Paraneoplastic cerebellar degeneration (PCD) is a common cause of subacute cerebellar ataxia caused by widespread loss of the Purkinje cells of the cerebellum. PCD diagnosis can be confirmed by detection of onconeural antibodies that may also indicate the underlying tumor type [1]. Most onconeural antibodies associated with PCD recognize intracellular antigens in Purkinje cells [2]. These antigens are supposed to induce, besides the antibody synthesis, an antigen-specific cytotoxic T-cell attack that probably is responsible of the Purkinje cell death and limited response to treatment [3,4]. The most common onconeural antibody in PCD is the Yo antibody that associates with breast and ovarian cancer [4]. In a previous case report, we identified a new antibody, targeting the intracellular Purkinje cell protein carbonic anhydrase-related protein VIII (CARPVIII), in a patient with PCD and melanoma [5]. We provide further evidence for the association of CARP VIII antibodies with PCD by demonstrating a second patient with these antibodies, who had an ovarian adenocarcinoma and developed cerebellar ataxia.
A 69-year-old female patient presented with an ovarian papillary serous cystadenocarcinoma with infiltration of the omentum and peritoneal carcinomatosis that was resected and treated with chemotherapy for one year. The patient had been in remission for 4 years when she was admitted to hospital with the clinical presentation of cerebellar ataxia since 2 weeks, with vertigo, instability, headache, and vomiting that did not improve upon symptomatic treatment. Neurological examination revealed vertical nystagmus, dysarthria, intention tremor of the upper extremities, and gait ataxia. The brain MRI was normal. Abdomen CT scan detected a paraaortic adenopathy that was confirmed in a biopsy as recurrency of the ovarian adenocarcinoma. Lumbar puncture showed pleocytosis (290 cells/µl, 94% lymphocytes) and elevated protein concentration (67mg/dl), with normal glucose levels, oligoclonal bands were not tested. The serum was investigated for onconeural and surface receptor antibodies by tissue-based assays and immunoblot [6]. The tissue-based screening revealed an anti-Purkinje cell antibody of the IgG1 subclass that stained the soma, dendrites, and axons of Purkinje cells as well as synaptic terminals in the deep cerebellar nuclei (Fig. 1A, B). Currently, three different anti-Purkinje cell antibodies are associated with this staining pattern and PCD: anti-PKCgamma, described in two patients with adenocarcinomas [7], anti-ARHGAP26, reported in four patients, including one with ovarian cancer [8,9,10], and anti-CARP VIII [5]. To further characterize our antibody, we performed an immunoblot with electrophoretically separated extracts of rat cerebellum. As controls we used serum of the first published CARP VIII patient [5] and a serum of a healthy individual. The patient’s antibody detected a protein band with a molecular mass of approximately 29 kDa and was identical to the band detected by the serum with CARP VIII antibodies (data not shown). Moreover, we stained filters with purified phage plaques resulting in the specific expression of CARP VIII, and control phage plaques (which do not result in expression of CARP VIII) with the patient’s serum, a commercial CARP VIII antibody (Millipore, Billerica, MA, USA), and serum from a healthy individual (Fig. 1C). The patient’s antibody and the commercial anti-CARP VIII antibody stained phage plaques expressing CARP VIII, whereas control plaques remained negative. In addition, we performed a competitive inhibition of immunohistochemistry, where we first incubated the rat brain section either with the patient’s serum (Fig. 1D) or a control serum (Fig. 1E) and subsequently with biotinylated anti-CARP VIII IgG, obtained from the first published case [5]. The biotinylated anti-CARP VIII antibody immunoreacted with the brain section preincubated with the negative control, but not with the section pre-incubated with the patient’s serum, indicating that the antibodies of this patient competed for the same epitopes recognized by the IgG of the patient with CARP VIII antibodies. Other antibodies such as GAD65 and classical onconeural antibodies Hu, Yo, Ri, Ma1/2, amphiphysin, SOX1, and CV2/CRMP5 screened by a commercial immunoblot (Ravo Diagnostika, GmbH, Freiburg, Germany) were negative. Moreover, no surface receptor antibodies (LGI1, CASPR2, NMDAR, AMPAR, GABA(B)R, DPPX, mGluR1, mGluR5, GlyR) were detectable. Immunohistochemical analysis of the biopsy material with a commercial anti-CARP VIII antibody demonstrated robust expression of CARP VIII in the tumor cells (Fig. 1F, G). Despite of treatment with chemotherapy and intravenous immunoglobulin the cerebellar syndrome worsened over the following 6 months and the patient became wheelchair restricted and developed a worsening of dysarthria. Brain MRI showed a progressive cerebellar atrophy compared to the previous image. The tumor remained in remission until last follow-up of 9 months.
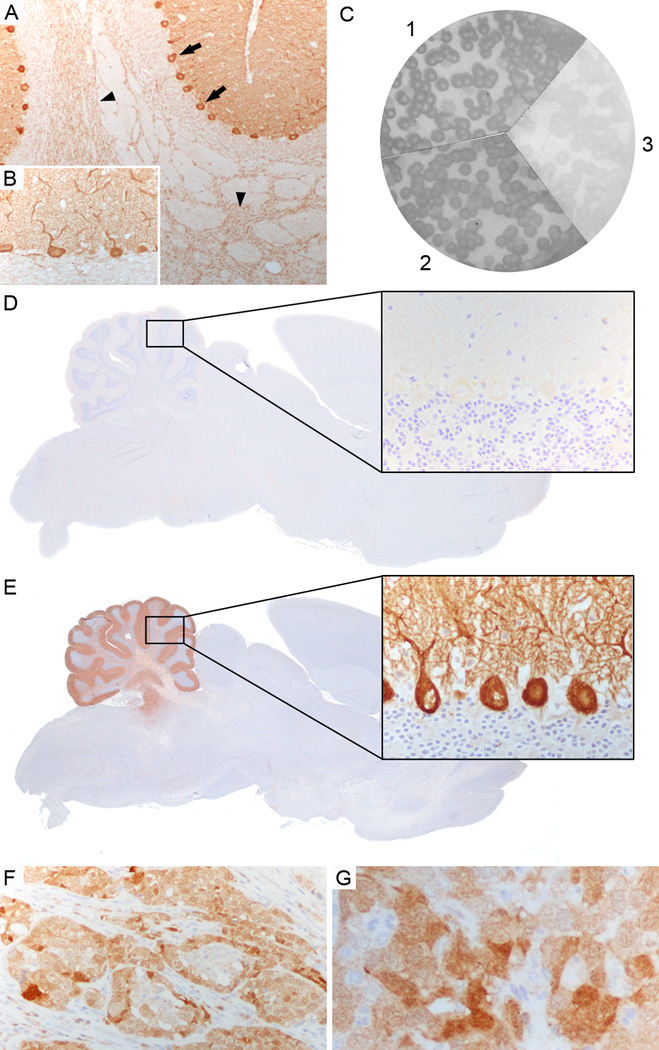
Figure 1. Characterization of CARP VIII antibody.
(A, B) Patient’s serum strongly labels Purkinje cell cytoplasm (arrows, A), axons (arrowheads, A), and dendrites (B) in rat cerebellum. (C) Filters with purified phage plaques expressing CARP VIII specifically reacted with a commercial CARP VIII antibody (1), and the patient’s serum (2) but not with serum from a healthy individual (3). (D, E) Competitive inhibition: Immunohistochemistry on rat brain, first incubated with the patient’s serum and subsequently labeled with a biotinylated CARP VIII antibody, is negative (D, rectangle in cerebellum enlarged in higher magnification) whereas immunohistochemistry on rat brain that was first incubated with a healthy control serum shows strong positive staining with the biotinylated CARP VIII antibody (E, rectangle in cerebellum enlarged in higher magnification). (F, G) Tumor biopsy of the ovarian adenocarcinoma shows expression of CARP VIII in the tumor cells. Magnification: A, F: x200; B, G, and enlarged rectangle in D, E: x400;
We report the second patient with CARP VIII antibodies who also developed PCD. However, unlike the previously reported patient the underlying tumor was an ovarian carcinoma that expressed CARP VIII. CARP VIII is related to the family of carbonic hydrases (CA), a group of enzymes that catalyze CO2 hydration. CARP VIII differs from this group in lacking the classical CA activity due to the absence of zinc-binding histidine residues [11]. Human CARP VIII is preferentially expressed in the brain, mainly in Purkinje cells [11,12]. Beyond Purkinje cells, little or no expression of CARP VIII has been described in adult human tissue. However, in cancer cells, the protein becomes strongly upregulated and has been described in different tumor types, including colorectal and non-small-cell lung carcinoma, where it was associated with higher proliferation and invasive properties, suggesting a role in tumor growth [12,13]. By immunohistochemistry, CARP VIII immunoreactivity has been detected in 15% of a series of melanomas and 3% of ovarian cancers (www.proteinatlas.org). The upregulation of the otherwise neuron-restricted CARP VIII in tumor cells likely contributes in breaking immune tolerance triggering the development of CARP VIII antibodies and the expression in different forms of cancer explains its association with more than one tumor type.
The cerebellar ataxia of our patient occurred at the time of tumor recurrence. Although PCD usually antedates the tumor diagnosis, it is not unusual that the clinical symptoms arise with the recurrence of the tumor, which might be explained by a dedifferentiation of tumor cells and/or change of antigen-presentation [4]. Unfortunately, in our case no tissue of the first tumor biopsy is available to test whether CARP VIII initially was negative but turned positive during tumor progression. The role of CARP VIII antibodies in disease evolution is unclear. CARP VIII is an intracellular protein, thus, the cerebellar degeneration is most likely T cell mediated and the patient’s antibodies, although of the IgG1 subclass and thus capable of activating complement, are not directly pathogenic. This is supported by the poor response to IVIg in both, the first and our CARP VIII patient, and probably neither plasma exchange nor other B cell targeting strategies might lead to a benefit, although more patients are needed to determine treatment response and prognosis [14]. However, the diagnosis of CARP VIII antibodies prompted early tumor diagnosis and removal. Unfortunately the clinical evolution was not favourable, as it usually happens in patients with anti-Hu and -Yo antibodies [4].
Our case confirms the association of CARP VIII antibodies with PCD and expands the spectrum of underlying tumors. Systematic screening for CARP VIII antibodies will detect more cases of this new paraneoplastic syndrome and can facilitate diagnosis and treatment of these patients. The possibility of CARPVIII antibodies should be considered in patients with strong suspicion of PCD and who do not have antibodies against other most common onconeural antibodies [1].
Acknowledgments
The authors thank Mercè Alba and Eva Caballero for technical assistance. This study was supported in part by grant PI12/00611 from the Fondo de Investigaciones Sanitarias, Madrid, Spain (FG), NIH RO1NS077851 (JD), RO1CA89054 (JD), a McKnight Neuroscience of Brain Disorders award (JD), Fondo de Investigaciones Sanitarias (FIS, Spain, 11/01780, JD), and Fundació la Marató de TV3 (JD). RH was funded by the Fonds zur Förderung der wissenschaftlichen Forschung, Austria, Project J3230.
Footnotes
Author contributions and disclosure:
Acquisition, analysis and interpretation of data: RH, LS, FV, RC, JD, FG; drafting/revising the manuscript: RH, LS, FV, RC, JD, FG; All authors give final approval of the version to be published. JD receives royalties from Athena Diagnostics for a patent for the use of Ma2 as autoantibody test, and licensing fees from Euroimmun for a patent for the use of NMDAR as autoantibody test.
References
- 1.Rosenfeld MR, Dalmau JO. Paraneoplastic disorders of the CNS and autoimmune synaptic encephalitis. Continuum (Minneap Minn) 2012;18:366–383. doi: 10.1212/01.CON.0000413664.42798.aa. [DOI] [PubMed] [Google Scholar]
- 2.Shams'ili S, Grefkens J, de Leeuw B, van den Bent M, Hooijkaas H, van der Holt B, Vecht C, Sillevis Smitt P. Paraneoplastic cerebellar degeneration associated with antineuronal antibodies: analysis of 50 patients. Brain. 2003;126:1409–1418. doi: 10.1093/brain/awg133. [DOI] [PubMed] [Google Scholar]
- 3.Sabater L, Hoftberger R, Boronat A, Saiz A, Dalmau J, Graus F. Antibody repertoire in paraneoplastic cerebellar degeneration and small cell lung cancer. PloS One. 2013;8:e60438. doi: 10.1371/journal.pone.0060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rojas I, Graus F, Keime-Guibert F, Rene R, Delattre JY, Ramon JM, Dalmau J, Posner JB. Long-term clinical outcome of paraneoplastic cerebellar degeneration and anti-Yo antibodies. Neurology. 2000;55:713–715. doi: 10.1212/wnl.55.5.713. [DOI] [PubMed] [Google Scholar]
- 5.Bataller L, Sabater L, Saiz A, Serra C, Claramonte B, Graus F. Carbonic anhydrase-related protein VIII: autoantigen in paraneoplastic cerebellar degeneration. Ann Neurol. 2004;56:575–579. doi: 10.1002/ana.20238. [DOI] [PubMed] [Google Scholar]
- 6.Hoftberger R, Dalmau J, Graus F. Clinical neuropathology practice guide 5-2012: Updated guideline for the diagnosis of antineuronal antibodies. Clin Neuropathol. 2012;31:337–341. doi: 10.5414/NP300545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoftberger R, Kovacs GG, Sabater L, Nagy P, Racz G, Miquel R, Dalmau J, Graus F. Protein kinase Cgamma antibodies and paraneoplastic cerebellar degeneration. J Neuroimmunol. 2013;256:91–93. doi: 10.1016/j.jneuroim.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Jarius S, Martinez-Garcia P, Hernandez AL, Brase JC, Borowski K, Regula JU, Meinck HM, Stocker W, Wildemann B, Wandinger KP. Two new cases of anti-Ca (anti-ARHGAP26/GRAF) autoantibody-associated cerebellar ataxia. J Neuroinflammation. 2013;10:7. doi: 10.1186/1742-2094-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarius S, Wandinger KP, Horn S, Heuer H, Wildemann B. A new Purkinje cell antibody (anti-Ca) associated with subacute cerebellar ataxia: immunological characterization. J Neuroinflammation. 2010;7:21. doi: 10.1186/1742-2094-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doss S, Nümann A, Ziegler A, Siebert E, Borowski K, Stöcker W, Prüss H, Wildemann B, Endres M, Jarius S. Anti-Ca/anti-ARHGAP26 antibodies associated with cerebellar atrophy and cognitive decline. J Neuroimmunol. 2013 doi: 10.1016/j.jneuroim.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Aspatwar A, Tolvanen ME, Parkkila S. Phylogeny and expression of carbonic anhydrase-related proteins. BMC Mol Biol. 2010;11:25. doi: 10.1186/1471-2199-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akisawa Y, Nishimori I, Taniuchi K, Okamoto N, Takeuchi T, Sonobe H, Ohtsuki Y, Onishi S. Expression of carbonic anhydrase-related protein CA-RP VIII in non-small cell lung cancer. Virchows Arch. 2003;442:66–70. doi: 10.1007/s00428-002-0721-y. [DOI] [PubMed] [Google Scholar]
- 13.Nishikata M, Nishimori I, Taniuchi K, Takeuchi T, Minakuchi T, Kohsaki T, Adachi Y, Ohtsuki Y, Ohishi S. Carbonic anhydrase-related protein VIII promotes colon cancer cell growth. Mol Carcinog. 2007;46:208–214. doi: 10.1002/mc.20264. [DOI] [PubMed] [Google Scholar]
- 14.Grisold W, Giometto B, Vitaliani R, Oberndorfer S. Current approaches to the treatment of paraneoplastic encephalitis. Ther Adv Neurol Disord. 2011;4:237–248. doi: 10.1177/1756285611405395. [DOI] [PMC free article] [PubMed] [Google Scholar]