Abstract
This paper presents an overview of historical advances and the current state of genetic psychophysiology, a rapidly developing interdisciplinary research linking genetics, brain, and human behavior, discusses methodological problems, and outlines future directions of research. The main goals of genetic psychophysiology are to elucidate the neural pathways and mechanisms mediating genetic influences on cognition and emotion, identify intermediate brain-based phenotypes for psychopathology, and provide a functional characterization of genes being discovered by large association studies of behavioral phenotypes. Since the initiation of this neurogenetic approach to human individual differences in the 1970s, numerous twin and family studies have provided strong evidence for heritability of diverse aspects of brain function including resting-state brain oscillations, functional connectivity, and event-related neural activity in a variety of cognitive and emotion processing tasks, as well as peripheral psychophysiological responses. These data indicate large differences in the presence and strength of genetic influences across measures and domains, permitting the selection of heritable characteristics for gene finding studies. More recently, candidate gene association studies began to implicate specific genetic variants in different aspects of neurocognition. However, great caution is needed in pursuing this line of research due to its demonstrated proneness to generate false-positive findings. Recent developments in methods for physiological signal analysis, hemodynamic imaging, and genomic technologies offer new exciting opportunities for the investigation of the interplay between genetic and environmental factors in the development of individual differences in behavior, both normal and abnormal.
Keywords: genetics, heritability, brain, endophenotype, EEG, ERP
1. Introduction
The past decade has seen remarkable development in neurosciences and genetics, which greatly facilitated research in genetic psychophysiology, an interdisciplinary field at the intersection of psychophysiology and human genetics. This review provides a survey of the main advances in this field as well as problems and caveats, some of which are well known to geneticists but may be less appreciated in the psychophysiologists and cognitive neuroscientists, and, finally, outlines future directions in genetic psychophysiology research in the light of recent advances in both genomic technologies and novel approaches to physiological signal analysis and multimodal neuroimaging.
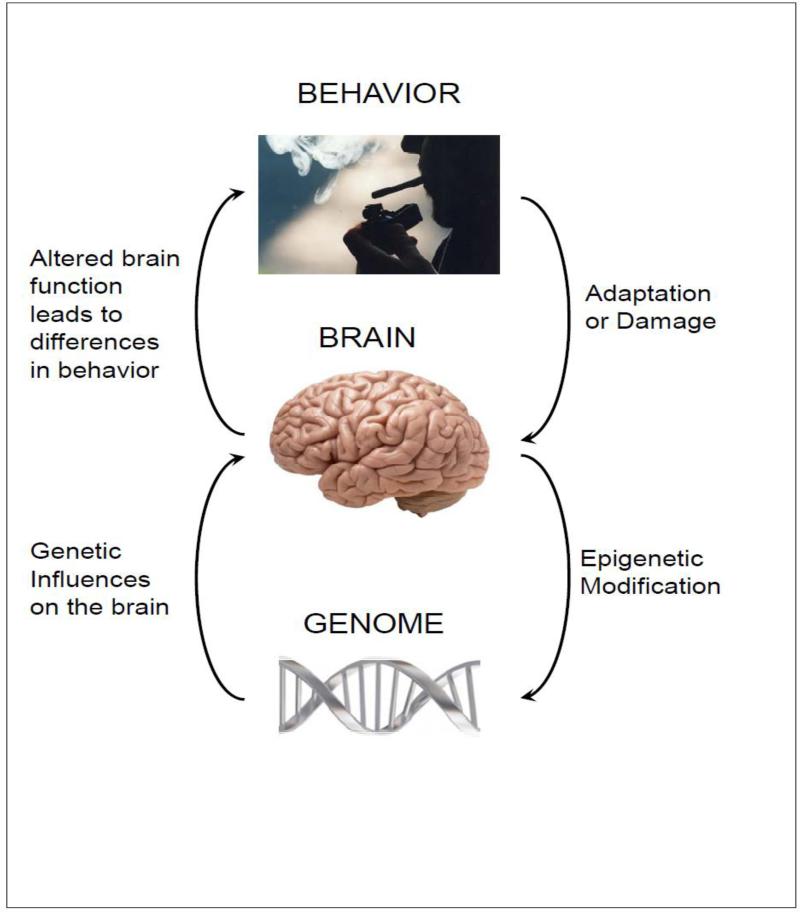
The issue of the relationships between genes and human behavior, both normal and abnormal, has a long and controversial history. Much progress has been made in understanding these relationships over recent decades, and the “nature versus nurture” debate is no longer relevant. Now, as the role of both genetic and environmental factors in shaping individual differences in human behavior is universally recognized, the focus of research has shifted towards the understanding of how genetic factors influence behavior. Because genes can influence behavior only to the extent that they influence the brain (Lomov and Ravich-Shcherbo, 1978), to address this question we need to elucidate the neural pathways and mechanisms that mediate the link between genes and behavior, including both normal individual differences and psychopathology. The main paths connecting genes, brain, and behavior are shown in Fig. 1. Psychophysiology is well positioned to address this question, thanks to its growing arsenal of methods for elucidating neural mechanisms underlying human behavior, from basic reflexes to complex social interactions.
Fig. 1.
Major causal paths linking genes, brain, and behavior. The “ascending” paths on the left show that genomic variation influences brain function which, in turn, leads to individual differences in behavior. The “descending” paths on the right show that behavior itself may lead to changes in the brain due to adaptation and learning or brain damage due to exposure to hazardous factors such as alcohol and drugs. These influences on the brain may also lead to epigenetic modifications affecting gene expression in brain cells and thus brain function. Each of these relationships is moderated by environmental factors (not shown for simplicity).
Why is genetic psychophysiology important? The significance of the field is at least three-fold: For psychophysiologists, genetic research can reveal the causal sources of individual variability in psychophysiological responses. For behavioral and psychiatric geneticists, genetic psychophysiology can provide intermediate phenotypes that can help to bridge the gap between genes and complex behaviors such as personality traits and psychopathology, as well as provide insight into the functional role of the genetic variants that have been associated with complex behavioral or psychiatric phenotypes in genome-wide association studies. Finally, by linking genetics, cerebral and autonomic functioning, and behavior, genetic psychophysiology contributes to integrative, systems-level understanding of the nature of individual psychological differences and psychopathology.
The goal of this review is to summarize the state of the field of genetic psychophysiology, identify current trends, discuss some methodological problems and caveats, and outline future directions in research. It is important to mention that this review is not intended to provide an exhaustive coverage of all genetic studies relevant to psychophysiology; for a detailed discussion review earlier studies, we refer the reader to several excellent reviews published previously (Boomsma et al., 1997; de Geus, 2002; van Beijsterveldt and van Baal, 2002). The present review is limited to traditional measurement modalities in psychophysiology and does not cover the field of so-called “imaging genetics” (genetic studies of fMRI phenotypes) as this line of research deserves a separate review. This review does not cover substantial literature on EEG abnormalities in hereditary neurological disorders such as epilepsy and genetic syndromes such as fragile X and chromosome 22q deletion as these are areas of more specialized clinical interest. Finally, this review does not include studies in animals such as EEG studies in genetic strains of rodents or gene knock-out models.
2. Historical highlights
Shortly after the first systematic description of the human EEG by Hans Berger in 1928, large individual differences in EEG pattern were noted: when Adrian and Matthews, other pioneers of the EEG research, presented their own EEGs to the members of the Physiological Society in Cambridge in 1934, it was found that Adrian's EEG displayed a regular alpha-rhythm, whereas Matthews produced “no regular waves” (Niedermeyer, 1999). The first twin studies of EEG followed shortly (see Box 1 for the interpretation of twin findings). Based on visual analysis of EEG patterns, Davis & Davis (1936) concluded that the degree of intrapair differences in MZ twins did not exceed the differences between repeated EEG recordings of the same person. In 1958, the first large-scale genetic study of the human EEG (100 MZ and 98DZ pairs) using multiple quantitative variables was published (Vogel, 1958). Using measures such as percent-time alpha and the dominant EEG frequency, Vogel has shown that MZ twin differences on these quantitative measures do not exceed average differences between the right and the left hemisphere of the same person. Because test-retest reliability serves as a theoretical upper limit for MZ correlations and heritability, these early studies suggested that the stable, trait-like variance in the human EEG is almost entirely determined by genetic factors.
The first twin study of event-related brain potentials (ERPs) was published by Dustman and Beck in 1965 (Dustman and Beck, 1965). They showed that the correlation between waveforms of sensory evoked potentials elicited by light flashes measured between MZ co-twins is of the same order as the correlations between two separate recordings made in the same person, whereas DZ twins showed markedly lower resemblance.
About the same time, systematic twin studies of the EEG and ERP phenotypes began in Ravich-Scherbo's Genetic Psychophysiology laboratory in Moscow. These studies, summarized in an edited book titled “Problems of Genetic Psychophysiology of Man” published in 1978 (Lomov and Ravich-Shcherbo, 1978), have demonstrated heritability of resting EEG including spectral band powers, amplitude variability, autoregressive parameters of EEG rhythms, as well as reactive EEG changes in response to sensory stimulation.
Subsequent studies have utilized increasingly larger samples of twins, employed more advanced genetic analysis methods based on linear structural equation modeling and investigated an increasingly broad spectrum of psychophysiological phenotypes. By now, several large-scale studies of psychophysiological phenotypes involving hundreds and even thousands of twins have been conducted, including Free University of Amsterdam (Dorret Boomsma and Eco de Geus), University of Minnesota (William Iacono), University of Southern California (Laura Baker), University of Queensland (Margaret Wright and Narelle Hansell), Washington University in St. Louis (Andrey Anokhin), and other research centers.
Most recently, with the advent of molecular genetics techniques allowing for direct measurement of DNA variation, we have seen an explosion of studies attempting to associate psychophysiological characteristics with polymorphic variants of various “candidate” genes that presumably play a role in specific aspects of the central nervous system functioning. Although the increasing availability and affordability of genotyping data offers new and exciting opportunities for genetic psychophysiology research, findings from association studies based on small samples are very prone to false-positive findings and should therefore be interpreted with great caution (this issue is addressed in greater detail below).
3. Methodological approaches
3.1. Overview
Below I introduce briefly methodological approaches and some key concepts relevant to genetic psychophysiology. Essentially, genetic psychophysiology is an application of human genetics methods to psychophysiological phenotypes; therefore, methodological approaches and problems are largely the same as in other areas of human genetic research dealing with complex phenotypes. Unlike classical “Mendelian” phenotypes that show a simple mode of inheritance and a relatively straightforward relationship between the phenotype and a single gene, a complex phenotype is a product of many genes and environmental factors, and the relationship between genotype and phenotype usually involves multiple mediating pathways and mechanisms. With rare exceptions, most psychophysiological indicators of individual differences are complex phenotypes.
There are two major directions of genetic investigation of complex phenotypes such as psychophysiological traits. The first line of research involves elucidation of genetic architecture of the phenotypes using the assessment of heritability and genetic correlations among different variables using biometrical genetic analysis of twin and family data. The second direction of research focuses on the identification of specific genes contributing to overall heritability using linkage and association studies. There are excellent reviews and primers on the genetic analyses of twin data (Boomsma et al., 2002; Evans et al., 2002; Posthuma et al., 2003; Rijsdijk and Sham, 2002; van Dongen et al., 2012) and genetic association analysis (Hardy and Singleton, 2009; Hirschhorn and Daly, 2005; Wang et al., 2005; Yang et al., 2013). A reader interested in these methodologies is advised to consult with these sources. A brief overview of these methods is provided below.
3.2. Assessment of heritability and genetic covariance using the twin method
The primary goal of twin studies is estimation of heritability, or the proportion of the total variance of the trait that can be explained by genetic variation. In other words, heritability is a quantitative measure of the extent to which observed individual differences in a trait of interest (e.g. ERP component amplitude) emerge as a result of genetic differences among individuals. Heritability can be expressed in percentage units and varies from 0 to 100%. By definition, the remainder of the variance in the trait is caused by non-genetic (environmental) factors that can be further subdivided into two categories: shared environmental factors representing those aspects of the environment that are common to co-twins (e.g. ethnicity, culture, family, neighborhood) and therefore tend to increase their similarity, and non-shared, or individual, environment that includes environmental factors and experiences that are unique to each of the co-twins and therefore tends to decrease twins’ similarity. In addition, genetic factors can be subdivided into additive (reflecting additive effects of genes contributing to the trait) of and non-additive (reflecting non-additive allelic interaction including within-locus dominance and between-loci epistatic interaction). These components of variance can be estimated by fitting linear structural equation models (SEM) to the observed twin data (see Box 1). Importantly, MZ twins share 100% of their segregating genes, whereas DZ twins share only 50% on the average, the same as non-twin siblings. However, both MZ and DZ twins reared together share their environment to the same extent (one of the key assumptions of the twin method). The model fitting approach provides tests of different models that explain the variance in the trait by some combination of genetic, shared environmental, and individual environmental factors. Goodness of fit of these models can be compared and parameter estimates obtained for the best-fitting model. These parameter estimates yield the measure of heritability.
Importantly, non-shared environmental variance also includes the measurement error, which is an important consideration for the selection of psychophysiological characteristics for genetic analysis. Because only stable, trait-like individual differences can be heritable, in most cases test-retest reliability can be regarded as the upper bound for heritability.
There are a few important attributes of heritability that should be taken into account when interpreting the results of twin studies. First, heritability is a characteristic of a population and cannot be applied to an individual. Second, heritability applies to a given population at a given moment in time (although most psychophysiological traits show a good convergence of heritability estimates obtained in different populations at different times).
Of course, it would be a simplification to say that assessment of heritability is the only goal of twin studies. Twin data provide rich information that can be used to address many additional questions. Multivariate analysis of twin data can be used to estimate genetic correlations between different traits, i.e. the extent to which genetic influences on two or more different psychophysiological characteristics are common or specific. For example, one may ask the question whether individual differences in novelty or oddball P3, or resting EEG and ERPs are influenced by same or different genetic factors. The application of this multivariate approach is illustrated below (section 4.2.8) by showing that substantial proportion of genetic variance in P3 amplitude is shared with resting-state EEG power. Another extension of the classical twin method is longitudinal genetic analysis that permits us to address such questions as whether the strength of genetic influences (heritability) changes with age, whether same or different genes influence the trait at different stages of development, and whether the rate of developmental changes is influenced by genetic factors (McArdle, 2006; Posthuma et al., 2003). Analysis of twin data can also reveal sex differences in genetic and environmental influences, i.e. whether heritability is different in males and females and whether the trait is influenced by the same or distinct genetic factor in the two sexes. Finally, data obtained from twins reared apart allow researchers to control for shared familial environment. For a more detailed overview of the twin method and its various extensions, the reader is referred to methodological reviews (Boomsma, 2002; Rijsdijk, 2002; Dongen, 2012).
The classical twin method provides important information about the genetic and environmental origin of individual differences, as well as commonality versus specificity of genetic influences on different phenotypes, but it does not specify genes influencing the trait. The latter goal can be achieved by genetic linkage and association studies.
3.3. Finding specific genes using genetic linkage and association methods
Genetic linkage refers to co-segregation of alleles of two different loci in families due to their physical proximity on a chromosome. If two loci reside very close on the same chromosome, they are unlikely to be segregated during the meiosis and will be transmitted together to the offspring. Thus, a linkage study is in essence an analysis of co-segregation of two loci in families, one of which is observed “marker” with known location on the chromosome, and the other is a latent locus containing a genetic variant underlying a phenotype of interest such as a disease. If a certain marker co-segregates with the phenotype, it can be inferred that a gene contributing to this phenotype is located in the same chromosomal region as the marker. Linkage analysis can provide the chromosomal location of the putative gene with a certain degree of confidence and resolution, but the identification of the gene requires further efforts such as DNA sequencing. Linkage studies are now becoming rare, as they are being surpassed by association studies.
Genetic association refers to the co-occurrence of a certain allele of a genetic marker and the phenotype of interest in the same individuals at above-chance level. Unlike linkage studies that require family data, genetic association studies of complex phenotypes can be conducted using samples of unrelated individuals, although related individuals can also be included, provided appropriate corrections are applied. Association studies fall into two broad categories: candidate gene association studies and genome-wide association studies (GWAS).
Candidate gene studies focus on genetic polymorphisms selected by their biological relevance to the studied phenotype. Usually, these are functional polymorphisms, i.e. their selection is based on the evidence that they produce functional effects at the molecular and cellular level such as changes in gene expression, enzyme activity, or receptor characteristics. Most of the association studies involving psychophysiology-relevant phenotypes were conducted using this approach. The Candidate gene approach has obvious strengths: it is hypothesis driven, utilizes genetic variants that are likely to be causal variants, and therefore has a strong potential to provide a mechanistic explanation for the observed association. Finally, it usually involves a limited number of statistical tests, thus mitigating the multiple comparisons problem. Consequently, this analysis does not require very large samples, which is an important consideration for phenotypes that are difficult and costly to measure, such as psychophysiological or neuroimaging phenotypes. However, in recent years, this approach has drawn much criticism for its inherently restrictive nature, i.e. limiting the search for genes involved in the determination of complex phenotype to a handful of apriori selected variants while neglecting the rest of the genome (other issues related to candidate gene association studies are discussed in greater detail in the “Problems” section of this review).
In contrast to the candidate gene approach, the GWAS approach is largely an exploratory approach without any prior knowledge or hypothesis about the genes or underlying biological mechanisms; it involves scanning the entire genome for possible associations using hundreds of thousands of markers providing a dense coverage of every chromosome. The obvious benefit of this approach is that it provides an exhaustive coverage of the whole genome. However, it also has some caveats. First, as in linkage studies, markers showing an association signal may not necessarily be causal variants, and the latter still need to be determined by fine mapping and sequencing of the region. Second, due to a massive multiple testing problem inherent to this approach and, consequently, the need to adjust the significance threshold, very large samples (of the order of thousands or even tens of thousands) are necessary to conduct a GWAS study with sufficient statistical power. In GWAS studies of psychopathology phenotypes conducted to date, this problem is further exacerbated by small effect sizes necessitating further increase in sample size in order to detect a significant effect. Although there is some hope that effect sizes for “brain-based” phenotypes may be larger, for the study of psychophysiology phenotypes the GWAS approach remains cost-prohibitive, though not impossible, especially if multi-center collaboration using a standardized set of measures is established. A compromise approach would be an extended version of the candidate gene design, with multiple genes selected based on bioinformatics evidence for their involvement in the biological pathway relevant to the studied phenotype. A sufficiently dense coverage of these genes may reduce the number of markers to thousands or perhaps even hundreds, which may be a realistic number for a study involving hundreds, rather than thousands, of subjects. For a more detailed description of genetic concepts and methods, as well a thorough discussion of the strengths and weaknesses of different approaches to the study of genetic association, the reader is referred to appropriate review articles (Attia et al., 2009a, b, c; Hardy and Singleton, 2009; Hirschhorn and Daly, 2005; McCarthy et al., 2008; Wang et al., 2005).
The application of genetic methods and approaches described above to psychophysiological characteristics has been increasing exponentially over recent years. These studies have generated a plethora of interesting findings that have greatly advanced our understanding of how genes influence the brain and, ultimately, shape individual differences in behavior, both normal and abnormal. Most notable of these advances are reviewed below, starting with the resting EEG as the most basic measure of brain functioning.
4. Advances
4.1. Resting-state EEG
4.1.1. Spectral band power
EEG recorded in the state of resting wakefulness is the most thoroughly studied psychophysiology-relevant phenotype. Early small-sample twin studies of the EEG (mentioned in Section 2) were followed by larger-scale studies using quantitative analysis of EEG time series and formal estimation of heritability.
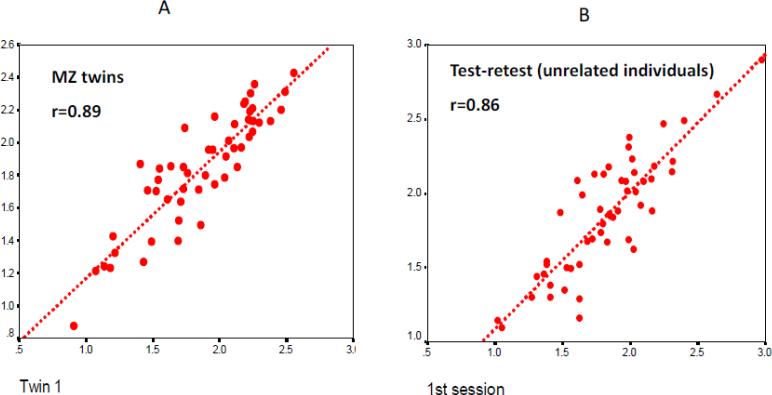
A twin study of EEG spectral characteristics (Lykken et al., 1974; Stassen et al., 1988b) found a striking similarity of EEG power spectra in MZ twins and much lower resemblance in DZ twin pairs. Intrapair MZ correlations were high and significant, with their magnitude approaching twin correlations for morphological characteristics such as height and weight (r>.8). Corroborating earlier observations, Lykken et al. demonstrated that across different quantitative characteristics of the resting EEG, the magnitude of MZ intrapair correlations is of the same order as the corresponding test-retest correlations within the same individual. Furthermore, this study employed a unique sample of MZ twins reared apart. Twin correlations for EEG characteristics in this sample were virtually at the same level as for MZ twins reared together (Lykken et al., 1974; Stassen et al., 1988a), suggesting that twin resemblance results from shared genetic, rather than shared environmental, factors. Because MZ twins reared apart share their genotypes but not environment, their phenotypic resemblance is due solely to their genetic commonality. Consequently, correlation between MZ twins reared apart can serve as a direct estimate of heritability. Another large twin study has yielded highly convergent results, indicating very high heritability of spectral band power (van Beijsterveldt et al., 1996). Collectively, these studies suggest that the stable (trait-like) part of EEG variance is almost entirely determined by genetic factors, with little contribution from the shared environmental factors. Sample sizes tended to increase in subsequent studies, providing more accurate estimates of heritability, and the results were remarkably consistent across studies, pointing to a very high heritability of the resting-state human EEG characteristics. Of different EEG frequency bands, alpha band power (8-13 Hz) tends to yield the highest heritability estimates (Anokhin et al., 2006a; Christian et al., 1996; Lykken et al., 1974; Smit et al., 2006; van Beijsterveldt et al., 1996; Vogel, 1970b). Fig. 2 illustrates intrapair twin correlations with respect to alpha-band power (unpublished data from the author's laboratory).
Fig. 2.
Twin resemblance for resting-state EEG alpha-band power. A. Scatterplot of intrapair correlation in monozygotic twin. One co-twin is plotted against the other co-twin. B. Scatterplot of test-retest correlations in unrelated individuals. Data from the first EEG recording session is plotted against the second EEG recording from the same individual, with the average interval between two sessions of about 2 weeks.
Furthermore, heritability estimates obtained using family data showed a good convergence with the results of twin studies. In one study, relative EEG power data obtained from 298 members of 45 families were subjected to principal component analysis. The alpha-power factor accounting for 38% of the total variance showed highly significant parent-offspring (r=.38) and sibling (r=.41) correlations, suggesting about 0% heritability (Anokhin, 1989). This convergence suggests that correlations between first-degree relatives obtained in family studies represent genetic transmission, rather than similarity arising from sharing the same environmental factors. A study of non-twin siblings has shown somewhat lower heritabilities of the resting EEG power assessed using both monopolar and digitally constructed bipolar derivations(Tang et al., 2007). Lower heritability estimates in the latter study can be explained by a very broad age range of 7 to 70 years which may lead to additional non-familial variance that is difficult to account for statistically. Both family (Anokhin, 1989) and twin (Zietsch et al., 2007) studies employing multivariate genetic analysis of absolute power measures of EEG frequency bands point to high phenotypic and genetic correlations among powers in different frequency bands, suggesting the existence of a common genetic factor influencing the overall amplitude of EEG oscillations. As mentioned above (section 3.1), bivariate genetic analysis permits a decomposition of covariance between two variables into genetic and environmental components and thus the assessment of the degree of overlap between genetic factors influencing two variables.
While most of twin EEG studies were conducted in young or middle-aged adult samples, several studies specifically addressed developmental aspects of EEG heritability. van Baal et al. (1996) examined heritability of absolute and relative powers in the main EEG spectral bands in 167 MZ and DZ pairs of 5 year old twins and found very high (80%) heritability for theta, alpha, and beta-1 bands, with somewhat lower heritabilities for delta and beta-2 bands. Another study from the same laboratory found even higher heritabilities (76-89%) of similar spectral EEG parameters in a sample of 16 years old adolescents (van Beijsterveldt et al., 1996). Both studies included opposite-sex DZ twins, which allowed the authors to test for possible differences in heritability between males and females, however, no significant sex differences were observed. One study investigated EEG in infant twins aged 7-12 months and found modest heritabilities and a contribution of shared environmental factors to the main EEG frequency bands; however, heritability tended to increase by the end of the first year of life (Orekhova et al., 2003). In summary, developmental twin studies suggest that the strength of genetic influences increases rapidly during development, and heritability reaches adult values already in young children. However, lower heritability in infants can be explained by larger measurement error due to movement and the difficulty of obtaining clean recordings. This alternative explanation can be tested by comparing heritability with test-retest heritability at different ages.
Very few studies addressed genetic influences on human EEG at the other end of the lifespan. One of the early twin studies of older adults (Heuschert, 1963) including 26 MZ twins at the age of 50-79 years noted a striking intra-pair similarity with respect to both general EEG characteristics and specific aging-related EEG changes such as slowing of the alpha-rhythm, increased variability of alpha-frequency, and diffuse slow-wave activity. This similarity was observed despite substantial differences in lifestyles and experiences of the twins. Interestingly, a blind matching of co-twins using their EEG was successful in 24 cases out of 26. This study, although based on a small sample, points to the importance of genetic factors in brain aging and suggests the possibility that quantitative EEG could potentially serve as a marker of genetically determined age-related changes in brain function, both normal and pathological (e.g. in Alzheimer's disease). This possibility should be explored in future twin EEG studies focused on the genetic determinants of individual differences in brain aging.
In summary, quantitative characteristics of the resting-state EEG are strongly influenced by genetic factors and, collectively, represent one of the most heritable human traits.
4.1.2. Frontal EEG asymmetry
Spectral band powers of the resting EEG reflect basic characteristics of neural oscillatory activity. Overall, there is a relative consensus that abundant alpha-band oscillations reflect cortical deactivation, whereas scarce or absent alpha oscillation and increased higher frequency activity reflects increased level of cortical arousal. However, functional interpretation of individual differences in spectral characteristics of the resting EEG is not very straightforward due to the lack of consistent evidence for their correlations with individual differences in behavior.
One notable exception is a special resting EEG phenotype, frontal EEG asymmetry (FA), which is expressed as the difference in alpha power between the left and right anterior scalp regions. A good test-retest stability of individual differences in FA was reported (Tomarken et al., 1992). Davidson et al. suggested that greater left than right alpha-band power presumably indicating lower left than right level of prefrontal activation is associated with stronger withdrawal motivation and increased vulnerability to depression, while the opposite pattern of frontal EEG asymmetry is associated with stronger approach motivation and low risk for depression (Davidson, 1998; Davidson and Fox, 1982). Over the past two decades, this attractive hypothesis has generated an extensive literature, however, evidence for the association between FA, depression, and relevant personality traits and behavioral measures remains somewhat mixed (Allen and Kline, 2004; Davidson, 1998; Hagemann, 2004), Nevertheless, FA is still considered by many researchers as an indicator of affective style and risk for internalizing psychopathology (Allen et al., 2004; Allen and Kline, 2004; De Pascalis et al., 2013; Gatzke-Kopp et al., 2012).
Family and twin studies suggest modest but significant heritability of FA in different age groups. Analysis of familial transmission using 27 families with EEG available from both parents and at least two offspring showed a significant correlation of r=.46 between mean parent and offspring values, which is a proxy measure for heritability, with the assumption that familial resemblance arises from genetic, rather than environmental commonality of family members (Anokhin and Rohrbaugh, 1998). This study also showed a significant familial association between FA and comorbid depression and alcoholism. However, generalizability of this study may be limited because the sample included families selected for high incidence of alcoholism, along with control families.
A small-scale twin study of FA yielded mixed results, with evidence for heritability obtained only under specific conditions (Allen et al., 1997; Coan et al., 2003). A subsequent twin study of FA has shown modest but significant heritability (27%) for asymmetry measured using F3 and F4 scalp sites but not more lateral (F7 and F8) sites, with no evidence for the contribution of shared familial environment (Anokhin et al., 2006a). Consistent with previous studies, heritability of alpha power at F3 and F4 locations was very high, 87% and 86%, respectively. Another twin study yielded slightly higher heritability estimates for FA in young adults (32% in males and 37% in females), however, genetic influences were non-significant in middle-aged adults. Smit et al. also found modest but significant correlations between FA and a “risk factor score” for anxiety and depression, a composite measure derived from several self-report assessments, with the relationship being mediated by genetic factors (Smit et al., 2007b). Finally, a recent study using a large sample of 9-10-year-old twins yielded low (11-28%) but significant heritability estimates for FA (Gao et al., 2009). More recently, studies implicating specific genetic variants in FA began to emerge. An association between FA and serotonin receptor-1a gene polymorphism (HTR1a) has been reported, although effect sizes were small, with d ranging from .03 to .06. (Bismark et al., 2010). Another study found that a variant of the dopamine receptor D4 gene (DRD4) moderated the relation between the pattern of resting frontal EEG asymmetry at 9 months and regulation and attention at 48 months in a sample of typically developing children (Schmidt et al., 2009).
In summary, twin, family and emerging genetic association studies consistently point to significant but modest genetic influences on FA, however, the utility of this EEG measure as an endophenotype for internalizing disorders may be limited due to its low heritability and somewhat inconsistent evidence for its association with depression and anxiety.
4.1.3. Other resting EEG measures: alpha rhythm frequency, non-linear dynamics, and functional connectivity
In addition to spectral band powers, spontaneous EEG oscillations can be quantified in a variety of other ways, with different methods emphasizing distinct aspects of neural dynamics, with specific functional significance attributed to many of them. These EEG characteristics also showed varying degree of heritability in twin studies.
One of such measures is the frequency of alpha-rhythm, the most prominent form of resting-state brain oscillations. Individual differences in alpha frequency have been shown to be associated with cognitive performance (Klimesch et al., 1993) and general intelligence (Anokhin and Vogel, 1996). Lykken et al. (1974) reported high heritability of individual alpha-rhythm frequency (84%). A more recent study of adolescent twins yielded remarkably consistent results, with heritability of individual alpha frequency estimated at 81%, suggesting that alpha frequency is a highly heritable index of brain functioning (Smit et al., 2006).
Other studies have focused on EEG measures of functional connectivity between distant cortical regions. A theory of spatiotemporal organization of cerebral function (Livanov, 1977) posits that dynamical functional networks supporting goal-directed behavior are formed by the synchronization of neural oscillations in distributed but functionally connected brain regions. One of the most frequently used metrics of large-scale synchronization of cortical oscillations is EEG coherence, a measure of phase consistency between two oscillatory processes at a given frequency which is analogous to a linear correlation.
A study of 5-6 year old twins showed significant heritability of inter-electrode EEG coherence, with stronger genetic influences (heritability around 60%) observed for long-distance frontal-parietal and central-parietal connections within the hemispheres, whereas most inter-hemispheric coherences showed lower heritability (Ibatoullina et al., 1994). These findings based on a moderate sample of twins were corroborated in a much larger scale study of 5-years old twins that also reported heritability estimates of 50-60% (van Baal et al., 1998), however, the latter study also found substantial heritability of inter-hemispheric connections. Furthermore, van Baal et al. have followed up their sample to age 7 and found that EEG coherence values changed significantly from age 5 to age 7, and their heritability increased for occipital cortical connections. Longitudinal genetic analysis indicated the emergence of new genetic influences on frontal-parietal coherences, consistent with available evidence for rapid cognitive development during this age period (van Baal et al., 2001). A study from the same group using an adolescent sample of twins (age 16) yielded very similar results, with around 60% of individual differences in inter-electrode coherence explained by genetic factors, except the delta band, where heritabilities were somewhat lower (van Beijsterveldt et al., 1998a).
Other measures of spatial connectivity can provide additional information about spatial synchronization of EEG oscillations. For example, synchronization likelihood (SL) provides a measure of the statistical relationships between two time series that is sensitive to both linear and non-linear interdependencies of the signal and unbiased after narrow band filtering (Stam, 2005). It is presumed that this measure reflects functional coupling between spatially distributed oscillating neuronal assemblies. Using a large sample of adult twins and extended twin families, Posthuma et al. (2005) found that SL is moderately to highly heritable (33–70%) especially in the alpha frequency range (8–13 Hz). Other studies by the same group (Smit et al., 2010; Smit et al., 2008) extended these findings to graph-theory based measures derived from synchronization likelihood. Two global network parameters, clustering coefficient (the proportion of interconnected local nodes) and average path length (average number of steps from a network node to other nodes) showed substantial heritabilities (38 and 46% on the average, respectively). Thus, the extent to which individual brain dynamics approaches the properties of the “small-world” network, i.e. combining modularity and global information integration, appears to be influenced by genetic factors.
In addition to the measures of oscillations in traditional frequency bands, one twin study addressed slower EEG dynamics using a measure of amplitude modulation, i.e. the temporal structure of the waxing and waning of the amplitudes of spontaneous alpha and beta EEG oscillations on time scale of 1 to 20 seconds using detrended fluctuation analysis (Linkenkaer-Hansen et al., 2007). There is evidence that correlations in brain oscillations on a large time scale (up to tens of seconds) may be important for performance of tasks that require coordination of neuronal activity on multiple time scales (Linkenkaer-Hansen et al., 2005). This study showed high (around 60%) heritability of “long-range temporal correlation measures” across the entire scalp; importantly, these measures were independent from the overall frequency-band power.
Another approach to EEG analysis and interpretation is based on the non-linear dynamic systems theory dealing with complex, aperiodic systems capable of self-organization (Elbert et al., 1994; Pritchard and Duke, 1995). From this perspective, the EEG time series can be viewed as a complex structure reflecting the complexity of the dynamics of the underlying neural generators. It has been shown that dimensional complexity of the EEG can depend on the number of independently oscillating neuronal networks in the cortex and reflect a fine-tuned balance between chaotic and non-chaotic neuronal dynamics, a property that has important implications for normal adaptive brain functioning. Evidence from different neuroimaging modalities suggests that the human brain is a self-organized, large-scale complex system that operates in a critical state on the edge of order and chaos, which provides optimal conditions for flexible transitions between mental states as well as information processing, storage, and retrieval, whereas dysregulation of this fine-tuned balance between chaotic and non-chaotic brain dynamics can lead to breakdown of behavior observed in psychopathology (Birbaumer et al., 1995; Elbert et al., 1994; Kitzbichler et al., 2009; Lutzenberger et al., 1995). A twin study using a pointwise dimension (PD2) measure showed that EEG complexity is highly heritable (65%) in the resting eyes closed condition, with somewhat lower heritability (about 50%) in the eyes open condition, suggesting that this complexity measure is a useful indicator of genetically transmitted characteristics of cortical dynamics (Anokhin et al., 2006b).
4.1.4. EEG in different functional states
While most of the twin studies of the human EEG focused on the resting state oscillations, some studies also examined heritability of EEG characteristics in other functional states, such as conditions following administration of psychoactive drugs and sleep. It is important to note that genetic influences on EEG responses to challenge (e.g. pharmacological or psychological) can be distinct from genetic influences on resting-state EEG because challenge can activate neural networks that may be “silent” during resting baseline and thus help to reveal genetic influences specific to these networks.
The twin design can be a useful tool in pharmacogenetics research, since it permits the detection and estimation of genetic influences on the psycho- and neuropharmacological effects of drugs. However, relatively few studies have investigated genetic influences on EEG changes produced by the administration of psychoactive drugs. Propping et al. (1977) examined individual differences in EEG changes after ethanol ingestion in twins. Acute alcohol produces a significant increase in theta- and alpha-power and decrease in beta-power, suggesting a sedative effect. Importantly, MZ twins showed greater similarity with respect to EEG response to alcohol than DZ twins, suggesting that individual differences in the acute effect of alcohol on the brain are at least partially genetically determined. Furthermore, alcohol-induced changes depended on individual differences in the baseline EEG: individuals with irregular, low amplitude alpha-rhythm showed the largest gain in alpha amplitude, while those with abundant, high amplitude alpha activity showed a blunted EEG response to alcohol. Based on the evidence that “desynchronized”, low-alpha EEG is indicative of higher level of tonic arousal and is prevalent among alcoholics, the authors proposed a model of alcoholism risk, according to which individuals with higher level of tonic central nervous system excitation may be predisposed to alcoholism because they experience greater relaxation after alcohol intake and therefore may be more sensitive to the rewarding and reinforcing effects of alcohol. Finally, MZ similarity on EEG measures tended to increase, while DZ similarity tended to decrease after alcohol intake, suggesting that genetic influences on brain oscillations are increased in the state of alcohol intoxication. These results have been corroborated in a later study (Sorbel et al., 1996) that, using a larger twin sample, showed increased heritability of EEG spectral band power in all frequency bands after alcohol intake, which was mainly due to decrease in within-pair differences of MZ twins. Taken together, these studies suggest that EEG under alcohol may be influenced by an additional set of genes specific to this condition, perhaps with non-additive affects, and these genetically determined differences in brain function may be important for the understanding of individual differences in the effects of alcohol on mood and behavior that can mediate susceptibility to alcohol use disorders.
Apart from alcohol, few other pharmacological challenges have been used in the context of genetic research on the human EEG. Hynek et al. (1978) examined the effects of chlorpromazine (a dopamine antagonist) on the EEG in twins and found that drug-induced EEG changes, most notably, an increase in beta-band power, are influenced by genetic factors. Although drug challenge studies using the twin design are very scarce, they demonstrate that this approach can be used to determine whether and to what extent individual differences in response to psychoactive drugs are influenced by genetic factors.
Several twin studies examined EEG recorded during sleep. In the first study of this kind, EEG was recorded from 6 pairs of twins for 4 consecutive nights. MZ twins were highly concordant with respect to periodic changes in sleep stages, whereas DZ twins were not (Zung and Wilson, 1966). Subsequent studies reported significant genetic influences on sleep stages 2, 3, 3+4, and 4, as well as rapid eye movement (REM) density, however, no genetic influences were found for total sleep period, period of sleep, total sleep time, sleep onset latency, and REM latency (Linkowski, 1999). A more recent study also involving EEG recording over four consecutive nights suggested a very high heritability (>90%) of the spectral power of the sleep EEG in the 8 to 15.75 Hz range (De Gennaro et al., 2008). Another study investigated genetic influences on EEG composition during sleep and found significant genetic influences on some aspects of sleep architecture including the duration of sleep stage 3 and REM sleep, as well as spectral composition of non-REM sleep, particularly in delta, alpha, and theta bands (Ambrosius et al., 2008). Finally, most recent studies have identified specific genetic variants contributing to individual differences in sleep EEG patterns, including functional polymorphisms of the clock gene PER3 and of genes contributing to signal transduction pathways involving adenosine (ADA, ADORA2A), brain-derived neurotrophic factor (BDNF), dopamine (COMT) and prion protein (PRNP) (reviewed inLandolt, 2011).
4.1.5. Familial transmission of special EEG variants
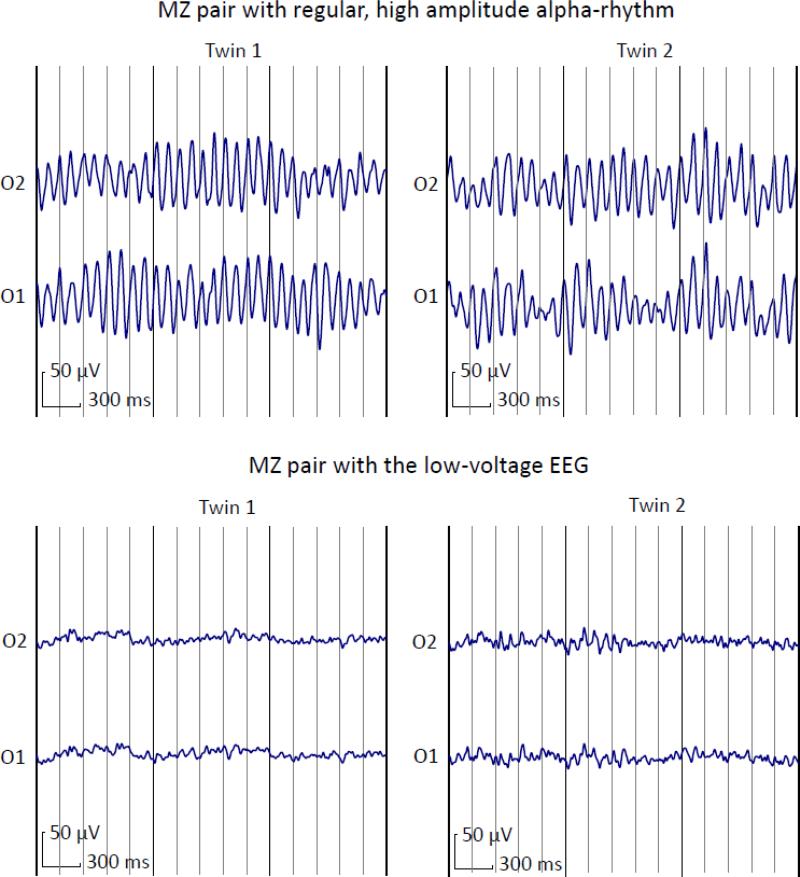
Individual differences in the EEG can be characterized using dimensional and categorical approaches. Although most genetic studies of the human EEG have been dealing with continuous variables such as frequency band power or coherence, other studies took a categorical approach focusing on the identification of distinct EEG types, or variants. This typological approach to the genetics of the human brain function was pioneered by F. Vogel, who has identified several specific discrete EEG variants including low-voltage (N), low-voltage borderline (NG); monotonous alpha waves (R); occipital fast alpha-variants (BO); fronto-precentral beta-groups (BG), and diffuse beta-waves (BD) (Vogel, 1970a). Most of these variants are peculiar enough to be distinguished by visual analysis, although quantitative measures were also proposed.
For example, the N variant (in later work also referred to as LVEEG) is characterized by the absence of alpha-rhythm, even in the eyes closed condition. Since alpha-rhythm is the most prominent form of oscillatory activity in most of the individual EEG recordings, this variant is readily recognizable by the naked eye, however, it can be objectively quantified using such measures as percent-time alpha (alpha-index) or alpha-band power. Family studies of these EEG variants conducted in different samples, one of them including large pedigrees (Anokhin et al., 1992; Vogel, 1970a), showed that familial transmission of some of these EEG variants, most notably, the LVEEG, is consistent with a simple Mendelian mode of inheritance, with about 50% of the offspring of a LVEEG carrier showing this EEG pattern and a clear segregation of the offspring into two categories, one with the LVEEG and the other showing varying degree of alpha-activity, with the distribution of quantitative alpha measures similar to that in the general population. Importantly, Vogel et al. have demonstrated modest but significant differences between these genetically transmitted EEG variants with respect to cognitive performance, emotional reactivity and personality(Vogel et al., 1979a; Vogel et al., 1979b).
4.1.6. Linkage and association studies of the resting-state EEG
The LVEEG phenotype described above was chosen for the first genetic linkage study of a human EEG phenotype (Anokhin et al., 1992; Steinlein et al., 1992). While no significant linkage was detected in the entire sample, admixture test for linkage heterogeneity was highly significant, indicating a strong linkage to chromosome 20q in a subset of families and thus suggesting a genetic heterogeneity of this EEG phenotype. Genetic heterogeneity refers to the possibility that the same phenotype may have different genetic underpinnings in different individuals and families. Different allelic variants in the same or different genes may lead to the same phenotypic effect, e.g. mutations in different genes causing disruptions at different stages of a metabolic chain can lead to the deficiency of the end product, and different mutation may be responsible for the transmission of the “deficient” phenotype in different families. Overall, these findings provide further support for the transmission of LVEEG phenotype in families as a discrete, categorical trait and point to the chromosomal location of the underlying genetic locus, however, specific genes contributing to the LVEEG phenotype remain to be determined. More recently, a significant association between alpha power and a serotonin receptor gene (HTR3B) polymorphism was reported, yet, the association was present in two samples (American Caucasians and Plain Indians) but not in the third and the largest of the studied samples (Finnish)(Ducci et al., 2009). Another study performed in American Indian population found evidence for significant linkage on chromosomes 1 and 6 (Ehlers et al., 2010).
These variable findings obtained in different populations support the notion of genetic heterogeneity of EEG variation suggested by the first linkage study (Anokhin et al., 1992). Importantly, other studies have provided evidence for functional significance of the LVEEG variant. Enoch et al. reported the association between the LVEEG phenotype, anxiety disorders, and alcoholism, with the strongest association observed for a subtype of alcoholism associated with anxiety disorders (Enoch et al., 1995; Enoch et al., 1999). Finally, it should be noted that the prevalence of the LVEEG phenotype shows considerable differences across different human populations and ethnic groups (Anokhin et al., 1992; Ehlers and Phillips, 2003; Ehlers et al., 2004). This observation has important implications for association studies, since group differences in both the frequency of the phenotype and the frequency of a genetic variant can lead to spurious association (see section 6.2.2). Thus, categorical approach to phenotypic characterization of the human EEG based on the identification of specific patterns of activity can be an alternative to the analysis of separate quantitative characteristics, however, there is a need for more accurate methods of objective classification of EEG patterns. Given the recent advances in quantitative methods of EEG analysis, source localization, and multivariate statistical techniques, this objective is quite tenable. A refinement of specific EEG phenotypes can lead to further progress in the identification of single genes affecting brain functioning.
Other linkage and association studies used quantitative phenotypes, mostly spectral band powers. A genome-wide association study (GWAS) performed in a sample of 322 Plains American Indians reported a significant association between theta-band EEG power and a SNP in the SGIP1 gene (SH3-domain GRB2-like endophilin-interacting protein 1). A similar trend was observed in North American Caucasian replication sample, but the association was non-significant after multiple comparisons correction (Hodgkinson et al., 2010).
A candidate gene association study in a sample of young adult females found that 10-repeat allele carriers of 5HTT (serotonin transporter) polymorphisms alleles conferring low transcription efficiency tended to have reduced EEG power, particularly in the gamma frequency band (Lee et al., 2011b). Another study by the same group (Lee et al., 2011a) reported a significant effect of the catechol-O-methyl-transferase (COMT), gene on the strength of the resting EEG spatial connectivity in the left frontal-temporal scalp area measured using the time-frequency mutual information method, such that connectivity strengths increased in carriers of the Val allele which causes a 3- to 4-fold increase in the enzyme activity and, consequently, reduced prefrontal DA levels (Bilder et al., 2004; Tunbridge et al., 2006). In a related analysis utilizing the same sample, the epsilon4 allele of the Apolipoprotein E (ApoE) gene, a known risk factor for cognitive impairments in Alzheimer's disease and normal aging, was associated with reduced EEG power, particularly in the alpha frequency band, as well as weaker inter-regional connectivity in the right hemisphere in a young population aged 19-21 (Lee et al., 2012). The the epsilon4 isoform variant is associated with reduced efficiency of proteolytic break-down of beta-amyloid, a peptide that builds up at abnormal levels in Alzheimer's disease (Jiang et al., 2008). Another study showed that among patients with Alzheimer disease, ApoE-4 allele carriers had reduced alpha activity in the left hemisphere (Canuet et al., 2012). A study involving younger and older adults showed that ApoE-4 carriers and non-carriers differed with respect to EEG reactivity to hyperventilation in an age-dependent manner, such that the younger ApoE-4 carriers had excessive reactivity of EEG to the HV, while older ApoE-4 carriers showed lower reactivity than non-carriers. Overall, ApoE-4 carriers showed more pronounced age-related decrease in EEG reactivity to hyperventilation, suggesting that ApoE-related pathology in old age may be mediated by vascular factors (Ponomareva et al., 2012).
A large study of sleep EEG (n=800) found a significant association between a single nucleotide polymorphism in the adenosine deaminase gene (ADA G22A) and sleep EEG characteristics, with carriers of the A allele showing higher power in delta and theta bands relative to non-carriers, suggesting higher sleep efficiency in A allele carriers (Mazzotti et al., 2012). Adenosin plays a role in sleep homeostasis, and A allele has been associated with decreased ADA activity and lower adenosine clearance (Battistuzzi et al., 1981), resulting in higher sleep efficiency.
Porjesz et al., using linkage analysis on a large sample mostly consisting of families selected for dense, multigenerational alcoholism, found evidence for linkage between the EEG beta-2 band (a quantitative trait derived by multivariate analyses of spectral band power) and a region on the short arm of chromosome 4 harboring a set of GABAA receptor genes. Since inhibitory GABA-ergic interneurons have been implicated in the generation of fast cortical oscillations, this finding lends itself to a plausible mechanistic interpretation. The authors concluded that, based on pharmacological data, the GABAA alpha-2 subunit may be the most likely candidate for their linkage findings (Porjesz et al., 2002a). Further analyses using improved genetic analytical approaches led to significant findings on chromosomes 1, 4, 5, and 15 with evidence of epistatic interaction between these loci(Ghosh et al., 2003). It is important to note that linkage analysis is just the first step to gene identification, since it only provides a probable chromosomal location of gene(s) influencing the phenotypic trait. Single-nucleotide polymorphisms (SNPs) offer an opportunity to test for association between the beta EEG traits and SNP located in the GABA receptor genes on chromosome 4. Subsequent linkage disequilibrium analyses of 69 SNPs within a cluster of four GABA(A) receptor genes performed by the same group found significant associations with both beta oscillations and alcoholism, yet, no coding differences were found between the high-risk and low-risk haplotypes, suggesting that the effect is mediated through gene regulation (Edenberg et al., 2004).
However, generalization of findings from COGA may be limited due to the selective nature of this sample containing a very high proportion of severe alcoholism cases and known effects of alcohol and other co-abused substances on the human EEG that often remain unaccounted for in genetic analyses; thus this interesting finding still awaits an independent replication in a general population-based sample. It is important that linkage analysis is just an important first step to gene identification, since it only provides a probable chromosomal location of gene(s) influencing the phenotypic trait. With the advent of SNPs, there is an opportunity to test for association between the beta EEG traits and SNP located in the GABA receptor genes on chromosome 4 using the same and other datasets.
4.2. Event-related brain potentials (ERPs)
Event-related potentials (ERPs) represent EEG changes in response to a discrete event relative to the baseline activity preceding that event. The ERP is averaged across trials and represents changes in the electric potential that are phase-locked to the even onset (evoked activity), and do not include activity that may be systematically related to the stimulus but does not show exact phase alignment with the stimulus. Historically, two types of evoked activity are distinguished: evoked potentials (EP) elicited by sensory stimuli and varying as a function of stimulus characteristics and event-related, or “endogenous” potentials that vary as a function of the subjective meaning of the stimulus, rather than its sensory characteristics.
4.2.1. Sensory evoked potentials
Earlier twin studies dealt mostly with evoked potentials elicited in different sensory modalities (for a more detailed review of genetic studies of evoked potentials, see van Beijsterveldt and van Baal (2002)). For example, Lewis et al. studied visual, auditory, and somatosensory evoked potentials in 44 MZ, 44 DZ, and 46 unrelated individuals and found that evoked response waveforms of MZ twins showed a consistently higher degree of similarity than those of the DZ or UR groups across all three sensory modalities and in most of the scalp areas studied. (Maryutina, 1994) used different types of stimuli and noted greater heritability for sensory stimuli relative to semantic stimuli, where shared environmental factors were also significant. Overall, studies of sensory evoked potentials show significant heritability of both amplitude and latency of early ERP components; however, differences in heritability between specific ERP parameters of conditions should be interpreted with caution because most of these earlier studies were based on relatively small samples of twins.
4.2.2. P3 component in oddball tasks
The P3 (P300) ER component is typically elicited in “oddball” tasks and associated with cognitive processing of relatively rare “target” task-relevant stimulus which is presented in the context of a more frequent “standard” stimuli that have to be ignored. Numerous studies have linked P3 to the processes of context updating, cognitive resource allocation, attention, and working memory (Polich, 2007). The oddball-P3 is the most extensively studied ERP phenomenon, perhaps because it was the first “endogenous” potential discovered (Sutton et al., 1965).
Genetic influences on P3 have been assessed in many twin studies that consistently showed significant genetic influences, although there was some variability in heritability estimates despite differences with respect to subject samples and ERP tasks across studies. Many of these studies are summarized in an excellent review and meta-analysis of twin studies of oddball-P3 conducted by van Beijsterveldt et al. (2002). In this analysis, the “AE” model including additive genetic (A) and non-shared environmental (E) factors was the best fitting one for both P3 amplitude and latency, and the overall estimate for heritability was 60% for P300 amplitude and 51% for P3 latency, with the remaining variance explained by unique environmental factors. It should be noted, however, that a study using repeated longitudinal assessments of P3 estimated heritability of the “stable” variance at 90% (Carlson and Iacono, 2006), suggesting that heritability of a single measurement is substantially attenuated by measurement error.
More recent studies have provided further evidence for genetic influences on P3 and addressed a number of related questions, including genetic covariation between P3 latency measured in a delayed response working memory task and working memory performance (Hansell et al., 2005), genetic specificity of P3 as evidenced by the lack of genetic overlap with other ERP measures such as MMN or P50 (Hall et al., 2006a), invariance of P3 heritability across the lifespan demonstrated using cross-sectional comparison of young and middle-aged adult cohorts (Smit et al., 2007a), genetic influences on age-related decrease of P3 amplitude using longitudinal analysis spanning ages from 17 to 23 (Carlson and Iacono, 2006) and genetic influences on neural oscillations contributing to P3 assessed using time-frequency decomposition of event-related EEG (Ethridge et al., 2012).
A number of linkage and association studies searched for specific genes contributing to heritability of P3 amplitude and other ERP characteristics documented by twin studies. Hill et al. (1998) reported an association between a polymorphic marker for D2 dopamine receptor gene (DRD2 Taq I, later localized in ANKK1 gene) and P3 amplitude in children of alcoholics, with A1 allele carriers showing reduced P3 amplitude. Another study (Anokhin et al., 1999b) has replicated this association in a sample of adults, mostly from families with alcoholism. Furthermore, this study found a significant interaction effect of this genetic polymorphism and tobacco smoking status on P300, such that smokers with A1 allele showed the largest P3 amplitude reduction. One possible explanation for this finding is that A1 allele increases the riskfor nicotine addiction, but this association is moderated by P3, with reduced P300 amplitudes facilitating addiction in A1 carriers and high P3 amplitude acting as a protective factor. According to this hypothesis, P3 is a moderator, rather than mediator, of genetic risk (Anokhin et al., 1999a). This hypothesis is consistent with the notion that P3 (which is ubiquitous across a variety of cognitive tasks) reflects the recruitment of cognitive control network, with higher P3 indicating higher cognitive control capacity and thus better impulse control. The proposed interpretation of P3 as an indicator of general, non-specific cognitive control capacity is supported by fMRI studies using oddball tasks(reviewed in Kiehl et al., 2005; Linden, 2005; Polich, 2007; Soltani and Knight, 2000) showing significant activation in frontal and parietal regions that are broadly consistent with regions implicated in a common, “superordinate” fronto-parietal system cognitive control network (Dosenbach et al., 2007; Naghavi and Nyberg, 2005; Niendam et al., 2012) as well as dorsal frontopriatal attention network supporting goal-directed (top-down) selection for stimuli and responses (Corbetta and Shulman, 2002).
Porjesz et al. (2002b) reported the results of linkage disequilibrium mapping of ERP phenotypes, including visual oddball P3 in a sample predominantly consisting of families with dense, multigenerational alcoholism. Of 351 highly polymorphic microsatellite markers tested for linkage separately with each of the EEG electrodes, none reached the nominal significance threshold (LOD score of 3), but several markers approached that value and were therefore considered “suggestive” of linkage; most notably, two markers on the chromosome 2 showed a trend to linkage with P3 amplitude at P4 and C4 locations. In addition to P3, the same study examined the N1 component obtained in the same VP3 paradigm and found significant linkage on chromosome 16. Although these results are very encouraging, drastic differences in the linkage results for neighboring electrodes (which typically show very high correlations with respect to P3 amplitude) raise important methodological question of whether P3 component at a given scalp location might reflect relatively independent processes influenced by distinct genetic factors. This issue should be addressed in future studies using e.g. independent component analysis and/or source localization in order to delineate genetic influences on dissociable neural processes contributing to the scalp-recorded P3 potential.
Apart from the traditional peak amplitude, several follow-up publications from the same group focused on event-related oscillations (EROs) assessed in the same ERP experiment. In contrast to the ERP measures that are derived from event-locked EEG signal in the time domain averaged across multiple trials, EROs represent the same signal in the frequency domain, allowing for the estimation of brain oscillations in different frequency bands that are associated with the event of interest. These studies reported significant genetic associations between EROs in the delta and theta band elicited by the target stimulus and various genetic polymorphisms including the cholinergic muscarinic receptor gene (CHRM2) (Jones et al., 2004), serotonin receptor gene HTR7 (Zlojutro et al., 2011), glutamate receptor gene (GRM8) (Chen et al., 2009), corticotropin releasing hormone receptor 1 gene (CRHR1) (Chen et al., 2010) and association with KCNJ6, a gene related a potassium inward rectifier channel demonstrated in a GWAS study at genome-wide significance level (Kang et al., 2012). These studies suggest that EROs can capture separate aspects of the neural response elicited by the target stimulus that are distinct from P3 amplitude and thus can serve as a promising phenotype for genetic studies. A study using coherence analysis of event-related EEG from an auditory oddball task (Winterer et al., 2003) found a significant association between polymorphism in exon 7 of the GABAB receptor 1 gene and EEG coherence in parietotemporal regions in both resting state and during the performance of auditory choice reaction time task.
In summary, twin studies consistently show substantial heritability of oddball-P3. Linkage and association studies have identified several genetic loci and candidate genes that may contribute to the variability in both traditional peak amplitude measure and more novel measures based on time-frequency decomposition of evoked and induced brain oscillations. However, the use of oddball P3 as an intermediate phenotype may be somewhat limited by the lack of specificity in its conceptual interpretation in terms of the underlying neural processes and cognitive correlates. Since multiple interpretations of P3 continue to exist focusing on context updating, attention, cortical inhibition, etc., it is difficult to link individual differences in P3 to variability in a specific cognitive process and its neural underpinnings. There is emerging evidence that P3 is composed of multiple overlapping oscillatory components (Rangaswamy et al., 2007), presumably originating from different sources. The location and time courses of neural generators contributing to P3 have been investigated using source reconstructions of scalp-recorded EEG, ERP recordings in patients with focal brain lesions, intracranial depth electrode recordings in humans and primates, fMRI registration during the performance of oddball tasks, and simultaneous EEG/fMRI registration (reviewed in (Linden, 2005; Polich, 2007; Soltani and Knight, 2000)) . These studies suggest that P3 generated by a distributed network of cortical regions including, most notably, the temporo-parietal junction (TPJ), medial temporal, medial frontal, and lateral prefrontal cortices, inferior parietal lobule, thalamus, as well as frontal-temporoparietal interaction and cortico-limbic interaction. Distinct processes contributing to P3 may have relatively independent genetic basis and functional significance, their associations with psychopathology need to be evaluated separately using paradigms emphasizing particular component processes (e.g. oddball or and novelty processing, visual and auditory modality), as well as advance signal analysis methods permitting the assessment of dissociable neural processes such as independent component analysis, time-frequency decomposition, connectivity measures, etc. It is possible that individual components contributing to P3 will show stronger associations with specific psychopathology phenotypes compared with the overall P3 response due to increased specificity of the neural processes represented by these components. Therefore, delineation of component processes giving rise to P3 potential holds great promise for both existing and future studies using P3 as an ERP endophenotype for psychopathology.
Apart from P3, however, other heritable ERP phenotypes with greater specificity with respect to underlying neurocognitive processes may be of great value. More recent studies have provided evidence for heritability of ERP characteristics measured in tasks focused on distinct aspects of cognitive processing and behavioral regulation. An overview of these studies follows below.
4.2.3. Mismatch negativity (MMN)
The mismatch negativity (MMN) is an automatic change-detection response of the brain elicited even in the absence of attention or behavioral task (Naatanen et al., 2012). MMN is typically elicited by auditory stimuli that deviate in some respect, such as frequency, intensity, or duration from the preceding more frequent stimuli and thus reflects the mismatch between the current sensory input and the memory representation of the preceding auditory stimuli. MMN is generated by the process of early change detection in the left and right auditory cortices and subsequent prefrontal activation. We are aware of only one published twin study of MMN (Hall et al., 2006b). In this study, both test-retest reliability and heritability of duration MMN amplitude was high (.66 and 68%, respectively), suggesting that the entire trait-like variance in MMN is genetically determined. However, this study was based on a modestly-sized sample (40 MZ pairs and 30 DZ pairs), therefore these point estimates should be interpreted with some caution. Importantly, a recent case-control study confirmed high test-retest reliability of MMN and has assigned this measure one of the highest ranks among other potential endophenotypes for schizophrenia, based on three criteria including state independence, long-term stability, and magnitude of deficits is patients relative to controls (Light et al., 2012). Unfortunately, heritability, a crucial criterion for endophenotype, was not included in this study's rating system. Evidence for heritability of MMN remains scarce, and replication on larger samples by independent laboratories is needed before a definitive conclusion about endophenotype value of this measure can be made.
4.2.4. Sensory gating (P50)
Sensory gating refers to the phenomenon of the suppression of response to the second of the two identical stimuli (usually, short clicks) when they are presented with a short inter-stimulus interval (less than 1 second). The degree of the suppression, or “gating”, of the second stimulus can be measured using the difference between ERP amplitudes elicited by the first and the second stimulus in the pair. Historically, this effect has been measured using the early P50 component reflecting largely pre-attentive sensory processing.
P50 suppression has been extensively studied as an endophenotype for schizophrenia (Freedman et al., 2003). The main assumption is that the gating deficit occurring at early stages of sensory processing can give rise to more complex cognitive deficits occurring at later processing stages, such as problems with attention and perception, cognitive fragmentation, hypervigilance, etc. (Braff and Geyer, 1990; Freedman et al., 2003).
There have been only a few twin studies of the P50 gating phenotype. Two earlier studies estimated twin correlations but did not provide formal heritability estimates, perhaps due to small sample size. One study found a .57 correlation in MZ and 0 in DZ twins (Young et al., 1996), while the other study reported intrapair correlations of .50 and .13 for MZ and DZ, respectively (Myles-Worsley et al., 1996). Another small-scale twin study suggested that the extent of alcohol-induced reduction in P50 suppression effect is a stable trait influenced by genetic factors (Freedman et al., 1987). A more recent study using 40 MZ pairs and 30 DZ pairs found intrapair twin correlations of .52 and .04 for MZ and DZ, respectively and estimated heritability at 68%.using the structural equations modeling (SEM) approach. However, another study (Anokhin et al., 2007b) based on a larger twin sample and using alternative methods for P50 quantification found substantially lower but significant heritability of P50 gating (27-36%, depending on the method for P50 measurement). The same study investigated the gating effect in relation to the ERP component immediately following P50, the much larger and robust N100 peak, and found significant heritability of the peak amplitude (73%) and N100 gating effect (57%). Notably, recent studies suggest that N100 gating can be an intermediate phenotype for schizophrenia (Light et al., 2012). What is the possible explanation of the discrepancy between these studies with respect to heritability estimates for P50 gating? Studies using repeated measurement of P50 gating ratio in the same individuals reported low test-retest reliabilities (Boutros et al., 1991; Cardenas et al., 1993; Smith et al., 1994), even within a single ERP session (Clementz et al., 1997), with a notable exception of the study by Hall et al. (2006a) that found high test-retest stability (.66) for the same measure.
A genetic linkage study has shown a significant linkage between P50 suppression index and the alpha7 neuronal nicotinic receptor subunit gene (CHRNA7)(Freedman et al., 1997). A subsequent association analysis showed association between an SNP in the linkage region and P50 phenotype (Leonard et al., 2002).
4.2.5. ERPs in response inhibition (Go-NoGo) tasks
Genetic and neurobiological underpinnings of response inhibition have received enormous attention in the past decade because deficits of response inhibition, or behavioral disinhibition, became a central concept in the studies of psychiatric disorders, particularly those belonging to the “externalizing” spectrum (Young et al., 2009). These behavioral phenotypes show very high heritability, however, relatively little is known about the neural mechanisms mediating these genetic influences. Individual differences in the neural processes underlying response inhibition can be studied using ERPs elicited in the Go-NoGo tasks, in which the subject is required to inhibit a prepotent motor response. Twin studies of response inhibition-related ERPs are scarce. Anokhin et al. (2004) investigated ERP components elicited in a Go-NoGo task and found significant heritability of peak amplitudes of two ERP components that are elicited by No-Go stimuli that required inhibition of a prepared response , the frontal N2 component (60%) and the “anteriorized” P3 component (58%), suggesting that individual differences in the neural correlate of response inhibition are strongly influenced by genetic factors. Previous studies suggest that the No-Go ERP effects are produced by a rapid activation of the network involving anterior cingulate and lateral prefrontal cortices subserving the detection and resolution of conflict between simultaneously active, but incompatible task-related representations, namely, execution and inhibition of a prepotent response (Nieuwenhuis et al., 2003; van Veen and Carter, 2002). The anteriorization of P3 can be an electrophysiological manifestation of the engagement of the prefrontally mediated cognitive control processes required for the resolution of the Go-NoGo conflict. Subsequent analyses of data from the same experiment using inter-trial phase-locking measure to assess neural synchrony showed that the degree of task-related phase synchronization of brain oscillations in the No-Go condition is modestly but significantly influenced by genetic factors (Mueller et al., 2007).
4.2.6. Error-Related Negativity (ERN)
Error-related negativity (ERN, or Ne) is electrophysiological index of error monitoring, or detection of the discrepancy between the desired and actually executed action. ERN is a sharp negative potential observed within 50-150 ms after incorrect responses in tasks that involve response selection or response inhibition and typically require a speeded response. ERN is followed by a slower positive potential termed Error Positivity (Pe). Correct responses in such tasks with a high level of response conflict elicit similar wave but of smaller amplitude, the “correct-related negativity” (CRN). Extant research has linked ERN to both automatic, largely pre-conscious error detection and conflict processing, while Pe has been associated with conscious awareness of the error and implementation of corrective adjustments of behavior following error detection. Converging evidence indicates that ERN is generated in the rostral part of the ACC (Debener et al., 2005). A study of 99 MZ and 151 DZ adolescent twins (Anokhin et al., 2008) has found a highly significant heritability of all three ERP components related to error monitoring: ERN (47%), CRN (60%) and Pe (52% in males, 39% in females). Furthermore, a significant genetic correlation was found between these components. Thus, neural substrates of error monitoring, a fundamental mechanism of behavioral regulation, appear to be under significant genetic control.
Several candidate gene association studies of ERN have been reported. Fallgatter et al. (2004), using a very small sample (n=27), found larger ERN amplitudes in the carriers of the low-activity short allele of the serotonin transporter gene (5-HTTLPR). Similar results were obtained in another study including a small group of children hat was very mixed with respect to psychiatric diagnoses and medication (Althaus et al., 2009). However, a subsequent study using a larger sample (n=89) and more detailed genotyping taking into account the A/G SNP in the long allele (Olvet et al., 2010), failed to replicate this finding. Another study found a highly significant effect of a functional polymorphism in the brain-derived neurotrophic factor (BDNF) gene on ERN amplitude and other characteristics, with Val/Val genotype associated with larger ERN response and stronger inter-trial phase locking (Beste et al., 2010).
4.2.7. Face-related ERPs
A growing number of ERP and fMRI studies is using images of human faces, with both neutral and emotional expressions, as stimuli for the investigation of the neural mechanisms of nonverbal social communication and their impairment in disease (Haxby et al., 2002). Event-related brain activity elicited by facial stimuli has proven itself as a useful indicator of individual differences in processing of socially relevant information and a marker of risk for a number of psychiatric disorders characterized by abnormalities in social cognition and behavior, most notably, autism and schizophrenia (Posamentier and Abdi, 2003). Thus, identification of heritable characteristics of the neural processing of socially relevant facial information can help to elucidate the pathways from genes to individual differences in social behavior, both normal and abnormal. Anokhin et al. (2010) examined genetic influences on the ERPs elicited by facial expressions of emotion using a paradigm in which neutral expression was instantaneously replaced by an emotional expression of the same face. Changes of emotional expression produced two distinct ERP components: N240 wave with a right temporoparietal maximum and a P300 wave with a centropariatal midline maximum. Genetic analyses revealed substantial heritability of both components (36–64% for N250 and 42–62% for P300 components, respectively), suggesting that neural processing of facial affect is strongly influenced by genetic factors.
4.2.8. EEG and ERP: Common and specific genetic influences
Traditionally, resting-state EEG and ERP measures have been studied independently. However, since early days of electroencephalography, it has been known that evoked potential waveforms emerge as a result of phase resetting of the ongoing EEG oscillations at different frequencies present in the resting EEG, as well as modulation of their amplitude (Livanov, 1934).
Studies of sensory evoked potentials in humans (Vogel et al., 1986) have shown that latency and amplitude of visual and auditory potentials depend on individual characteristics of the resting-state EEG: subjects with abundant, high-amplitude alpha-rhythm showed higher amplitudes of most peaks of the visually and auditory evoked potentials compared to individuals with the low-voltage EEG type who showed reductions in ERP amplitudes (described above in greater detail). This study provided the first evidence that genetically transmitted differences in the resting EEG can influence the characteristics of brain potentials elicited by sensory stimulation. However, the conclusions of that early work were limited to differences between two relatively rare EEG variants.
Using data from the Amsterdam twin study cited above (van Beijsterveldt et al., 1996, 1998b) Anokhin et al. (2001), investigated the structure of genetic and environmental relationships between visual oddball P300 amplitude and spectral band powers of resting EEG recorded independently in the same subjects. A multivariate genetic analysis of twin data revealed substantial genetic overlap between the spectral content of the resting EEG and P3 amplitude: individuals with greater delta- and theta-band activity at rest showed larger P300 components in the visual oddball task, and vice versa. Importantly, this relationship was largely due to common genetic factors influencing both resting EEG and ERP. Since resting-state brain oscillation can be considered as baseline relative to event-related perturbations of that activity giving rise to ERPs, these results suggest that genetic influences on P3 amplitude are mediated in part by spectral properties of baseline, resting-state EEG. However, the results also indicated a significant residual genetic influence on P3 amplitude that was not shared with the resting EEG, with genetic factors specific to P300 accounting for 26% of the total P300 variance.
Substantial overlap between genetic influences on P300 amplitude and resting EEG has important implications for the functional interpretation of individual differences in P3 amplitude. First, this finding suggests that substantial proportion of genetic variability in P3 reflects some baseline characteristics of brain neurophysiology rather than differences in cognitive processing. Second, if individual differences in task related cognitive processing are of primary interest, it can be recommended that resting-state EEG characteristics be included as covariates in order to isolate the “true” ERP-specific variance.
4.3. Startle reflex
Startle reflex is a rapid automatic defensive response to abrupt sensory stimulation. It is ubiquitous in mammalian species, and its biological role is to protect the organism from a sudden attack or injury (Lang et al., 1993). In humans, one of the most pronounced components of the startle reflex is the eyeblink that can be elicited by strong abrupt sound and quantified using the measurement of electromyographic activity of the muscle responsible for the eyeblink (m. orbicularis oculi). Startle magnitude reflects individual difference in the overall defensive reactivity, and exaggerated startle response has been associated with different pathological conditions, most notably posttraumatic stress disorder. Most of the startle research, however, was concerned with startle modulation effects, most notably, pre-pulse inhibition and emotion-modulated startle.
Prepulse inhibition (PPI) refers to the suppression of the startle reflex that occurs when an intense startling stimulus is preceded by a weaker ‘prepulse’ stimulus. PPI is considered a measure of sensorimotor gating, automatic inhibitory regulation of sensory input and/or motor response that allows for an undisturbed processing of relatively weak sensory events. Gating deficits are considered to be a possible neurobiological mechanism mediating cognitive abnormalities in schizophrenia and some other disorders (Braff et al., 2001). PPI is considered one of the potential endophenotypes for schizophrenia (Light et al., 2012).
A twin study using the PPI paradigm has shown high heritability of the overall startle response magnitude (68%) and slightly lower heritability (58%) of the PPI index (Anokhin et al., 2003). Importantly, a separate analysis of the first and the second half of the experiment showed attenuation of heritability, which can be explained by overall startle habituation in the course of the experiment leading to the “floor effect” in the assessment of startle amplitude. Thus, contrary to the more common case when larger number of trials usually leads to more reliable estimate of the response, in the case of PPI a shorter experiment can yield more heritable measures.
Another widely used paradigm involving startle response is based on the well-established fact that the intensity of startle reflex depends on the ongoing motivational and affective state of the subject. Both animals and humans studies have shown that this obligatory defensive reflex is facilitated by aversive/defensive motivational states and attenuated by appetitive states (Lang et al., 1993). In humans, startle modulation is typically achieved by pictures with different affective content: pleasant, neutral, and unpleasant. Individual differences in the extent of startle suppression by pleasant pictures and/or potentiation by unpleasant, especially, threatening pictures is viewed as an objective measure of relative sensitivity of the appetitive and aversive motivational systems respectively. The first twin study of emotion-modulated startle (17 MZ and 12 DZ pairs) estimated twin correlations that were suggestive of significant genetic influences on the degree of emotional modulation of startle, but a formal genetic analysis was not performed due to the small sample size (Carlson et al., 1997). However, a subsequent larger study (Anokhin et al., 2007a) failed to show genetic or shared environmental influences on the amount of startle modulation, although heritability of the overall startle magnitude in all three valence conditions was high (59-61%). Thus, this study found no evidence for familial influences, and individual differences in startle modulation could be attributed entirely to individually unique environmental factors.
One possible explanation of this negative finding is the possibility of high developmental plasticity of the neural organization of emotional reactivity which allows the organism to adapt flexibly to the changing environment. However, there is another, more parsimonious explanation: test-retest assessments suggest very low individual stability of emotion-modulated startle (Larson et al., 2005; Lee et al., 2009), which casts doubt on the notion that it can serve as an indicator of stable, trait-like individual differences. However, these negative findings do not preclude the possibility that individual differences in emotion-modulated startle are influenced by genetic factors. First, the study by Anokhin et al. (2007a) included only females, and there is a possibility that emotion-modulated startle is heritable in males but not females. Second, measures of startle modulation in other experimental paradigms may be under greater genetic influences. For example, twin studies of startle modulation by affective facial expressions or fear-potentiated startle can help to clarify this issue.
Overall, characteristics psychophysiological indices of emotional processing.such as frontal EEG asymmetry and emotion modulation of startle reflex showed somewhatlower reliability relative to measures of cognitive processing. This may seem somewhat counterintuitive because, from the evolutionary perspective, emotional response systems are older and more directly related to survival; consequently, one can expect a greater degree of genetic determination of such responses compared to e.g. executive functions the development of which may be to a larger extent mediated by social environment. On the other hand, however, developmental plasticity of emotional reactivity is an adaptive feature that permits a flexible adaptation to individually specific environment (which automatically decreases heritability at the population level). Importantly, twin research designs can provide important information about the role of environmental factors, both shared and non-shared. For example, the co-twin control studies based on the comparison of MZ twins who are discordant for a certain environmental exposure allows for a direct estimation of the effects of specific environmental factors by providing an ideal control for genetic factors and environment shared by twins.
5. Implications for understanding gene-brain-behavior relationships in health and disease
5.1. Intermediate phenotypes, or “endophenotypes”
How can the knowledge gained in genetic psychophysiology research help us to advance the broader field of behavior and psychiatric genetics? The first important contribution can be facilitation of gene finding. During the past two decades, enormous resources have been allocated to finding genes that confer liability to highly heritable psychiatric disorders and addictive behaviors such as schizophrenia, autism, ADHD, alcoholism, nicotine addiction, and other conditions. There was a great hope that the application of high-density arrays of genetic markers made available by the rapid progress in molecular genetics and the use of gigantic samples would lead to a quick progress in the identification of the genes responsible for these disorders. The results of early genome-wide association studies (GWAS) were largely disappointing because first positive findings were often refuted by subsequent studies based on even larger samples. For example, numerous large scale linkage and association studies of alcoholism have been conducted, but the agreement across studies was very poor and, by now, there is no single well-replicated finding of a gene conferring risk for alcoholism, except the genes encoding the key enzymes of alcohol metabolism (ADHs and ALDHs), that have been known long before the GWAS era (Edenberg, 2012). However, the situation started to change in the last few years as independently replicated GWAS findings have emerged for nicotine addiction(Wang et al., 2012), schizophrenia(van Dongen and Boomsma, 2013)and other disorders, although these genetic variants account only for a very small portion of heritability of these phenotypes.
One of the issues that used to be frequently mentioned in relation to GWAS has been so called “missing heritability”, i.e. the fact that genetic variants identified by GWAS can account only for a small portion of heritability. However, more recent GWAS studies were able to capture an increasingly large proportion of the phenotypic variation, especially when all SNPs, rather than only GWAS “hits”, are considered. As GWAS efforts continue and sample sizes grow to tens or even hundreds of thousands, it becomes clear that the perceived failure or disappointment about GWAS was overstated (Visscher et al., 2012). Vissher et al. underscore that the aim of GWAS is to detect loci that are associated with complex traits, rather than explain all genetic variation, and this aim is being successfully achieved by GWAS studies that have led to new discoveries about genes and pathways involved in common diseases and other complex traits (Visscher et al., 2012). However, most of these recent successes concerned “somatic” phenotypes such as auto-immune or metabolic diseases, while the progress in the identification of loci associated with behavioral phenotypes such as psychiatric disorders was relatively modest, with a few exceptions (e.g. Rietveld et al., 2013; Smoller et al., 2013). The root of the problem lies in the complexity, variability, and possible etiological heterogeneity of the diagnostic phenotypes, as well as the large “distance” between the predisposing genes and their phenotypic expression. Genetic influences on complex behavioral phenotypes are mediated by numerous pathways and mechanisms that interact in a highly non-linear fashion and are strongly modulated by environmental influences. The second source of the lies in the large number of genes and their small effect sizes. At the early stages of behavioral and psychiatric genetics there was a hope that substantial portion of variance of complex phenotypes can be explained by “oligogenes”, i.e. a few genes with relatively large effects. However, this assumption has been largely refuted by recent GWAS studies, as it became clear that many genes with very small effects are likely to be involved in the determination of complex human traits. Due to this complexity of pathways linking genes to behavior and small gene effects, attempts to directly associate individual genes with a complex behavioral phenotype turn out to be a formidable, if not impossible, task.
One possible solution for this seemingly intractable problem is to shift the focus of genetic studies from the complex phenotype to its “building blocks”, or component processes of liability, and to trace them all the way up to the behavioral phenotype. The first step is to delineate these components, which can be represented by heritable individual differences in brain function and psychophysiological responses. The next step is to identify common and process-specific genes influencing these components. The final step is to determine how interaction between these components gives rise to the complex behavioral phenotype, i.e. elucidation of etiological mechanisms.
This brain-based approach to understanding genetic influences on behavior was proposed by Vogel (Vogel et al., 1979b). He suggested and implemented a research strategy that first focuses on the identification of genetic variability in brain function and then explores how this variability influences behavior. In psychiatric genetics, such intermediate brain-based phenotypes were labeled as “endophenotypes”(Gottesman and Gould, 2003).
One of the initial arguments in favor of the focus on intermediate phenotypes was that variability at the neurobiological level is “closer” to immediate gene effects, such as change of a protein function than complex behavior and, due to this proximity to gene products, the effects of single genes on neurophenotypes can be relatively large and therefore readily detectable. However, this turned out not to be the case, as neurophenotypes, at least those that can be measured non-invasively in humans and likely to play a causal role in behavioral differences, are also quite complex and are therefore unlikely to demonstrate simpler genetic architecture (de Geus, 2010; Flint and Munafo, 2007). Various models linking genes, endophenotypes, and clinical phenotypes are discussed by Rommelse et al. (2011). However, this complexity does not diminish the value of neurophenotypes, since with the advent of GWAS and growing number of genetic variants contributing to behavioral variation, there is an increasing need in the investigation of the pathways mediating the effects of these variants on behavior. In fact, functional significance of many of genetic loci being identified by GWAS is not readily clear. Therefore, De Geus (2010) argued that EEG endophenotypes can serve as an important tool for the elucidation of the functional meaning of candidate genetic variants that derive from association studies by helping us to understand where in the brain, at which stage and during what type of information processing these genetic variants have a role (de Geus, 2010).
A number of criteria for psychophysiological endophenotypes were proposed (de Geus, 2002, 2010), including 1) association with disorder, even during remission; 2) temporal stability including developmental stability; 3) heritability; 4) familial association with disorder as indicated by the presence of the same abnormality in the first degree relatives of probands. Additional criteria may include a) association with dimensional phenotypes indicating liability such as temperamental traits, for example, harm avoidance and trait negative affect for internalizing spectrum disorders and impulsivity for externalizing spectrum; b) suitability for repeated administration, i.e. relative resistance to learning and practice effects; c) availability of animal models, which allows for a more detailed investigation of both genetic and neurobiological mechanisms at more elementary levels such as gene expression in specific brain regions and its modification by strictly controlled environmental influences and neurophysiological investigation and the level of single neurons and local circuits; d) construct validity in the sense that psychophysiological measure is a specific and valid indicator for a certain cognitive process; e) knowledge of the underlying genetic architecture and specific genes involved, i.e. heritability “explained”.
Of course, in reality, very few candidate endophenotypes would meet all these criteria. A question arises, what of the criteria listed above are most imperative in order for a measure to be an intermediate phenotype. Since, by definition, it is imperative that a measure or construct mediating genetic influences on behavior is genetically determined, heritability appears to be the main criterion. Furthermore, a demonstration of heritability automatically serves as a proof of reliability, since test-retest reliability theoretically serves as the upper limit for heritability. The latter pertains to the stable, trait-like variance; consequently, a non-stable measure cannot be heritable. It is important to note that the reverse is not true: high test-retest stability does not necessarily imply heritability, because stable individual differences can also be shaped by environmental factors. The intermediate phenotype approach is illustrated below using the example of the oddball P3 ERP component as a marker of risk for alcoholism and associated externalizing behaviors syndrome.
5.2. An example: P3 as intermediate phenotype for externalizing disorders
Numerous studies have shown that oddball P3 amplitude is reduced in alcoholics and, most importantly, in unaffected biological relatives of alcoholics(Begleiter and Porjesz, 1990; Hill et al., 1995; Hill et al., 1988; Iacono et al., 2003, 2008; Polich et al., 1994; Porjesz and Begleiter, 1991); this evidence summarized in a recent meta-analysis (Euser et al., 2012). The robust finding of familial association between P3 and alcoholism is corroborated by the evidence for high heritability of individual differences in P3 amplitude in the general population(Anokhin et al., 2001; Katsanis et al., 1997; van Beijsterveldt et al., 1998b; van Beijsterveldt and van Baal, 2002). Furthermore, reduced P3 was found to be associated not only with AUDs but also with nicotine dependence (Anokhin et al., 2000), drug dependence (Bauer, 1997), conduct disorder (Bauer and Hesselbrock, 1999), suggesting that diminished P3 indicates an inherited predisposition for a spectrum of addictive behaviors and comorbid externalizing psychopathology (Gilmore et al., 2010; Iacono et al., 2003). Multivariate genetic analyses of twin data demonstrated significant genetic correlations between P3 and latent externalizing factor, thus supporting the notion that P3 indicates genetic diathesis for externalizing disorders (Hicks et al., 2007). Importantly, a converging line of evidence suggests that reduced P3 indicates a genetically transmitted deficit predating alcohol use and abuse, rather than a consequence of heavy drinking: the reduction of P3 is present in high-risk (i.e. family history positive) adolescents was present before the onset of drinking (Hill and Shen, 2002; Hill et al., 1999; Hill et al., 2000); P3 is not significantly affected by alcohol use (Perlman et al., 2009); in longitudinal studies reduced P3 prospectively predicted the onset of alcohol abuse(Gilmore et al., 2012) as well as aggression and crime (Gao and Raine, 2009); in non-alcohol dependent social drinkers without family history of alcoholism P3 amplitude was unrelated to lifetime alcohol intake(Bijl et al., 2005); finally, a co-twin control study(Carlson et al., 2002) has shown that in twin pairs discordant for AUDs the alcohol abusing/dependent twins' amplitude did not differ from that of non-alcoholic co-twins, providing further evidence that P3 amplitude reduction is a genetically transmitted marker of risk, rather than consequence of drinking. Interestingly, animal models show a pattern of ERP differences that parallels the human findings described above: selectively bred alcohol-preferring rats show reduced P3 amplitude in the parietal cortex and increased fast-frequency EEG activity compared with and non-preferring rats(Criado and Ehlers, 2010). Thus, high heritability of P3 (and associated event-related theta oscillations) in the general population, its robust familial association with alcoholism, genetic correlations with a broader externalizing trait, and little modification by alcohol use, makes it a good intermediate phenotype (endophenotype) for genetically transmitted vulnerability to alcoholism.
However, exact neurocognitive mechanisms by which the reduction of P3 amplitude contributes to the risk for externalizing spectrum behaviors remains unclear. One possible interpretation based on the evidence that electrocortical positivity is associated with inhibitory regulation of cortical excitability (Birbaumer et al., 1990), is that reduced P3 reflects a tonic elevation of cortical excitability, with the latter being a risk factor for poor inhibitory control of behavior (Anokhin et al., 1999b; Begleiter and Porjesz, 1999). However, this cortical disinhibition hypothesis does not seem to be in good agreement with the widely accepted notion that dysregulated behaviors result from poor cortical control over subcortically generated drives and impulses. According to this model, a deficit, rather than excess, of cortical activation would be a risk factor for externalizing behaviors. Apart from oddball tasks, P3 potentials are elicited across a variety of other cognitive tasks by goal-relevant stimuli that require some sort of decision making and response. On the other hand, meta-analysis of functional neuroimaging studies indicated that diverse executive functions tend to engage a very similar set of brain regions, suggesting the existence of a common, super-ordinate fronto-cingulo-parietal cognitive control network (Niendam et al., 2012). Moreover, these regions show substantial overlap with regions identified as possible P3 generators (Eichele et al., 2005; Mantini et al., 2009; Mulert et al., 2004). It is therefore reasonable to hypothesize that P3 reflects the engagement of this frontopariatal cognitive control network (CCN), and reduced P3 indicates insufficient recruitment of this network, perhaps due to poor integration of its components. Due to non-specificity of CCN, it is more likely to act as a moderator, rather than mediator of more specific risk factors, such that high level of CCN functioning will suppress the manifestation of specific predisposing factors, while low level of CCN functioning will facilitate the development of abnormal behavior by reducing threshold for the expression of disease-specific liability. This proposed model can explain why P3 amplitude reduction is observed across diverse psychiatric disorders.
This review focused only on a single example of intermediate phenotype for psychiatric disorders. Genetic psychophysiology research has identified potential intermediate phenotypes for a broad spectrum of other disorders, such as enlarged error-related negativity in obsessive-compulsive disorder, abnormalities of face processing autism, etc. , however, detailed discussion of these findings is beyond the scope of the present review.
6. Challenges facing genetic psychophysiology
Despite substantial progress achieved in genetic psychophysiology and increasing interest in this field as evidenced by rapid growth of the number of publications over the past 10 years, some old challenges remain and new ones have emerged. Below we summarize the main challenges facing the field.
6.1. Is psychophysiology still relevant in the fMRI era?
One frequently discussed question is whether EEG and ERP methods remain relevant in the time of increasing proliferation and availability of fMRI. The answer is a definite “yes”, and there are many important arguments in favor of electrophysiology methods - some of them are quite general, while others are more specific to the field of genetic psychophysiology.
The first important argument is that EEG is a direct measure of neuronal activity, whereas the blood oxygenation-dependent (BOLD) signal is not. Modeling the relationship between BOLD signal and neural activity hinges on a number of assumptions, the validity of which may be unclear in particular cases. The exact nature of neural activity reflected by BOLD signal is also poorly understood. Although it has been established that the BOLD signal is associated with gradual postsynaptic potentials, rather than action potentials of the neurons, the relative contribution of inhibitory (IPSP) and excitatory (EPSP) postsynaptic potentials to the BOLD signal remains to be determined. An increase in the BOLD signal is commonly interpreted as “activation”, however, if IPSPs make a substantial contribution to the increase in BOLD signal, the validity of this straightforward interpretation may be challenged. Furthermore, it cannot be excluded apriori that functionally meaningful changes in neuronal activity may occur without significant changes in local metabolism, for example when some neurons reduce while others increase their activity within the same region, or when synchronization of activity changes without the change in the overall number of active neurons. For example, a recent study has shown that the BOLD response is insensitive to substantial changes in neural activity associated with certain aspects of visual processing (Swettenham et al., 2013). Conversely, another study suggested that, in addition to haemodynamic response related to a visual stimuli, additional trial-related haemodynamic signal emerged as an anticipatory adaptation to pending processing demands, and this latter signal was not directly associated with neuronal activity (Cardoso et al., 2012; Sirotin and Das, 2009). Thus, functionally significant changes in local neuronal activity and changes in BOLD signal may not always be related in a straightforward, linear fashion, which underscores the importance of electrophysiology methods.
The second important advantage of electrophysiology methods is their excellent temporal resolution permitting the delineation of distinct stages of information processing in the brain (stimulus detection, categorization, response selection, error detection, etc.) that unfold within a period of less than on second. In contrast, the BOLD signal reaches its peak only about 5 seconds after the stimulus and takes 10 more seconds to return to baseline. Due to their ability to capture specific, short-living neural processes, electrophysiology methods can provide a better mechanistic understanding of cognitive processing and its impairment of disease. While fMRI is better suited to answer the question, where the information is processed, modern methods of EEG/ERP analysis are better suited to address the question of how the information is processed by the brain (Lorig, 2009). It is possible that some psychiatric disorders result from the impairment of mechanisms, such as precise temporal and spatial integration and coordination of activity in distributed networks, rather than impairment of a particular brain region.
Of course, coarse spatial resolution is the main disadvantage of electrophysiology methods. Yet, higher cognitive functions are subserved by highly dynamic, rapidly forming and dissipating distributed functional networks involving dynamic interaction among distant brain regions. This raises the question of whether we really need a very fine spatial resolution in order to localize processes that may inherently lack a strict anatomical localization, as suggested by both electrophysiology and fMRI data (e.g. substantial variability of regional BOLD “activation” across subjects in the same task and variability within subject across sessions or even within a single session). Finally, the neural substrates of well-established ERP components are being increasingly clarified by studies integrating ERP and fMRI data (see e.g.Debener et al., 2005; Ford et al., 2004), which provides neuroanatomical validation for ERP phenotypes.
Other two arguments in favor of EEG/ERP are more specific to genetic psychophysiology research. First, Most EEG/ERP measures show high intraindividual stability, a necessary prerequisite for genetic research: test-retest correlations are in the range of .6-.9 for resting EEG characteristics and .5-.7 for many ERP paradigms, although reliability of other popular psychophysiological indices such as auditory P50 gating and emotion-modulated startle remains questionable. In contrast, a review of test-retest studies of fMRI-based measures of task-related regional activation has revealed modest test-retest reliability (Bennett and Miller, 2010). The problem is exacerbated by the fact that the less reliable the measure is, the larger samples are needed for genetic studies to detect a significant effect, which is particularly undesirable in the case of neuroimaging studies because of the high cost of assessments. Accordingly, another advantage of electrophysiology methods is their low cost and relative portability permitting the assessment of large samples required for genetic research.
6.2. Methodological issues
6.2.1 General Issues
Genetic association studies using psychophysiology measures face many of the same problems as psychiatric genetic and behavior genetic research, including relatively small effect sizes of individual genes and complexity of phenotypes. Moreover, these problems are further exacerbated by such factors as lack of standardized assessments which hinders replication efforts and pooling data across laboratories, multiplicity of phenotype description (a single ERP experiment can generate a host of variables), and small sample sizes (because psychophysiology assessments require a lab visit). Clearly, the combination of multiple phenotypes, multiple genes, and small sample size is prone to produce false positive findings (Type I error). Apart from these general problems, there are a number of problems that are specific to the measurement of the phenotype and to certain aspects of the genetic analyses.
6.2.2. Issues related to psychophysiological measurements
A critical issue in genetic research of complex phenotypes, including psychophysiological responses, is test-retest reliability. Clearly, only individually stable, trait-like characteristics can be heritable. As already mentioned above, test-retest reliability can be regarded as upper boundary for heritability. Psychophysiological variables show a very broad range of reliability, from very high such as resting EEG, to very low such as emotion-modulated startle reflex. Therefore, one important but often neglected step in genetic studies should be the selection of a reliable indicator of the function or process of interest. Some pilot studies may be needed to compare the utility of different psychophysiological indices, but this investment will pay off because a properly conducted genetic study requires large samples, and the availability of a reliable measure may reduce the sample size required to achieve the desired statistical power.
Another important issue related to the likelihood of gene finding and the interpretation of results is the specificity of psychophysiological phenotypes. It is reasonable to expect that measures that load strongly on a single specific aspect or mechanisms of neural processing and are less affected by other processes would have a simpler neural and genetic basis. On the other hand, measures reflecting a more integrative level of processing may be a product of multiple interacting processes and have a more complex genetic basis. For example, oddball P3 has been extensively used in genetic psychophysiology research related to psychopathology, particularly alcoholism and schizophrenia, but the degree to which it has contributed to the understanding of the mechanisms mediating genetic influences on these disorders has been limited by its low specificity with respect to distinct neurocognitive processes that might be conceptually implicated in the pathophysiology of these disorders. Given multiple brain regions and mechanism implicated in P3 generation and the diversity of its functional interpretations, oddball P3 is itself a very complex phenotype, for example, fMRI studies have identified 34 regions that are significantly activated during target detection in the auditory oddball task (Kiehl et al., 2005). Therefore, more focused tasks may be needed, with greater specificity with respect to separable neurocognitive processes. For example, error-related negativity (ERN) appears more selective with respect to both cognitive processes and neural substrates than oddball P3, and therefore may be a more tenable target for the identification of genes affecting functionally and anatomically specific neural circuit.
An important consideration for genetic association studies of psychophysiological phenotypes is standardization of assessments that should enable the replication of results, meta-analyses, as well as pooling data from different studies in order to increase statistical power. Historically, there is a large variability in laboratory protocols, including task design, specific stimuli used, recording parameters, and instructions to the subjects. Even the “classic” oddball P3 amplitude can be measured in a variety of ways. First, paradigms may be different, leading to different cognitive processes engaged by the task (e.g. Hill et al., 1998; Noble et al., 1994). Second, quantification of the neural response may be different, varying from simple peak amplitude of the averaged ERP to more sophisticated algorithms such as event-related band power.
Addressing the issues of reliability, specificity, and standardization can be greatly facilitated by following the general guidelines for ERP research (Duncan et al., 2009). A proper quantification of event-related brain activity (e.g., using principal or independent components analysis to separate overlapping components of response when needed) is essential for the identification of genetic factors influencing neural activity associated with specific cognitive processes. While the use of alternative representation of event-related response may be well justified, it can be recommended that traditional peak and latency data are also presented to ensure comparability across studies.
Age range of the sample is another important consideration. Many EEG and ERP phenotypes show substantial age-related changes, and large variability in age within the sample may inflate twin correlation both in MZ and DZ pairs, leading to an overestimation of the shared environmental influences. Therefore, it can be recommended that age-related trend is removed in regression analyses before entering the data in genetic analyses or, alternatively, age effects should be included in the biometrical model. Of note, a linear regression will likely be insufficient in many cases due to nonlinear shape of age-related trends for many variables such as resting EEG band powers and P3 amplitude.
Finally, it is important to test and account for gender effects by testing “sex limitation” models that allow one to test for gender differences in the degree of heritability and gender-specific genetic effects, i.e. to determine whether the same or different genetic factors influence the trait in males and females.
6.2.3. Issues related to genetic analysis
The most serious problem is related to candidate gene association studies and is concerned with high probability of false positive findings. The main source of this problem is a dangerous combination of two factors known to increase the rate of Type I error: small sample size and multiple comparisons. This problem is not limited to genetic psychophysiology research and has already raised substantial concern in other fields employing candidate gene approach.
This problem is further exacerbated by the well-known publication bias towards reporting positive findings. Recent meta-analytic studies have demonstrated that this ubiquitous problem is especially pervasive in genetic research of complex neuropsychiatric and behavioral phenotypes. The existence of this bias is indicated by a higher rate of positive results among novel findings compared to replication attempts, since journals are more likely to accept a paper reporting a novel genetic association than a paper reporting a negative finding (Duncan and Keller, 2011; Flint and Munafo, 2012). Smaller studies are more likely to be published if they yield positive results because researchers are typically more reluctant to publish negative results, moreover, if a study reporting a negative result is underpowered, it is less likely to be accepted for publication than a similarly underpowered study reporting a positive finding.
Perhaps the most notorious example is the excess of positive findings in “imaging genetics” studies that has been revealed in several recent analyses of publications in this field (Flint and Munafo, 2012; Ioannidis, 2011; Ioannidis and Trikalinos, 2007). These analyses estimated the expected proportion of positive findings of association given statistical power and the reported effect size, and compared it with the observed rate of positive reports. The results indicated strong biases towards reporting positive findings, probably due to selective outcome reporting and selective analyses reporting.
Another important factor leading to increased rate of false-positive findings is multiple testing combined with selective reporting of only those statistical comparisons that yielded positive findings, for example, multiple measures can be derived from an ERP experiment and tested for association with multiple genetic polymorphisms, but only one test that yielded a significant finding may be described in the published report. Recent analyses of published studies support the notion that many more tests have been conducted than reported in the literature (Duncan and Keller, 2011; Flint and Munafo, 2012).
Analyses of the literature also showed that low power increases the proportion of false-positive to true-positive findings among those studies that achieve nominal statistical significance (Munafo and Flint, 2010). Finally, genetic association studies published in journals with a high impact factor tend to be based on smaller sample sizes while reporting larger effect sizes and are therefore more likely to provide an overestimate of the true effect size (Munafo et al., 2009). In light of evidence for high rates of reports containing false positive findings, a number of journals in the field of psychiatric and behavior genetics have adopted more restrictive editorial policies for the acceptance of studies reporting candidate gene association findings.
What can be learned from these examples? First, there is no reason to believe that the situation with candidate gene association studies in genetic psychophysiology is any different from other fields using the same approach. Therefore, a sizable portion of the reported positive findings based on small samples studies are likely to be false positives and it can be expected that the replication rate will be very modest. Next, we need to adjust strategies and standards in this area of research and, perhaps, editorial policies towards a more conservative approach. In particular, studies reporting negative findings should be given at least the same weight as studies reporting positive findings.
Studies reporting candidate gene associations should apply appropriate correction for multiple testing and explicitly disclose the total number of tests performed. Data reduction may be necessary to avoid multiple testing, for example, a dataset including EEG spectral power measures obtained for multiple frequency bands and multiple electrodes can be subjected to principal components analysis in order to extract 3or 4 factor scores that explain the great bulk of variance in the original variables. Second, studies should use adequate sample sizes that are justified by power calculations and realistic estimation of the expected effect size. Given that effect sizes are likely to be small (Flint and Munafo, 2012; McCarthy et al., 2008), quite large samples may be needed by the standards of psychophysiology (not genetics!). Even when a single measure is tested for association with a single genetic variant, samples of the order of hundreds may be needed, especially, if there are covariates that have to be controlled for. A GWAS study of the human EEG (Hodgkinson et al., 2010) has reported effects sizes that are substantially larger relative to other human phenotypes that have been investigated using the GWAS approach much more extensively. It remains to be seen whether these effects will be replicated in subsequent studies.
Another issue of potential concern is population stratification. It is generally assumed that association between a genetic variant and the phenotype of interest arises due to linkage disequilibrium (close location of two genes on the chromosome resulting in their co-inheritance), however, association can also result from spurious sources. Most commonly, spurious association is caused by population stratification, when the studied population is composed of subgroups that differ with respect to both the genetic marker allele frequency and the studied phenotype (see Hamer and Sirota (2000) for a nice didactic example). A number of ways to deal with this issue have been proposed, the most simple of which is to ensure maximum homogeneity of the studied sample with respect to racial and ethnic ancestry. Detailed discussion of these and other methodological issues is outside the scope of this review; interested reader can consult more specialized literature on this subject (Tiwari et al., 2008).
7. Future directions
Despite the challenges discussed above, genetic psychophysiology research holds great promise for the elucidation of the pathways and mechanisms by which genes influence human behavior(Boomsma et al., 1997; de Geus, 2010; Rommelse et al., 2011). New exciting developments can be anticipated in the coming years, many of them driven by methodological improvements both in psychophysiology and genetics.
The application of novel approaches to the analysis of physiological signals will likely lead to identification of genes affecting distinct aspects of the brain function. In the past decade, advanced methods for the analysis of spontaneous and event-related EEG activity have received widespread acceptance, such as principal- and independent-component analyses, time-frequency decomposition of event-related oscillations, measures of dynamical complexity, assessment of local neural synchrony using phase-locking measures, methods for the assessment of functional connectivity, including directional and causal measures such as Granger causality, source-level connectivity, and other advanced techniques that allow researchers to extract information about conceptually important aspects of brain functioning. Collectively, these measures allow for a better utilization of the rich information contained in the EEG and ERP data. Recent studies underscore the importance of various forms of neural synchrony as biological substrate for cognitive processing, including such measures as event-related coherence, phase-locking, and cross-frequency coupling. However, these task-related characteristics have been little studied from the genetic perspective. So far genetic studies of EEG-based connectivity measures were mostly limited to the resting state. Resting EEG characteristics have obvious advantage as candidate endophenotypes: first, they show stronger heritability relative to event-related measures; second, resting EEG recordings require minimal participation of the subject and can therefore be used in clinical samples where administration of cognitive tasks would be problematic. These studies provided important insights into the genetic underpinnings of some general characteristics of brain organization such as “small world network” properties (Smit et al., 2010) and suggested potentially useful endophenotypes. Nonetheless, extending this approach to event-related changes in neuronal synchrony and connectivity in cognitive tasks can take this line of research to a new level by adding more specificity in regard to dissociable cognitive processes and neural circuits.
The potential of multivariate genetic methods (see section 3.1) demonstrated in behavior genetic research (Boomsma et al., 2002; van Dongen et al., 2012) has not yet been fully utilized in genetic psychophysiology. In particular, these methods can reveal whether different characteristics are influenced by overlapping or independent genetic factors. This approach can be applied to psychophysiological measures from the same domain (e.g. novelty and oddball P3), across measurement domains (e.g. ERP and startle response or measures of autonomic reactivity), and even to covariances between psychophysiological and psychometric measures (e.g. ERPs and personality or psychopathology). Information gained by such analyses can substantially further our understanding of many psychophysiological phenomena.
On the molecular genetics side, several recent developments offer new opportunities for genetic psychophysiology research. There is an exponential increase in the number of identified copy number variants (CNVs), genomic variation resulting from deletions and duplications of DNA fragments. The cost of genome sequencing is rapidly dropping and this technology may soon become available at affordable prices. In addition, progress in bioinformatics will permit the selection of broader arrays of candidate genes by their relevance to the biological pathways hypothesized to play causal role in shaping individual differences in brain function and behavior.
The coming years will probably see more GWAS studies of psychophysiology phenotypes, perhaps through collaboration among multiple laboratories, because such studies require very large samples (of the order of thousands). For example, resting EEG is being routinely recorded in many laboratories in addition to ERP experiments. In most cases, EEG data can be retroactively standardized by applying re-referencing, re-sampling, filtering, and other transformations when necessary, and such data can be pooled to conduct an adequately powered full genome scan.
Another important direction of research for genetic psychophysiology in coming years will be functional characterization of genes implicated in psychological and psychiatric phenotypes. Generally speaking, two alternative approaches to the study of the genotype-phenotype relationships can be distinguished. The first one is the currently dominating top-down, phenotype-centered approach that identifies a phenotype of interest and asks the question: what genes influence this phenotype? In contrast, the bottom-up, gene-centered approach starts with a specific genetic polymorphism and asks the question: what are the phenotypic effects of this genetic variation? Few studies have taken the latter strategy, but it can be anticipated that the ongoing GWAS studies of psychiatric phenotypes will identify important causal variants conferring susceptibility to disorder, but very little may be known about the mechanisms of their action. de Geus has recently proposed that testing the association of the newly identified risk alleles with EEG and ERP endophenotypes can help us understand “where in the brain, in which stage, and during what type of information processing the genetic variant has a role”(de Geus, 2010). Importantly, such testing would require substantially smaller samples than a GWAS study (de Geus, 2010). Genetic variants can be screened for functional effects using a broad battery of psychophysiological and behavioral tests, and the resulting “functional profile” can provide important insights into the neurocognitive mechanisms mediating the effects of these genes on behavior.
Another rapidly developing area of genetic research is concerned with epigenetic modifications of gene expression through DNA methylation and histone modifications that can lead to long-lasting changes of the phenotype. These epigenetic processes can be triggered by exposure to both physical and psychosocial factors (Fraga et al., 2005; Wong et al., 2010). Epigenetic differences have been documented in MZ twins (Kaminsky et al., 2009); these differences are already present in children (Wong et al., 2010) and tend to accumulate with age (Fraga et al., 2005; Poulsen et al., 2007). Investigation of epigenetic characteristics in twin pairs discordant for a phenotype of interest (e.g. EEG or specific ERP response) can shed light on the role of epigenetic factors in brain development and shaping individual differences in neurocognitive functioning.
One promising area of research for genetic psychophysiology with a strong translational potential is psychopharmacogenetic research focused on the elucidation of the mechanisms behind drug X gene interaction. Here, at least two distinct directions can be identified. The first direction involves the investigation of genetically determined differences in acute psychophysiological responses to drugs of abuse, which can facilitate the understanding of the mechanisms of addiction to alcohol, nicotine, and other drugs. For example, a twin study of acute alcohol effects on the EEG revealed heritable individual differences in acute tolerance and sensitization to alcohol (O'Connor et al., 1999). Another study suggested that the effect of nicotine of P50 sensory gating is moderated by functional variation in the dopamine transporter gene (DAT) (Millar et al., 2011). The second direction is concerned with genetically determined differences in psychophysiological responses to medication. For a better understanding of variability in treatment efficacy and outcomes, it is important to know what specific neurocognitive mechanisms are affected by a given medication in genetically different individuals.
Finally, recent years have seen proliferation of methods for modification of brain function by electric or magnetic brain stimulation (George and Aston-Jones, 2010), including Transcranial Magnetic Stimulation (TMS) and re-emergence (after almost 100 years) of Transcranial Direct Current Stimulation (tDCS). These methods offer an opportunity for the investigation of the interplay between genetic determination and plasticity in brain function. Can genetically determined deficits in specific aspects of neurocognitive processing be ameliorated by brain stimulation? Will these modifications be long-lasting? Conversely, how responses to brain stimulation are modulated by the individual's genotype?
8. Summary and conclusions
The past decade has seen a remarkable increase in studies at the intersection of genetic and neurosciences, including psychophysiology. Substantial progress has been made in the understanding of the role that genetic factors play in shaping individual differences in pychophysiological responses across different measurement domains and modalities. Twin studies have documented high heritability of diverse aspects of brain functioning assessed using resting–state EEG recordings, and substantial genetic influences on event-related neuronal activity registered in a variety of tasks emphasizing distinct aspects of cognitive and emotional processing. However, some of the studied psychophysiological phenotypes showed low heritability, primarily due to their poor test-retest reliability. Overall, characteristics related to cognitive functioning showed higher heritability than psychophysiological indices of emotional processing. Emerging candidate gene association studies are beginning to suggest specific genetic variants responsible for individual differences in brain function and various psychophysiological responses. However, this line of research should be pursued with great caution due to its demonstrated proneness to generate false-positive findings and overestimate the real effect size. Recent developments in methods for physiological signal analysis, multimodal brain imaging, and genomic technologies offer new exciting opportunities for the investigation of the brain mechanisms mediating genetic and environmental influences on behavior, both normal and abnormal.
Highlights.
Twin and family studies indicate strong heritability of brain function
Association studies point to specific genes affecting brain physiology
Genetic psychophysiology can provide intermediate phenotypes for psychopathology
Box: Assessment of heritability using twin data.
Fig. 3.
EEG phenotypes in twins. Upper panel: regular alpha variant; lower panel: Low-voltage EEG.
Table. 1.
Candidate gene association studies of event-related brain potentials (ERPs).
| Source | Sample | Age | ERP Paradigm | ERP measures | Genes | Main Findings |
|---|---|---|---|---|---|---|
| Blum et al., 1994 | 155 psychiatric patients (m+f) | Adults | oddball | P3 amplitude, latency | DRD2/ANKK1 Taq Ia | ↑ P3 latency in A1A1; no effect on amplitude |
| Noble et al., 1994 | 98 (males,incl. sons of alc) | 12.5 (10-14) | Visual oddball, 1-back version | P3 amplitude, latency | DRD2/ANKK1 TaqIa | ↑ P3 latency in A1A1(43ms difference); no effect on amplitude |
| Freedman et al., 1997 | 104 (9 families with at least 2 cases of sz) | Not reported | P50 gating | P50 ratio | Linkage analysis in families, 9 selected markers | ↓P50 gating is linked to the site of α7-nic receptor (15q13-14) |
| Hill et al., 1998 | 58 (33f), families with alcoholism | 11.3 | Visual oddball | Amplitude, Latency | DRD2/ANKK1 Taq Ia | ↓P3 amplitude in A1 carriers; no effect on latency |
| Anokhin et al., 1999 | 591 (105 families with alcoholism) | 18-48 | Visual oddball | Amplitude, Latency | DRD2/ANKK1 Taq Ia | ↓P3 amplitude in A1 carriers in smokers but not in non-smokers |
| Winterer et al., 2000 | 95 (59f) | 35.8 | Auditory choice reaction time task | N1/P2 complex | GABRG2 (GABAA γ2 gene 5q33 | Direction not reported |
| Porjesz et al., 2002 (COGA study) | 1334 (253 dense alcoholic families) | Not reported | Visual oddball | P3 and N1 amplitudes | Linkage analysis in families, genome-wide (351 markers) | Significant linkage of P3 amplitude to Chr 2 and “suggestive” linkage on Chr. 5, 6, and 17 |
| Gallinat et al., 2003 | 49 (12f) schizophrenic patients and 170 (85f) HC | 32.8(sz) and 43.9(HC) | Auditory oddball | P3 amplitude | COMT (Val108/158Met) | ↓frontal P3 amplitude in Met allele homozygotes in schizophrenic patients but not in HC |
| Lin et al., 2001 | 134 females | 19-21 | Auditory oddball | P3 latency, amplitude | DRD2/ANKK1 Taq Ia | No Association |
| Tsai et al., 2003 | 120 females | 19-21 | Auditory oddball | P3 latency, amplitude | DRD3, DRD4, DAT1 | No Association |
| Jones et al., 2004 | 1067 members of 210 families with alcoholism | Visual oddball | Event-related oscillations | CHRM2, muscarinic cholinergic receptor gene | Significant association with SNPs located within and near the gene | |
| Reinvang et al., 2005 | 29 Patients with memory problems | 50-76 | MMN (duration deviants); auditory oddball | MMN amplitude (difference wave); N1, N2, and P3 amplitudes | ApoE (ε3 and ε4 alleles) | ↓N1 and N2 amplitude in ε4 allele carriers (with allele dose effect); no effect on P3a or P3b |
| Yu et al., 2005 | 191 females | 19-21 | Auditory oddball | P3 latency, amplitude | MAO-A, functional VNTR | No Association |
| Winterer et al., 2006 | 112 HC, 83 schizophrenic patients, 87 unaffected siblings | 18-60 | Auditory oddball | prefrontal “noise” (variability of response across trials) | COMT (Val108/158Met) | ↑ prefrontal “noise” in val/val homozygotes |
| Kramer et al., 2007 | 53 (36f) | 21 | Flanker+stop-signal task | Incompatibility N2,ERN, stop-N2 and –P3 | COMT, DRD4 -521 | ↑ERN in T/T homozygotes of DRD4; ↑stop-N2 and P3 in val/val homozygotes of COMT |
| Wright et al., 2008 | 647 (twins/siblings) | Spatial working memory task | P3 | Genome-wide linkage study | Significant linkage to chromosome 7q | |
| Chen et al., 2009 | 1,049 (209 families with alcoholism) | Visual oddball | Event-related theta band power (target) | Glutamate receptor gene GRM8 | Association with multiple SNPs | |
| Marco-Pallares et al., 2009 | 40 (ca. 30f) | 18-35 | Gambling task | ERPs to wins and losses, event-related beta power | COMT; DRD4 -521 | ↑MFN, ↑beta power for gains in Val/Val homozygotes relative to Met/Met homozygotes |
| Schofield et al., 2009 | 475 (51%f) | 32 | Auditory oddball | Target P3 | BDNF | ↑P3 latency in Met/Met homozygotes |
| Althaus et al., 2009 | 65 (14 f), including 45 children with psychiatric disorders and 20 HC | 10-13 | Probabilistic learning task | ERN | 5-HTTLPR; DRD2/ANKK1 | ↑ negative feedback P3 and ↓FRN habituation in the S-allele carriers; ↑ negative feedback P3 and ↓ positive feedback P3 in A1 carriers |
| Althaus et al., 2010 | Same sample as above | 10-13 | Probabilistic learning task | ERN, Feedback P3, Error positivity (Pe), SPN preceding feedback | Dopamine transporter gene DAT1 (SLC6A3) | ↓Pe, ↓SPN in 10/10 repeat genotype carriers |
| Beste et al., 2010 | 57 (40f) | 25 | Go-NoGo task | No-Go N2, Go- and No-Go P3 | BDNF | ↑N2 in met allele carriers |
| Marco-Pallares et al., 2010 | 48 (31f) | 18-35 | Auditory oddball with novel stimuli | Novelty P3a, target P3b | COMT; DRD4 -521 | ↑P3a in Met/Met homozygotes of COMT, and ↑novelty-related theta power in Met/Met and T/T homozygotes |
| Olvet et al., 2010 | 81 (44f) | undergraduates | Flanker task | ERN | 5-HTTLPR + rs25531 SNP | No association |
| Zlojutro et al., 2011 | 1064+1095 (replication); COGA study | Visual oddball | Theta event-related oscillations | GWAS | ↓ Theta EROs in T/T homozygotes of an SNP in HTR7 serotonin receptor gene | |
| Biehl et al., 2011 | 160 (90f) | 27 | Flanker task | ERN | DAT1(SLC6A3); DRD4 | ↓ERN in 7-repeat cariers (DRD4); ↑Pe in 10/10 homozygotes (DAT1) |
| Enge et al., 2011 | 72 (46f) | 22 | Auditory oddball, novelty | N1 | 5-HTTLPR | ↑N1 in S-allele carriers with high scores on self-reported “cognitive effort” |
| Garcia-Garcia et al., 2011 | 40 | 22 | Cued task switching | P3 to cues | COMT, DRD2/ANKK1 | Combined effect of two genotypes on P3: ↑ P3 in Val/A1+ and Met/A1− carriers |
| Mueller et al., 2011 | 169 males | 24 | Flanker task | ERN (ICA-derived) | COMT | ↑ERN and related delta- and theta-band power in Val carriers; reversed by D2 receptor blocker sulpiride |
| Bender et al., 2012 | 195(91f) | 15 | AX-CPT | Early CNV | COMT; DAT1(SLC6A3) - 2 VNTR polymorphisms; | ↑ Early posterior CNV in carriers Met/Met genotype and 6-repeat-10-repeat haplotype |
| Heinzel et al., 2013 | 181 adult ADHD patients, 114 HC | 35 (approx.) | Go-NoGo version of a CPT task | No-Go related “anteriorization” of P3 potential | DRD4, COMT | Complex interaction between genotypes with non-linear (U-shaped) effects |
| Bertoletti et al., 2012 | 49 children (23f) | 8-10 | Affective faces | N170 | μ-opioid receptor gene OPRM1, A118G polymorphism | ↑N170 in response to facial expressions in G allele carriers |
| Osinsky et al., 2012 | 65 (46f) | 22 | Facial Stroop task | Conflict-related N400; ERN | COMT | ↓ N400, ↓ ERN in Met/Met carriers |
| Beste et al., 2013 | 97 (67f) | 25 | Flanker/No-Go task | No-Go N2, P3; ERN | Neuropeptide S receptor (NPSR1) gene | ↑No-Go P3 and ↑ERN in T allele carriers |
| Spronk et al., 2013 | 320 (148f) | 18-70 | Auditory Oddball | Target P3 | COMT; DBH - 1021C>T | No association |
Abbreviations: f=females, m=males; HC=Healthy Controls; ERP measures are amplitudes of components indicated, if not specified otherwise. Indication of increased and decreased ERP components in specific genotype groups (↑ and ↓, respectively) refers to the absolute amplitude of the component regardless of its polarity; COGA: Collaborative Study on the Genetics of Alcoholism.
Acknowledgement
Supported by NIH grants DA027096 and DA01889 from the National Institute on Drug Abuse. The author thanks Tara Tinnin, M.S.W. for her help with the preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen JJ, Coan JA, Nazarian M. Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biol. Psychol. 2004;67:183–218. doi: 10.1016/j.biopsycho.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Allen JJ, Kline JP. Frontal EEG asymmetry, emotion, and psychopathology: the first, and the next 25 years. Biol. Psychol. 2004;67:1–5. doi: 10.1016/j.biopsycho.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Allen JJB, Reiner J, Katsanis J, Iacono WG. When it is and when it is not: the heritability of frontal EEG asymmetry. Psychophysiology. 1997;34:S17. [Google Scholar]
- Althaus M, Groen Y, Wijers AA, Mulder LJ, Minderaa RB, Kema IP, Dijck JD, Hartman CA, Hoekstra PJ. Differential effects of 5-HTTLPR and DRD2/ANKK1 polymorphisms on electrocortical measures of error and feedback processing in children. Clin. Neurophysiol. 2009;120:93–107. doi: 10.1016/j.clinph.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Ambrosius U, Lietzenmaier S, Wehrle R, Wichniak A, Kalus S, Winkelmann J, Bettecken T, Holsboer F, Yassouridis A, Friess E. Heritability of sleep electroencephalogram. Biol. Psychiatry. 2008;64:344–348. doi: 10.1016/j.biopsych.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Anokhin A, Steinlein O, Fischer C, Mao YP, Vogt P, Schalt E, Vogel F. A Genetic-Study of the Human Low-Voltage Electroencephalogram. Hum. Genet. 1992;90:99–112. doi: 10.1007/BF00210751. [DOI] [PubMed] [Google Scholar]
- Anokhin A, Vogel F. EEG alpha rhythm frequency and intelligence in normal adults. Intelligence. 1996;23:1–14. [Google Scholar]
- Anokhin AP. THE GENETIC-ASPECTS OF HUMAN-BRAIN FUNCTIONING EVIDENCE FROM EEG MULTIVARIATE-ANALYSIS. Int. J. Psychophysiol. 1989;7:120–121. [Google Scholar]
- Anokhin AP, Golosheykin S, Heath AC. Genetic and environmental influences on emotion-modulated startle reflex: a twin study. Psychophysiology. 2007a;44:106–112. doi: 10.1111/j.1469-8986.2006.00486.x. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Golosheykin S, Heath AC. Heritability of frontal brain function related to action monitoring. Psychophysiology. 2008;45:524–534. doi: 10.1111/j.1469-8986.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Golosheykin S, Heath AC. Heritability of individual differences in cortical processing of facial affect. Behav. Genet. 2010;40:178–185. doi: 10.1007/s10519-010-9337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Heath AC, Myers E. Genetics, prefrontal cortex, and cognitive control: A twin study of event-related potentials in a response inhibition task. Neurosci. Lett. 2004;368:314–318. doi: 10.1016/j.neulet.2004.07.036. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Heath AC, Myers E. Genetic and environmental influences on frontal EEG asymmetry: a twin study. Biol. Psychol. 2006a;71:289–295. doi: 10.1016/j.biopsycho.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Heath AC, Myers E, Ralano A, Wood S. Genetic influences on prepulse inhibition of startle reflex in humans. Neurosci. Lett. 2003;353:45–48. doi: 10.1016/j.neulet.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Muller V, Lindenberger U, Heath AC, Myers E. Genetic influences on dynamic complexity of brain oscillations. Neurosci. Lett. 2006b;397:93–98. doi: 10.1016/j.neulet.2005.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Rohrbaugh JW. Frontal EEG asymmetry in families. Psychophysiology. 1998;35:S17. [Google Scholar]
- Anokhin AP, Rohrbaugh JW, Todorov AA, Vedeniapin AB. The P300 event-related brain potential in neuropsychiatric disorders: A moderator of genetic risk? Behav. Genet. 1999a;29:349–349. [Google Scholar]
- Anokhin AP, Todorov AA, Madden PA, Grant JD, Heath AC. Brain event-related potentials, dopamine D2 receptor gene polymorphism, and smoking. Genet. Epidemiol. 1999b;17(Suppl 1):S37–42. doi: 10.1002/gepi.1370170707. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, van Baal GC, van Beijsterveldt CE, de Geus EJ, Grant J, Boomsma DI. Genetic correlation between the P300 event-related brain potential and the EEG power spectrum. Behav. Genet. 2001;31:545–554. doi: 10.1023/a:1013341310865. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Vedeniapin AB, Heath AC, Korzyukov O, Boutros NN. Genetic and environmental influences on sensory gating of mid-latency auditory evoked responses: a twin study. Schizophr. Res. 2007b;89:312–319. doi: 10.1016/j.schres.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Vedeniapin AB, Sirevaag EJ, Bauer LO, O'Connor SJ, Kuperman S, Porjesz B, Reich T, Begleiter H, Polich J, Rohrbaugh JW. The P300 brain potential is reduced in smokers. Psychopharmacology (Berl) 2000;149:409–413. doi: 10.1007/s002130000387. [DOI] [PubMed] [Google Scholar]
- Attia J, Ioannidis JP, Thakkinstian A, McEvoy M, Scott RJ, Minelli C, Thompson J, Infante-Rivard C, Guyatt G. How to use an article about genetic association: A: Background concepts. JAMA. 2009a;301:74–81. doi: 10.1001/jama.2008.901. [DOI] [PubMed] [Google Scholar]
- Attia J, Ioannidis JP, Thakkinstian A, McEvoy M, Scott RJ, Minelli C, Thompson J, Infante-Rivard C, Guyatt G. How to use an article about genetic association: B: Are the results of the study valid? JAMA. 2009b;301:191–197. doi: 10.1001/jama.2008.946. [DOI] [PubMed] [Google Scholar]
- Attia J, Ioannidis JP, Thakkinstian A, McEvoy M, Scott RJ, Minelli C, Thompson J, Infante-Rivard C, Guyatt G. How to use an article about genetic association: C: What are the results and will they help me in caring for my patients? JAMA. 2009c;301:304–308. doi: 10.1001/jama.2008.993. [DOI] [PubMed] [Google Scholar]
- Battistuzzi G, Iudicone P, Santolamazza P, Petrucci R. Activity of adenosine deaminase allelic forms in intact erythrocytes and in lymphocytes. Ann Hum Genet. 1981;45:15–19. doi: 10.1111/j.1469-1809.1981.tb00301.x. [DOI] [PubMed] [Google Scholar]
- Bauer LO. Frontal P300 decrements, childhood conduct disorder, family history, and the prediction of relapse among abstinent cocaine abusers. Drug Alcohol Depend. 1997;44:1–10. doi: 10.1016/s0376-8716(96)01311-7. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. Subtypes of family history and conduct disorder: effects on P300 during the stroop test. Neuropsychopharmacology. 1999;21:51–62. doi: 10.1016/S0893-133X(98)00139-0. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Neuroelectric processes in individuals at risk for alcoholism. Alcohol Alcohol. 1990;25:251–256. doi: 10.1093/oxfordjournals.alcalc.a044998. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcohol. Clin. Exp. Res. 1999;23:1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Bennett CM, Miller MB. How reliable are the results from functional magnetic resonance imaging? Ann Ny Acad Sci. 2010;1191:133–155. doi: 10.1111/j.1749-6632.2010.05446.x. [DOI] [PubMed] [Google Scholar]
- Beste C, Kolev V, Yordanova J, Domschke K, Falkenstein M, Baune BT, Konrad C. The role of the BDNF Val66Met polymorphism for the synchronization of error-specific neural networks. J. Neurosci. 2010;30:10727–10733. doi: 10.1523/JNEUROSCI.2493-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijl S, de Bruin EA, Kenemans JL, Verbaten MN, Bocker KB. Effects of chronic alcohol consumption in a visual attention task and an auditory oddball task: an event-related potential study. Alcohol. Clin. Exp. Res. 2005;29:2029–2038. doi: 10.1097/01.alc.0000187163.52577.0d. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Elbert T, Canavan AG, Rockstroh B. Slow potentials of the cerebral cortex and behavior. Physiol. Rev. 1990;70:1–41. doi: 10.1152/physrev.1990.70.1.1. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Flor H, Lutzenberger W, Elbert T. Chaos and order in the human brain. Electroencephalogr. Clin. Neurophysiol. Suppl. 1995;44:450–459. [PubMed] [Google Scholar]
- Bismark AW, Moreno FA, Stewart JL, Towers DN, Coan JA, Oas J, Erickson RP, Allen JJ. Polymorphisms of the HTR1a allele are linked to frontal brain electrical asymmetry. Biol. Psychol. 2010;83:153–158. doi: 10.1016/j.biopsycho.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma D, Anokhin A, de Geus E. Genetics of electrophysiology: Linking genes, brain, and behavior. Current Directions in Psychological Science. 1997;6:106–110. [Google Scholar]
- Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nature reviews. 2002;3:872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Overall J, Zouridakis G. Test-retest reliability of the P50 mid-latency auditory evoked response. Psychiatry Res. 1991;39:181–192. doi: 10.1016/0165-1781(91)90086-5. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch. Gen. Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Canuet L, Tellado I, Couceiro V, Fraile C, Fernandez-Novoa L, Ishii R, Takeda M, Cacabelos R. Resting-state network disruption and APOE genotype in Alzheimer's disease: a lagged functional connectivity study. PLoS One. 2012;7:e46289. doi: 10.1371/journal.pone.0046289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Gerson J, Fein G. The reliability of P50 suppression as measured by the conditioning/testing ratio is vastly improved by dipole modeling. Biol. Psychiatry. 1993;33:335–344. doi: 10.1016/0006-3223(93)90322-5. [DOI] [PubMed] [Google Scholar]
- Cardoso MM, Sirotin YB, Lima B, Glushenkova E, Das A. The neuroimaging signal is a linear sum of neurally distinct stimulus- and task-related components. Nat. Neurosci. 2012;15:1298–1306. doi: 10.1038/nn.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SR, Iacono WG. Heritability of P300 amplitude development from adolescence to adulthood. Psychophysiology. 2006;43:470–480. doi: 10.1111/j.1469-8986.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Iacono WG, McGue M. P300 amplitude in adolescent twins discordant and concordant for alcohol use disorders. Biol. Psychol. 2002;61:203–227. doi: 10.1016/s0301-0511(02)00059-5. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Katsanis J, Iacono WG, McGue M. Emotional modulation of the startle reflex in twins: preliminary findings. Biol. Psychol. 1997;46:235–246. doi: 10.1016/s0301-0511(97)00014-8. [DOI] [PubMed] [Google Scholar]
- Chen AC, Manz N, Tang Y, Rangaswamy M, Almasy L, Kuperman S, Nurnberger J, Jr., O'Connor SJ, Edenberg HJ, Schuckit MA, Tischfield J, Foroud T, Bierut LJ, Rohrbaugh J, Rice JP, Goate A, Hesselbrock V, Porjesz B. Single-nucleotide polymorphisms in corticotropin releasing hormone receptor 1 gene (CRHR1) are associated with quantitative trait of event-related potential and alcohol dependence. Alcohol. Clin. Exp. Res. 2010;34:988–996. doi: 10.1111/j.1530-0277.2010.01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Tang Y, Rangaswamy M, Wang JC, Almasy L, Foroud T, Edenberg HJ, Hesselbrock V, Nurnberger J, Jr., Kuperman S, O'Connor SJ, Schuckit MA, Bauer LO, Tischfield J, Rice JP, Bierut L, Goate A, Porjesz B. Association of single nucleotide polymorphisms in a glutamate receptor gene (GRM8) with theta power of event-related oscillations and alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:359–368. doi: 10.1002/ajmg.b.30818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian JC, Morzorati S, Norton JA, Jr., Williams CJ, O'Connor S, Li TK. Genetic analysis of the resting electroencephalographic power spectrum in human twins. Psychophysiology. 1996;33:584–591. doi: 10.1111/j.1469-8986.1996.tb02435.x. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Geyer MA, Braff DL. P50 suppression among schizophrenia and normal comparison subjects: a methodological analysis. Biol. Psychiatry. 1997;41:1035–1044. doi: 10.1016/S0006-3223(96)00208-9. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB, Malone S, Iacono WG. The heritability of trait midfrontal EEG asymmetry and negative emotionality: Sex differences and genetic nonadditivity. Psychophysiology. 2003;40:S34–S34. [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Criado JR, Ehlers CL. Event-related oscillations in the parietal cortex of adult alcohol-preferring (P) and alcohol-nonpreferring rats (NP). Alcohol. 2010;44:335–342. doi: 10.1016/j.alcohol.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Anterior electrophysiological asymmetries, emotion, and depression: conceptual and methodological conundrums. Psychophysiology. 1998;35:607–614. doi: 10.1017/s0048577298000134. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Fox NA. Asymmetrical brain activity discriminates between positive and negative affective stimuli in human infants. Science. 1982;218:1235–1237. doi: 10.1126/science.7146906. [DOI] [PubMed] [Google Scholar]
- Davis H, Davis P. Action potentials of the brain. Arch. Neurol. 1936;36:1214–1224. [Google Scholar]
- De Gennaro L, Marzano C, Fratello F, Moroni F, Pellicciari MC, Ferlazzo F, Costa S, Couyoumdjian A, Curcio G, Sforza E, Malafosse A, Finelli LA, Pasqualetti P, Ferrara M, Bertini M, Rossini PM. The electroencephalographic fingerprint of sleep is genetically determined: a twin study. Ann. Neurol. 2008;64:455–460. doi: 10.1002/ana.21434. [DOI] [PubMed] [Google Scholar]
- de Geus EJ. Introducing genetic psychophysiology. Biol. Psychol. 2002;61:1–10. doi: 10.1016/s0301-0511(02)00049-2. [DOI] [PubMed] [Google Scholar]
- de Geus EJ. From genotype to EEG endophenotype: a route for post-genomic understanding of complex psychiatric disease? Genome medicine. 2010;2:63. doi: 10.1186/gm184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pascalis V, Cozzuto G, Caprara GV, Alessandri G. Relations among EEG-alpha asymmetry, BIS/BAS, and dispositional optimism. Biol. Psychol. 2013;94:198–209. doi: 10.1016/j.biopsycho.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J. Neurosci. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U. S. A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci F, Enoch MA, Yuan Q, Shen PH, White KV, Hodgkinson C, Albaugh B, Virkkunen M, Goldman D. HTR3B is associated with alcoholism with antisocial behavior and alpha EEG power--an intermediate phenotype for alcoholism and co-morbid behaviors. Alcohol. 2009;43:73–84. doi: 10.1016/j.alcohol.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan CC, Barry RJ, Connolly JF, Fischer C, Michie PT, Naatanen R, Polich J, Reinvang I, Van Petten C. Event-related potentials in clinical research: guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin. Neurophysiol. 2009;120:1883–1908. doi: 10.1016/j.clinph.2009.07.045. [DOI] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am. J. Psychiatry. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustman RE, Beck EC. The visually evoked potential in twins. Electroencephalogr. Clin. Neurophysiol. 1965;19:570–575. doi: 10.1016/0013-4694(65)90242-7. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ. Genes contributing to the development of alcoholism: an overview. Alcohol research : current reviews. 2012;34:336–338. doi: 10.35946/arcr.v34.3.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, Jr., O'Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am. J. Hum. Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Gizer IR, Phillips E, Wilhelmsen KC. EEG alpha phenotypes: linkage analyses and relation to alcohol dependence in an American Indian community study. BMC Med Genet. 2010;11:43. doi: 10.1186/1471-2350-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E. EEG low-voltage alpha and alpha power in African American young adults: relation to family history of alcoholism. Alcohol. Clin. Exp. Res. 2003;27:765–772. doi: 10.1097/01.ALC.0000065439.09492.67. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Schuckit MA. EEG alpha variants and alpha power in Hispanic American and white non-Hispanic American young adults with a family history of alcohol dependence. Alcohol. 2004;33:99–106. doi: 10.1016/j.alcohol.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Eichele T, Specht K, Moosmann M, Jongsma ML, Quiroga RQ, Nordby H, Hugdahl K. Assessing the spatiotemporal evolution of neuronal activation with single-trial event-related potentials and functional MRI. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17798–17803. doi: 10.1073/pnas.0505508102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert T, Ray WJ, Kowalik ZJ, Skinner JE, Graf KE, Birbaumer N. Chaos and physiology: deterministic chaos in excitable cell assemblies. Physiol. Rev. 1994;74:1–47. doi: 10.1152/physrev.1994.74.1.1. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Rohrbaugh JW, Davis EZ, Harris CR, Ellingson RJ, Andreason P, Moore V, Varner JL, Brown GL, Eckardt MJ, et al. Relationship of genetically transmitted alpha EEG traits to anxiety disorders and alcoholism. Am. J. Med. Genet. 1995;60:400–408. doi: 10.1002/ajmg.1320600510. [DOI] [PubMed] [Google Scholar]
- Enoch MA, White KV, Harris CR, Robin RW, Ross J, Rohrbaugh JW, Goldman D. Association of low-voltage alpha EEG with a subtype of alcohol use disorders. Alcohol. Clin. Exp. Res. 1999;23:1312–1319. [PubMed] [Google Scholar]
- Ethridge LE, Malone SM, Iacono WG, Clementz BA. Genetic influences on composite neural activations supporting visual target identification. Biol. Psychol. 2012;92:329–341. doi: 10.1016/j.biopsycho.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euser AS, Arends LR, Evans BE, Greaves-Lord K, Huizink AC, Franken IH. The P300 event-related brain potential as a neurobiological endophenotype for substance use disorders: a meta-analytic investigation. Neurosci. Biobehav. Rev. 2012;36:572–603. doi: 10.1016/j.neubiorev.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Evans DM, Gillespie NA, Martin NG. Biometrical genetics. Biol. Psychol. 2002;61:33–51. doi: 10.1016/s0301-0511(02)00051-0. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Herrmann MJ, Roemmler J, Ehlis AC, Wagener A, Heidrich A, Ortega G, Zeng Y, Lesch KP. Allelic variation of serotonin transporter function modulates the brain electrical response for error processing. Neuropsychopharmacology. 2004;29:1506–1511. doi: 10.1038/sj.npp.1300409. [DOI] [PubMed] [Google Scholar]
- Flint J, Munafo MR. The endophenotype concept in psychiatric genetics. Psychol. Med. 2007;37:163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J, Munafo MR. Candidate and non-candidate genes in behavior genetics. Curr. Opin. Neurobiol. 2012 doi: 10.1016/j.conb.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Gray M, Whitfield SL, Turken AU, Glover G, Faustman WO, Mathalon DH. Acquiring and inhibiting prepotent responses in schizophrenia: event-related brain potentials and functional magnetic resonance imaging. Arch. Gen. Psychiatry. 2004;61:119–129. doi: 10.1001/archpsyc.61.2.119. [DOI] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suner D, Cigudosa JC, Urioste M, Benitez J, Boix-Chornet M, Sanchez-Aguilera A, Ling C, Carlsson E, Poulsen P, Vaag A, Stephan Z, Spector TD, Wu YZ, Plass C, Esteller M. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. U. S. A. 2005;102:10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc. Natl. Acad. Sci. U. S. A. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Ross RG, Waldo MC, Stevens KE, Adler LE, Leonard S. The genetics of sensory gating deficits in schizophrenia. Current psychiatry reports. 2003;5:155–161. doi: 10.1007/s11920-003-0032-2. [DOI] [PubMed] [Google Scholar]
- Freedman R, Waldo M, Waldo CI, 3rd, Wilson JR. Genetic influences on the effects of alcohol on auditory evoked potentials. Alcohol. 1987;4:249–253. doi: 10.1016/0741-8329(87)90019-x. [DOI] [PubMed] [Google Scholar]
- Gao Y, Raine A. P3 event-related potential impairments in antisocial and psychopathic individuals: a meta-analysis. Biol. Psychol. 2009;82:199–210. doi: 10.1016/j.biopsycho.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Gao Y, Tuvblad C, Raine A, Lozano DI, Baker LA. Genetic and environmental influences on frontal EEG asymmetry and alpha power in 9-10-year-old twins. Psychophysiology. 2009;46:787–796. doi: 10.1111/j.1469-8986.2009.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzke-Kopp LM, Jetha MK, Segalowitz SJ. The role of resting frontal EEG asymmetry in psychopathology: Afferent or efferent filter? Dev. Psychobiol. 2012 doi: 10.1002/dev.21092. [DOI] [PubMed] [Google Scholar]
- George MS, Aston-Jones G. Noninvasive techniques for probing neurocircuitry and treating illness: vagus nerve stimulation (VNS), transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). Neuropsychopharmacology. 2010;35:301–316. doi: 10.1038/npp.2009.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Begleiter H, Porjesz B, Chorlian DB, Edenberg HJ, Foroud T, Goate A, Reich T. Linkage mapping of beta 2 EEG waves via non-parametric regression. Am J Med Genet B Neuropsychiatr Genet. 2003;118B:66–71. doi: 10.1002/ajmg.b.10057. [DOI] [PubMed] [Google Scholar]
- Gilmore CS, Malone SM, Iacono WG. Brain electrophysiological endophenotypes for externalizing psychopathology: a multivariate approach. Behav. Genet. 2010;40:186–200. doi: 10.1007/s10519-010-9343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore CS, Malone SM, Iacono WG. Is the P3 amplitude reduction seen in externalizing psychopathology attributable to stimulus sequence effects? Psychophysiology. 2012;49:248–251. doi: 10.1111/j.1469-8986.2011.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Hagemann D. Individual differences in anterior EEG asymmetry: methodological problems and solutions. Biol. Psychol. 2004;67:157–182. doi: 10.1016/j.biopsycho.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Hall MH, Schulze K, Bramon E, Murray RM, Sham P, Rijsdijk F. Genetic overlap between P300, P50, and duration mismatch negativity. Am J Med Genet B Neuropsychiatr Genet. 2006a;141B:336–343. doi: 10.1002/ajmg.b.30318. [DOI] [PubMed] [Google Scholar]
- Hall MH, Schulze K, Rijsdijk F, Picchioni M, Ettinger U, Bramon E, Freedman R, Murray RM, Sham P. Heritability and reliability of P300, P50 and duration mismatch negativity. Behav. Genet. 2006b;36:845–857. doi: 10.1007/s10519-006-9091-6. [DOI] [PubMed] [Google Scholar]
- Hamer D, Sirota L. Beware the chopsticks gene. Mol. Psychiatry. 2000;5:11–13. doi: 10.1038/sj.mp.4000662. [DOI] [PubMed] [Google Scholar]
- Hansell NK, Wright MJ, Luciano M, Geffen GM, Geffen LB, Martin NG. Genetic covariation between event-related potential (ERP) and behavioral non-ERP measures of working-memory, processing speed, and IQ. Behav. Genet. 2005;35:695–706. doi: 10.1007/s10519-005-6188-2. [DOI] [PubMed] [Google Scholar]
- Hardy J, Singleton A. Genomewide association studies and human disease. N. Engl. J. Med. 2009;360:1759–1768. doi: 10.1056/NEJMra0808700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biol. Psychiatry. 2002;51:59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- Heuschert D. [Eeg Studies on Uniovular Twins in Oldage]. Zeitschrift fur menschliche Vererbungs- und Konstitutionslehre. 1963;37:128–172. [PubMed] [Google Scholar]
- Hicks BM, Bernat E, Malone SM, Iacono WG, Patrick CJ, Krueger RF, McGue M. Genes mediate the association between P3 amplitude and externalizing disorders. Psychophysiology. 2007;44:98–105. doi: 10.1111/j.1469-8986.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Locke J, Zezza N, Kaplan B, Neiswanger K, Steinhauer SR, Wipprecht G, Xu J. Genetic association between reduced P300 amplitude and the DRD2 dopamine receptor A1 allele in children at high risk for alcoholism. Biol. Psychiatry. 1998;43:40–51. doi: 10.1016/s0006-3223(97)00203-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S. Neurodevelopmental patterns of visual P3b in association with familial risk for alcohol dependence and childhood diagnosis. Biol. Psychiatry. 2002;51:621–631. doi: 10.1016/s0006-3223(01)01301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke J, Steinhauer SR, Konicky C, Lowers L, Connolly J. Developmental delay in P300 production in children at high risk for developing alcohol-related disorders. Biol. Psychiatry. 1999;46:970–981. doi: 10.1016/s0006-3223(99)00032-3. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Lowers L, Locke J. Factors predicting the onset of adolescent drinking in families at high risk for developing alcoholism. Biol. Psychiatry. 2000;48:265–275. doi: 10.1016/s0006-3223(00)00841-6. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer S, Locke J. Event-related potentials in alcoholic men, their high-risk male relatives, and low-risk male controls. Alcohol. Clin. Exp. Res. 1995;19:567–576. doi: 10.1111/j.1530-0277.1995.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR, Zubin J, Baughman T. Event-related potentials as markers for alcoholism risk in high density families. Alcohol. Clin. Exp. Res. 1988;12:545–554. doi: 10.1111/j.1530-0277.1988.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Hirschhorn JN, Daly MJ. Genome-wide association studies for common diseases and complex traits. Nature reviews. 2005;6:95–108. doi: 10.1038/nrg1521. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Enoch MA, Srivastava V, Cummins-Oman JS, Ferrier C, Iarikova P, Sankararaman S, Yamini G, Yuan Q, Zhou Z, Albaugh B, White KV, Shen PH, Goldman D. Genome-wide association identifies candidate genes that influence the human electroencephalogram. Proc. Natl. Acad. Sci. U. S. A. 2010;107:8695–8700. doi: 10.1073/pnas.0908134107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynek K, Zvolsky P, Drabkova H. Chlorpromazine on the EEG in twins. Act. Nerv. Super. (Praha) 1978;20:48–49. [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. Int. J. Psychophysiol. 2003;48:147–178. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu Rev Clin Psychol. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Ibatoullina AA, Vardaris RM, Thompson L. Genetic and Environmental-Influences on the Coherence of Background and Orienting Response Eeg in Children. Intelligence. 1994;19:65–78. [Google Scholar]
- Ioannidis JP. Excess significance bias in the literature on brain volume abnormalities. Arch. Gen. Psychiatry. 2011;68:773–780. doi: 10.1001/archgenpsychiatry.2011.28. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP, Trikalinos TA. An exploratory test for an excess of significant findings. Clinical trials. 2007;4:245–253. doi: 10.1177/1740774507079441. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Lee CY, Mandrekar S, Wilkinson B, Cramer P, Zelcer N, Mann K, Lamb B, Willson TM, Collins JL, Richardson JC, Smith JD, Comery TA, Riddell D, Holtzman DM, Tontonoz P, Landreth GE. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut L, Goate A, Wang JC, Dick DM, Hinrichs A, Kwon J, Rice JP, Rohrbaugh J, Stock H, Wu W, Bauer LO, Chorlian DB, Crowe RR, Edenberg HJ, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J, Jr, O'Connor SJ, Schuckit MA, Stimus AT, Tischfield JA, Reich T, Begleiter H. Linkage and linkage disequilibrium of evoked EEG oscillations with CHRM2 receptor gene polymorphisms: implications for human brain dynamics and cognition. Int. J. Psychophysiol. 2004;53:75–90. doi: 10.1016/j.ijpsycho.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Kaminsky ZA, Tang T, Wang SC, Ptak C, Oh GH, Wong AH, Feldcamp LA, Virtanen C, Halfvarson J, Tysk C, McRae AF, Visscher PM, Montgomery GW, Gottesman II, Martin NG, Petronis A. DNA methylation profiles in monozygotic and dizygotic twins. Nat. Genet. 2009;41:240–245. doi: 10.1038/ng.286. [DOI] [PubMed] [Google Scholar]
- Kang SJ, Rangaswamy M, Manz N, Wang JC, Wetherill L, Hinrichs T, Almasy L, Brooks A, Chorlian DB, Dick D, Hesselbrock V, Kramer J, Kuperman S, Nurnberger J, Jr., Rice J, Schuckit M, Tischfield J, Bierut LJ, Edenberg HJ, Goate A, Foroud T, Porjesz B. Family-based genome-wide association study of frontal theta oscillations identifies potassium channel gene KCNJ6. Genes Brain Behav. 2012;11:712–719. doi: 10.1111/j.1601-183X.2012.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsanis J, Iacono WG, McGue MK, Carlson SR. P300 event-related potential heritability in monozygotic and dizygotic twins. Psychophysiology. 1997;34:47–58. doi: 10.1111/j.1469-8986.1997.tb02415.x. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Stevens MC, Laurens KR, Pearlson G, Calhoun VD, Liddle PF. An adaptive reflexive processing model of neurocognitive function: supporting evidence from a large scale (n = 100) fMRI study of an auditory oddball task. NeuroImage. 2005;25:899–915. doi: 10.1016/j.neuroimage.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Kitzbichler MG, Smith ML, Christensen SR, Bullmore E. Broadband criticality of human brain network synchronization. PLoS Comput Biol. 2009;5:e1000314. doi: 10.1371/journal.pcbi.1000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Schimke H, Pfurtscheller G. Alpha frequency, cognitive load and memory performance. Brain Topogr. 1993;5:241–251. doi: 10.1007/BF01128991. [DOI] [PubMed] [Google Scholar]
- Landolt HP. Genetic determination of sleep EEG profiles in healthy humans. Prog. Brain Res. 2011;193:51–61. doi: 10.1016/B978-0-444-53839-0.00004-1. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN, Patrick CJ. Emotion and psychopathology: a startle probe analysis. Prog. Exp. Pers. Psychopathol. Res. 1993;16:163–199. [PubMed] [Google Scholar]
- Larson CL, Ruffalo D, Nietert JY, Davidson RJ. Stability of emotion-modulated startle during short and long picture presentation. Psychophysiology. 2005;42:604–610. doi: 10.1111/j.1469-8986.2005.00345.x. [DOI] [PubMed] [Google Scholar]
- Lee H, Shackman AJ, Jackson DC, Davidson RJ. Test-retest reliability of voluntary emotion regulation. Psychophysiology. 2009;46:874–879. doi: 10.1111/j.1469-8986.2009.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TW, Yu YW, Hong CJ, Tsai SJ, Wu HC, Chen TJ. The effects of catechol-O-methyl-transferase polymorphism Val158Met on functional connectivity in healthy young females: a resting EEG study. Brain Res. 2011a;1377:21–31. doi: 10.1016/j.brainres.2010.12.073. [DOI] [PubMed] [Google Scholar]
- Lee TW, Yu YW, Hong CJ, Tsai SJ, Wu HC, Chen TJ. The influence of serotonin transporter polymorphisms on cortical activity: a resting EEG study. BMC Neurosci. 2011b;12:33. doi: 10.1186/1471-2202-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TW, Yu YW, Hong CJ, Tsai SJ, Wu HC, Chen TJ. The influence of apolipoprotein E Epsilon4 polymorphism on qEEG profiles in healthy young females: a resting EEG study. Brain Topogr. 2012;25:431–442. doi: 10.1007/s10548-012-0229-y. [DOI] [PubMed] [Google Scholar]
- Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Short M, Drebing C, Berger R, Venn D, Sirota P, Zerbe G, Olincy A, Ross RG, Adler LE, Freedman R. Association of promoter variants in the alpha7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch. Gen. Psychiatry. 2002;59:1085–1096. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR, Rissling AJ, Radant A, Sugar CA, Sprock J, Pela M, Geyer MA, Braff DL. Characterization of neurophysiologic and neurocognitive biomarkers for use in genomic and clinical outcome studies of schizophrenia. PLoS One. 2012;7:e39434. doi: 10.1371/journal.pone.0039434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DE. The p300: where in the brain is it produced and what does it tell us? Neuroscientist. 2005;11:563–576. doi: 10.1177/1073858405280524. [DOI] [PubMed] [Google Scholar]
- Linkenkaer-Hansen K, Monto S, Rytsala H, Suominen K, Isometsa E, Kahkonen S. Breakdown of long-range temporal correlations in theta oscillations in patients with major depressive disorder. J. Neurosci. 2005;25:10131–10137. doi: 10.1523/JNEUROSCI.3244-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkenkaer-Hansen K, Smit DJ, Barkil A, van Beijsterveldt TE, Brussaard AB, Boomsma DI, van Ooyen A, de Geus EJ. Genetic contributions to long-range temporal correlations in ongoing oscillations. J. Neurosci. 2007;27:13882–13889. doi: 10.1523/JNEUROSCI.3083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkowski P. EEG sleep patterns in twins. J. Sleep Res. 1999;8(Suppl 1):11–13. doi: 10.1046/j.1365-2869.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- Livanov MN. [Analysis of bioelectrical oscillations in the cerebral cortex of rabbit]. Contemporary neuropathology, psychiatry, and psychohygiene. 1934;3:98–115. [Google Scholar]
- Livanov MN. Spatial Organization of Cerebral Processes. John Wiley & Sons; New York Toronto: 1977. [Google Scholar]
- Lomov BF, Ravich-Shcherbo IV. [Problems of Genetic Psychophysiology of Man] Nauka; Moscow: 1978. [Google Scholar]
- Lorig TS. What was the question? fMRI and inference in psychophysiology. Int. J. Psychophysiol. 2009;73:17–21. doi: 10.1016/j.ijpsycho.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Lutzenberger W, Preissl H, Pulvermuller F. Fractal dimension of electroencephalographic time series and underlying brain processes. Biol. Cybern. 1995;73:477–482. doi: 10.1007/BF00201482. [DOI] [PubMed] [Google Scholar]
- Lykken DT, Tellegen A, Thorkelson K. Genetic determination of EEG frequency spectra. Biol. Psychol. 1974;1:245–259. doi: 10.1016/0301-0511(74)90001-5. [DOI] [PubMed] [Google Scholar]
- Mantini D, Corbetta M, Perrucci MG, Romani GL, Del Gratta C. Large-scale brain networks account for sustained and transient activity during target detection. NeuroImage. 2009;44:265–274. doi: 10.1016/j.neuroimage.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryutina TM. Genetic and Environmental-Factors in Interindividual Verp Variability. Personality and Individual Differences. 1994;17:531–537. [Google Scholar]
- Mazzotti DR, Guindalini C, de Souza AA, Sato JR, Santos-Silva R, Bittencourt LR, Tufik S. Adenosine deaminase polymorphism affects sleep EEG spectral power in a large epidemiological sample. PLoS One. 2012;7:e44154. doi: 10.1371/journal.pone.0044154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ. Latent curve analyses of longitudinal twin data using a mixed-effects biometric approach. Twin Res Hum Genet. 2006;9:343–359. doi: 10.1375/183242706777591263. [DOI] [PubMed] [Google Scholar]
- McCarthy MI, Abecasis GR, Cardon LR, Goldstein DB, Little J, Ioannidis JP, Hirschhorn JN. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nature reviews. 2008;9:356–369. doi: 10.1038/nrg2344. [DOI] [PubMed] [Google Scholar]
- Millar A, Smith D, Choueiry J, Fisher D, Albert P, Knott V. The moderating role of the dopamine transporter 1 gene on P50 sensory gating and its modulation by nicotine. Neuroscience. 2011;180:148–156. doi: 10.1016/j.neuroscience.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller V, Anokhin AP, Lindenberger U. Heritability of phase synchrony of brain oscillations induced by response inhibition in a Go/No-Go task. Behav. Genet. 2007;37:777–778. [Google Scholar]
- Mulert C, Pogarell O, Juckel G, Rujescu D, Giegling I, Rupp D, Mavrogiorgou P, Bussfeld P, Gallinat J, Moller HJ, Hegerl U. The neural basis of the P300 potential. Focus on the time-course of the underlying cortical generators. Eur. Arch. Psychiatry Clin. Neurosci. 2004;254:190–198. doi: 10.1007/s00406-004-0469-2. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Flint J. How reliable are scientific studies? Br. J. Psychiatry. 2010;197:257–258. doi: 10.1192/bjp.bp.109.069849. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Stothart G, Flint J. Bias in genetic association studies and impact factor. Mol. Psychiatry. 2009;14:119–120. doi: 10.1038/mp.2008.77. [DOI] [PubMed] [Google Scholar]
- Myles-Worsley M, Coon H, Byerley W, Waldo M, Young D, Freedman R. Developmental and genetic influences on the P50 sensory gating phenotype. Biol. Psychiatry. 1996;39:289–295. doi: 10.1016/0006-3223(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Kujala T, Escera C, Baldeweg T, Kreegipuu K, Carlson S, Ponton C. The mismatch negativity (MMN)--a unique window to disturbed central auditory processing in ageing and different clinical conditions. Clin. Neurophysiol. 2012;123:424–458. doi: 10.1016/j.clinph.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration? Conscious. Cogn. 2005;14:390–425. doi: 10.1016/j.concog.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E. The Normal EEG of the Waking Adult. In: Niedermeyer E, Lopes da Silva F, editors. Electroencephalography: Basic Principles, Clinical Applications and Related Fields. Lippincott Williams & Wilkins; Baltimore MD: 1999. pp. 149–173. [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, affective & behavioral neuroscience. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cognitive, affective & behavioral neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Noble EP, Berman SM, Ozkaragoz TZ, Ritchie T. Prolonged P300 latency in children with the D2 dopamine receptor A1 allele. Am. J. Hum. Genet. 1994;54:658–668. [PMC free article] [PubMed] [Google Scholar]
- O'Connor S, Sorbel J, Morzorati S, Li TK, Christian JC. A twin study of genetic influences on the acute adaptation of the EEG to alcohol. Alcohol. Clin. Exp. Res. 1999;23:494–501. [PubMed] [Google Scholar]
- Olvet DM, Hatchwell E, Hajcak G. Lack of association between the 5-HTTLPR and the error-related negativity (ERN). Biol. Psychol. 2010;85:504–508. doi: 10.1016/j.biopsycho.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Posikera IN, Malykh SB. Heritability and “environmentability” of electroencephalogram in infants: the twin study. Psychophysiology. 2003;40:727–741. doi: 10.1111/1469-8986.00073. [DOI] [PubMed] [Google Scholar]
- Perlman G, Johnson W, Iacono WG. The heritability of P300 amplitude in 18-year- olds is robust to adolescent alcohol use. Psychophysiology. 2009;46:962–969. doi: 10.1111/j.1469-8986.2009.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin. Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychol. Bull. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Ponomareva NV, Goltsov AY, Kunijeva SS, Scheglova NS, Malina DD, Mitrofanov AA, Boikova TI, Rogaev EI. Age- and genotype-related neurophysiologic reactivity to oxidative stress in healthy adults. Neurobiol. Aging. 2012;33:839, e811–821. doi: 10.1016/j.neurobiolaging.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Almasy L, Edenberg HJ, Wang K, Chorlian DB, Foroud T, Goate A, Rice JP, O'Connor SJ, Rohrbaugh J, Kuperman S, Bauer LO, Crowe RR, Schuckit MA, Hesselbrock V, Conneally PM, Tischfield JA, Li TK, Reich T, Begleiter H. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proc. Natl. Acad. Sci. U. S. A. 2002a;99:3729–3733. doi: 10.1073/pnas.052716399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Neurophysiological factors in individuals at risk for alcoholism. Recent Dev. Alcohol. 1991;9:53–67. [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Wang K, Almasy L, Chorlian DB, Stimus AT, Kuperman S, O'Connor SJ, Rohrbaugh J, Bauer LO, Edenberg HJ, Goate A, Rice JP, Reich T. Linkage and linkage disequilibrium mapping of ERP and EEG phenotypes. Biol. Psychol. 2002b;61:229–248. doi: 10.1016/s0301-0511(02)00060-1. [DOI] [PubMed] [Google Scholar]
- Posamentier MT, Abdi H. Processing faces and facial expressions. Neuropsychol. Rev. 2003;13:113–143. doi: 10.1023/a:1025519712569. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Beem AL, de Geus EJ, van Baal GC, von Hjelmborg JB, Iachine I, Boomsma DI. Theory and practice in quantitative genetics. Twin Res. 2003;6:361–376. doi: 10.1375/136905203770326367. [DOI] [PubMed] [Google Scholar]
- Posthuma D, de Geus EJ, Mulder EJ, Smit DJ, Boomsma DI, Stam CJ. Genetic components of functional connectivity in the brain: the heritability of synchronization likelihood. Hum. Brain Mapp. 2005;26:191–198. doi: 10.1002/hbm.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen P, Esteller M, Vaag A, Fraga MF. The epigenetic basis of twin discordance in age-related diseases. Pediatr. Res. 2007;61:38R–42R. doi: 10.1203/pdr.0b013e31803c7b98. [DOI] [PubMed] [Google Scholar]
- Pritchard WS, Duke DW. Measuring “chaos” in the brain: a tutorial review of EEG dimension estimation. Brain Cogn. 1995;27:353–397. doi: 10.1006/brcg.1995.1027. [DOI] [PubMed] [Google Scholar]
- Propping P. Genetic control of ethanol action on the central nervous system. An EEG study in twins. Hum. Genet. 1977;35:309–334. doi: 10.1007/BF00446623. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Jones KA, Porjesz B, Chorlian DB, Padmanabhapillai A, Kamarajan C, Kuperman S, Rohrbaugh J, O'Connor SJ, Bauer LO, Schuckit MA, Begleiter H. Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. Int. J. Psychophysiol. 2007;63:3–15. doi: 10.1016/j.ijpsycho.2006.10.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld CA, Medland SE, Derringer J, Yang J, Esko T, Martin NW, Westra HJ, Shakhbazov K, Abdellaoui A, Agrawal A, Albrecht E, Alizadeh BZ, Amin N, Barnard J, Baumeister SE, Benke KS, Bielak LF, Boatman JA, Boyle PA, Davies G, de Leeuw C, Eklund N, Evans DS, Ferhmann R, Fischer K, Gieger C, Gjessing HK, Hagg S, Harris JR, Hayward C, Holzapfel C, Ibrahim-Verbaas CA, Ingelsson E, Jacobsson B, Joshi PK, Jugessur A, Kaakinen M, Kanoni S, Karjalainen J, Kolcic I, Kristiansson K, Kutalik Z, Lahti J, Lee SH, Lin P, Lind PA, Liu Y, Lohman K, Loitfelder M, McMahon G, Vidal PM, Meirelles O, Milani L, Myhre R, Nuotio ML, Oldmeadow CJ, Petrovic KE, Peyrot WJ, Polasek O, Quaye L, Reinmaa E, Rice JP, Rizzi TS, Schmidt H, Schmidt R, Smith AV, Smith JA, Tanaka T, Terracciano A, van der Loos MJ, Vitart V, Volzke H, Wellmann J, Yu L, Zhao W, Allik J, Attia JR, Bandinelli S, Bastardot F, Beauchamp J, Bennett DA, Berger K, Bierut LJ, Boomsma DI, Bultmann U, Campbell H, Chabris CF, Cherkas L, Chung MK, Cucca F, de Andrade M, De Jager PL, De Neve JE, Deary IJ, Dedoussis GV, Deloukas P, Dimitriou M, Eiriksdottir G, Elderson MF, Eriksson JG, Evans DM, Faul JD, Ferrucci L, Garcia ME, Gronberg H, Guethnason V, Hall P, Harris JM, Harris TB, Hastie ND, Heath AC, Hernandez DG, Hoffmann W, Hofman A, Holle R, Holliday EG, Hottenga JJ, Iacono WG, Illig T, Jarvelin MR, Kahonen M, Kaprio J, Kirkpatrick RM, Kowgier M, Latvala A, Launer LJ, Lawlor DA, Lehtimaki T, Li J, Lichtenstein P, Lichtner P, Liewald DC, Madden PA, Magnusson PK, Makinen TE, Masala M, McGue M, Metspalu A, Mielck A, Miller MB, Montgomery GW, Mukherjee S, Nyholt DR, Oostra BA, Palmer LJ, Palotie A, Penninx BW, Perola M, Peyser PA, Preisig M, Raikkonen K, Raitakari OT, Realo A, Ring SM, Ripatti S, Rivadeneira F, Rudan I, Rustichini A, Salomaa V, Sarin AP, Schlessinger D, Scott RJ, Snieder H, St Pourcain B, Starr JM, Sul JH, Surakka I, Svento R, Teumer A, Tiemeier H, van Rooij FJ, Van Wagoner DR, Vartiainen E, Viikari J, Vollenweider P, Vonk JM, Waeber G, Weir DR, Wichmann HE, Widen E, Willemsen G, Wilson JF, Wright AF, Conley D, Davey-Smith G, Franke L, Groenen PJ, Hofman A, Johannesson M, Kardia SL, Krueger RF, Laibson D, Martin NG, Meyer MN, Posthuma D, Thurik AR, Timpson NJ, Uitterlinden AG, van Duijn CM, Visscher PM, Benjamin DJ, Cesarini D, Koellinger PD. GWAS of 126,559 individuals identifies genetic variants associated with educational attainment. Science. 2013;340:1467–1471. doi: 10.1126/science.1235488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Brief Bioinform. 2002;3:119–133. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- Rommelse NN, Geurts HM, Franke B, Buitelaar JK, Hartman CA. A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and attention-deficit/hyperactivity disorder and facilitate the search for pleiotropic genes. Neurosci. Biobehav. Rev. 2011;35:1363–1396. doi: 10.1016/j.neubiorev.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Perez-Edgar K, Hamer DH. Linking gene, brain, and behavior: DRD4, frontal asymmetry, and temperament. Psychol Sci. 2009;20:831–837. doi: 10.1111/j.1467-9280.2009.02374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotin YB, Das A. Anticipatory haemodynamic signals in sensory cortex not predicted by local neuronal activity. Nature. 2009;457:475–479. doi: 10.1038/nature07664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit CM, Wright MJ, Hansell NK, Geffen GM, Martin NG. Genetic variation of individual alpha frequency (IAF) and alpha power in a large adolescent twin sample. Int. J. Psychophysiol. 2006;61:235–243. doi: 10.1016/j.ijpsycho.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Smit DJ, Boersma M, van Beijsterveldt CE, Posthuma D, Boomsma DI, Stam CJ, de Geus EJ. Endophenotypes in a dynamically connected brain. Behav. Genet. 2010;40:167–177. doi: 10.1007/s10519-009-9330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit DJ, Posthuma D, Boomsma DI, de Geus EJ. Genetic contribution to the P3 in young and middle-aged adults. Twin Res Hum Genet. 2007a;10:335–347. doi: 10.1375/twin.10.2.335. [DOI] [PubMed] [Google Scholar]
- Smit DJ, Posthuma D, Boomsma DI, De Geus EJ. The relation between frontal EEG asymmetry and the risk for anxiety and depression. Biol. Psychol. 2007b;74:26–33. doi: 10.1016/j.biopsycho.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Smit DJ, Stam CJ, Posthuma D, Boomsma DI, de Geus EJ. Heritability of “small-world” networks in the brain: a graph theoretical analysis of resting-state EEG functional connectivity. Hum. Brain Mapp. 2008;29:1368–1378. doi: 10.1002/hbm.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA, Boutros NN, Schwarzkopf SB. Reliability of P50 auditory event-related potential indices of sensory gating. Psychophysiology. 1994;31:495–502. doi: 10.1111/j.1469-8986.1994.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Craddock N, Kendler K, Lee PH, Neale BM, Nurnberger JI, Ripke S, Santangelo S, Sullivan PF. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani M, Knight RT. Neural origins of the P300. Crit. Rev. Neurobiol. 2000;14:199–224. [PubMed] [Google Scholar]
- Sorbel J, Morzorati S, O'Connor S, Li TK, Christian JC. Alcohol effects on the heritability of EEG spectral power. Alcohol. Clin. Exp. Res. 1996;20:1523–1527. doi: 10.1111/j.1530-0277.1996.tb01694.x. [DOI] [PubMed] [Google Scholar]
- Stam CJ. Nonlinear dynamical analysis of EEG and MEG: Review of an emerging field. Clin. Neurophysiol. 2005;116:2266–2301. doi: 10.1016/j.clinph.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Stassen HH, Lykken DT, Bomben G. The within-pair EEG similarity of twins reared apart. Eur. Arch. Psychiatry Neurol. Sci. 1988a;237:244–252. doi: 10.1007/BF00449914. [DOI] [PubMed] [Google Scholar]
- Stassen HH, Lykken DT, Propping P, Bomben G. Genetic determination of the human EEG. Survey of recent results on twins reared together and apart. Hum. Genet. 1988b;80:165–176. doi: 10.1007/BF00702862. [DOI] [PubMed] [Google Scholar]
- Steinlein O, Anokhin A, Yping M, Schalt E, Vogel F. Localization of a gene for the human low-voltage EEG on 20q and genetic heterogeneity. Genomics. 1992;12:69–73. doi: 10.1016/0888-7543(92)90408-k. [DOI] [PubMed] [Google Scholar]
- Sutton S, Braren M, Zubin J, John ER. Evoked-potential correlates of stimulus uncertainty. Science. 1965;150:1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- Swettenham JB, Muthukumaraswamy SD, Singh KD. BOLD Responses in Human Primary Visual Cortex are Insensitive to Substantial Changes in Neural Activity. Front Hum Neurosci. 2013;7:76. doi: 10.3389/fnhum.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Chorlian DB, Rangaswamy M, O'Connor S, Taylor R, Rohrbaugh J, Porjesz B, Begleiter H. Heritability of bipolar EEG spectra in a large sib-pair population. Behav. Genet. 2007;37:302–313. doi: 10.1007/s10519-006-9133-0. [DOI] [PubMed] [Google Scholar]
- Tiwari HK, Barnholtz-Sloan J, Wineinger N, Padilla MA, Vaughan LK, Allison DB. Review and evaluation of methods correcting for population stratification with a focus on underlying statistical principles. Hum Hered. 2008;66:67–86. doi: 10.1159/000119107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Kinney L. Psychometric properties of resting anterior EEG asymmetry: temporal stability and internal consistency. Psychophysiology. 1992;29:576–592. doi: 10.1111/j.1469-8986.1992.tb02034.x. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol. Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- van Baal GC, Boomsma DI, de Geus EJ. Longitudinal genetic analysis of EEG coherence in young twins. Behav. Genet. 2001;31:637–651. doi: 10.1023/a:1013357714500. [DOI] [PubMed] [Google Scholar]
- Van Baal GC, De Geus EJ, Boomsma DI. Genetic architecture of EEG power spectra in early life. Electroencephalogr. Clin. Neurophysiol. 1996;98:502–514. doi: 10.1016/0013-4694(96)95601-1. [DOI] [PubMed] [Google Scholar]
- van Baal GC, de Geus EJ, Boomsma DI. Genetic influences on EEG coherence in 5-year-old twins. Behav. Genet. 1998;28:9–19. doi: 10.1023/a:1021400613723. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Heritability of human brain functioning as assessed by electroencephalography. Am. J. Hum. Genet. 1996;58:562–573. [PMC free article] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Genetic and environmental influences on EEG coherence. Behav. Genet. 1998a;28:443–453. doi: 10.1023/a:1021637328512. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Individual differences in P300 amplitude: a genetic study in adolescent twins. Biol. Psychol. 1998b;47:97–120. doi: 10.1016/s0301-0511(97)00025-2. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, van Baal GC. Twin and family studies of the human electroencephalogram: a review and a meta-analysis. Biol. Psychol. 2002;61:111–138. doi: 10.1016/s0301-0511(02)00055-8. [DOI] [PubMed] [Google Scholar]
- van Dongen J, Boomsma DI. The evolutionary paradox and the missing heritability of schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:122–136. doi: 10.1002/ajmg.b.32135. [DOI] [PubMed] [Google Scholar]
- van Dongen J, Slagboom PE, Draisma HH, Martin NG, Boomsma DI. The continuing value of twin studies in the omics era. Nature reviews. 2012;13:640–653. doi: 10.1038/nrg3243. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol. Behav. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. Am. J. Hum. Genet. 2012;90:7–24. doi: 10.1016/j.ajhg.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel F. Über die Erblichkeit des normalen Elektroencephalogramms [On heritability of the normal electroencephalogram] Thieme Verlag; Stuttgart: 1958. [Google Scholar]
- Vogel F. Genetic Basis of Normal Human Electroencephalogram (Eeg). Humangenetik. 1970a;10:91–&. doi: 10.1007/BF00295509. [DOI] [PubMed] [Google Scholar]
- Vogel F. The genetic basis of the normal human electroencephalogram (EEG). Humangenetik. 1970b;10:91–114. doi: 10.1007/BF00295509. [DOI] [PubMed] [Google Scholar]
- Vogel F, Kruger J, Hopp HP, Schalt E, Schnobel R. Visually and auditory evoked EEG potentials in carriers of four hereditary EEG variants. Hum. Neurobiol. 1986;5:49–58. [PubMed] [Google Scholar]
- Vogel F, Schalt E, Kruger J. Electroencephalogram (Eeg) as a Research Tool in Human-Behavior Genetics - Psychological Examinations in Healthy Males with Various Inherited Eeg Variants .2. Results. Hum. Genet. 1979a;47:47–80. doi: 10.1007/BF00295570. [DOI] [PubMed] [Google Scholar]
- Vogel F, Schalt E, Kruger J, Propping P, Lehnert KF. Electroencephalogram (Eeg) as a Research Tool in Human-Behavior Genetics - Psychological Examinations in Healthy Males with Various Inherited Eeg Variants .1. Rationale of the “Study.Material.Methods.Heritability of Test Parameters. Hum. Genet. 1979b;47:1–45. doi: 10.1007/BF00295569. [DOI] [PubMed] [Google Scholar]
- Wang JC, Kapoor M, Goate AM. The genetics of substance dependence. Annual review of genomics and human genetics. 2012;13:241–261. doi: 10.1146/annurev-genom-090711-163844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WY, Barratt BJ, Clayton DG, Todd JA. Genome-wide association studies: theoretical and practical concerns. Nature reviews. 2005;6:109–118. doi: 10.1038/nrg1522. [DOI] [PubMed] [Google Scholar]
- Winterer G, Smolka M, Samochowiec J, Ziller M, Mahlberg R, Gallinat J, Rommelspacher HP, Herrmann WM, Sander T. Association of EEG coherence and an exonic GABA(B)R1 gene polymorphism. Am J Med Genet B Neuropsychiatr Genet. 2003;117B:51–56. doi: 10.1002/ajmg.b.10031. [DOI] [PubMed] [Google Scholar]
- Wong CC, Caspi A, Williams B, Craig IW, Houts R, Ambler A, Moffitt TE, Mill J. A longitudinal study of epigenetic variation in twins. Epigenetics : official journal of the DNA Methylation Society. 2010;5:516–526. doi: 10.4161/epi.5.6.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Lee SH, Goddard ME, Visscher PM. Genome-wide complex trait analysis (GCTA): methods, data analyses, and interpretations. Methods Mol. Biol. 2013;1019:215–236. doi: 10.1007/978-1-62703-447-0_9. [DOI] [PubMed] [Google Scholar]
- Young DA, Waldo M, Rutledge JH, 3rd, Freedman R. Heritability of inhibitory gating of the P50 auditory-evoked potential in monozygotic and dizygotic twins. Neuropsychobiology. 1996;33:113–117. doi: 10.1159/000119260. [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. J. Abnorm. Psychol. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zietsch BP, Hansen JL, Hansell NK, Geffen GM, Martin NG, Wright MJ. Common and specific genetic influences on EEG power bands delta, theta, alpha, and beta. Biol. Psychol. 2007;75:154–164. doi: 10.1016/j.biopsycho.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Zlojutro M, Manz N, Rangaswamy M, Xuei X, Flury-Wetherill L, Koller D, Bierut LJ, Goate A, Hesselbrock V, Kuperman S, Nurnberger J, Jr., Rice JP, Schuckit MA, Foroud T, Edenberg HJ, Porjesz B, Almasy L. Genome-wide association study of theta band event-related oscillations identifies serotonin receptor gene HTR7 influencing risk of alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:44–58. doi: 10.1002/ajmg.b.31136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zung WW, Wilson WP. Sleep and dream patterns in twins. Markov analysis of a genetic trait. Recent Adv. Biol. Psychiatry. 1966;9:119–130. doi: 10.1007/978-1-4684-8228-7_8. [DOI] [PubMed] [Google Scholar]