Abstract
Plasmacytoid dendritic cells (pDC) constitute the body’s principal source of type I interferon (IFN) and are comparatively abundant in the liver. Among various cytokines implicated in liver ischemia and reperfusion (I/R) injury, type I IFNs have been described recently as playing an essential role in its pathogenesis. Moreover, type I IFNs have been shown to up-regulate hepatocyte expression of IFN regulatory factor 1 (IRF-1), a key transcription factor that regulates apoptosis and induces liver damage after I/R. Our aim was to ascertain the capacity of IFN-α released by liver pDC to induce liver damage through hepatic IRF-1 up-regulation after I/R injury. Our findings show that liver pDC mature and produce IFN-α in response to liver I/R. Liver pDC isolated after I/R induced elevated levels of IRF-1 production by hepatocytes compared with liver pDC isolated from sham-operated mice. Notably, hepatic IRF-1 expression was reduced significantly by neutralizing IFN-α. In vivo, IFN-α neutralization protected the liver from I/R injury by reducing hepatocyte apoptosis. This was associated with impaired expression of IRF-1 and pro-apoptotic molecules such as Fas ligand, its receptor (Fas) and death receptor 5 which are regulated by IRF-1. Furthermore, pDC-depleted mice failed to up-regulate hepatic IFN-α and displayed less liver injury associated with reduced levels of hepatic IL-6, tumor necrosis factor-α and hepatocyte apoptosis after I/R compared with controls. Conclusion: these data support the hypothesis that IFN-α derived from liver pDC plays a key role in the pathogenesis of liver I/R injury by enhancing apoptosis as a consequence of induction of hepatocyte IRF-1 expression.
Keywords: Type I interferon, transplantation, ischemia, interferon regulatory factor-1, liver
Introduction
Hepatocellular injury caused by ischemia/reperfusion (I/R) is the result of a highly complex cascade of events that is triggered when the liver is exposed transiently to hypoxia then reperfused with oxygenated blood. I/R injury is unavoidable during transplant surgery, and adversely affects patient and graft outcomes.1, 2 In a recent large study of living- and deceased-donor liver transplant patients, extended cold ischemic time was associated with elevated rates of early organ failure and acute cellular rejection.3 Moreover, because of the greater susceptibility of extended criteria liver grafts to ischemic insult, I/R injury exacerbates the donor organ shortage problem. No specific treatment is available to prevent or reduce hepatic I/R injury and current management is based on supportive care. Thus, extensive efforts are warranted to better understand the mechanisms that underlie liver I/R injury.
Type I interferons (IFNs; IFN-α and IFN-β) are multifunctional cytokines, that activate or modulate immune responses.4 Type I IFNs bind to type 1 IFN receptors (IFNAR) and activate Jak1 and Tyk2 that, in turn, phosphorylate signal transducer and activator of transcription (STAT)1 and STAT2 and promote the formation of the IFN-stimulated gene factor (ISGF) 3 complex. The ISGF3 complex binds to nuclear IFN-stimulated response elements (ISREs) and induces transcription of IFN-stimulated genes (ISGs)4, 5 that encode factors responsible for antiviral, antiproliferative and immunoregulatory responses.6 Due to their critical role in innate immunity, type I IFNs are used as therapeutic agents in viral hepatitis, leukemia, renal cell carcinoma and multiple sclerosis.7 On the other hand, type I IFNs can play pathogenic roles in some autoimmune diseases, such as psoriasis and systemic lupus erythematosus (SLE).4
Neutralization of IFN-α is a potential therapeutic approach in type I IFN-related diseases and therapeutic effects of IFN-α neutralizing antibody (Ab) have been reported in mouse models of HIV encephalitis and septic shock.8, 9 Recently, the safety of an anti-IFN-α monoclonal antibody (mAb) (MEDI-545) has been confirmed in a chronic psoriasis phase I trial.10 Type I IFNs are regarded as critical factors in both warm and cold liver I/R injury. Thus, in mice, IFNAR KO livers are protected from damage,11, 12 suggesting that IFN-α neutralization might be a therapeutic option for inhibition of liver I/R injury.
The transcription factor IFN regulatory factor (IRF)-1 is induced by cytokines (type I IFNs, IFN-γ, tumor necrosis factor-α [TNF-α], IL-1), viruses and retinoic acid.13, 14 It regulates cell proliferation, apoptosis and immune responses. Overexpression of IRF-1 promotes apoptosis by up-regulation of Fas ligand (CD95L), while IRF-1-deficient hepatocytes are resistant to apoptosis.15, 16 IRF-1 is known to play a key role in liver I/R injury since high mobility group box 1 (HMGB-1) released from dying/dead cells up-regulates IRF-1 in hepatocytes through Toll-like receptor (TLR) 4 ligation and promotes liver injury.17 Furthermore, IRF-1-deficient livers exhibit less I/R injury correlated with the reduced apoptosis due to diminished expression of death ligands, including Fas ligand and death receptor (DR)5.18, 19
Although type I IFNs are produced by almost all cells, IFN-α is produced mainly by plasmacytoid dendritic cells (pDC) while IFN-β is produced by fibroblasts, synoviocytes and stromal cells.20 pDC are an unconventional subset of bone marrow-derived DCs and produce large amounts of IFN-α following TLR7 and TLR9 ligation.21, 22 Although rare cells, pDC are recognized as important players in protecting the host from viral infection by producing IFN-α. By contrast, pDC that sense self DNA through TLR9 and produce high amounts of IFN-α, drive autoimmunity and exacerbate SLE and psoriasis.23 In addition to IFN-α, pDC produce pro- (IL-6, IL-12 and TNF-α) and anti-inflammatory cytokines (IL-10) following TLR ligation.24 Thus, like conventional DCs, pDCs play key roles in innate and adaptive immunity in the liver.25 While conventional DC have been implicated in the regulation of liver I/R injury.26, 27 to date, no data are available concerning the role of pDC in hepatic I/R injury.
In this study, we hypothesized that IFN-α derived from pDC might be involved in the pathogenesis of liver I/R injury, and that IFN-α blockade might protect the liver from damage by inhibiting IRF-1 expression in hepatocytes and consequently reducing their apoptosis. Our data show that pDC are activated and produced IFN-α after liver I/R and that IFN-α promotes IRF-1 expression by hepatocytes in vitro. In vivo, IFN-α neutralization significantly reduces liver I/R-induced damage, with associated reduction in IRF-1, pro-inflammatory cytokine (IL-6 and TNF-α) and death receptor (Fas and DR5) expression in the liver. Moreover, depletion of pDC in vivo reduces hepatic IFN-α and protects the liver from I/R injury.
Materials and Methods
Experimental Animals
Male C57BL/6J (B6; H-2b) and IRF-1 KO (B6 background) mice, 8–12 weeks old, were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in the specific pathogen-free central animal facility of University of Pittsburgh School of Medicine. Experiments were conducted in accordance with the National Institutes of Health publication “Guide for the Care and Use of Laboratory Animals, under an Institutional Animal Care and Use Committee-approved protocol. Mice received Purina rodent chow (Ralston Purina, St. Louis, MO) and tap water ad libitum.
Liver Ischemia and Reperfusion Injury Model
A non-lethal model of segmental (70%) hepatic warm ischemia was used, as described.27 Mouse livers were exposed to 1 hour ischemia by clamping the portal triad. The animals were euthanized after 6 hours reperfusion.
IFN-α neutralization and pDC Depletion
For IFN-α neutralization experiments, anti-IFN-α mAb (clone F18; 50µg/mouse iv, Abcam Inc, Cambridge, MA) was injected 30 minutes before I/R injury. For in vivo pDC depletion, mice were injected for 3 consecutive days with anti-mPDCA-1 pure-functional grade mAb (500µg/mouse, ip; Miltenyi Biotec, Auburn, CA).
Isolation and Stimulation of Liver pDC
mPDCA1+pDC were isolated from livers of mice given the endogenous DC poietin fms-like tyrosine kinase 3 ligand (Flt3L; CHO cell derived recombinant human Flt3L; 10 µg/mouse/day i.p., for 10 days), as described.28 Thus, bulk liver DC were enriched by density centrifugation using Nycodenz (Sigma, St, Louis, MO). For pDC purification (consistently >95%), mPDCA1+ cells were positively selected from the DC-enriched fraction using immunomagnetic beads and a paramagnetic LS column (MiltenyiBiotec).
Flow Cytometry (Cell Surface Staining)
Liver pDC, liver non-parenchymal cells (NPC) and spleen cells were treated with FcγR-blocking rat anti-mouse CD16/32 mAb (2.4G2) to prevent non-specific Ab binding. They were then incubated for 30 minutes with FITC-, PE-, APC-, PE-Cy5-, or PE-Cy7-conjugated mAbs to detect expression of CD11c (HL3), B220/CD45R (RA3-6B2), Siglec-H (eBio440c), I-Abβ-chain (25-9-17) (eBioscience, San Diego, CA), CD40 (3/23), CD80 (16-10A1), CD86 (GL1) and B7-H1 (CD274) [MIH5] (BD Biosciences, San Diego, CA) and APC-mPDCA-1 (JF05-1C2.4.1; Miltenyi Biotec). All mAbs and appropriate Ig isotype controls were obtained from BD Pharmingen (San Diego, CA), unless specified. Flow analysis was performed using a LSR II flow cytometer (BD Bioscience) and results expressed as mean fluorescence intensity (MFI).
RT-PCR
Messenger RNA (mRNA) expression was quantified by SYBER Green real-time reverse transcription polymerase chain reaction (RT-PCR) with an ABI-Prism 7000 sequence detection system (PE Applied Biosystems, Foster City, CA) and primers specific for IFN-α (F: 5’-GCAACCCTCCTAGACTCATTCT-3’; R: 5’-CCAGCAGGGCGTCTTCCT-3’), TNF-α ( F: 5’-CATCTTCTCAAAATTCGAGTGA-3’; R; 5’-TGGGAGTAGACAAGGTACAAC-3’), IL-6 (F: 5’-TCAATTCCAGAAACCGCTATGA-3’; R: 5’-CACCAGCATCAGTCCCAAGA-3’), IRF-1 ( F: 5’-TTAGCCCGGACACTTTCTCTGATGG-3’; R: 5’-GTCCCCTCGAGGGCTGTCAATCTCT-3’), Fas (F: 5’-CTGCGATGAAGAGCATGGTTT-3’; R: 5’-CCATAGGCGATTTCTGGGAC-3’), FasL (F: 5’-TGAATTACCCATGTCCCCAG-3’; 5’-AAACTGACCCTGGAGGAGCC-3‘), DR5 (F: 5’-TGACGGGGAAGAGGAACTGA-3’; R: 5’-GGCTTTGACCATTTGGATTTGA-3’) or β-actin (F: 5’-AGAGGGAAATCGTGCGTGAC-3’; R: 5’-CAATAGTGATGACCTGGCCGT-3’). The expression of each gene was normalized to that of β-actin mRNA, as described.29
Hepatocyte Isolation and Culture
Hepatocytes (B6 mouse) were isolated by an in situ collagenase (type IV, Sigma) perfusion technique, as described.17 Hepatocyte purity exceeded 98%, and viability exceeded 95% as determined by standard testing. Liver pDC from sham or I/R injury mice were incubated for 18hr, without stimulation, to accumulate IFN-α in the supernatant. The pDC and their respective supernatants were then applied to hepatocyte cultures with/without anti-IFN-α mAb. Mouse IFN-α 4 (PBL Interferon Source, Piscataway, NJ) was used as a positive control. After 3 hr coculture, the hepatocytes were harvested for RT-PCR.
MTT assay
Hepatocytes (B6 WT or IRF1KO) were cultured with or without IFN-α (1000 U/ml) for 4 hr. Hepatocyte viability were determined by MTT assay (Roche Applied Science, Mannheim, Germany) following the manufacturer’s instructions.
ELISA
Levels of IFN-α in culture supernatants were determined using commercial ELISA kits from PBL Interferon Source following the manufacturer’s instructions.
Alanine Aminotransferase (ALT) Levels
Serum ALT levels were measured using the Opera Clinical Chemistry System (Bayer Co., Tarrytown, NY).
Routine and Immunohistopathology
Liver tissue samples were fixed in 10% formalin, embedded in paraffin, sectioned (6 µm), and stained with H&E and TUNEL (terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling) as described.19
Statistical analysis
Data are expressed as means ± 1 SD. Significances of differences between means were determined by the non-parametric Mann-Whitney U-test. A ‘p’ value < 0.05 was considered significant.
Results
IFN-α Production by Liver pDC is Up-Regulated During Liver I/R Injury
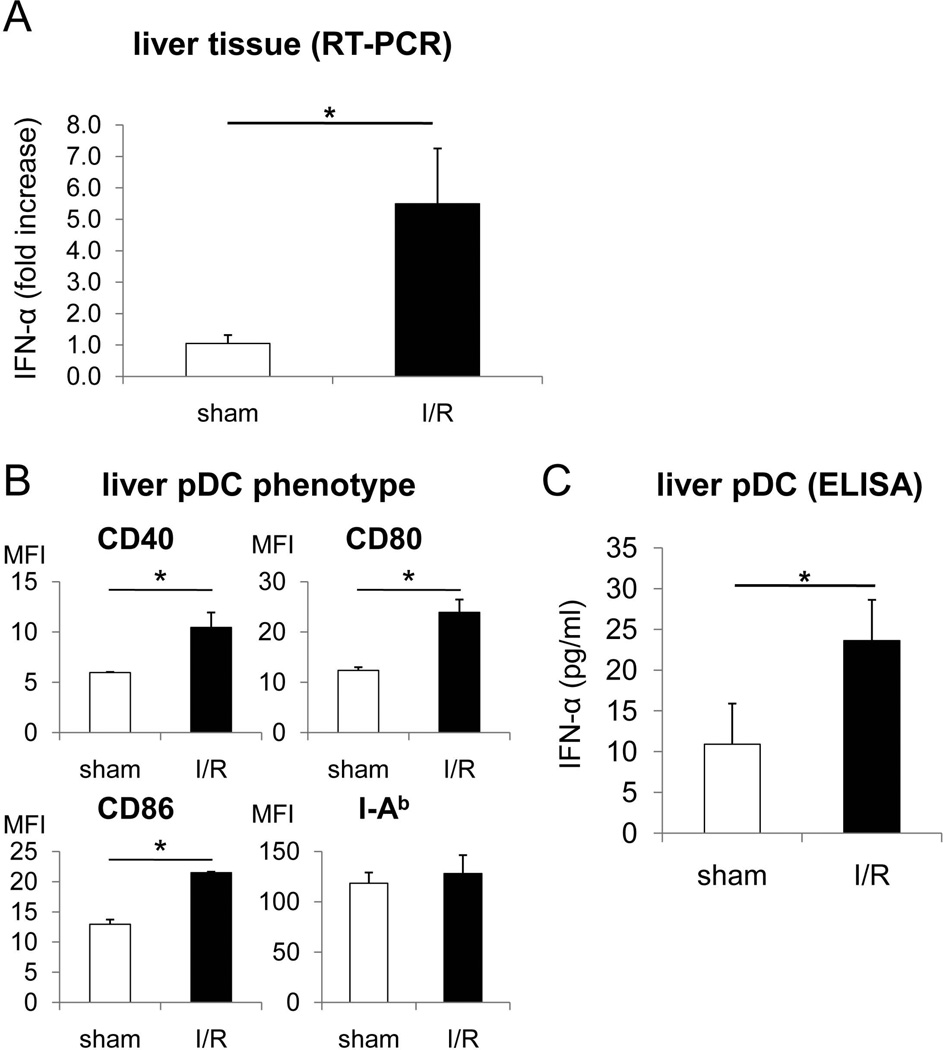
To verify the level of IFN-α expression in B6 mouse liver following warm I/R, real-time RT-PCR was performed on whole liver tissue. As shown in Fig. 1A, IFN-α gene transcription was markedly and significantly up-regulated (approximately 5-fold) after 6 hours I/R compared with sham-operated control mice. We also examined the phenotype and function of liver pDC freshly-isolated after liver I/R. As shown in Fig. 1B, liver pDC from I/R injury mice displayed significant increases in the intensity of expression (MFI) of cell surface co-stimulatory molecules (CD40, CD80, CD86) compared with pDC from sham-operated controls, whereas similar levels of major histocompatibility complex (MHC) class II (I-Ab) were expressed by both groups. Moreover, freshly-isolated liver pDC from I/R injury mice secreted enhanced quantities of IFN-α following 18 hours culture (Fig. 1C).
Fig. 1.
Hepatic I/R injury stimulates phenotypic maturation and IFN-α production by liver interstitial pDC. B6 mice underwent I/R injury as described in the Materials and Methods. After 6 hours reperfusion, liver pDC were isolated by immunomagnetic bead sorting and phenotypic and functional analyses performed. (A) Analysis of whole liver tissue for IFN-α message by real-time RT-PCR. (B) Cell surface phenotype of liver pDC (CD11cint B220+) from I/R injury and sham-operated control mice analyzed by mAb staining and flow cytometry. C) IFN-α production by freshly-isolated liver pDC isolated 6 hours after I/R injury measured by ELISA. (n=4 mice per group; * p<0.01).
IRF-1, that Promotes Hepatocyte Apoptosis, is Upregulated in Hepatocytes by IFN-α Secreted From pDC as the Result of I/R Injury
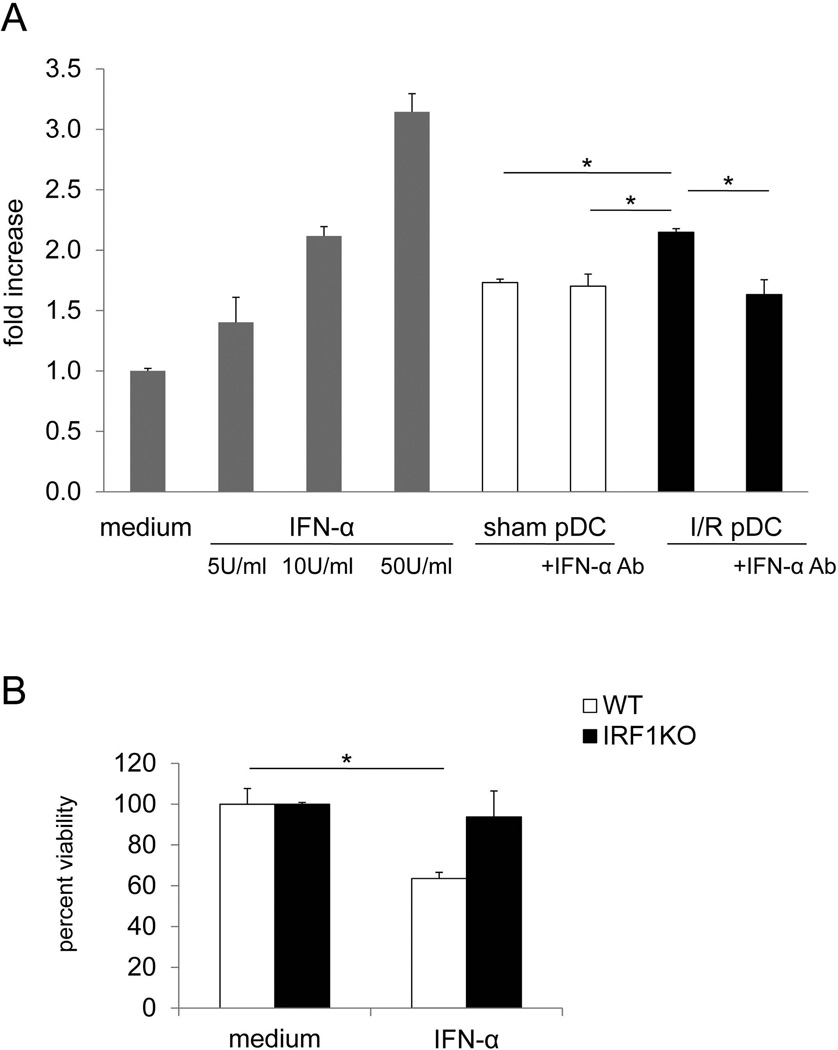
IRF-1 is induced in hepatocytes by stimulation with pro-inflammatory cytokines, such as type I IFNs, IFN-γ, TNF-α and IL-1.13, 14 Therefore we next examined whether IFN-α released by liver pDC after liver I/R injury might upregulate IRF-1 expression by cultured hepatocytes in vitro.30 23 22 Liver pDC were isolated from sham operated or I/R-injured mice and cultured overnight without stimulation. Liver pDC and their accompanying supernatants were transferred to mouse hepatocyte cultures for 3 hours, with or without addition of neutralizing anti-IFN-α mAb at the start of co-cultures. IRF-1 production by hepatocytes was then measured by RT-PCR. As shown in Fig. 2A, liver pDC isolated from sham-operated mice and to a significantly greater extent from I/R-injured mice, up-regulated IRF-1 production by mouse hepatocytes. Notably, neutralization of IFN-α significantly reduced the ability of liver pDC of I/R-injured mice to induce IRF-1 production by hepatocytes. To confirm the involvement of IRF-1 in IFN-α-induced hepatocyte apoptosis, the viability of hepatocytes from WT or IRF-1 KO mice in the presence of IFN-α was examined. Whereas the viability of WT hepatocytes was significantly decreased after 4hr incubation with IFN-α, that of IRF-1 KO hepatocytes was not affected (Figure 2B).
Fig. 2.
During I/R injury, activated liver pDC promote IRF-1 production by hepatocytes. (A) Liver pDCs, isolated from sham-operated or I/R injury B6 mice were incubated for 18 hours to accumulate IFN-α in the culture supernatant. Thereafter, pDC and their accompanying supernatants were transferred to normal mouse hepatocytes and cultured for 3 hours, with or without neutralizing anti-IFN-α mAb (5µg/ml) added at the start of culture. IRF-1 production was assessed by RT-PCR. Mouse hepatocytes cultured with or without IFN-α were used as positive and negative controls, respectively. Overall analysis of n=3 independent experiments. *, p<0.01. (B) Hepatocytes from WT or IRF-1 KO were cultured with or without IFN-α (1000 U/ml) for 4 hr. Hepatocyte viability was determined by MTT assay. *, p<0.05
In Vivo Blocking of IFN-α Reduces Liver I/R Injury, Associated With Reduced In Situ IL-6 and TNF-α Expression
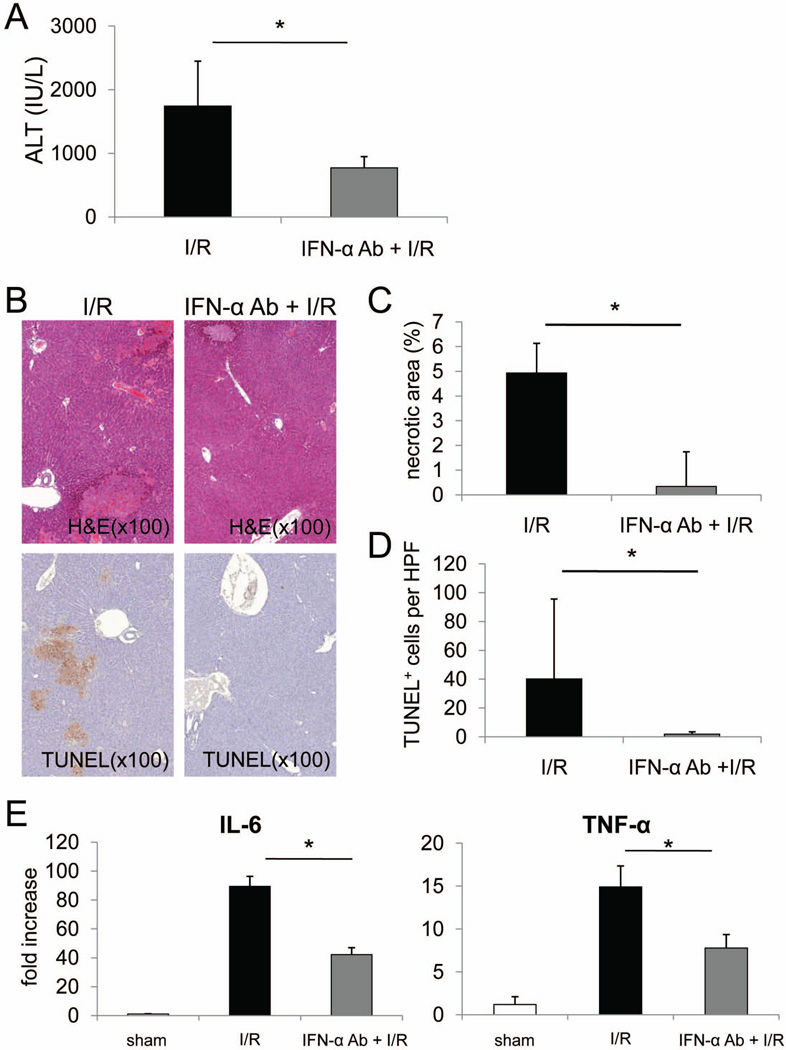
As shown in Fig. 1, IFN-α is upregulated during mouse liver I/R and is thought to be involved early in the pathogenesis of tissue injury.11 To further evaluate the role of IFN-α in liver I/R, we injected mice with neutralizing anti-IFN-α mAb or isotype control Ig immediately before liver I/R injury. As is evident in Fig. 3A, serum ALT levels after 6 hours reperfusion were significantly lower (approx 50%) in the mAb-treated mice compared with the control group (Fig. 3A). Consistent with these reductions in ALT levels, the extent of tissue necrosis and the incidence of TUNEL+ (apoptotic) cells (hepatocytes) in the anti-IFN-α-treated group were much lower than in the untreated I/R injury group (Fig. 3B–D). At the same time, gene transcripts for pro-inflammatory cytokines (IL-6 and TNF-α) in liver tissue were reduced significantly in the IFN neutralizing mAb-injected group (Fig. 3E).
Fig. 3.
In vivo neutralization of IFN-α reduces liver I/R injury, associated with reduced IL-6 and TNF-α gene expression. B6 mice were injected iv with neutralizing IFN-α mAb (50 µg/mouse; iv) or isotype control Ig, 30 minutes before being submitted to I/R injury. After 6 hours reperfusion, serum and livers were harvested. (A) Serum ALT levels; overall analysis of 4 mice per group; *p<0.05. (B) Representative H&E- and TUNEL-stained sections of liver tissue. (C) Analysis of the extent of necrosis. (D) Incidence of apoptotic (TUNEL+) cells per high power field. (E) mRNA levels of IL-6 and TNF-α in whole liver tissue. C–E, overall analysis of n=4 mice per group. *p<0.01.
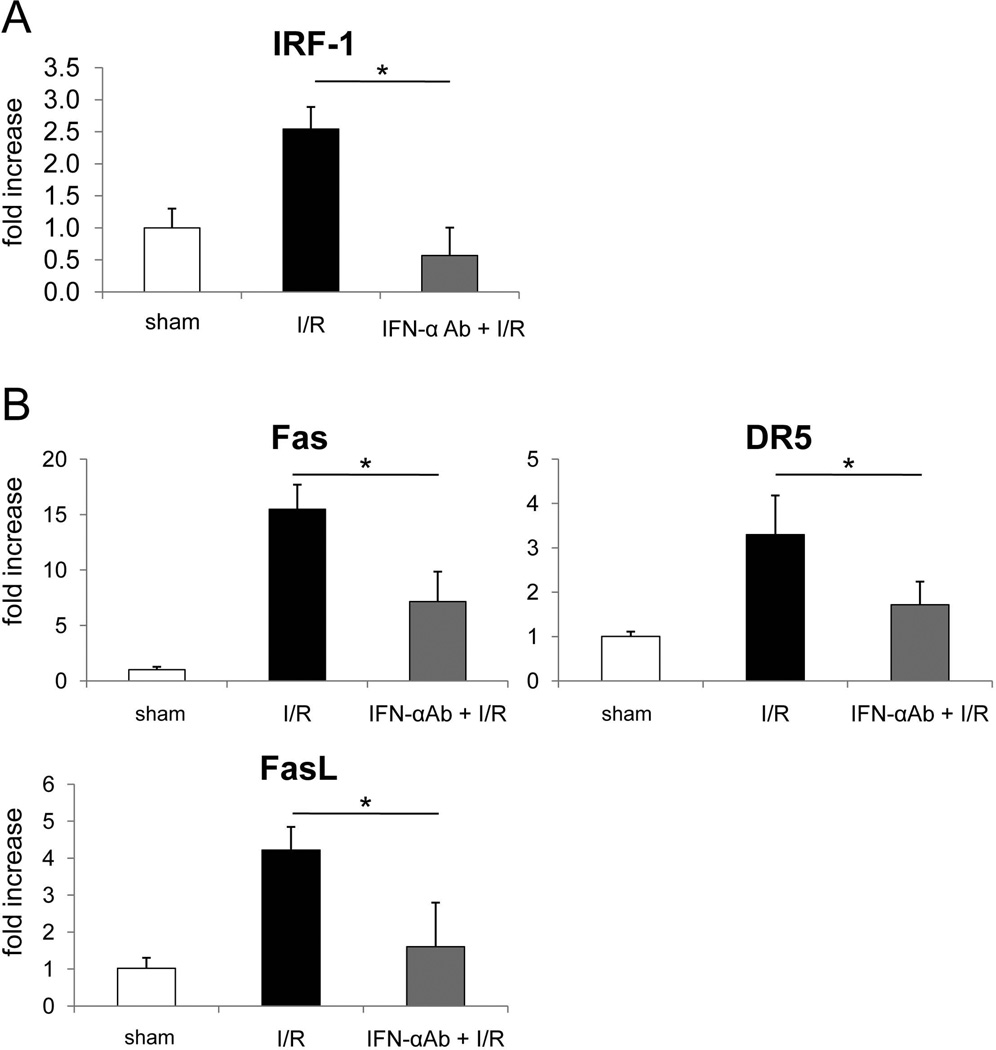
In Vivo Blocking of IFN-α Down-Regulates IRF-1 Gene Transcription During Liver I/R Injury
IFN-α is a potent inducer of IRF-113 that promotes cell apoptosis in a Fas- related manner.15 To ascertain whether the protective effect of IFN-α neutralization in vivo that was associated with reduced hepatocyte apoptosis was due to down-regulation of IRF-1 expression in liver tissue, we performed real-time RT-PCR for IRF-1, death ligand (FasL) and the death receptors Fas and DR5. As shown in Fig. 4A, IRF-1 gene transcription levels in livers of anti-IFN-α-mAb-treated mice were much lower (approx 80%) than in isotype-treated control mice. Consistent with the IRF-1 mRNA levels, Fas, DR5 and FasL gene transcription that is regulated by IRF-1 and determines the extent of apoptosis, was also reduced significantly in anti-IFN-α-mAb-treated mouse livers (Fig. 4B). These data suggest that the protective effect of IFN-α neutralization in liver I/R injury is due to reduced apoptosis as the result of less IFN-α-mediated induction of IRF-1 and consequently less induction of both death receptors and death ligands that are regulated by IRF-1.
Fig. 4.
The protective effect of IFN-α neutralization is associated with down-regulation of IRF-1 gene transcription and death molecule expression. B6 (n=4 per group) mice were injected iv with IFN-α neutralizing mAb (50 µg/mouse; iv) or isotype control Ig, 30 minutes before being submitted to I/R injury. After 6 hours reperfusion, liver tissue was harvested. (A) IRF-1 and (B) death ligands (FasL) and death receptor (Fas and DR5) mRNA expression in liver tissue were evaluated by real-time RT-PCR. *p<0.01.
In Vivo pDC Depletion Reduces IFN-α Gene Transcription and Protects Livers From I/R Injury
pDC are regarded as the main source of IFN-α in the body.20 To further investigate their role in IFN-α production and liver I/R injury, we depleted pDC in vivo before surgery using anti-PDCA1 mAb. Mice were injected i.p. for three consecutive days with anti-mPDCA-1 pure-functional grade Ab (500µg), and 24 hours after the last injection underwent I/R. Fig. 5A shows the profound depletion of liver pDC (Siglec-H+B220+) achieved by mAb administration. IFN-α induction in injured livers was markedly inhibited (~70% reduction) by pDC depletion (Fig. 5B). As shown in Fig. 5C–F, the livers of pDC-depleted mice were protected from I/R injury, as evidenced by reductions in serum ALT (C) and tissue damage (areas of necrosis and numbers of TUNEL+ cells; D–F). Taken together, these data suggest that liver pDC are an important source of IFN-α during liver I/R injury and play a key role in the pathogenesis of I/R injury.
Fig. 5.
In vivo pDC depletion protects the liver from I/R injury. B6 mice were injected for 3 consecutive days with anti-mPDCA-1 pure-functional grade mAb (500µg, i.p.) or isotype control Ig. (A) To assess the depleting efficiency of the Ab, freshly-isolated liver non-parenchymal cells were stained for CD11c, B220, Siglec-H and mPDCA-1 and analyzed by flow cytometry. CD11cintB220+Siglec-H+mPDCA-1+ cells (pDC) were gated and their frequency calculated. Data are representative of 3 mice per group. (B–F) Twenty-four hours after the final mAb injection, the vasculature supplying the left and median lobes of the liver was occluded for 60 minutes. The liver was then reperfused for 6 hours. (B) IFN-α mRNA expression was measured by real-time RT-PCR. (C) Serum ALT levels (n=5; *p<0.05). (D) H&E and TUNEL staining were performed to assess the extent of liver damage and parenchymal cell apoptosis. Representative histopathological images of ischemic lobes are shown. (E) Extent of necrotic area and (F) TUNEL-positive cell number per high power field (HPF) were analyzed in liver tissue. depl = depletion.
Discussion
Type I IFNs (IFN-α/β) are pleiotropic cytokines produced by DC, macrophages, other immune cells, fibroblasts and virally-infected cells, that contribute both to viral clearance and disease pathogenesis. They have been shown recently to play a pathogenic role in warm and cold liver I/R injury. Thus, in mice, IFNAR KO livers are protected from such injury.11, 12 Specific cytokine (e.g. TNF-α and IL-6) neutralization has already proved an effective approach for disease therapy in the clinic31, 32 and an IFN-α neutralizing Ab (MEDI-5) is already in phase I clinical trials in psoriasis that have confirmed its safety.10 Here, we show marked reduction in murine liver warm I/R injury either by selective depletion of pDC or IFN-α neutralization and a possible underlying protective mechanism of impaired production of IRF-1, a key downstream molecule of IFN-α signaling13 in the liver.
IRF-1 is a transcription factor induced by viruses, double-stranded RNA, retinoic acid and cytokines, including IFN-α. It regulates cell proliferation, apoptosis and immune responses.33 We show in this study that IFN-α neutralization reduces IRF-1 induction by hepatocytes in vitro and in the liver after I/R injury. IRF-1 is known to induce apoptosis by increasing expression of FasL.15, 19 We also found that IFN-α neutralization reduced hepatocyte apoptosis after liver I/R injury, consistent with lower expression of death ligand (FasL) and death receptors (Fas and DR5) within the liver. These results suggest that IFN-α neutralization attenuates the induction of IRF-1 in hepatocytes and consequently Fas-FasL and DR5 expression, resulting in reduced apoptosis following liver I/R injury.
Recently, other IRF members have been shown to contribute to the pathogenesis of liver warm I/R injury. IRF-2 is structurally similar to IRF-1 and competes with IRF-1 as an antagonist. Klune et al have reported that overexpression of IRF-2 protects the liver from I/R injury by an IRF-1-dependent mechanism.34 IRF-3 is another transcription factor that is involved in type-I IFN and other pro-inflammatory cytokine production after TLR stimulation. Interestingly, IRF-3-deficient mice exhibit severe warm liver injury due to greater IL-17 induction and enhanced neutrophil infiltration.35
Type I IFNs have immunostimulatory capacity and can activate immune cells, including T cells, natural killer (NK) cells and DCs.36 IFN-α-primed DCs produce enhanced levels of pro-inflammatory cytokines, such as IL-1β, IL-6, IL-12, IFN-γ and TNF-α, and promote T helper type-1 cell responses.37 In this study, we have shown that IFN-α neutralization decreases key pro-inflammatory cytokines,- IL-6 and TNF-α, after liver I/R injury. This finding is consistent with recent observations using IFNAR KO mice.11, 12 Although we did not identify the sources of these pro-inflammatory cytokines, our data suggest that IFN-α is involved in promotion of pro-inflammatory cytokine production during liver I/R injury.
pDC are comparatively abundant in the liver compared with secondary lymphoid tissue38 and regarded as the main source of IFN-α.20, 39 These non-conventional DCs predominantly express TLR7/TLR9 and produce high amounts of IFN-α in response to their ligation. Although these TLR receptors recognize single-stranded RNA and double-stranded DNA as pathogen-associated molecular patterns during viral infection,40 TLR7/TLR8/TLR9 on pDC can sense self-RNA (TLR7/8)41 and self-DNA (TLR9)23 as damage-associated molecular patterns and produce IFN-α. In this study, we found that hepatic warm I/R injury rapidly up-regulated IFN-α gene transcription in the liver after 6 hours reperfusion. This finding is consistent with an earlier report that type I, but not type II IFN receptor KO mice are protected from warm liver I/R damage.11 Moreover, very recently, using a clinically-relevant mouse model of extended hepatic cold preservation followed by orthotropic liver transplantation (OLT), Shen et al12 have shown that liver graft but not recipient type I IFN receptor KO deficiency is required to ameliorate I/R injury in OLT.
Notably, in the present study, mice profoundly depleted of pDC exhibited less tissue damage after liver I/R compared with control mice. The decreased liver injury in pDC-depleted mice was associated with a significant reduction in IFN-α (Fig. 5B) in hepatic tissue. Interestingly, after 6 hours reperfusion, the extent of hepatic IFN-α message in pDC-depleted animals was >70% lower than in controls, suggesting that pDC are the main source of type I-IFNs in the liver. We did not compare IFN-α production by liver pDC and Kupffer cells that are much more abundant in the liver than pDC and play a role in regulation of hepatic IRI.42, 43 However, since pDC depletion resulted in such profound reduction in hepatic IFN-α expression, it may be concluded that liver macrophages are not a major source of IFN-α. Since IFN-β, another type I IFN, may be produced by macrophages during liver I/R injury,11 and shares downstream STAT1 and IRF-1 signaling pathways with IFN-α, it would be of interest in future studies, to address the in vivo function of both IFNs in this model.
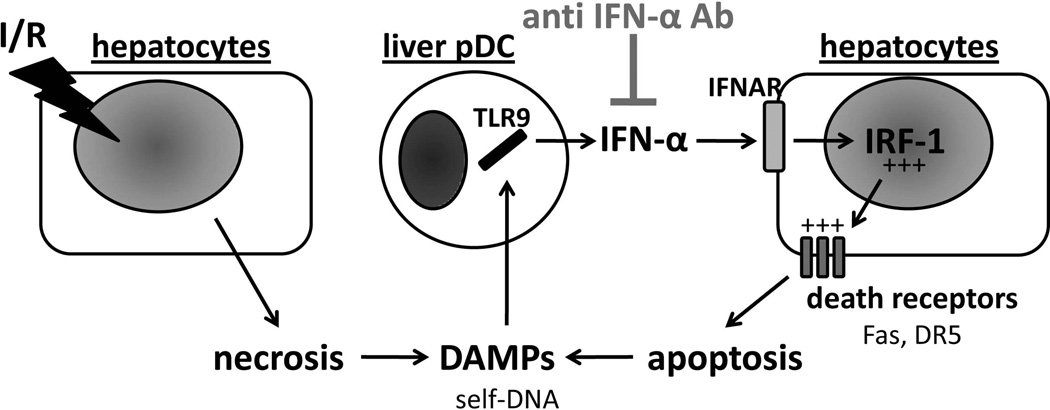
Here we also show that, following reperfusion, liver pDC acquire a more mature phenotype and secrete enhanced amounts of IFN-α that are associated with higher levels of IFN-α expression in the liver. Liver pDC isolated after warm I/R (activated pDC) secreted IFN-α that contributed to enhanced IRF-1 production by hepatocytes. Taken together, these observations suggest that during I/R, liver pDC may be activated by self-DNA to produce IFN-α that plays a key role in the pathogenesis of I/R injury. A schematic outline of the proposed mechanisms suggested by our findings is shown in Fig. 6.
Fig. 6.
Schematic representation of the proposed role of pDC and type I IFN in hepatic I/R. Vascular occlusion involving 70% of the liver for 1 hour followed by 6 hours reperfusion induces hepatocyte necrosis and release of damage-associated molecular patterns (DAMPs), such as the TLR9 ligand self-DNA. Self-DNA engages TLR9 in hepatic pDC that, in turn, are activated and release comparatively large amounts of type I IFN (IFN-α). Thereafter, IFN-α engages its receptor (IFNAR) on hepatocytes and induces transcription of IRF-1, a key pro-inflammatory factor involved in the pathogenesis of I/R injury, able to perpetuate liver damage and release new DAMPs. Blocking of type I IFN using neutralizing anti-IFN-α mAb down-regulates hepatocyte IRF-1 with a concomitant reduction in hepatocyte apoptosis (decreased levels of Fas and DR5) and pro-inflammatory cytokines, resulting in reduced liver damage. +++, elevated expression.
Conventional myeloid DC that are important regulators of innate and adaptive immunity, have been implicated previously in the regulation of inflammation and tissue injury after liver I/R,26, 27, 37, 44 with both inhibitory and enhancing effects reported. Our recent observations27 suggest that the local liver microenvironment plays an important role in determining DC function during I/R injury. However, no previous reports have focused on the role of pDC in hepatic I/R injury. pDC are believed to exhibit immunoregulatory properties due to their poor immunostimulatory function, Treg inducing ability and IL-10 production45, 46 Depletion of these cells in vivo exacerbates viral infection,47 asthma48 and transplant rejection.49 On the other hand, the present study shows that pDC depletion can protect the liver from warm I/R injury and reveals an underlying pathological role of these cells. Similarly to our results, pDC have been shown to play a pathological role in experimental allergic encephalomyelitis (EAE) in mice, with pDC depletion reducing disease activity.50 Type I IFNs derived from pDC are important for inhibition of viral replication and clearance,21 but type I IFNs are also pathogenic in the early phase of EAE50 and in our liver warm I/R injury model. Overall, these observations suggest that the specific role of type I IFNs in each experimental model may determine the role of pDC (protective or pathogenic).
Taken together, our novel findings demonstrate that IFNα secreted by activated pDC is involved in the promotion of hepatic I/R injury and in modulating the expression of pro-inflammatory cytokines such as IL-6 and TNF-α and most importantly, IRF-1 which regulates hepatocyte apoptosis. The data provide significant new insight into mechanisms that promote liver I/R injury via a pDC-IFN-α-IRF-1 pathway and support the therapeutic potential of IFN-α neutralization for amelioration of liver I/R injury.
Acknowledgment
We thank Ms. Miriam Freeman for excellent administrative support.
Financial support: This work was supported by National Institutes of Health (NIH) Grant P01 AI81678 and by The Roche Organ Transplantation Research Foundation (grant # 874279717). AC was supported by a Basic Science Fellowship from The American Society of Transplantation, an American Liver Foundation Sunflowers for Holli Fellowship, a Starzl Transplantation Institute Young Investigator Grant and a travel grant (63500/DS) from the Policlinico di Bari, Bari, Italy. OY was supported by NIH T32 AI74490 and by a non-concurrent Basic Science Fellowship from the American Society of Transplantation.
Abbreviations
- DR5
death receptor 5
- IFN
interferon
- IFNAR
type 1 interferon receptor
- I/R
ischemia/reperfusion
- ALT
alanine aminotransferase
- NPC
non-parenchymal cell(s)
- DC
dendritic cell(s)
- pDC
plasmacytoid dendritic cell(s)
References
- 1.Huang Y, Rabb H, Womer KL. Ischemia-reperfusion and immediate T cell responses. Cell Immunol. 2007;248:4–11. doi: 10.1016/j.cellimm.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641–1654. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaked A, Ghobrial RM, Merion RM, Shearon TH, Emond JC, Fair JH, Fisher RA, et al. Incidence and severity of acute cellular rejection in recipients undergoing adult living donor or deceased donor liver transplantation. Am J Transplant. 2009;9:301–308. doi: 10.1111/j.1600-6143.2008.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I interferons. Nat Rev Immunol. 2012;12:125–135. doi: 10.1038/nri3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diamond MS, Farzan M. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat Rev Immunol. 2013;13:46–57. doi: 10.1038/nri3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piehler J, Thomas C, Garcia KC, Schreiber G. Structural and dynamic determinants of type I interferon receptor assembly and their functional interpretation. Immunol Rev. 2012;250:317–334. doi: 10.1111/imr.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George PM, Badiger R, Alazawi W, Foster GR, Mitchell JA. Pharmacology and therapeutic potential of interferons. Pharmacol Ther. 2012;135:44–53. doi: 10.1016/j.pharmthera.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Sas AR, Bimonte-Nelson H, Smothers CT, Woodward J, Tyor WR. Interferon-alpha causes neuronal dysfunction in encephalitis. J Neurosci. 2009;29:3948–3955. doi: 10.1523/JNEUROSCI.5595-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshi VD, Kalvakolanu DV, Chen W, Zhang L, Kang TJ, Thomas KE, Vogel SN, et al. A role for Stat1 in the regulation of lipopolysaccharide-induced interleukin-1beta expression. J Interferon Cytokine Res. 2006;26:739–747. doi: 10.1089/jir.2006.26.739. [DOI] [PubMed] [Google Scholar]
- 10.Bissonnette R, Papp K, Maari C, Yao Y, Robbie G, White WI, Le C, et al. A randomized, double-blind, placebo-controlled, phase I study of MEDI-545, an anti-interferon-alfa monoclonal antibody, in subjects with chronic psoriasis. J Am Acad Dermatol. 2010;62:427–436. doi: 10.1016/j.jaad.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 11.Zhai Y, Qiao B, Gao F, Shen X, Vardanian A, Busuttil RW, Kupiec-Weglinski JW. Type I, but not type II, interferon is critical in liver injury induced after ischemia and reperfusion. Hepatology. 2008;47:199–206. doi: 10.1002/hep.21970. [DOI] [PubMed] [Google Scholar]
- 12.Shen XD, Ke B, Ji H, Gao F, Freitas MC, Chang WW, Lee C, et al. Disruption of Type-I IFN pathway ameliorates preservation damage in mouse orthotopic liver transplantation via HO-1 dependent mechanism. Am J Transplant. 2012;12:1730–1739. doi: 10.1111/j.1600-6143.2012.04021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroger A, Koster M, Schroeder K, Hauser H, Mueller PP. Activities of IRF-1. J Interferon Cytokine Res. 2002;22:5–14. doi: 10.1089/107999002753452610. [DOI] [PubMed] [Google Scholar]
- 14.Geller DA, Nguyen D, Shapiro RA, Nussler A, Di Silvio M, Freeswick P, Wang SC, et al. Cytokine induction of interferon regulatory factor-1 in hepatocytes. Surgery. 1993;114:235–242. [PubMed] [Google Scholar]
- 15.Chow WA, Fang JJ, Yee JK. The IFN regulatory factor family participates in regulation of Fas ligand gene expression in T cells. J Immunol. 2000;164:3512–3518. doi: 10.4049/jimmunol.164.7.3512. [DOI] [PubMed] [Google Scholar]
- 16.Kano A, Haruyama T, Akaike T, Watanabe Y. IRF-1 is an essential mediator in IFN-gamma-induced cell cycle arrest and apoptosis of primary cultured hepatocytes. Biochem Biophys Res Commun. 1999;257:672–677. doi: 10.1006/bbrc.1999.0276. [DOI] [PubMed] [Google Scholar]
- 17.Dhupar R, Klune JR, Evankovich J, Cardinal J, Zhang M, Ross M, Murase N, et al. Interferon regulatory factor 1 mediates acetylation and release of high mobility group box 1 from hepatocytes during murine liver ischemia-reperfusion injury. Shock. 2011;35:293–301. doi: 10.1097/SHK.0b013e3181f6aab0. [DOI] [PubMed] [Google Scholar]
- 18.Tsung A, Stang MT, Ikeda A, Critchlow ND, Izuishi K, Nakao A, Chan MH, et al. The transcription factor interferon regulatory factor-1 mediates liver damage during ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1261–G1268. doi: 10.1152/ajpgi.00460.2005. [DOI] [PubMed] [Google Scholar]
- 19.Ueki S, Dhupar R, Cardinal J, Tsung A, Yoshida J, Ozaki KS, Klune JR, et al. Critical role of interferon regulatory factor-1 in murine liver transplant ischemia reperfusion injury. Hepatology. 2010;51:1692–1701. doi: 10.1002/hep.23501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crow MK. Type I interferon in organ-targeted autoimmune and inflammatory diseases. Arthritis Res Ther. 2010;12(Suppl 1):S5. doi: 10.1186/ar2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234:142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y-J. IPC: Professional Type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 23.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, Cao W, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 24.Castellaneta A, Sumpter TL, Chen L, Tokita D, Thomson AW. NOD2 ligation subverts IFN-alpha production by liver plasmacytoid dendritic cells and inhibits their T cell allostimulatory activity via B7-H1 up-regulation. J Immunol. 2009;183:6922–6932. doi: 10.4049/jimmunol.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753–766. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 26.Bamboat ZM, Ocuin LM, Balachandran VP, Obaid H, Plitas G, Dematteo RP. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. J Clin Invest. 2010;120:559–569. doi: 10.1172/JCI40008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M, Ueki S, Kimura S, Yoshida O, Castellaneta A, Ozaki KS, Demetris AJ, et al. Roles of dendritic cells in murine hepatic warm and liver transplantation-induced cold ischemia/reperfusion injury. Hepatology. 2013;57:1585–1596. doi: 10.1002/hep.26129. [DOI] [PubMed] [Google Scholar]
- 28.Tokita D, Sumpter TL, Raimondi G, Zahorchak AF, Wang Z, Nakao A, Mazariegos GV, et al. Poor allostimulatory function of liver plasmacytoid DC is associated with pro-apoptotic activity, dependent on regulatory T cells. J Hepatol. 2008;49:1008–1018. doi: 10.1016/j.jhep.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueki S, Castellaneta A, Yoshida O, Ozaki K, Zhang M, Kimura S, Isse K, et al. Hepatic B7 homolog 1 expression is essential for controlling cold ischemia/reperfusion injury after mouse liver transplantation. Hepatology. 2011;54:216–228. doi: 10.1002/hep.24360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 31.Olsen NJ, Stein CM. New drugs for rheumatoid arthritis. N Engl J Med. 2004;350:2167–2179. doi: 10.1056/NEJMra032906. [DOI] [PubMed] [Google Scholar]
- 32.Melton L, Coombs A. Actemra poised to launch IL-6 inhibitors. Nat Biotechnol. 2008;26:957–959. doi: 10.1038/nbt0908-957. [DOI] [PubMed] [Google Scholar]
- 33.Romeo G, Fiorucci G, Chiantore MV, Percario ZA, Vannucchi S, Affabris E. IRF-1 as a negative regulator of cell proliferation. J Interferon Cytokine Res. 2002;22:39–47. doi: 10.1089/107999002753452647. [DOI] [PubMed] [Google Scholar]
- 34.Klune JR, Dhupar R, Kimura S, Ueki S, Cardinal J, Nakao A, Nace G, et al. Interferon regulatory factor-2 is protective against hepatic ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2012;303:G666–G673. doi: 10.1152/ajpgi.00050.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loi P, Yuan Q, Torres D, Delbauve S, Laute MA, Lalmand MC, Petein M, et al. Interferon regulatory factor 3 deficiency leads to interleukin-17-mediated liver ischemia-reperfusion injury. Hepatology. 2013;57:351–361. doi: 10.1002/hep.26022. [DOI] [PubMed] [Google Scholar]
- 36.Ludigs K, Parfenov V, Du Pasquier RA, Guarda G. Type I IFN-mediated regulation of IL-1 production in inflammatory disorders. Cell Mol Life Sci. 2012;69:3395–3418. doi: 10.1007/s00018-012-0989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farkas A, Kemeny L. Monocyte-derived interferon-alpha primed dendritic cells in the pathogenesis of psoriasis: new pieces in the puzzle. Int Immunopharmacol. 2012;13:215–218. doi: 10.1016/j.intimp.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172:1009–1017. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 39.Matta BM, Raimondi G, Rosborough BR, Sumpter TL, Thomson AW. IL-27 production and STAT3-dependent upregulation of B7-H1 mediate immune regulatory functions of liver plasmacytoid dendritic cells. J Immunol. 2012;188:5227–5237. doi: 10.4049/jimmunol.1103382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 41.Ganguly D, Chamilos G, Lande R, Gregorio J, Meller S, Facchinetti V, Homey B, et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med. 2009;206:1983–1994. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18:891–902. doi: 10.1046/j.1440-1746.2003.03056.x. [DOI] [PubMed] [Google Scholar]
- 43.Devey L, Ferenbach D, Mohr E, Sangster K, Bellamy CO, Hughes J, Wigmore SJ. Tissue-resident macrophages protect the liver from ischemia reperfusion injury via a heme oxygenase-1-dependent mechanism. Mol Ther. 2009;17:65–72. doi: 10.1038/mt.2008.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsung A, Zheng N, Jeyabalan G, Izuishi K, Klune JR, Geller DA, Lotze MT, et al. Increasing numbers of hepatic dendritic cells promote HMGB1-mediated ischemia-reperfusion injury. J Leukoc Biol. 2007;81:119–128. doi: 10.1189/jlb.0706468. [DOI] [PubMed] [Google Scholar]
- 45.Matta BM, Castellaneta A, Thomson AW. Tolerogenic plasmacytoid DC. Eur J Immunol. 2010;40:2667–2676. doi: 10.1002/eji.201040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogers NM, Isenberg JS, Thomson AW. Plasmacytoid dendritic cells: no longer an enigma and now key to transplant tolerance? Am J Transplant. 2013;13:1125–1133. doi: 10.1111/ajt.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cervantes-Barragan L, Zust R, Weber F, Spiegel M, Lang KS, Akira S, Thiel V, et al. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood. 2007;109:1131–1137. doi: 10.1182/blood-2006-05-023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 50.Isaksson M, Ardesjo B, Ronnblom L, Kampe O, Lassmann H, Eloranta ML, Lobell A. Plasmacytoid DC promote priming of autoimmune Th17 cells and EAE. Eur J Immunol. 2009;39:2925–2935. doi: 10.1002/eji.200839179. [DOI] [PubMed] [Google Scholar]