Abstract
The diversity of synapses within the simple modular structure of the cerebellum has been crucial for study of the phasic extrasynaptic signaling by fast neurotransmitters collectively referred to as ‘spillover.’ Additionally, the accessibility of cerebellar components for in vivo recordings and their recruitment by simple behaviors or sensory stimuli has allowed for both direct and indirect demonstrations of the effects of transmitter spillover in the intact brain. The continued study of spillover in the cerebellum not only promotes our understanding of information transfer through cerebellar structures but also how extrasynaptic signaling may be regulated and interpreted throughout the CNS.
Introduction
Extrasynaptic actions of the fast neurotransmitters glutamate and GABA in the central nervous system have been a well-studied topic in neurophysiological research over the last two decades. Despite the initial skepticism towards its prevalence in the intact brain and the perception that neurotransmitter spillover represents a breakdown of point-to-point synaptic transmission, there is mounting evidence that spillover forms an extra layer of communication between neurons, at times even in the absence of underlying synaptic connections. Studies in many brain regions including the hippocampus (1–3), olfactory bulb (4), and cortex (5, 6) have detailed circumstances when spillover of glutamate or GABA from the synaptic cleft leads to significant signals in downstream neurons. But perhaps more than any other region, the cerebellum has offered the most fertile environment for the progress of this story from theory to mechanism to function over successive in vitro and in vivo studies. In this review we will highlight the structural and functional mechanisms that foster spillover in the cerebellum (7) with updates regarding the contribution spillover makes to local circuit processes.
In contrast to tonic signaling from ambient levels of extrasynaptic neurotransmitter (8, 9) or aberrant extrasynaptic glutamate signaling that drives excitotoxicity and neurodegeneration (10–12), spillover occurs in a phasic manner and exhibits common features across disparate brain environments. Spillover is most often triggered by stimuli that recruit a dense group of release sites to increase cooperativity between independent sites (13) or by high frequency repetitive stimuli leading to a buildup of extracellular transmitter (1, 2). As the resulting extrasynaptic concentration of glutamate is much lower than in the cleft, spillover detection typically requires the presence of high affinity receptors such as NMDARs (13, 14), mGluRs (15), or GABABRs (3, 16). The lower transmitter concentrations also result in slow-rising and -decaying currents that may transmit different information than their fast synaptic counterparts (17). Finally, spillover is highly regulated by transmitter uptake, such that it is uncovered or potentiated by transporter blockade (18–20). Despite these general themes, individual examples of GABA and glutamate spillover in the cerebellum appear to subserve markedly different purposes depending on their context (Figure 1).
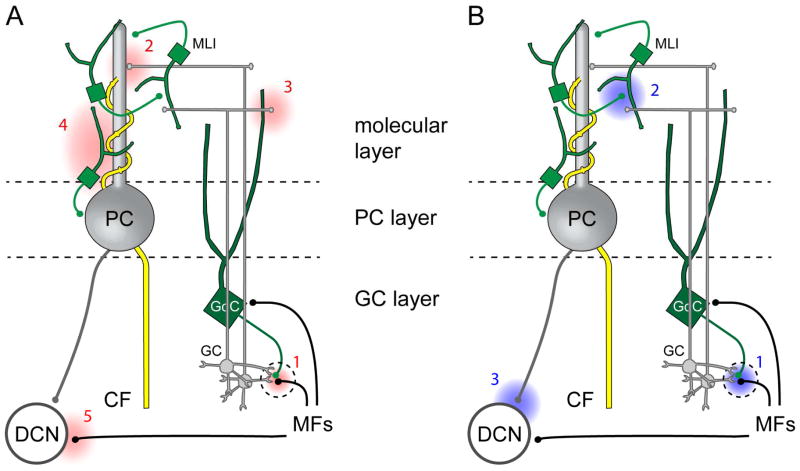
Figure 1. Diverse sites of fast neurotransmitter spillover in local cerebellar circuits.
(A) Glutamate spillover (red) occurs at the mossy fiber-granule cell (MF-GC) glomerulus including signaling to Golgi cell (GoC; 1) axons, following high frequency parallel fiber (PF) stimulation onto Purkinje cells (PCs; 2), molecular layer interneurons (MLIs; 2) and GoCs (3), heterosynaptically from climbing fiber (CF)-to-PC synapses that activate MLIs (4), and following MF stimulation onto deep cerebellar nuclei (DCN; 5) neurons.
(B) GABA spillover (blue) occurs from Golgi cells (GoC; 1), molecular layer interneurons (MLI) to Purkinje cells (PC; 2) and to MLIs (2), and PC axons that terminate on deep cerebellar nuclei (DCN; 3) neurons. See text for references.
Mossy Fiber Input Pathway
Mossy fibers (MFs) arise from a variety of locations in the spinal cord and brain stem to form one of only two projection pathways into the cerebellar cortex. Their glutamatergic terminals in the granule cell (GC) layer form specialized glomerular structures that represent one of the most complex arrangements of synaptic contacts in the CNS. Each MF terminal at the core of the glomerulus makes closely spaced synaptic contacts with the dendrites of ~50 GCs (21). GC dendrites within the glomerulus also receive inhibitory synapses from the main interneuron within the GC layer, the Golgi cell (GoC). The large MF terminal may serve as a barrier to prevent dissipation of neurotransmitters by diffusion and to exclude glial membranes, thereby reducing transmitter uptake (22). The plexus of dendritic processes that surround the MF terminal is ensheathed by astrocytes that express GLT-1 and GLAST subtypes of glutamate transporters. These structural features, in combination with the high frequency burst firing of MFs (up to 700 Hz; ref. 23) set the stage for physiological transmitter spillover.
Glutamate spillover was suggested by Silver and colleagues (24) to explain the speeding of MF-GC excitatory postsynaptic current (EPSC) decay times in response to lower release probability (Pr). The presence of multiple closely aligned release sites allows postsynaptic receptors to sense glutamate spillover from neighboring synapses, resulting in a slowing of the EPSC decay that is absent when release site density is reduced under conditions of low Pr. Subsequent studies uncovered the presence of distinct fast- and slow-rising EPSCs at single MF-GC connections, representing direct synaptic responses to high glutamate concentrations and indirect spillover responses to lower glutamate concentrations (17, 25). Summation of slower spillover with fast synaptic responses increased the probability of GC spiking suggesting that spillover increases the reliability of synaptic transmission (17, 26). Although pooling of transmitter following spillover could desensitize AMPARs on GCs and thus limit synaptic responses to subsequent release events, desensitization under most conditions is modest and EPSC amplitudes can be maintained at high frequencies (22, 27).
Activation of low-affinity AMPARs by spillover indicated that spillover signaling was robust at MF glomeruli, so it is not surprising that spillover at these synapses is also sensed by high-affinity NMDARs. Extrasynaptic NMDARs were activated during evoked but not quantal release, displaying a requirement for spillover from multiple sites suggesting that NMDARs were located outside the synapse (28, 29). Interestingly, both AMPAR- and NMDAR-spillover behave similarly in facilitating temporal integration of subsequent signals in a burst (30), indicating that however it is sensed, spillover not only strengthens the MF-GC connection, but also increases its dynamic range. This is achieved through summation of the slower NMDAR and AMPAR spillover-mediated conductances that are resistant to desensitization (22, 27). This idea is supported by the work of Hausser and colleagues that used in vivo MF terminal recordings in response to sensory stimulation (23). Stimulation in the form of an air puff directed at a rat’s whiskers induced bursts of spiking (at least 500 Hz) in MF terminals that were followed reliably by GC spiking. Interestingly, while GC EPSC amplitudes depressed during sensory-evoked MF bursts, the charge transfer accompanying each release event continued to summate due to the recruitment of slow, non-depressing spillover EPSCs. The conclusions from Rancz et al. (23) are not only consistent with evidence for glutamate spillover in vitro, but also suggest that spillover extends the ability of synapses to exchange information at the upper range of physiological frequencies.
Transmitter spillover also regulates the MF-GC connection indirectly, via regulation of local inhibition. In addition to its actions at GC dendrites, glutamate spillover interacts with GoC terminals that project into the active glomerulus (15). MF burst stimulation (> 10-Hz) decreases GABA release by recruiting presynaptic mGluRs, disinhibiting the active glomerulus. Heterosynaptic modulation also occurs in the opposite direction with phasic GABA spillover regulating glutamate release from MFs. Metabotropic GABABRs on MF axons are not activated by baseline GoC activity, but can be recruited with higher frequency GoC stimulation (16). Spillover activation of GABABRs decreased MF glutamate release through local control of terminal Ca2+ influx, such that release may even be modulated on a sub-glomerular scale (31). GABA spillover also plays a significant role in the direct inhibition of GCs through dendritic GABAARs. Both fast- and slow-components of GC inhibitory postsynaptic currents (IPSCs) have been detailed, mirroring the synaptic and spillover components seen for GC EPSCs (32). The slower spillover component formed ~90% of the phasic inhibitory charge transfer in mixed responses (33), and in some individual GoC-GC connections formed 100% of the response, demonstrating pure GABA spillover transmission (34). Furthermore, dynamic clamp studies modeling fast and slow inhibition found the spillover component to be extremely effective at blocking spiking during the later portions of a one second MF stimulus train, suggesting that the presence of GABA spillover shapes GC responses to prolonged MF activity (34).
Spillover of both glutamate and GABA is thus facilitated at MF inputs by a combination of the glomerular structure and high rates of activity experienced with sensory stimulation. Slow glutamate spillover as well as local disinhibition by mGluR-dependent dampening of GoC GABA release promotes the reliable transfer of this signal to GCs for the next stage of information processing. On the other hand, activation of GoCs by afferent signals can lead to phasic inhibition strengthened by GABA spillover within the glomerulus, an effect that may be magnified by concurrent depression of MF transmitter release through spillover activation of presynaptic GABABRs. Together, transmitter spillover provides multiple mechanisms to regulate signaling within the glomerulus, allowing the MF-GC connection to function over the range of its physiological frequencies.
Parallel Fiber Relay Pathway
Cerebellar GCs relay integrated MF information into the cerebellar cortex by projecting axons that ascend through the molecular layer and bifurcate to run long distances (~5 mm; ref. 35) in the longitudinal plane. These parallel fibers (PFs) collectively provide dense innervation throughout the molecular layer, but each individual fiber extends only sparse glutamatergic synapses to the many Purkinje cells (PCs) and molecular layer interneurons (MLIs) along its course (36, 37). PF spillover can occur during high-frequency GC activity (23) or multivesicular release (38–40), resulting in glutamate concentration transients that can overwhelm the moderate glial coverage near these synapses (41). However, the sparse and low Pr synapses (42) made by individual PFs suggests coincident activation of beams of PFs is required to achieve significant spillover (but see 39, 43, 44).
Glutamate transporters limit spillover during PF beam stimulation (45); however, burst stimulation of PFs seems to overcome this barrier, recruiting perisynaptic mGluR1 receptors (46). When paired with activation of the climbing fiber input pathway, PF burst stimulation can trigger mGluR1-dependent long-term depression of synaptic receptors that may underlie cerebellum-dependent learning (47–49). Differential expression of the neuronal glutamate transporter EAAT4 in PC dendrites regulates spillover signaling to PCs (50–52) as well as to nearby glial processes (53).
A similar pattern of receptor activation follows burst stimulation in the other known targets of PFs: MLIs and GoCs. In acute slices (54) as well as in the intact brain (55), PFs burst stimulation activates dendritic mGluR2s on GoCs. This glutamatergic signal paradoxically decreases GoC activity through a G-protein coupled potassium conductance (54), resulting in a pause in spiking. This might be a complimentary mechanism to the gap junction-mediated pause that occurs in GoC networks following sparse physiological activation (56). At PF-MLI synapses, high-frequency stimulation results in spillover activation of AMPARs at neighboring synapses (57), extrasynaptic NMDARs (57, 58) and group I mGluRs (59). All three signals converge to increase MLI activity: AMPAR/NMDAR activation increases the gain of the PF-MLI signal, while mGluR activation signals for a short term increase in MLI excitability (59, 60). Interestingly, high-frequency signaling through the MLI network also results in elevated extrasynaptic GABA. GABA spillover closely follows PF burst stimulation to signal back to PFs, activating both axonal GABAARs (61–63) and GABABRs (64, 65). While axonal GABAAR activation increases [Ca2+] and excitability (62), GABABR activation decreases [Ca2+] and Pr (after ~300 ms, 65). These effects might work in concert to produce a biphasic regulation of PF release, but as each of the cited works included antagonists of the opposing GABAR subtype, no direct data exists to test this speculation.
Although high-frequency burst protocols used in most PF-spillover studies are modeled after firing rates measured in GCs responding to sensory input (23), but results in slices could be biased towards spillover due to the recruitment of dense beams of PFs resulting from electrical stimulation in the molecular layer. Studies by Attwell and colleagues (66, 67) demonstrated that changing the site of stimulation from the molecular layer to the GC layer greatly reduced the spillover component of PF signaling due to the sparse GC activation (45). While molecular layer burst stimulation results in mGluR- and endocannabinoid-dependent LTD of PF glutamate release, GC layer burst stimulation failed to induce such plasticity due to the lack of effective spillover signaling (66). These results indicate a requirement not only for high-frequency, but also dense activation of PFs to induce significant spillover signaling.
Together these results indicate that spillover in the PF pathway represents a salient signal that may underlie forms of cerebellar learning through extrasynaptic mGluR signaling at PC dendrites while simultaneously providing feedback regulation of PF release through disynaptic GABA spillover or PC retrograde signaling. PF spillover may also provide a feedback signal to the cerebellar input stage by triggering an mGluR-dependent decrease in GoC spiking.
Climbing Fiber Input Pathway
In contrast to the divergent MF-PF pathway, each branch of a climbing fiber (CF) from the inferior olivary nucleus forms hundreds of glutamatergic synapses onto a single PC. These high Pr synapses exhibit multivesicular release, where simultaneous or near simultaneous release of multiple vesicles per site generates a very high concentration of synaptic glutamate (68, 69). The activation of a dense group of release sites sets the stage for spillover signaling. However, CFs do not approach the high firing rates of MFs or PFs, with average rates around 1 Hz and short sensory-induced bursts of ~15 Hz (70, 71), decreasing the likelihood of frequency-dependent spillover. Near-complete glial ensheathment (41) and postsynaptic glutamate transporter expression (51, 72, 73) further restrain potential extrasynaptic glutamate signaling, as inhibition of glial and neuronal uptake uncovers significant activation of perisynaptic mGluRs (74) as well as spillover between neighboring PCs (75).
Although it was accepted that CFs synapse exclusively to PCs providing a privileged connection, recent studies have uncovered other direct (76) and indirect signaling pathways. For example, MLIs do not receive CF synapses (77, 78), yet in vivo studies have demonstrated plasticity at MLI synapses following coincident CF-PF activation (79) as well as slow inward postsynaptic responses following inferior olive stimulation (80). A subsequent study in acute brain slices showed the CF-MLI response occurs concurrently with CF-PC responses, is mediated by long duration, low concentration glutamate transients and is strongly regulated by glutamate transporters - all indicators that slow MLI responses are the result of CF-mediated glutamate spillover (78). Two recent studies have directly examined the implications of this connection for ongoing cerebellar cortical activity. Viral delivery of channelrhodopsin to inferior olivary neurons demonstrated that synchronous activation of multiple CFs induced spillover-mediated MLI spiking that contributed to a pause in PC activity regardless of whether or not they were targets of the active CF (81). This stimulation paradigm could be relevant to cerebellar function following recent demonstrations of sensory-induced synchronous CF activity across broad patches of neighboring PCs in vivo (82, 83), albeit at the relatively slow time resolution of the Ca2+-imaging techniques used in those studies.
Our group showed that CF-MLI spillover results in a long-lasting feedforward inhibition between MLIs following stimulation of a single CF (84). This agrees with in vivo recordings of prolonged MLI spiking following CF stimulation, supporting its relevance in the intact brain (85). Interestingly, simultaneously excited MLIs did not inhibit each other’s spiking, but rather worked in concert to send strong inhibitory signals to other interneurons and PCs not excited by the active CF. This spillover-mediated feedforward inhibition ultimately resulted in a biphasic regulation of neighboring PC excitability, inducing inhibition followed by a subsequent period of disinhibition (84). These data may explain in vivo accounts of biphasic regulation of neighboring PCs spiking following CF stimulation (71, 86, 87), and complement observations that CF signaling is a strong regulator of simple spike activity (88–90).
Taken together, these studies indicate two distinct modes of operation for CF glutamate spillover in routing information through the PC layer. During non-synchronous firing, individual CF activation can regulate multiple neighboring PCs while the target PC experiences a complex spike. Neighboring PCs could be either inhibited or disinhibited depending on the net effect of MLIs that are excited or inhibited by CF spillover. During synchronized CF release; however, MLIs excitation extends post-complex spike pauses to targeted PCs (81).
Deep Cerebellar Nuclei
CF and MF activity is processed and relayed by PCs as an inhibitory projection to the deep cerebellar nuclei (DCN), the final output structure of the cerebellum. Spontaneously active glutamatergic, GABAergic, and glycinergic projection neurons in the DCN (91) are modulated by PC GABA release that occurs at rates up to 100 Hz spontaneously and 300 Hz during movement (92, 93). The interaction of convergent PC inputs to individual DCN neurons can lead to powerful control of their activity patterns (94) and gate the induction of synaptic plasticity (95, 96). PC-DCN synapses are often somatic, with large presynaptic boutons housing ~10 individual release sites (97). Glia surround boutons but do not seem to separate release sites, suggesting that individual PC-DCN connections operate independently from each other but yet have the capacity for spillover between release sites. This intra-bouton spillover is reminiscent of glutamate-spillover at MF glomeruli (17), and was shown to counteract synaptic depression during high frequency signaling due to transmitter pooling even in spite of presynaptic vesicle depletion (97).
In addition to PC inhibitory synapses, DCN neurons also receive direct excitatory input from MFs. This connection is thought to be an important site for cerebellar-dependent learning (98, 99) during tasks that activate both MFs and PCs (100). Not surprisingly, transmitter spillover is implicated in multiple forms of MF-DCN plasticity. Reminiscent of PF-PC responses, synaptic MF responses are mediated by AMPARs- and NMDARs, but physiological burst stimulation recruits perisynaptic mGluRs (101). Pairing mGluR1 activation with postsynaptic depolarization induces LTD of MF responses that may correspond to extinction during associative eyelid conditioning (98). Conversely, pairing MF burst stimulation with either step hyperpolarization or PC burst stimulation induces an NMDAR-dependent LTP of MF synapses (95, 100). Such a protocol mimics DCN activation patterns during classical eyelid conditioning, and is dependent on the timing of the bursts of PC inhibition (100) to control Ca2+ entry and rebound firing (94). As GABA spillover maintains the influence of PC-DCN inhibition during high frequency trains, it likely provides an essential mechanism that could underlie the acquisition phase of cerebellar classical conditioning. Thus, much like in the MF or PF pathway, transmitter spillover may represent an important mechanism that allows DCN neurons to process salient high-frequency signals.
Common Themes of Mechanism and Function
Two non-mutually exclusive conditions support spillover signaling during cerebellar synaptic transmission: 1) Structural restrictions to diffusion and uptake promote spatially-specific transmitter pooling from nearby release sites 2) Activity-dependent spillover resulting from high frequency stimulation that elevate extrasynaptic transmitter concentrations. Such high frequency firing occurs during sensory induced MF or PF signaling in the cortex or MF signaling in the DCN. Dense, synchronous release site activation as with PF beam stimulation could also be considered a form of activity-dependent spillover, as it represents a specific pattern of fiber activation. Interestingly, these conditions are good predictors of how the spillover signal might function within the local circuit. Structurally restricted spillover seems to participate in the main signal being transmitted, extending and complementing the fast excitation or inhibition it accompanies. Activity-dependent spillover generally functions as a teaching signal that a salient stimulus is occurring by inducing pre- or postsynaptic plastic changes in synaptic strength. CF spillover represents something of an anomaly within this classification scheme, as it results from dense synchronous release that is the sole mode of transmission for the pathway. Due to the specific nature of CF spillover to MLIs but not nearby oligodendrocyte precursor cells or neighboring PCs, it seems possible that some as yet unspecified structural specializations predispose MLIs to receive that signal.
Conclusions
The study of spillover in the cerebellum not only promotes our understanding of information transfer through cerebellar structures, but will also provide insight into the potential for non-synaptic signaling throughout the CNS. Establishing the mechanisms and consequences of spillover in the cerebellum will lay the foundation for a deeper functional understanding of anatomically-defined neural maps.
Acknowledgments
We would like to thank Linda Overstreet-Wadiche for her help and comments.
Footnotes
Conflict of Interest Statement:
The authors have no conflicts of interest associated with this manuscript.
References
- 1.Scanziani M, Malenka R, Nicoll R. Role of intercellular interactions in heterosynaptic long-term depression. Nature. 1996;380(6573):446–50. doi: 10.1038/380446a0. [DOI] [PubMed] [Google Scholar]
- 2.Asztely F, Erdemli G, Kullmann D. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron. 1997;18(2):281–93. doi: 10.1016/s0896-6273(00)80268-8. [DOI] [PubMed] [Google Scholar]
- 3.Scanziani M. GABA spillover activates postsynaptic GABA(B) receptors to control rhythmic hippocampal activity. Neuron. 2000;25(3):673–81. doi: 10.1016/s0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 4.Isaacson JS. Glutamate spillover mediates excitatory transmission in the rat olfactory bulb. Neuron. 1999;23(2):377–84. doi: 10.1016/s0896-6273(00)80787-4. [DOI] [PubMed] [Google Scholar]
- 5.Harris AZ, Pettit DL. Recruiting extrasynaptic NMDA receptors augments synaptic signaling. J Neurophysiol. 2008;99(2):524–33. doi: 10.1152/jn.01169.2007. [DOI] [PubMed] [Google Scholar]
- 6.Chalifoux JR, Carter AG. Glutamate spillover promotes the generation of NMDA spikes. J Neurosci. 2011;31(45):16435–46. doi: 10.1523/JNEUROSCI.2777-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szapiro G, Barbour B. Parasynaptic signalling by fast neurotransmitters: the cerebellar cortex. Neuroscience. 2009;162(3):644–55. doi: 10.1016/j.neuroscience.2009.03.077. [DOI] [PubMed] [Google Scholar]
- 8.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6(3):215–29. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 9.Sah P, Hestrin S, Nicoll R. Tonic activation of NMDA receptors by ambient glutamate enhances excitability of neurons. Science. 1989;246(4931):815–8. doi: 10.1126/science.2573153. [DOI] [PubMed] [Google Scholar]
- 10.Rothstein J, Patel S, Regan M, Haenggeli C, Huang Y, Bergles D, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–7. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 11.Pitt D, Werner P, Raine CS. Glutamate excitotoxicity in a model of multiple sclerosis. Nature medicine. 2000;6(1):67–70. doi: 10.1038/71555. [DOI] [PubMed] [Google Scholar]
- 12.Buckingham SC, Campbell SL, Haas BR, Montana V, Robel S, Ogunrinu T, et al. Glutamate release by primary brain tumors induces epileptic activity. Nature medicine. 2011;17(10):1269–74. doi: 10.1038/nm.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnth-Jensen N, Jabaudon D, Scanziani M. Cooperation between independent hippocampal synapses is controlled by glutamate uptake. Nat Neurosci. 2002;5(4):325–31. doi: 10.1038/nn825. [DOI] [PubMed] [Google Scholar]
- 14.Kullmann DM, Erdemli G, Asztely F. LTP of AMPA and NMDA receptor-mediated signals: evidence for presynaptic expression and extrasynaptic glutamate spill-over. Neuron. 1996;17(3):461–74. doi: 10.1016/s0896-6273(00)80178-6. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell S, Silver R. Glutamate spillover suppresses inhibition by activating presynaptic mGluRs. Nature. 2000;404(6777):498–502. doi: 10.1038/35006649. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell S, Silver R. GABA spillover from single inhibitory axons suppresses low-frequency excitatory transmission at the cerebellar glomerulus. J Neurosci. 2000;20(23):8651–8. doi: 10.1523/JNEUROSCI.20-23-08651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiGregorio D, Nusser Z, Silver R. Spillover of glutamate onto synaptic AMPA receptors enhances fast transmission at a cerebellar synapse. Neuron. 2002;35(3):521–33. doi: 10.1016/s0896-6273(02)00787-0. [DOI] [PubMed] [Google Scholar]
- 18.Otis T, Wu Y, Trussell L. Delayed clearance of transmitter and the role of glutamate transporters at synapses with multiple release sites. J Neurosci. 1996;16(5):1634–44. doi: 10.1523/JNEUROSCI.16-05-01634.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Overstreet L, Kinney G, Liu Y, Billups D, Slater N. Glutamate transporters contribute to the time course of synaptic transmission in cerebellar granule cells. J Neurosci. 1999;19(21):9663–73. doi: 10.1523/JNEUROSCI.19-21-09663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diamond J. Neuronal glutamate transporters limit activation of NMDA receptors by neurotransmitter spillover on CA1 pyramidal cells. J Neurosci. 2001;21(21):8328–38. doi: 10.1523/JNEUROSCI.21-21-08328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakab RL, Hamori J. Quantitative morphology and synaptology of cerebellar glomeruli in the rat. Anat Embryol (Berl) 1988;179(1):81–8. doi: 10.1007/BF00305102. [DOI] [PubMed] [Google Scholar]
- 22.Xu-Friedman M, Regehr W. Ultrastructural contributions to desensitization at cerebellar mossy fiber to granule cell synapses. J Neurosci. 2003;23(6):2182–92. doi: 10.1523/JNEUROSCI.23-06-02182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rancz EA, Ishikawa T, Duguid I, Chadderton P, Mahon S, Häusser M. High-fidelity transmission of sensory information by single cerebellar mossy fibre boutons. Nature. 2007;450(7173):1245–8. doi: 10.1038/nature05995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silver R, Colquhoun D, Cull-Candy S, Edmonds B. Deactivation and desensitization of non-NMDA receptors in patches and the time course of EPSCs in rat cerebellar granule cells. J Physiol. 1996;493 (Pt 1):167–73. doi: 10.1113/jphysiol.1996.sp021372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen T, DiGregorio D, Silver R. Modulation of glutamate mobility reveals the mechanism underlying slow-rising AMPAR EPSCs and the diffusion coefficient in the synaptic cleft. Neuron. 2004;42(5):757–71. doi: 10.1016/j.neuron.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Sargent P, Saviane C, Nielsen T, DiGregorio D, Silver R. Rapid vesicular release, quantal variability, and spillover contribute to the precision and reliability of transmission at a glomerular synapse. J Neurosci. 2005;25(36):8173–87. doi: 10.1523/JNEUROSCI.2051-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiGregorio D, Rothman J, Nielsen T, Silver R. Desensitization properties of AMPA receptors at the cerebellar mossy fiber granule cell synapse. J Neurosci. 2007;27(31):8344–57. doi: 10.1523/JNEUROSCI.2399-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cathala L, Misra C, Cull-Candy S. Developmental profile of the changing properties of NMDA receptors at cerebellar mossy fiber-granule cell synapses. J Neurosci. 2000;20(16):5899–905. doi: 10.1523/JNEUROSCI.20-16-05899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cathala L, Brickley S, Cull-Candy S, Farrant M. Maturation of EPSCs and intrinsic membrane properties enhances precision at a cerebellar synapse. J Neurosci. 2003;23(14):6074–85. doi: 10.1523/JNEUROSCI.23-14-06074.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz EJ, Rothman JS, Dugue GP, Diana M, Rousseau C, Silver RA, et al. NMDA receptors with incomplete Mg(2)(+) block enable low-frequency transmission through the cerebellar cortex. J Neurosci. 2012;32(20):6878–93. doi: 10.1523/JNEUROSCI.5736-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomsen LB, Jorntell H, Midtgaard J. Presynaptic calcium signalling in cerebellar mossy fibres. Frontiers in neural circuits. 2010;4:1. doi: 10.3389/neuro.04.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossi DJ, Hamann M. Spillover-mediated transmission at inhibitory synapses promoted by high affinity alpha6 subunit GABA(A) receptors and glomerular geometry. Neuron. 1998;20(4):783–95. doi: 10.1016/s0896-6273(00)81016-8. [DOI] [PubMed] [Google Scholar]
- 33.Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33(4):625–33. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- 34.Crowley JJ, Fioravante D, Regehr WG. Dynamics of fast and slow inhibition from cerebellar golgi cells allow flexible control of synaptic integration. Neuron. 2009;63(6):843–53. doi: 10.1016/j.neuron.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harvey RJ, Napper RM. Quantitative study of granule and Purkinje cells in the cerebellar cortex of the rat. J Comp Neurol. 1988;274(2):151–7. doi: 10.1002/cne.902740202. [DOI] [PubMed] [Google Scholar]
- 36.Palay SL, Chan-Palay V. Cerebellar cortex: cytology and organization. 1974. p. 348. [Google Scholar]
- 37.Napper RM, Harvey RJ. Number of parallel fiber synapses on an individual Purkinje cell in the cerebellum of the rat. J Comp Neurol. 1988;274(2):168–77. doi: 10.1002/cne.902740204. [DOI] [PubMed] [Google Scholar]
- 38.Bender V, Pugh J, Jahr C. Presynaptically expressed long-term potentiation increases multivesicular release at parallel fiber synapses. J Neurosci. 2009;29(35):10974–8. doi: 10.1523/JNEUROSCI.2123-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nahir B, Jahr CE. Activation of extrasynaptic NMDARs at individual parallel fiber-molecular layer interneuron synapses in cerebellum. J Neurosci. 2013;33(41):16323–33. doi: 10.1523/JNEUROSCI.1971-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foster KA, Crowley JJ, Regehr WG. The influence of multivesicular release and postsynaptic receptor saturation on transmission at granule cell to Purkinje cell synapses. J Neurosci. 2005;25(50):11655–65. doi: 10.1523/JNEUROSCI.4029-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu-Friedman M, Harris K, Regehr W. Three-dimensional comparison of ultrastructural characteristics at depressing and facilitating synapses onto cerebellar Purkinje cells. J Neurosci. 2001;21(17):6666–72. doi: 10.1523/JNEUROSCI.21-17-06666.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu-Friedman M, Regehr W. Probing fundamental aspects of synaptic transmission with strontium. J Neurosci. 2000;20(12):4414–22. doi: 10.1523/JNEUROSCI.20-12-04414.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valera AM, Doussau F, Poulain B, Barbour B, Isope P. Adaptation of granule cell to Purkinje cell synapses to high-frequency transmission. J Neurosci. 2012;32(9):3267–80. doi: 10.1523/JNEUROSCI.3175-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt H, Brachtendorf S, Arendt O, Hallermann S, Ishiyama S, Bornschein G, et al. Nanodomain coupling at an excitatory cortical synapse. Current biology : CB. 2013;23(3):244–9. doi: 10.1016/j.cub.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Marcaggi P, Billups D, Attwell D. The role of glial glutamate transporters in maintaining the independent operation of juvenile mouse cerebellar parallel fibre synapses. J Physiol. 2003;552(Pt 1):89–107. doi: 10.1113/jphysiol.2003.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tempia F, Miniaci MC, Anchisi D, Strata P. Postsynaptic current mediated by metabotropic glutamate receptors in cerebellar Purkinje cells. J Neurophysiol. 1998;80(2):520–8. doi: 10.1152/jn.1998.80.2.520. [DOI] [PubMed] [Google Scholar]
- 47.Ichise T, Kano M, Hashimoto K, Yanagihara D, Nakao K, Shigemoto R, et al. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288(5472):1832–5. doi: 10.1126/science.288.5472.1832. [DOI] [PubMed] [Google Scholar]
- 48.Brasnjo G, Otis T. Neuronal glutamate transporters control activation of postsynaptic metabotropic glutamate receptors and influence cerebellar long-term depression. Neuron. 2001;31(4):607–16. doi: 10.1016/s0896-6273(01)00377-4. [DOI] [PubMed] [Google Scholar]
- 49.Hansel C, Linden DJ, D’Angelo E. Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat Neurosci. 2001;4(5):467–75. doi: 10.1038/87419. [DOI] [PubMed] [Google Scholar]
- 50.Takayasu Y, Iino M, Kakegawa W, Maeno H, Watase K, Wada K, et al. Differential roles of glial and neuronal glutamate transporters in Purkinje cell synapses. J Neurosci. 2005;25(38):8788–93. doi: 10.1523/JNEUROSCI.1020-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wadiche JI, Jahr CE. Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nat Neurosci. 2005;8(10):1329–34. doi: 10.1038/nn1539. [DOI] [PubMed] [Google Scholar]
- 52.Wadiche JI, Tzingounis AV, Jahr CE. Intrinsic kinetics determine the time course of neuronal synaptic transporter currents. Proc Natl Acad Sci USA. 2006;103(4):1083–7. doi: 10.1073/pnas.0510476103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsai M-C, Tanaka K, Overstreet-Wadiche L, Wadiche JI. Neuronal glutamate transporters regulate glial excitatory transmission. J Neurosci. 2012;32(5):1528–35. doi: 10.1523/JNEUROSCI.5232-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watanabe D, Nakanishi S. mGluR2 postsynaptically senses granule cell inputs at Golgi cell synapses. Neuron. 2003;39(5):821–9. doi: 10.1016/s0896-6273(03)00530-0. [DOI] [PubMed] [Google Scholar]
- 55.Holtzman T, Sivam V, Zhao T, Frey O, van der Wal PD, de Rooij NF, et al. Multiple extra-synaptic spillover mechanisms regulate prolonged activity in cerebellar Golgi cell-granule cell loops. J Physiol. 2011;589(Pt 15):3837–54. doi: 10.1113/jphysiol.2011.207167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vervaeke K, Lorincz A, Gleeson P, Farinella M, Nusser Z, Silver RA. Rapid desynchronization of an electrically coupled interneuron network with sparse excitatory synaptic input. Neuron. 2010;67(3):435–51. doi: 10.1016/j.neuron.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carter A, Regehr W. Prolonged synaptic currents and glutamate spillover at the parallel fiber to stellate cell synapse. J Neurosci. 2000;20(12):4423–34. doi: 10.1523/JNEUROSCI.20-12-04423.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clark B, Cull-Candy S. Activity-dependent recruitment of extrasynaptic NMDA receptor activation at an AMPA receptor-only synapse. J Neurosci. 2002;22(11):4428–36. doi: 10.1523/JNEUROSCI.22-11-04428.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karakossian MH, Otis TS. Excitation of cerebellar interneurons by group I metabotropic glutamate receptors. J Neurophysiol. 2004;92(3):1558–65. doi: 10.1152/jn.00300.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Collin T, Franconville R, Ehrlich BE, Llano I. Activation of metabotropic glutamate receptors induces periodic burst firing and concomitant cytosolic Ca2+ oscillations in cerebellar interneurons. J Neurosci. 2009;29(29):9281–91. doi: 10.1523/JNEUROSCI.1865-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stell BM, Rostaing P, Triller A, Marty A. Activation of presynaptic GABA(A) receptors induces glutamate release from parallel fiber synapses. J Neurosci. 2007;27(34):9022–31. doi: 10.1523/JNEUROSCI.1954-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pugh JR, Jahr CE. Axonal GABAA Receptors Increase Cerebellar Granule Cell Excitability and Synaptic Activity. J Neurosci. 2011;31(2):565–74. doi: 10.1523/JNEUROSCI.4506-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dellal SS, Luo R, Otis TS. GABAA receptors increase excitability and conduction velocity of cerebellar parallel fiber axons. J Neurophysiol. 2012;107(11):2958–70. doi: 10.1152/jn.01028.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dittman J, Regehr W. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J Neurosci. 1996;16(5):1623–33. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dittman J, Regehr W. Mechanism and kinetics of heterosynaptic depression at a cerebellar synapse. J Neurosci. 1997;17(23):9048–59. doi: 10.1523/JNEUROSCI.17-23-09048.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marcaggi P, Attwell D. Endocannabinoid signaling depends on the spatial pattern of synapse activation. Nat Neurosci. 2005;8(6):776–81. doi: 10.1038/nn1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marcaggi P, Attwell D. Short- and long-term depression of rat cerebellar parallel fibre synaptic transmission mediated by synaptic crosstalk. J Physiol. 2007;578(Pt 2):545–50. doi: 10.1113/jphysiol.2006.115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wadiche JI, Jahr CE. Multivesicular release at climbing fiber-Purkinje cell synapses. Neuron. 2001;32(2):301–13. doi: 10.1016/s0896-6273(01)00488-3. [DOI] [PubMed] [Google Scholar]
- 69.Rudolph S, Overstreet-Wadiche L, Wadiche JI. Desynchronization of multivesicular release enhances Purkinje cell output. Neuron. 2011;70(5):991–1004. doi: 10.1016/j.neuron.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Armstrong D, Rawson J. Activity patterns of cerebellar cortical neurones and climbing fibre afferents in the awake cat. J Physiol (Lond) 1979;289:425–48. doi: 10.1113/jphysiol.1979.sp012745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schwarz C, Welsh J. Dynamic modulation of mossy fiber system throughput by inferior olive synchrony: a multielectrode study of cerebellar cortex activated by motor cortex. J Neurophysiol. 2001;86(5):2489–504. doi: 10.1152/jn.2001.86.5.2489. [DOI] [PubMed] [Google Scholar]
- 72.Otis T, Kavanaugh M, Jahr C. Postsynaptic glutamate transport at the climbing fiber-Purkinje cell synapse. Science. 1997;277(5331):1515–8. doi: 10.1126/science.277.5331.1515. [DOI] [PubMed] [Google Scholar]
- 73.Dehnes Y, Chaudhry F, Ullensvang K, Lehre K, Storm-Mathisen J, Danbolt N. The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate-gated chloride channel concentrated near the synapse in parts of the dendritic membrane facing astroglia. J Neurosci. 1998;18(10):3606–19. doi: 10.1523/JNEUROSCI.18-10-03606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dzubay J, Otis T. Climbing fiber activation of metabotropic glutamate receptors on cerebellar purkinje neurons. Neuron. 2002;36(6):1159–67. doi: 10.1016/s0896-6273(02)01052-8. [DOI] [PubMed] [Google Scholar]
- 75.Takayasu Y, Iino M, Shimamoto K, Tanaka K, Ozawa S. Glial glutamate transporters maintain one-to-one relationship at the climbing fiber-Purkinje cell synapse by preventing glutamate spillover. J Neurosci. 2006;26(24):6563–72. doi: 10.1523/JNEUROSCI.5342-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin SC, Huck JH, Roberts JD, Macklin WB, Somogyi P, Bergles DE. Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron. 2005;46(5):773–85. doi: 10.1016/j.neuron.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 77.Kollo M, Holderith NB, Nusser Z. Novel subcellular distribution pattern of A-type K+ channels on neuronal surface. J Neurosci. 2006;26(10):2684–91. doi: 10.1523/JNEUROSCI.5257-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szapiro G, Barbour B. Multiple climbing fibers signal to molecular layer interneurons exclusively via glutamate spillover. Nat Neurosci. 2007;10(6):735–42. doi: 10.1038/nn1907. [DOI] [PubMed] [Google Scholar]
- 79.Jörntell H, Ekerot C-F. Reciprocal bidirectional plasticity of parallel fiber receptive fields in cerebellar Purkinje cells and their afferent interneurons. Neuron. 2002;34(5):797–806. doi: 10.1016/s0896-6273(02)00713-4. [DOI] [PubMed] [Google Scholar]
- 80.Jörntell H, Ekerot C-F. Receptive field plasticity profoundly alters the cutaneous parallel fiber synaptic input to cerebellar interneurons in vivo. J Neurosci. 2003;23(29):9620–31. doi: 10.1523/JNEUROSCI.23-29-09620.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mathews PJ, Lee KH, Peng Z, Houser CR, Otis TS. Effects of climbing fiber driven inhibition on Purkinje neuron spiking. J Neurosci. 2012;32(50):17988–97. doi: 10.1523/JNEUROSCI.3916-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ozden I, Sullivan MR, Lee HM, Wang SS-H. Reliable coding emerges from coactivation of climbing fibers in microbands of cerebellar Purkinje neurons. J Neurosci. 2009;29(34):10463–73. doi: 10.1523/JNEUROSCI.0967-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schultz SR, Kitamura K, Post-Uiterweer A, Krupic J, Häusser M. Spatial pattern coding of sensory information by climbing fiber-evoked calcium signals in networks of neighboring cerebellar Purkinje cells. J Neurosci. 2009;29(25):8005–15. doi: 10.1523/JNEUROSCI.4919-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coddington LT, Rudolph S, Vande Lune P, Overstreet-Wadiche L, Wadiche JI. Spillover-mediated feedforward inhibition functionally segregates interneuron activity. Neuron. 2013;78(6):1050–62. doi: 10.1016/j.neuron.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jirenhed DA, Bengtsson F, Jorntell H. Parallel fiber and climbing fiber responses in rat cerebellar cortical neurons in vivo. Frontiers in systems neuroscience. 2013;7:16. doi: 10.3389/fnsys.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bloedel JR, Ebner TJ, Yu QX. Increased responsiveness of Purkinje cells associated with climbing fiber inputs to neighboring neurons. J Neurophysiol. 1983;50(1):220–39. doi: 10.1152/jn.1983.50.1.220. [DOI] [PubMed] [Google Scholar]
- 87.Bosman LW, Koekkoek SK, Shapiro J, Rijken BF, Zandstra F, van der Ende B, et al. Encoding of whisker input by cerebellar Purkinje cells. J Physiol. 2010;588(Pt 19):3757–83. doi: 10.1113/jphysiol.2010.195180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barmack NH, Yakhnitsa V. Cerebellar climbing fibers modulate simple spikes in Purkinje cells. J Neurosci. 2003;23(21):7904–16. doi: 10.1523/JNEUROSCI.23-21-07904.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barmack NH, Yakhnitsa V. Microlesions of the inferior olive reduce vestibular modulation of Purkinje cell complex and simple spikes in mouse cerebellum. J Neurosci. 2011;31(27):9824–35. doi: 10.1523/JNEUROSCI.1738-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Badura A, Schonewille M, Voges K, Galliano E, Renier N, Gao Z, et al. Climbing fiber input shapes reciprocity of Purkinje cell firing. Neuron. 2013;78(4):700–13. doi: 10.1016/j.neuron.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 91.Uusisaari M, De Schutter E. The mysterious microcircuitry of the cerebellar nuclei. J Physiol. 2011;589(Pt 14):3441–57. doi: 10.1113/jphysiol.2010.201582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thach WT. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J Neurophysiol. 1968;31(5):785–97. doi: 10.1152/jn.1968.31.5.785. [DOI] [PubMed] [Google Scholar]
- 93.Thach WT. Discharge of cerebellar neurons related to two maintained postures and two prompt movements. I. Nuclear cell output. J Neurophysiol. 1970;33(4):527–36. doi: 10.1152/jn.1970.33.4.527. [DOI] [PubMed] [Google Scholar]
- 94.Person AL, Raman IM. Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nature. 2011 doi: 10.1038/nature10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pugh JR, Raman IM. Potentiation of mossy fiber EPSCs in the cerebellar nuclei by NMDA receptor activation followed by postinhibitory rebound current. Neuron. 2006;51(1):113–23. doi: 10.1016/j.neuron.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 96.Person AL, Raman IM. Deactivation of L-type Ca current by inhibition controls LTP at excitatory synapses in the cerebellar nuclei. Neuron. 2010;66(4):550–9. doi: 10.1016/j.neuron.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Telgkamp P, Padgett DE, Ledoux VA, Woolley CS, Raman IM. Maintenance of high-frequency transmission at purkinje to cerebellar nuclear synapses by spillover from boutons with multiple release sites. Neuron. 2004;41(1):113–26. doi: 10.1016/s0896-6273(03)00802-x. [DOI] [PubMed] [Google Scholar]
- 98.McCormick DA, Thompson RF. Cerebellum: essential involvement in the classically conditioned eyelid response. Science. 1984;223(4633):296–9. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- 99.Medina JF, Nores WL, Ohyama T, Mauk MD. Mechanisms of cerebellar learning suggested by eyelid conditioning. Curr Opin Neurobiol. 2000;10(6):717–24. doi: 10.1016/s0959-4388(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 100.Pugh JR, Raman IM. Mechanisms of potentiation of mossy fiber EPSCs in the cerebellar nuclei by coincident synaptic excitation and inhibition. J Neurosci. 2008;28(42):10549–60. doi: 10.1523/JNEUROSCI.2061-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang W, Linden DJ. Long-term depression at the mossy fiber-deep cerebellar nucleus synapse. J Neurosci. 2006;26(26):6935–44. doi: 10.1523/JNEUROSCI.0784-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]