safranin-O staining in cartilage explants and increased GAGs release into the medium (Figures 5E,F). This catabolic effect of palmitate was significantly (p<0.05) enhanced in explants stimulated with IL-1β.
Figure 5.
Free fatty acids effects in bovine and human cartilage explants. Full-thickness cartilage explants were treated with palmitate (0.5 mM), oleate (0.5 mM), or IL-1β (1 ng/ml) as indicated for 72 hours. (A) Chondrocyte viability visualized by live/dead assay and (B) percentage of live chondrocytes in bovine cartilage explants. Magnification: 40X. (C) Cleaved poly (ADP-ribose) polymerase (PARP) detection by immunohistochemistry and (D) percentage of PARP positive chondrocytes in bovine cartilage explants. Magnification 100X. (E) Safranin O staining of glycosaminoglycans (GAGs) and (F) GAG release into the supernatants in bovine cartilage explants. Magnification: 40X. (G) GAGs and (H) IL-6 concentration in the supernatants of cultured human cartilage explants. Images are representative of two different experiments performed in triplicate. Values are expressed as mean ± SEM. *, p<0.05.
Palmitate induces matrix degradation and IL-6 production in human cartilage explants
Next, we tested potential proinflammatory and catabolic effects of palmitate in human cartilage explants. As shown in Figures 5G-H, palmitate significantly (p<0.05) increased GAGs and IL-6 levels in the media. These effects were significantly (p<0.05) increased in explants treated with IL-1β.
Caspase or TLR-4 signaling inhibition decreases palmitate and IL-1β–induced matrix degradation on bovine cartilage explants
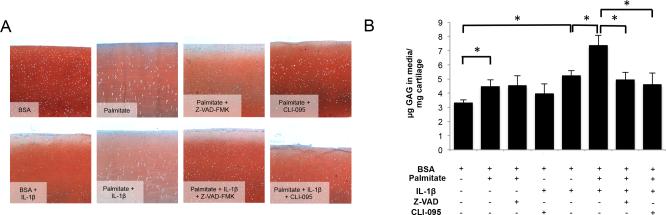
To evaluate whether caspase inhibition can prevent catabolic actions of palmitate and IL-1β in articular cartilage, we measured ECM degradation in bovine cartilage explants treated with palmitate, alone or in combination with IL-1β, in the presence of Z-VAD-FMK or CLI-095. As shown in Figure 6, Z-VAD-FMK and CLI-095 reduced matrix breakdown induced by palmitate and IL-1β co-treatment as evidenced by an increase in Safranin-O staining and a significant (p<0.05) decrease in GAG release.
Figure 6.
Effects of Z-VAD-FMK and CLI-095 in bovine cartilage explants treated with palmitate and IL-1β. Full-thickness cartilage explants were treated with palmitate (0.5 mM), IL-1β (1 ng/ml), Z-VAD-FMK (100 μM) or CLI-095 (1 μg/ml) as indicated for 72 hours. (A) Safranin O staining of glycosaminoglycans (GAGs). Magnification: 40X. (B) GAG release into the supernatants. Images are representative of two different experiments performed in triplicate. Values are expressed as mean ± SEM. *, p<0.05.
DISCUSSION
The increasing prevalence of metabolic OA has demanded a better understanding of the complex mechanisms by which excess adiposity may induce joint damage. In obese patients, especially those with MetS, white adipose tissue releases inflammatory factors to the bloodstream, such as cytokines, adipokines and FFA (31). Here, we report for the first time that the saturated FFA palmitate exerts pro-apoptotic and pro-inflammatory actions in articular cartilage, acting synergistically with IL-1β to promote chondrocyte death and cartilage destruction.
No studies have yet compared FFA levels in synovial fluid of normal and OA patients. However, there is indirect evidence suggesting increased FFA concentrations in OA synovial fluid. In a pioneering study conducted by Lipiello and colleagues, elevated FA levels were observed in osteoarthritic articular cartilage, and were positively associated with histological severity (25). More recently, Cillero-Pastor also reported significant FA accumulation in the surface of OA articular cartilage using mass spectrometry (32). Chondrocytes can synthetize and accumulate endogenous lipids and also are able to internalize exogenous FA and store them in lipid droplets (33). While both mechanisms can be altered in OA, the observations that extracellular lipids are more prominent in the cartilage surface (34, 35) and that high blood levels of FFA lead to FA accumulation in extravascular spaces (34, 35) might be indicative of increased FFA concentration in synovial fluid. Future studies comparing lipid profiles of normal and OA synovial fluids are needed.
The present paper shows that palmitate causes cell death and production of inflammatory mediators in articular chondrocytes, two main features of OA cartilage. Chondrocytes are responsible for preserving articular cartilage integrity by synthetizing and maintaining extracellular matrix, and a reduction of cartilage cellularity is a hallmark of OA (36). Several insults can trigger chondrocyte death, such as reactive oxygen species, death receptor activators, mitochondrial dysfunction, and mechanical stress (36). Our results show that palmitate induces apoptotic cell death in human articular chondrocytes and in bovine cartilage explants. Furthermore, palmitate and IL-1β co-incubation synergistically exacerbated chondrocyte death in both models. The mechanism underlying this synergy is unclear, since IL-1β alone does not induce apoptosis in chondrocytes (36, 37). Interestingly, ER stress has been shown to sensitize beta cells to IL-1β-induced apoptosis by disrupting the balance between pro- and anti-apoptotic BCL-2 proteins (38). There is evidence for ER stress in OA cartilage (Liu-Bryan R, Terkeltaub R, 2013; personal communication) and the contribution of palmitate to this change and its role in palmitate-mediated chondrocyte death remains to be investigated.
Inflammation has been proven to be a driver of the OA process and synovitis is now accepted as a critical feature of OA (9). We report here a proinflammatory response of cultured articular chondrocytes and fibroblast-like synoviocytes to palmitate treatment, evidenced by a rapid upregulation of il6 and cox2 expression that is enhanced by low concentrations of IL-1β. These results indicate that high FFA levels in synovial fluid can initiate and/or aggravate joint inflammation, potentially contributing to OA development and progression.
In agreement with previous reports (39), palmitate proinflammatory activity was dependent on TLR-4 signaling, while, as expected, inhibition of TLR-4 signaling did not block IL-1β-induced il6 and cox2 gene expression. TRL-4 and IL-1 receptors converge in the activation of downstream transcription factors, such as NFkB, to induce expression of proinflammatory mediators (40). Our findings are in agreement with this notion, since palmitate and IL-1β synergistically increased the expression of proinflammatory factors in human articular chondrocytes and fibroblast-like synoviocytes, and encourage future studies aimed to understand the role of TLR-4 signaling in metabolic OA.
Loss of ECM homeostasis is a central event in OA pathogenesis that results from increased expression and activity of proteolytic enzymes, such as MMP-9, MMP-13, ADAMTS-4 or ADAMTS-5, and decreased expression of ECM proteins, such as aggrecan or collagen type 2 (41). Chondrocyte death and matrix degradation are mechanistically linked, since matrix degradation results in the loss of survival mechanisms and cell death can contribute to matrix degradation (42). In cultured chondrocytes, palmitate treatment did not modify gene expression of ECM proteins or ECM-degrading proteases, whereas IL-1β significantly upregulated MMP-13 and ADAMTS-4 expression. On the other hand, either palmitate or IL-1β alone induced proteoglycan loss and GAG release in bovine and human cartilage explants. However, whereas IL-1β induced cartilage loss without decreasing cell viability, palmitate treatment markedly induced both chondrocyte death and matrix breakdown, indicating that palmitate catabolic effects on articular cartilage are in part mediated by chondrocyte apoptosis. Notably, palmitate and IL-1β synergistic effect on ECM destruction was associated with enhanced chondrocyte death. These results collectively suggest that catabolic effects of palmitate and IL-1β co-treatment in articular cartilage are likely mediated by a combination of increased chondrocyte apoptosis and increased expression of cartilage-degrading enzymes.
The use of caspase inhibitors has proven to be an effective therapy in different animal models of OA (42). Pan-caspase inhibitors, such as Z-VAD-FMK, are of particular interest because they not only inhibit apoptosis but also prevent pro-IL-1β proteolytic activation by caspase 1 (43). In the present study, Z-VAD-FMK treatment significantly decreased matrix breakdown in bovine cartilage explants co-treated with palmitate and IL-1β. In a similar fashion, blockage of TLR-4 signaling protected bovine cartilage explants from adverse effects induced by palmitate and IL-1β. These results support the above-mentioned notion that palmitate and IL-1β catabolic effects are mediated by chondrocyte death and proinflammatory mediators, and suggest that pharmacological inhibition of both processes might be an effective therapy to protect cartilage from saturated FFA and IL-1β. However, one limitation of our study is that some ex vivo experiments were performed only in bovine cartilage explants. Due to low availability of normal human cartilage, and high intra-sample variability, only key experiments were carried out using human samples in order to confirm the proinflammatory and procatabolic effect of palmitate in human articular cartilage. Whether caspase or TLR-4 inhibition is effective reducing saturated FFA effects on human articular cartilage remains to be tested.
It is worth mentioning that MetS is not the only scenario where FFA and inflammation might have an impact on OA development. After an intra-articular fracture, lipid infiltrations in the synovial fluid are often found concomitant with acute synovial inflammation (44, 45). According to our results, saturated FFA might contribute to the initial chondrocyte death and matrix degradation observed in affected areas immediately following joint injury. Therefore, early treatment with caspase or TLR-4 inhibitors could ameliorate cell death and inflammation, limiting the initial lesion caused to cartilage, and thus preventing the development of post-traumatic OA.
Our data show clear differential effects of palmitate and oleate in chondrocytes and cartilage. Monounsaturated FFA have been reported to attenuate some of the adverse effects of saturated FFA (29), so the possibility that palmitate effects in cartilage could be attenuated by co-incubation with oleate cannot be ruled out. In this regard, treatment of cartilage explants with a 1:1 mixture of palmitate and oleate led to a 70% increase in GAG release (46), indicating that co-incubation with oleate does not abolish palmitate-induced matrix degradation of articular cartilage. However, whether oleate can modulate other palmitate-associated effects in cartilage remains to be elucidated.
In summary, we report that palmitate acts as a pro-inflammatory and catabolic factor that, in synergy with IL-1β, induces chondrocyte apoptosis and articular cartilage breakdown. These findings thus provide a new link between obesity, inflammation and OA and open new doors to future studies aimed to find effective therapies for patients with metabolic OA.
Acknowledgments
The authors acknowledge Merissa Olmer, Lilo Creighton, Melissa Szeto and Nick Glembotski for technical assistance.
Funding This study was supported by National Institutes of Health grants AG007996 and AR050631, and the Sam and Rose Stein Endowment Fund.
Footnotes
Contributors ML had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study design: OAG, NHR and RGS. Acquisition of data: OAG. Analysis and interpretation of data: OAG, NHR and RGS. Manuscript preparation and approval: OAG, NHR and RGS.
Competing interests None.
Ethics approval This study was conducted with the approval of the Scripps Human Subjects Committee.
Contributor Information
Oscar Alvarez-Garcia, Department of Molecular and Experimental Medicine. The Scripps Research Institute. La Jolla, CA. USA.
Nicole H Rogers, California Institute for Biomedical Research (Calibr). La Jolla, CA. USA.
Roy G Smith, Department of Metabolism and Aging. The Scripps Research Institute. Jupiter, FL. USA.
Martin K Lotz, Department of Molecular and Experimental Medicine. The Scripps Research Institute. La Jolla, CA. USA.
REFERENCES
- 1.Dillon CF, Rasch EK, Gu QP, Hirsch R. Prevalence of knee osteoarthritis in the United States: Arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. 2006;33(11):2271–9. [PubMed] [Google Scholar]
- 2.Cooper C, Snow S, McAlindon TE, Kellingray S, Stuart B, Coggon D, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000;43(5):995–1000. doi: 10.1002/1529-0131(200005)43:5<995::AID-ANR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115–26. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 4.Zhuo Q, Yang W, Chen J, Wang Y. Metabolic syndrome meets osteoarthritis. Nature reviews Rheumatology. 2012;8(12):729–37. doi: 10.1038/nrrheum.2012.135. [DOI] [PubMed] [Google Scholar]
- 5.Losina E, Walensky RP, Reichmann WM, Holt HL, Gerlovin H, Solomon DH, et al. Impact of obesity and knee osteoarthritis on morbidity and mortality in older Americans. Annals of internal medicine. 2011;154(4):217–26. doi: 10.1059/0003-4819-154-4-201102150-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felson DT, Goggins J, Niu JB, Zhang YQ, Hunter DJ. The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis Rheum. 2004;50(12):3904–9. doi: 10.1002/art.20726. [DOI] [PubMed] [Google Scholar]
- 7.Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Cl Rh. 2011;25(6):815–23. doi: 10.1016/j.berh.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yusuf E, Nelissen RG, Ioan-Facsinay A, Stojanovic-Susulic V, DeGroot J, van Osch G, et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis. 2010;69(4):761–5. doi: 10.1136/ard.2008.106930. [DOI] [PubMed] [Google Scholar]
- 9.Berenbaum F, Eymard F, Houard X. Osteoarthritis, inflammation and obesity. Current opinion in rheumatology. 2013;25(1):114–8. doi: 10.1097/BOR.0b013e32835a9414. [DOI] [PubMed] [Google Scholar]
- 10.Sowers M, Karvonen-Gutierrez CA, Palmieri-Smith R, Jacobson JA, Jiang YB, Ashton-Miller JA. Knee Osteoarthritis in Obese Women With Cardiometabolic Clustering. Arthrit Care Res. 2009;61(10):1328–36. doi: 10.1002/art.24739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Issa RI, Griffin TM. Pathobiology of obesity and osteoarthritis: integrating biomechanics and inflammation. Pathobiology of aging & age related diseases. 2012;2(2012) doi: 10.3402/pba.v2i0.17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stienstra R, Tack CJ, Kanneganti TD, Joosten LA, Netea MG. The inflammasome puts obesity in the danger zone. Cell metabolism. 2012;15(1):10–8. doi: 10.1016/j.cmet.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nature medicine. 2012;18(3):363–74. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 14.McNulty AL, Miller MR, O'Connor SK, Guilak F. The effects of adipokines on cartilage and meniscus catabolism. Connective tissue research. 2011;52(6):523–33. doi: 10.3109/03008207.2011.597902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bastiaansen-Jenniskens YM, Clockaerts S, Feijt C, Zuurmond AM, Stojanovic-Susulic V, Bridts C, et al. Infrapatellar fat pad of patients with end-stage osteoarthritis inhibits catabolic mediators in cartilage. Ann Rheum Dis. 2012;71(2):288–94. doi: 10.1136/ard.2011.153858. [DOI] [PubMed] [Google Scholar]
- 16.Ku JH, Lee CK, Joo BS, An BM, Choi SH, Wang TH, et al. Correlation of synovial fluid leptin concentrations with the severity of osteoarthritis. Clin Rheumatol. 2009;28(12):1431–5. doi: 10.1007/s10067-009-1242-8. [DOI] [PubMed] [Google Scholar]
- 17.Huang K, Du G, Li L, Liang HS, Zhang B. Association of chemerin levels in synovial fluid with the severity of knee osteoarthritis. Biomarkers. 2012;17(1):16–20. doi: 10.3109/1354750X.2011.634028. [DOI] [PubMed] [Google Scholar]
- 18.Yusuf E, Ioan-Facsinay A, Bijsterbosch J, Klein-Wieringa I, Kwekkeboom J, Slagboom PE, et al. Association between leptin, adiponectin and resistin and long- term progression of hand osteoarthritis. Ann Rheum Dis. 2011;70(7):1282–4. doi: 10.1136/ard.2010.146282. [DOI] [PubMed] [Google Scholar]
- 19.Koskinen A, Juslin S, Nieminen R, Moilanen T, Vuolteenaho K, Moilanen E. Adiponectin associates with markers of cartilage degradation in osteoarthritis and induces production of proinflammatory and catabolic factors through mitogen-activated protein kinase pathways. Arthritis Res Ther. 2011;13(6) doi: 10.1186/ar3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massengale M, Reichmann WM, Losina E, Solomon DH, Katz JN. The relationship between hand osteoarthritis and serum leptin concentration in participants of the Third National Health and Nutrition Examination Survey. Arthritis Res Ther. 2012;14(3) doi: 10.1186/ar3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Boer TN, van Spil WE, Huisman AM, Polak AA, Bijlsma JWJ, Lafeber FPJG, et al. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthr Cartilage. 2012;20(8):846–53. doi: 10.1016/j.joca.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Boden G. Obesity and free fatty acids. Endocrinology and metabolism clinics of North America. 2008;37(3):635–46, viii-ix. doi: 10.1016/j.ecl.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gkretsi V, Simopoulou T, Tsezou A. Lipid metabolism and osteoarthritis: Lessons from atherosclerosis. Prog Lipid Res. 2011;50(2):133–40. doi: 10.1016/j.plipres.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Bonner WMJH, Malanos C, Bryant M. Changes in the lipids of human articular cartilage with age. Arthritis Rheum. 1975 doi: 10.1002/art.1780180505. [DOI] [PubMed] [Google Scholar]
- 25.Lippiello L, Walsh T, Fienhold M. The association of lipid abnormalities with tissue pathology in human osteoarthritic articular cartilage. Metabolism: clinical and experimental. 1991;40(6):571–6. doi: 10.1016/0026-0495(91)90046-y. [DOI] [PubMed] [Google Scholar]
- 26.Carames B, Kiosses WB, Akasaki Y, Brinson DC, Eap W, Koziol J, et al. Glucosamine Activates Autophagy In Vitro and In Vivo. Arthritis Rheum. 2013;65(7):1843–52. doi: 10.1002/art.37977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu J, Wang QH, Huang LH, Dong HY, Lin LJ, Lin N, et al. Palmitate Causes Endoplasmic Reticulum Stress and Apoptosis in Human Mesenchymal Stem Cells: Prevention by AMPK Activator. Endocrinology. 2012;153(11):5275–84. doi: 10.1210/en.2012-1418. [DOI] [PubMed] [Google Scholar]
- 28.Carames B, Taniguchi N, Seino D, Blanco FJ, D'Lima D, Lotz M. Mechanical Injury Suppresses Autophagy Regulators and Pharmacologic Activation of Autophagy Results in Chondroprotection. Arthritis Rheum. 2012;64(4):1182–92. doi: 10.1002/art.33444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watt MJ, Hoy AJ, Muoio DM, Coleman RA. Distinct roles of specific fatty acids in cellular processes: implications for interpreting and reporting experiments. Am J Physiol-Endoc M. 2012;302(1):E1–E3. doi: 10.1152/ajpendo.00418.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ii M, Matsunaga N, Hazeki K, Nakamura K, Takashima K, Seya T, et al. A novel cyclohexene derivative, ethyl (6R)-6-[N-(2-chloro-4-fluorophenyl) sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol Pharmacol. 2006;69(4):1288–95. doi: 10.1124/mol.105.019695. [DOI] [PubMed] [Google Scholar]
- 31.Gregor MF, Hotamisligil GS. Inflammatory Mechanisms in Obesity. Annu Rev Immunol. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 32.Cillero-Pastor B, Eijkel G, Kiss A, Blanco FJ, Heeren RMA. Time-of-Flight Secondary Ion Mass Spectrometry-Based Molecular Distribution Distinguishing Healthy and Osteoarthritic Human Cartilage. Anal Chem. 2012;84(21):8909–16. doi: 10.1021/ac301853q. [DOI] [PubMed] [Google Scholar]
- 33.Lippiello L, Fienhold M, Grandjean C. Metabolic and Ultrastructural-Changes in Articular-Cartilage of Rats Fed Dietary-Supplements of Omega-3-Fatty-Acids. Arthritis Rheum. 1990;33(7):1029–36. doi: 10.1002/art.1780330716. [DOI] [PubMed] [Google Scholar]
- 34.Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50(7):1612–7. doi: 10.2337/diabetes.50.7.1612. [DOI] [PubMed] [Google Scholar]
- 35.Samuel VT, Shulman GI. Mechanisms for Insulin Resistance: Common Threads and Missing Links. Cell. 2012;148(5):852–71. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhn K, D'Lima DD, Hashimoto S, Lotz M. Cell death in cartilage. Osteoarthr Cartilage. 2004;12(1):1–16. doi: 10.1016/j.joca.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Oliver BL, Cronin CG, Zhang-Benoit Y, Goldring MB, Tanzer ML. Divergent stress responses to IL-1beta, nitric oxide, and tunicamycin by chondrocytes. Journal of cellular physiology. 2005;204(1):45–50. doi: 10.1002/jcp.20261. [DOI] [PubMed] [Google Scholar]
- 38.Miani M, Barthson J, Colli ML, Brozzi F, Cnop M, Eizirik DL. Endoplasmic reticulum stress sensitizes pancreatic beta cells to interleukin-1beta-induced apoptosis via Bim/A1 imbalance. Cell death & disease. 2013;4:e701. doi: 10.1038/cddis.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, et al. Saturated fatty acid and TLR signaling link beta cell dysfunction and islet inflammation. Cell metabolism. 2012;15(4):518–33. doi: 10.1016/j.cmet.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 40.Kawai T, Akira S. Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity. 2011;34(5):637–50. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Goldring MB, Marcu KB. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;11(3) doi: 10.1186/ar2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D'lima D, Hermida J, Hashimoto S, Colwell C, Lotz M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 2006;54(6):1814–21. doi: 10.1002/art.21874. [DOI] [PubMed] [Google Scholar]
- 43.Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Vanness K, Greenstreet TA, et al. Molecular-Cloning of the Interleukin-1-Beta Converting Enzyme. Science. 1992;256(5053):97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- 44.Aponte EM, Novik JI. Identification of Lipohemarthrosis with Point-of-Care Emergency Ultrasonography: Case Report and Brief Literature Review. J Emerg Med. 2013;44(2):453–6. doi: 10.1016/j.jemermed.2012.07.062. [DOI] [PubMed] [Google Scholar]
- 45.Furman BD, Olson SA, Guilak F. The development of posttraumatic arthritis after articular fracture. J Orthop Trauma. 2006;20(10):719–25. doi: 10.1097/01.bot.0000211160.05864.14. [DOI] [PubMed] [Google Scholar]
- 46.Griffin TM, Fermor B, Huebner JL, Kraus VB, Rodriguiz RM, Wetsel WC, et al. Diet-induced obesity differentially regulates behavioral, biomechanical, and molecular risk factors for osteoarthritis in mice. Arthritis Res Ther. 2010;12(4) doi: 10.1186/ar3068. [DOI] [PMC free article] [PubMed] [Google Scholar]