Summary
Objectives
The rapid emergence, spread, and disease severity of avian influenza A(H7N9) in China has prompted concerns about a possible pandemic and regional spread in the coming months. The objective of this study was to predict the risk of future human infections with H7N9 in China and neighboring countries by assessing the association between H7N9 cases at sentinel hospitals and putative agricultural, climatic, and demographic risk factors.
Methods
This cross-sectional study used the locations of H7N9 cases and negative cases from China’s influenza-like illness surveillance network. After identifying H7N9 risk factors with logistic regression, we used Geographic Information Systems (GIS) to construct predictive maps of H7N9 risk across Asia.
Results
Live bird market density was associated with human H7N9 infections reported in China from March-May 2013. Based on these cases, our model accurately predicted the virus’ spread into Guangxi autonomous region in February 2014. Outside China, we find there is a high risk that the virus will spread to northern Vietnam, due to the import of poultry from China.
Conclusions
Our risk map can focus efforts to improve surveillance in poultry and humans, which may facilitate early identification and treatment of human cases.
Keywords: chickens, influenza in birds, influenza A virus – H7N9 subtype, international health problems, surveillance
Introduction
Avian influenza A (H7N9) virus has generated concern due to its rapid spread, high case fatality, virological factors that suggest adaption to mammalian hosts, lack of population immunity, and potential for person-to-person transmission. Within five months of the first reported case in eastern China in March 2013, the virus spread to the northern and southern parts of the country1. The fatality rate in 379 cases as of 3 March 2014 has been 30% with most patients presenting with symptoms of severe pneumonia1, 2. Although poultry exposure in live bird markets (LBMs) is the most significant risk factor for H7N9 infection in humans3, the virus may transmit from person-to-person in very occasional cases when there is prolonged, unprotected contact with an index case4. H7N9 strains have also been reported to develop resistance in cases treated with oseltamivir, complicating treatment and leading to worse clinical outcomes5, 6.
As of 3 March 2014, 82% of cases have occurred in eastern China (Shanghai and the neighboring provinces of Jiangsu and Zhejiang) and southern China (Guangdong province). However, in April and December 2013, two patients were hospitalized in Taiwan after being infected with H7N9 in Jiangsu7, 8, and an additional patient was hospitalized in Malaysia in February 2014 following infection in Guangdong9, highlighting the potential for long distance spread via air travel. After peaking in March and early April 2013, the number of new cases decreased significantly in late April and May following the closure of LBMs in the affected areas in early April and no cases were reported in June10. The sharp reduction in cases could be due to many reasons, including the fact that seasonal and avian influenza in humans historically reach low levels during the summer in China11. However, new cases were detected in in July, August, October–December 2013, and January–March 20141 raising the question of when and where human infections with H7N9 might occur next.
The objective of this paper is to predict the risk of future human infections with H7N9 in China and adjacent countries in East Asia. We did this by identifying agricultural, climatic, and demographic risk factors for current H7N9 infections in humans and constructing a regression model to predict future risk. The surveillance of H7N9 in poultry cannot rely on bird die-offs or illness of poultry since H7N9 infection in poultry is asymptomatic12. Therefore, a risk map is needed to most effectively target and conduct animal surveillance to prioritize areas where circulation and spread among poultry in farms and LBMs is most likely. Such a risk map will focus efforts to improve surveillance in poultry and humans, which may facilitate early identification and treatment of human cases as well as timely implementation of intervention measures.
Materials and methods
Surveillance for H7N9
We used a cross-sectional study design to identify putative risk factors for H7N9 infections in humans reported by surveillance between 4 March and 28 May 2013. We used cases reported from July to December to validate the model. The analysis was carried out at the scale of the county (xian), an administrative unit below the provincial level. County size reflects provincial history and politics13. Cities may contain several counties. For example, cities reporting H7N9 encompass 6.77 counties on average (Fig. A1).
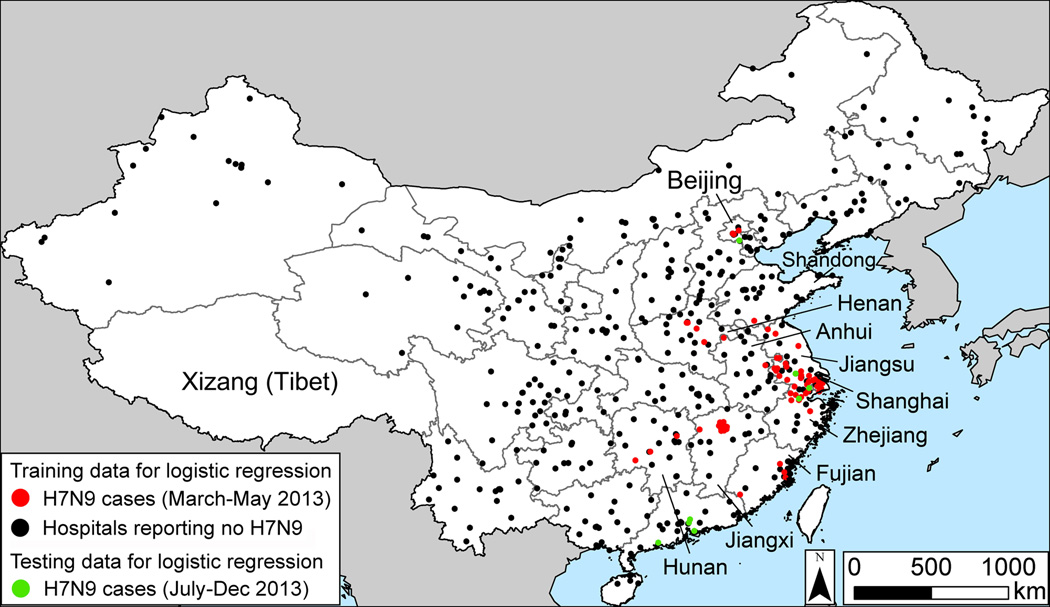
To construct a map of the risk of H7N9 in each county in China, we first required positive H7N9 cases. We used the geographic location of positive H7N9 cases reported to the Chinese Center for Disease Control and Prevention (Fig. 1), and assigned cases to counties based on the six digit postal code of the patient’s home. In addition, the model required the locations of negative cases. Due to surveillance bias, we could not assume that counties reporting no H7N9 had no human infections. For this reason, we utilized data from China’s influenza-like illness (ILI) surveillance network14, which tested individuals and found negatives. We classified a county as negative if all of the samples collected at hospitals in the county from March to May tested negative for H7N9. The logistic regression model described below was fitted to data from counties that reported H7N9 cases and counties that submitted samples to the ILI network and tested negative. Counties that did not submit samples to the ILI network (n=2012, which is 83% of China’s counties) were not used to fit the model in the main text. In the Supplementary Material, we construct a model that assumes that counties reporting no cases were negative (Table A2, Fig. A2).
Fig. 1.
Geographic locations of influenza A (H7N9) infections in humans in China, March-May 2013. During this period, the samples were collected samples from all provinces except Xizang (Tibet).
For each county in the surveillance data set, we calculated the value of four predictor variables hypothesized to be drivers of H7N9 infections in humans (Table A1 lists the data sources and A3 the median and quartile values among the counties).
LBM density
Most patients infected with H7N9 reported exposure to poultry at LBMs3; in addition, the prevalence of antibodies against H7N9 may be higher in LBM workers than in the general population15. H7N9 has been isolated from chickens in LBMs as well as ducks and pigeons16. We used data on market locations in 201217 to test whether LBM density predicted H7N9 in humans.
Temperature and relative humidity
Since cold, dry conditions increase the transmission of the influenza A virus in animal models18, we hypothesized that counties with low temperatures and relative humidity would be more likely to have H7N9 infections in humans. Although absolute humidity is a more significant determinant of influenza transmission efficiency than relative humidity19, spatial data were only available for the latter.
Human population density
We hypothesized that high human population density could serve as a proxy for the intensity of poultry consumption, farming, or trading.
Distance from past H7N9 cases
Poultry farmers in China occasionally bypass nearby retail markets to sell to wholesale markets thousands of kilometers away20, 21. Large wholesale hubs such as those in Shanghai, Hunan province, and the Guangxi autonomous region supply retail markets where consumers purchase birds. As of 3 March 2014, H7N9 has been detected in birds and environmental samples at retail and wholesale markets but not farms22. A case-control study indicates that exposure to chickens at retail markets is the most risk important factor for H7N9 infection in humans3. We assume that a county close to a reported case has high exposure to H7N9 because the virus has entered the local retail market chain.
Logistic regression
All predictor variables except distance were included in a logistic regression to predict the occurrence of H7N9 infections in humans at the county scale. We utilized logistic regression because this approach has been used to quantify the risk of other zoonotic infections such as H5N1 avian influenza23, 24. The model included an offset term to account for the fact that sampling effort was greater in more populous counties. After fitting the regression model to the positive and negative counties in China (Fig. 1), we applied the model to counties in China that did not submit samples to the ILI network and to adjacent countries in Asia.
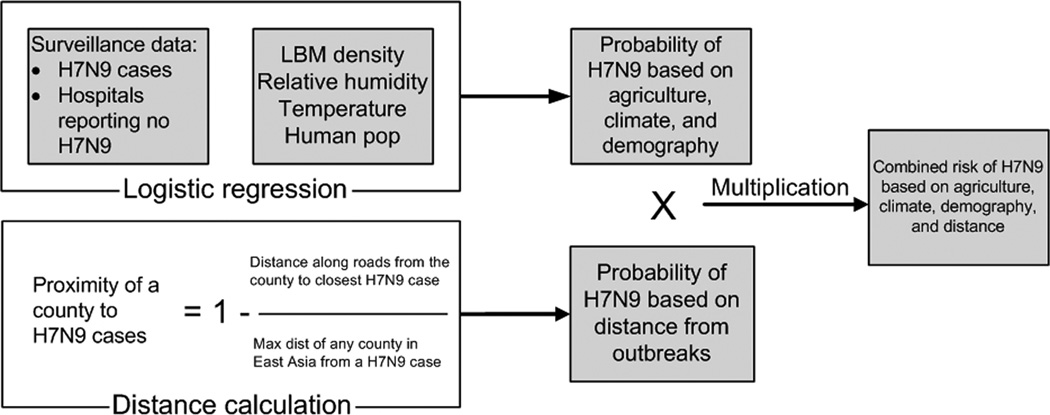
Combined risk model
The logistic regression predicts the risk of H7N9 infection in humans based on the counties’ agricultural, climatic, and demographic characteristics. To account for the effect of geographic proximity on H7N9 risk, we calculated the distance along roads from each county in East Asia to the nearest county in China where H7N9 has been reported in humans. After creating a map of the risk of infected poultry movement and a map of the risk of H7N9 in humans based on agricultural, climatic, and demographic characteristics, we multiplied the maps by one another (Fig. 2). This provides a composite measure of risk that incorporates suitability for H7N9 based on environmental characteristics as well as exposure to H7N9 based on geographic proximity.
Fig. 2.
Calculation of a county’s risk of H7N9 infection in humans based on agricultural, climatic, and demographic variables and distance from reported cases.
Results
Risk factors for H7N9 infections in humans in China (March–May 2013)
Of 2411 counties in China, 373 counties were negative for H7N9 and 56 were positive in our surveillance data set. The logistic regression model based on the surveillance data identified LBM density as the most significant risk factor (Table 1). The odds ratio for LBM density was 1.08, meaning that the risk of H7N9 infection in humans increases 8% for each additional LBM per km2. Temperature also approached significance (p=0.056). When we assumed that counties reporting no H7N9 were negative, LBM density was highly significant and temperature was slightly significant (Table A2). There was no relationship between H7N9 infections in humans and relative humidity or human population density (Table 1). We assessed the goodness of fit of the model via a Hosmer-Lemeshow test, in which the null hypothesis is that the logistic regression fits the data well25. We failed to reject the null hypothesis, indicating that our model explains a significant amount of variation in H7N9 risk (Hosmer-Lemeshow Ĉ=8.89, df=8, p=0.35). However, we cannot rule out that other variables not considered here are important drivers of H7N9.
Table 1.
Risk factors for H7N9 infections in humans based on surveillance data, March–May 2013.
| Variable | Odds ratio (95% C.I.) | Coefficient | SE | p value |
|---|---|---|---|---|
| Intercept | Not applicable | −3.939 | 0.404 | <.0001 |
| LBM density (markets per km2) | 1.08 (1.04–1.12) | 0.0733 | 0.0184 | <.0001 |
| Relative humidity (%) | 0.99 (0.98–1.01) | −3.13 × 10−3 | 7.1 × 10−3 | 0.6595 |
| Temperature (°C) | 1.04 (0.99–1.08) | 0.0378 | 0.0198 | 0.0563 |
| Human population density (people per km2) | 1 (1–1) | 4.5 × 10−5 | 1.72 × 10−4 | 0.7918 |
Accuracy assessment of the spatial model
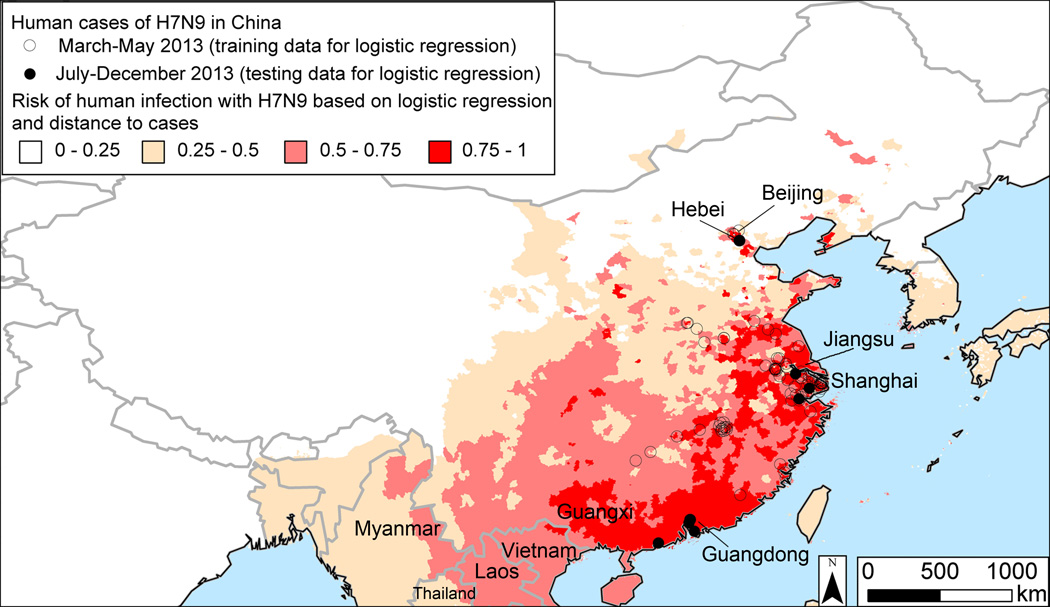
To validate the logistic regression model, we first partitioned the surveillance data into a training set comprising 75% of the positive and 75% of the negative counties and tested the model’s accuracy on the withheld 25%. The partitioning was done randomly and with replication (n=10,000). The model constructed from the training set had high predictive power when applied to the test set (AUC = 0.843, ROC contrast test chi-square = 1.15 × 105, df = 1, p < .0001). The model was constructed using human cases from March to May 2013. We further validated the model by assessing its ability to predict new human cases that occurred in Guangdong province in August-December, Hebei province in July, Jiangsu in December, and Zhejiang province in October-December that the model classified as having a high risk of human infection with H7N9. The predicted probability of H7N9 was 0.82 in Guangdong, 0.56 in Hebei, 0.89 in Jiangsu, and 0.94 in Zhejiang. The model constructed from cases reported between March and May 2013 predicts a high risk of H7N9 in the Guangxi autonomous region, which borders Guangdong (Fig. 3). As predicted, two human cases were reported in Guangxi in February 201426. The model that classifies counties reporting no H7N9 as negative also predicts a hotspot in this region (Fig A2). In general, this model predicts fewer areas to have a high risk of H7N9 because the large number of counties assumed to be negative swamps the small number of positives.
Fig. 3.
Future risk of H7N9 in East Asia based on cases reported in China from March-May 2013.
Predicting future hotspots of H7N9 in East Asia
Outside China, the model predicts a high risk of H7N9 infections in humans in northern Vietnam. Guangdong, where H7N9 has been isolated from chickens and humans, is within 200 km of the Vietnamese border. Since our model does not include a time parameter, we cannot predict when future outbreaks might occur. Developing spatio-temporal predictions would several seasons of data27, which is currently unavailable as H7N9 is an emerging infection. Other possible future sites of H7N9 outbreaks in Southeast Asia identified by the model include northern Laos and eastern Myanmar.
Discussion
Like the present study, Fang et al.17 mapped the risk of H7N9 using the locations of human cases in China during the spring of 2013. The model presented here was developed in collaboration with Fang et al. but differed from the earlier model in several respects. First, the current model incorporates a county’s proximity to reported cases as a predictor of the spread of H7N9. In addition, rather than using random negatives, the current model includes individuals who were tested during ILI surveillance and found to be negative (potential biases of ILI surveillance for detecting negatives are discussed below). Furthermore, the current risk map was based on a logistic regression model that included an offset term to account for the fact that H7N9 surveillance was more intensive in populous areas. Finally, whereas the previous risk map covered China, the present risk map was constructed for China and neighboring countries in East Asia.
Since the two models were constructed from H7N9 infections in humans in the first half of 2013, how well did they predict cases in the second half of the year? Jiangsu and Zhejiang provinces reported cases in the spring of 2013 and have also reported new cases since June. In both provinces, the two models predicted a risk of future cases of 75% or greater. During the second half of 2013, Hebei and Guangdong provinces reported a total of eight cases but neither had reported cases in the spring. In these two provinces, both models predict some risk of H7N9. However, the magnitude of the risk predicted by the current model is higher (Hebei: 20% in Fang et al.17 vs. 56% in present model; Guangdong: 40–60% in Fang et al. vs. 75–100% in this study). To this extent, the present model appears to provide somewhat more accurate predictions when validated on out-of-sample data. Our map was constructed using cases reported during the virus’ initial emergence in eastern China from March-May 2013. However, the model accurately predicted the virus’ spread into the Guangxi autonomous region in southern China, which occurred during the second wave of H7N9 infections (December 2013–February 2014).
Since our sampling design was cross-sectional, it is not possible to conclude that there is a causal relationship between H7N9 infections in humans in China and the variables that were significant in our statistical model. However, our results suggest hypotheses that should be tested more specifically in future studies. The significant association between LBM density and H7N9 cases in our model provides further support for the hypothesis that LBMs are an important source of H7N9 transmission to humans3, 10, 28. A previous study found a tight coupling between climatic variables and seasonal influenza in humans in Hong Kong29. We did not detect a significant relationship between H7N9 and climate, though temperature approached significance. This may reflect differences in transmission between seasonal and avian influenza. In the case of seasonal influenza, humidity affects the distance that virus particles travel30. However, given that H7N9 is a novel infection, we do not yet fully understand the routes of transmission and have not yet observed the impact of seasonality on its emergence.
Since most H7N9 cases have been reported in large cities, why was there no relationship between H7N9 and human population density according to our model? We found that the surveillance effort to detect H7N9 cases was much greater in densely populated cities (Spearman’s rho = 0.35, p = 2.71 × 10−16). When the logistic regression model was adjusted to account for the intensity of sampling, there was no effect of population on the risk of H7N9 infection in humans. Our results suggest that LBM density, which was uncorrelated with population, is the most important risk factor among those analyzed here.
Controlling H7N9 in China will require multi-pronged policies involving the public health, food safety, and veterinary sectors. Closing LBMs appears to be an effective approach for eradicating or reducing H7N9 infections in humans10. In poultry, policies to control H7N9 include culling on positive farms and vaccination. Human vaccines against H7N9 are under development31, 32. However, no H7N9 vaccine for poultry is currently available. Although vaccination of poultry against H5N1 is widespread in China, there may be little incentive for poultry producers to vaccinate against H7N9 due to its low pathogenicity in domestic birds and vaccine-induced antibodies may confuse surveillance efforts. Measures that have been effective for controlling other subtypes of avian influenza at LBMs in China include mandatory monthly rest days and bans on keeping live birds overnight33. These utility of these approaches for controlling H7N9 merits investigation.
Our results support strengthening LBM and human surveillance in northern Vietnam, whose poultry trade with China and proximity to sites where H7N9 has been detected in Guangdong and Guangxi make it the area outside China where the virus is most likely to spread next. In northern Vietnam, every day 100 tons of hens that are past their peak egg productivity called “spent hens” are imported from China into Quang Ninh and Lang Son, where they are sold at higher prices as meat chickens34. There is also considerable cross-border trade of day-old chicks and ducklings35. Poultry sellers routinely transport live birds long distances to wholesale markets within northern Vietnam36, which could enable H7N9 to spread extensively if it is introduced. H5N1, which is endemic in Vietnamese poultry, is believed to have spread to Vietnam from China37. Other H7N9 hotspots predicted by the model in Southeast Asia include northern Laos and eastern Myanmar. However, these areas are not known to import chickens from provinces of China where H7N9 has been detected.
Among the limitations of this study was that our surveillance data were collected from patients who presented with ILI in urban areas rather than from individuals selected at random. The ILI surveillance system may have failed to detect H7N9 due to the biased age distribution of individuals sampled and geographic biases in screening. Concerning the first bias, although most H7N9 cases are 60 or older, only 7% of individuals screened by the ILI system were in this age group14. Concerning the second, H7N9 causes severe disease rather than subclinical infections14, 38, 39, but the frequency of severe disease reporting varies across China10. For example, from March to May, the ILI system screened 100 samples per week from hospitals in northern China but 200 per week from hospitals in the rest of the country. In addition, since most sentinel hospitals are in urban areas, the surveillance network may have failed to detect H7N9 cases in rural populations. Due to geographic variation in testing efforts and age-biased sampling, the ILI surveillance system likely failed to detect cases of severe respiratory disease caused by H7N9. For instance, from March to May 2013, 8% of cases of pneumonia of unknown etiology (PUE) in China were confirmed to be H7N910. In our data set, 37% of the negative counties were located in provinces that reported PUE. Thus, some of the putatively negative counties used to construct our risk map may have had cases of H7N9.
Due to these limitations of the surveillance data, our risk map may have low accuracy in northern China outside of areas with intensive H7N9 screening such as Beijing. The model appeared to perform well in Beijing to the extent that it classifies the municipality, which has reported five human cases of H7N9, as having a risk of 75–100% of H7N9 infections in humans. However, the Beijing CDC has had more aggressive surveillance and testing than some other parts of China, so the model had extensive training data for the city. Thus, the performance of the model in Beijing likely reflects surveillance bias rather than the ability of the model to make accurate predictions when confronted with new data.
Future work should refine our risk map to account for genetic differences among strains of H7N9. In order to have sample size sufficiently large to construct a reliable logistic regression model, we conflated all strains of H7N9. However, some strains may be of more concern from a public health standpoint. For example, strains A/Anhui/1/2013 (H7N9) and A/Hangzhou/1/2013 (H7N9) carry residues at position 226 that allow them to bind to human respiratory cells, whereas the A/Shanghai/1/2013 (H7N9) strain lacks this specificity6. If H7N9 continues to be detected in humans, future research could identify areas where there is a high risk of human infection with strains that are adapted to human respiratory cells, or carry other mutations that affect human transmissibility, virulence, and sensitivity to antivirals. Another important area for future research is developing chicken density maps that cover all of Southeast Asia and distinguish between LBMs and farms, as the former have higher risk of H7N9 spillover to humans. The southern border of available chicken density maps is 18°N40, which excludes Cambodia and southern Vietnam. Although a global map of poultry density is available from the UN Food & Agriculture Organization, it does not distinguish chickens from other domestic birds or LBMs from farms. Finally, validating our model in the field is an important task for future work, which could confirm the occurrence of H7N9 in predicted hotspots including northern Vietnam. If a large number of new H7N9 cases occurs, or if there are major changes in the input parameters of our regression model, such as the number of LBMs per county, the model could be rerun to provide updated predictions about the risk of infection in humans.
Supplementary Material
Acknowledgments
We thank two reviewers whose comments improved the manuscript and the 554 sentinel hospitals and 408 network laboratories participating in the Chinese National Influenza Surveillance Network for data collection and laboratory testing. Thanks are also due to the Chinese Center for Disease Control and Prevention and the Ministry of Health, China, for coordinating and supporting the Chinese National Influenza Surveillance Network, and the China-US Collaborative Program on Emerging and Re-emerging Infectious Diseases for support of epidemiological experts. The Chinese National Influenza-like Illness Surveillance Network was supported by the Chinese central government. Fuller and Smith were supported by a National Institutes of Health/National Science Foundation award, “Ecology and Evolution of Infectious Diseases,” from the Fogarty International Center 3R01-TW005869.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics statement:
This work was approved by the appropriate ethical committees at the University of California, Los Angeles, the Chinese Center for Disease Control and Prevention, and the US Centers for Disease Control and Prevention.
Potential conflicts of interest:
All authors: No reported conflict.
References
- 1.WHO. Geneva: WHO; 2014. Human infection with avian influenza A(H7N9) virus – update 3 2014. [Google Scholar]
- 2.Gao H-N, Lu H-Z, Cao B, Du B, Shang H, Gan J-H, et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. New England Journal of Medicine. 2013;368:2277–2285. doi: 10.1056/NEJMoa1305584. [DOI] [PubMed] [Google Scholar]
- 3.Ai J, Huang Y, Xu K, Ren D, Qi X, Ji H, et al. Case-control study of risk factors for human infection with influenza A(H7N9) virus in Jiangsu Province, China. Eurosurveillance. 2013;18:20510. doi: 10.2807/1560-7917.es2013.18.26.20510. [DOI] [PubMed] [Google Scholar]
- 4.Qi X, Qian Y-H, Bao C-J, Guo X-L, Cui L-B, Tang F-Y, et al. Probable person to person transmission of novel avian influenza A (H7N9) virus in Eastern China, 2013: epidemiological investigation. British Medical Journal. 2013;347:f4752. doi: 10.1136/bmj.f4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu YW, Lu SH, Song ZG, Wang W, Hao P, Li JH, et al. Association between adverse clinical outcome in human disease caused by novel influenza A H7N9 virus and sustained viral shedding and emergence of antiviral resistance. Lancet. 2013;381:2273–2279. doi: 10.1016/S0140-6736(13)61125-3. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe T, Kiso M, Fukuyama S, Nakajima N, Imai M, Yamada S, et al. Characterization of H7N9 influenza A viruses isolated from humans. Nature. 2013;501:551–555. doi: 10.1038/nature12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang S-Y, Lin P-H, Tsai J-C, Hung C-C, Chang S-C. The first case of H7N9 influenza in Taiwan. Lancet. 2013;381:1621. doi: 10.1016/S0140-6736(13)60943-5. [DOI] [PubMed] [Google Scholar]
- 8.Hong Kong Centre for Health Protection. Issued at HKT. Vol. 23. Hong Kong: Center for Health Protection; 2013. CHP closely monitor a human case of avian influenza A(H7N9) in Taiwan. Press Release, December 31, 2013; p. 17. [Google Scholar]
- 9.WHO. Geneva: WHO; 2014. Human infection with avian influenza A(H7N9) virus – update 17 February 2014. [Google Scholar]
- 10.Xiang N, Havers F, Chen T, Song Y, Tu W, Li L, et al. Assessment of utility of national pneumonia surveillance to accurately describe Influenza H7N9 epidemiology in China. Emerging Infectious Diseases. 2013;19:1784–1790. doi: 10.3201/eid1911.130865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamerius J, Nelson MI, Zhou SZ, Viboud C, Miller MA, Alonso WJ. Global influenza seasonality: reconciling patterns across temperate and tropical regions. Environ Health Perspect. 2011;119:439–445. doi: 10.1289/ehp.1002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uyeki T, Cox NJ. Global concerns regarding novel influenza A (H7N9) virus infections. New England Journal of Medicine. 2013;20:1862–1864. doi: 10.1056/NEJMp1304661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veeck G, Pannell CW, Smith CJ, Huang Y. Globalization and the Dynamics of Political, Economic, and Social Change. Lanham, Maryland: Rowman & Littlefield; 2011. China's Geography. [Google Scholar]
- 14.Xu C, Havers F, Wang L, Chen T, Shi J, Wang D, et al. Monitoring avian influenza A(H7N9) virus through national Influenza-like Illness surveillance, China. Emerging Infectious Diseases. 2013;19:1289–1292. doi: 10.3201/eid1908.130662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang S, Chen Y, Cui D, Yao H, Lou J, Huo Z, et al. Avian-origin H7N9 virus infection in H7N9-affected areas of China: a serological study. Journal of Infectious Diseases. 2013;209:265–269. doi: 10.1093/infdis/jit430. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Wang J, Su W, Gao S, Luo J, Zhang M, et al. Relationship between domestic and wild birds in live poultry market and a novel H7N9 virus in China. Journal of Infectious Diseases. 2013;209:34–37. doi: 10.1093/infdis/jit478. [DOI] [PubMed] [Google Scholar]
- 17.Fang L-Q, Li X-L, Lui K, Li Y-J, Yao H-W, Liang S, et al. Mapping the spread and risk of avian influenza A (H7N9) in China. Scientific Reports. 2013;3:2722. doi: 10.1038/srep02722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowen AC, Mubareka S, Steel J, Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathogens. 2007;3:1470–1476. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaman J, Kohn M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natl Acad Sci U S A. 2009;106:3243–3248. doi: 10.1073/pnas.0806852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magalhaes RJS, Zhou XY, Jia BB, Guo FS, Pfeiffer DU, Martin V. Live poultry trade in southern China provinces and HPAIV H5N1 infection in humans and poultry: the role of Chinese New Year festivities. PLoS One. 2012;7:e49712. doi: 10.1371/journal.pone.0049712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin V, Zhou X, Marshall E, Jia B, Fusheng G, Franco Dixon M, et al. Risk-based surveillance for avian influenza control along poultry market chains in South China: the value of social network analysis. Prev Vet Med. 2011;102:196–205. doi: 10.1016/j.prevetmed.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z. Paris: World Organization for Animal Health; 2013. [May 21, 2013]. Low pathogenic avian influenza (poultry), People's Republic of China, Report to the World Organization for Animal Health. Follow-up Report 9. [Google Scholar]
- 23.Fang LQ, de Vlas SJ, Liang S, Looman CWN, Gong P, Xu B, et al. Environmental factors contributing to the spread of H5N1 avian influenza in mainland China. PLoS One. 2008;3:e2268. doi: 10.1371/journal.pone.0002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin V, Pfeiffer DU, Zhou XY, Xiao XM, Prosser DJ, Guo FS, et al. Spatial distribution and risk factors of Highly Pathogenic Avian Influenza (HPAI) H5N1 in China. Plos Pathogens. 2011;7:e1001308. doi: 10.1371/journal.ppat.1001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. Third Edition. Hoboken, New Jersey: Wiley; 2013. [Google Scholar]
- 26.WHO. Geneva: WHO; 2014. Number of confirmed human cases of avian influenza A(H7N9) reported to WHO. Report 12 - data in WHO/HQ as of 14 February 2014, 12:00 GMT. [Google Scholar]
- 27.Schabenberger O, Gotway CA. Statistical Methods for Spatial Data Analysis. Boca Raton: Chapman & Hall/CRC; 2005. [Google Scholar]
- 28.Yu H, Wu JT, Cowling BJ, Liao Q, Fang VJ, Zhou SZ, et al. Effect of closure of live poultry markets on poultry-to-person transmission of avian influenza A H7N9 virus: an ecological study. Lancet. 2013 doi: 10.1016/S0140-6736(13)61904-2. Online early publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soebiyanto RP, Adimi F, Kiang RK. Modeling and predicting seasonal influenza transmission in warm regions using climatological parameters. PLoS One. 2010;5:e9450. doi: 10.1371/journal.pone.0009450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang W, Marr LC. Dynamics of airborne influenza a viruses indoors and dependence on humidity. PLoS One. 2011;6:e21481. doi: 10.1371/journal.pone.0021481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith GE, Flyer DC, Raghunandan R, Liu Y, Wei Z, Wu Y, et al. Development of influenza H7N9 virus like particle (VLP) vaccine: homologous A/Anhui/1/2013 (H7N9) protection and heterologous A/chicken/Jalisco/CPA1/2012 (H7N3) cross-protection in vaccinated mice challenged with H7N9 virus. Vaccine. 2013;31:4305–4313. doi: 10.1016/j.vaccine.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 32.Fries LF, Smith GE, Glenn GM. A recombinant viruslike particle influenza A (H7N9) vaccine. New England Journal of Medicine. 2013;369:2564–2566. doi: 10.1056/NEJMc1313186. [DOI] [PubMed] [Google Scholar]
- 33.Leung YHC, Lau EHY, Zhang LJ, Guan Y, Cowling BJ, Peiris JSM. Avian influenza and ban on overnight poultry storage in live poultry markets, Hong Kong. Emerging Infectious Diseases. 2012;18:1339–1341. doi: 10.3201/eid1808.111879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desvaux S. Epidemiology of the Highly Pathogenic Avian Influenza H5N1 in Northern Vietnam: Applications for Surveillance and Control. Perth, Australia: Ph.D. Thesis, Murdoch University; 2012. [Google Scholar]
- 35.FAO. Animal Production and Health Paper No. 171. Rome: Food and Agriculture Organization of the United Nations; 2011. Approaches to Controlling, Preventing and Eliminating H5N1 Highly Pathogenic Avian Influenza in Endemic Countries. [Google Scholar]
- 36.Magalhaes RJS, Ortiz-Pelaez A, Kim LLT, Quoc HD, Otte J, Pfeiffer DU. Associations between attributes of live poultry trade and HPAI H5N1 outbreaks: a descriptive and network analysis study in northern Vietnam. BMC Vet Res. 2010;6:10. doi: 10.1186/1746-6148-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wan XF, Nguyen T, Davis CT, Smith CB, Zhao ZM, Carrel M, et al. Evolution of highly pathogenic H5N1 avian influenza viruses in Vietnam between 2001 and 2007. PLoS One. 2008;3:e3462. doi: 10.1371/journal.pone.0003462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Q, Zhou L, Zhou M, Chen Z, Li F, Wu H, et al. Preliminary report: epidemiology of avian influenza A(H7N9) outbreak in China. New England Journal of Medicine. 2013 Apr 24; 2013 Epub. [Google Scholar]
- 39.Xu W, Lu L, Shen B, Li J, Xu J, JIang S. Serological investigation of subclinical influenza A(H7N9) infection among healthcare and non-healthcare workers in Zhejiang province, China. Clinical Infectious Diseases. 2013;57:919–921. doi: 10.1093/cid/cit396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prosser D, Wu J, Ellis EC, Gale F, Van Boeckel TP, Wint W, et al. Modelling the distribution of chickens, ducks, and geese in China. Agriculture Ecosystems & Environment. 2011;141:281–289. doi: 10.1016/j.agee.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.