Abstract
Objective
Acquired epilepsy is frequently associated with structural lesions following trauma, stroke and infections. While seizures are often difficult to treat, there is no clinically applicable strategy to prevent the development of epilepsy in patients at risk. We have recently shown that vascular injury is associated with activation of albumin-mediated transforming growth factor β (TGF-β) signaling, and followed by local inflammatory response and epileptiform activity ex vivo. Here we investigated albumin-mediated TGF-β signaling and tested the efficacy of blocking the TGF-β pathway in preventing epilepsy.
Methods
We addressed the role of TGF-β signaling in epiletogenesis in two different rat models of vascular injury, combining in vitro and in vivo biochemical assays, gene expression, magnetic resonance and direct optical imaging for blood-brain barrier (BBB) permeability and vascular reactivity. Long-term electrocorticographic (ECoG) recordings were acquired in freely behaving animals.
Results
We demonstrate that serum-derived albumin preferentially induces activation of the activin receptor-like kinase 5 (ALK5) pathway of TGF-β receptor I in astrocytes. We further show that the angiotensin II type 1 receptor antagonist (AT1), losartan, previously identified as a blocker of peripheral TGF-β signaling, effectively blocks albumin-induced TGF-β activation in the brain. Most importantly, losartan prevents the development of delayed recurrent spontaneous seizures, an effect that persists weeks after drug withdrawal.
Interpretation
TGF-β signaling, activated in astrocytes by serum-derived albumin, is involved in epileptogenesis. We propose losartan, an FDA-approved drug, as an efficient anti-epileptogenic therapy for epilepsy associated with vascular injury.
Introduction
Brain insults, including traumatic brain injuries, stroke and infections are associated with a significantly increased risk of early seizures and delayed epilepsy1–3. Importantly, these conditions are also frequently associated with dysfunction of the blood-brain barrier (BBB) and the extravasation of serum proteins into the brain4–9.
We have recently shown that induction of BBB breakdown leads to neuronal hyperexcitability and epileptiform activity in the treated area, recorded ex vivo from neocortical brain slices10. Moreover, we identified the abundant serum protein albumin as sufficient to induce similar epileptiform activity11. Upon brain exposure to albumin, the protein binds to the type II receptor of the transforming growth factor β (TGF-β) and activates a transcriptional program12.
The TGF-β family of proteins is a group of cytokines that play a role in intercellular communication and regulates cellular processes, including cell growth, migration, differentiation, apoptosis, inflammation and expression of extracellular matrix proteins13,14. TGF-β signals via a complex of TGF-β membrane receptor types I and II (TGF-βRI and II, respectively). There are 7 known variants of type I receptors (activin-like kinase, ALK, 1–7), of which two are expressed in the brain; ALK1 and ALK515. Upon ligand binding, the TGF-β signaling intracellular mediators, the Smad proteins, are phosphorylated. ALK1 phosphorylates Smad1, 5 and 8, while ALK5 phosphorylates Smad2 and 3. The down-stream signaling involves interaction with other Smad proteins to form specific complexes that accumulate in the nucleus and promote transcriptional activity13,14,16,17.
The activation of brain TGF-β signaling by albumin induces rapid transcriptional programming, that includes astrocytic transformation12,18, inflammatory signaling and delayed down-regulation of gamma-amino-butyric acid (GABA)-related genes. Importantly, the transformation of astrocytes and the associated down-regulation of the inward-rectifier potassium channel (Kir 4.1), glutamate transporters and gap junction proteins (connexins 30 and 43) resulted in accumulation of extracellular potassium and glutamate upon neuronal activation, further increasing network excitability18.
In the present study, we aimed to identify the detailed molecular mechanisms underlying albumin-induced TGF-β signaling in different cell populations of the brain, to allow the design of targeted preventive treatments. We identified the FDA approved angiotensin II type 1 receptor (AT1) antagonist, losartan, as a potential TGF-β signaling blocker and tested its efficacy in the prevention of epilepsy following vascular injury and brain exposure to albumin.
Materials and Methods
Experimental procedures were approved by the animal care and use ethical committees at the University of California, Berkeley, and Ben-Gurion University of the Negev, Beer-Sheva.
Reagents
Earle’s Balanced Salt Solution (EBSS), Hank’s Balanced Salt Solution (HBSS), Dulbecco’s Modified Eagle’s Media (DMEM), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), Modified Eagle’s Media (MEM), Neurobasal Media, GlutaMAX, B27 supplement, Trypsin-EDTA and Penicillin-Streptomycin were obtained from Invitrogen. Papain enzyme, poly-L-lysine, cytosine β-D-arabinofuranoside (Ara-C), losartan potassium, bovine serum albumin (BSA, minimum 98%), fluorescein isothio-cyanate conjugate bovine albumin, sodium deoxycholate (DOC, minimum 97%) and Evans blue (EB) were all obtained from Sigma Aldrich. Serum Extender was obtained from BD Biosciences. TGF-β type 1 receptor ALK4, 5 and 7 selective inhibitor SB431542 and the ALK5 selective inhibitor SJN2511 were obtained from Tocris Biosciences. Recombinant TGF-β1 was obtained from Peprotech and recombinant BMP6 was obtained from R&D Biosystems.
Cell Culture
Primary neuronal cortical cultures were prepared from embryonic day 18 rats, as reported previously 19. Briefly, cells were dissociated and plated; Culture medium was replaced after 4 hours with neurobasal medium supplemented (2% B27 supplement and 0.5mM GlutaMAX™), (5% CO2, 37°C). After 7 days, cytosine arabinofuranoside (AraC, 10 μM) was added to the cultures. At 10–11 days, cells were incubated with 0.2 mM BSA (fraction V), 10 ng/ul TGF-β1, or 50 ng/ul Bmp6 (4 or 24 hours, 37°C). For astrocytic cultures, cells from the cerebral cortices of P1-2 rat pups were dissociated with papain and mechanical trituration, and cultured in high-glucose Dulbecco’s modified eagle medium (supplemented with 10% FBS and 1% penicillin/streptomycin, 37°C and 5% CO2). After 10 days the cells were shaken (37°C, 3 hours) to remove microglia and passaged onto plates. When confluent, culture medium was replaced with serum-free high-glucose DMEM (containing 1% penicillin/streptomycin) for 18 hours. Cells were then incubated in serum-free medium containing BSA (fraction V), TGF-β1, or Bmp6 as above. TGF-βR blockers, SB431542 (30μM) or SJN2511 (30μM) were added 1 hour prior to BSA (0.2mM).
Alexa488-albumin was used to measure albumin uptake for all time points. Quantification was achieved using a two-step process as reported 20 against a set of known standards (using a micro-plate reader). Albumin uptake was normalized to total protein (quantified using a bicinchoninic acid assay kit (Pierce)). TGF-β1 levels were measured in conditioned media using enzyme-linked immunosorbent assay (R&D systems) according to the manufacturer’s guidelines.
Antibodies and Western Blot analysis
Lysate samples were separated by SDS-PAGE and transferred onto a nitrocellulose membrane (brains) or a polyvinylidene fluoride (PVDF) membrane (cultures). For ALK1 and ALK5, membranes were blocked with 5% nonfat milk (1 hour, room temperature), incubated (overnight, 4°C) with primary antibody, and with a peroxidase-conjugated secondary antibody (1 hour, room temperature). For p-Smad1/5/8 and p-Smad2/3 western blots were incubated with primary antibody for 48 hours and with a peroxidase-conjugated secondary antibody for 2 hours. Immuno-blots were then visualized using ECL Plus Western blotting detection reagents (Amersham Biosciences). β-actin expression levels served as a loading control. Antibodies used: rabbit anti-ALK1 (Epitomics 2940-1), rabbit anti-ALK5 (Cell Signaling 3712), rabbit anti-p-Smad1/5/8, rabbit anti-p-Smad2/3 (Cell Signaling Technology 9511 and 3101, respectively) and mouse anti-actin (Sigma Aldrich A1978). Anti-rabbit and mouse secondary antibodies conjugated to HRP were obtained from Jackson ImmunoResearch (711-035-152 and 715-035-150, respectively).
Real-time RT-PCR
Total RNA was isolated using the TRIzol reagent (Invitrogen). mRNA expression levels were determined using a real-time kinetic analysis (iQ5 detection system, Bio-Rad). Data were analyzed using the PCR Miner program21. 18S/RPLP mRNA levels were used as internal controls. The following primer sequences were used:
GFAP:
(5′-AGAAAACCGCATCACCATTC-3′, 5′-TCCTTAATGACCTCGCCATC-3′)
18S rRNA (reference):
(5′-CCATCCAATCGGTAGTAGCG-3′, 5′-GTAACCCGTTGAACCCCATT-3′)
TGF-β1:
(5′-GGACTCTCCACCTGCAAGAC-3′, 5′-GCGAGCCTTAGTTTGGACAG-3′)
RPLP (reference):
(5′-ATCTACTCCGCCCTCATCCT-3′, 5′-GCAGATGAGGCTTCCAATGT-3′)
In vivo experiments
79 adult male Wistar rats (160–240gr) were anesthetized and a cranial window was performed as reported10. The exposed brain was perfused with artificial cerebral spinal fluid (ACSF) containing (in mM): 129 NaCl, 21 NaHCO3, 1.25 NaH2PO4, 1.8 MgSO4, 1.6 CaCl2, 3 KCl, and 10 glucose. Following treatment the bone was placed back and the skin was sutured. Animals were randomly divided into 5 groups with the following treatments: (i) BSA (0.2mM in ACSF)11, (ii) BSA and losartan (10μM), (iii) DOC (2mM)10, (iv) DOC followed by a single dose of losartan (IP, 100mg/kg) and 3 weeks of oral administration (2gr/L in drinking water) or (v) ACSF (serving as sham operated controls). Brain exposure to BSA, losartan, DOC and ACSF was performed using 40 minutes perfusion.
Electrocorticographic recordings and seizure analysis
Electrocorticographic (ECoG) activity was recorded using two epidural electrodes, placed posterior and anterior to the cranial window (Fig 4C), and a telemetric recording system (Data Science International, United States), as previously reported22–24. Blinded ECoG analysis was performed off-line using an in-house automated seizure detection algorithm, based on feature extraction and artificial neural network (ANN) clustering; For classifier training, a 1.5 hour long dataset was comprised, consisting of background activity, noise, artifacts and 24 seizures (lasting 10–60 seconds each) recorded from genetic25, status epilepticus-induced26 and albumin-induced27 models of epilepsy. The data was band-pass filtered (2–100Hz) and segmented into 2 second long epochs (1 second overlap). A reference vector of ANN values was created, assigning ‘seizure’ and ‘non-seizure’ epochs with target values of ‘1’ and ‘−1’, respectively. Considering the potential benefit of a multi-feature ECoG representation, 22 features (previously suggested for brain signal analysis) were extracted from each epoch. A forwards sequential selection algorithm28 was applied to reveal a feature combination yielding optimal trade-off between classification accuracy and computational complexity. A five feature subset (energy, relative power in the slow gamma frequency band, curve length, standard deviation and relative power in the beta frequency band) was selected and subsequently used for training the final ANN (single hidden layer (n=50) back-propagation ANN29 with Powell-Beale conjugate gradient training30). Favoring high sensitivity at the expense of occasional misclassifications, the ANN threshold was set to 0.85, based on receiver operating characteristic curve optimization. Seizures were defined as events lasting a minimum of 5 seconds, thus a sliding window thresholding procedure was applied for detecting consecutive ‘positive’ ANN outputs. System performance was assessed by analyzing over 2800 hours of ECoG from 15 animals (pilocarpine, n=6; albumin, n=3; and synapsin triple knockout, n=3) and 3 non-epileptic controls. To overcome diversity in signal properties, arising from the differences between animals and/or electrode placements, features of each recording were independently normalized to their values during baseline (i.e. noise- and seizure-free activity). Performance evaluation resulted in overall sensitivity and positive predictive value above 98% in unedited signals containing noise, artifacts and interictal discharges.
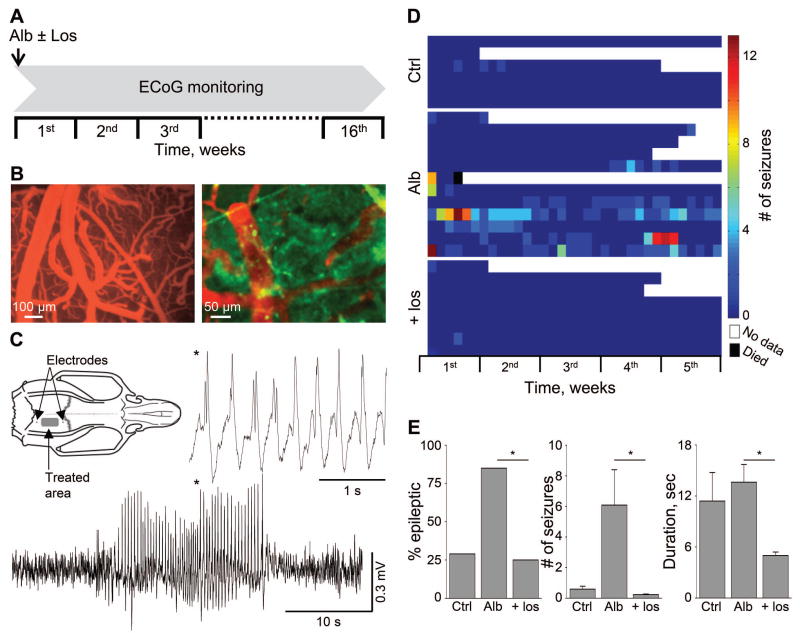
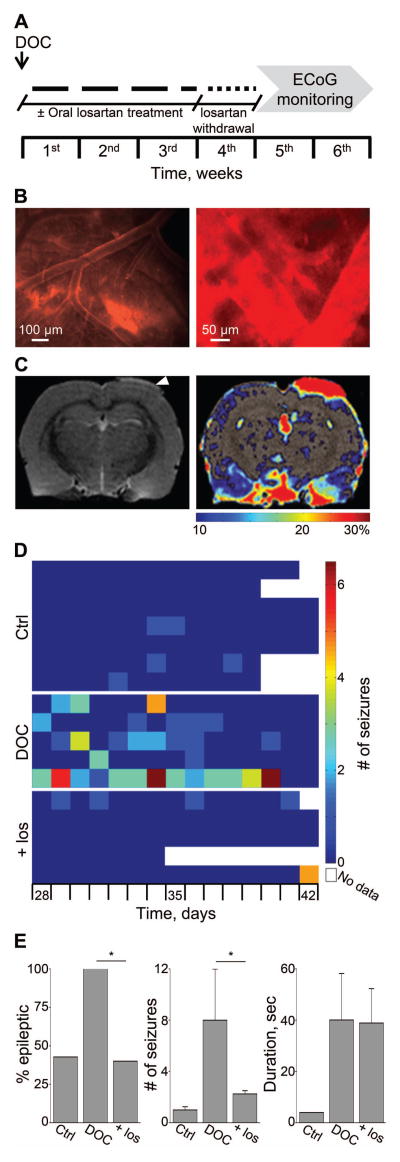
Figure 4.
Losartan prevents albumin-induced epilepsy. (A) Treatment protocol; animals were treated with local application of albumin (Alb) in the presence or absence of losartan (Los), added to the ACSF. (B) In-vivo imaging of cortical vessels during local application of fluorescently labeled albumin (green) and Evans blue (EB, red) intravenous injection, indicating no EB extravasation, consistent with normally functioning BBB. (C) The development of epilepsy was assessed through the occurrence of spontaneous electrocorticographic seizures (see exemplary seizure of an albumin-treated rat). (D) Color coded table showing the number of seizures per day is presented for each rat in albumin- (Alb) compared to ACSF- (Ctrl) and losartan- (Los) treated animals (see scale bar). (E) Bar graphs represent the percent of epileptic animals, number of seizures per week and the duration of seizures. Overall, compared to the albumin group, losartan treatment significantly lowered the percent of epileptic animals, and the number and duration of seizures among animals that did develop epilepsy *p ≤ 0.05.
Measuring vascular diameter and blood-brain barrier permeability
In vivo vascular imaging was performed as reported24,31. BBB permeability was estimated based on direct cortical imaging [following Evans blue injection (EB, 2% in 0.9% NaCl, 2.4ml/kg, IV)]. Images were acquired using (i) Lumar microscope (Carl Zeiss) and EMCCD camera (658×496 resolution, Andor Technology, DL-658 M-TIL), and (ii) fibered confocal microscopy (CellVisio, Mauna Kea Technologies). In addition, contrast enhanced MRI (CE-MRI) was performed (Pharmascan 70/16 AS, Bruker Biospin, Ettlingen, Germany) using T1-weighted imaging before and 5 minutes following administration of gadolinium-diethylenetriamine penta-acetic acid (Gd-DTPA, 0.5M, 2.5ml/kg, IV) as reported32.
Statistical Analyses
The effect of albumin in the presence or absence of TGF-βR blockers on protein and mRNA levels in cultures and in cortical tissue was compared using the Mann-Whitney test. The differences in the percent of epileptic animals among groups were compared using the Chi square test, and the number of seizures and their duration were compared using Mann-Whitney (SPSS statistics, SPSS Inc.). p≤0.05 was determined as statistical significance.
Results
Albumin uptake into astrocytes induces TGF-β1 up-regulation
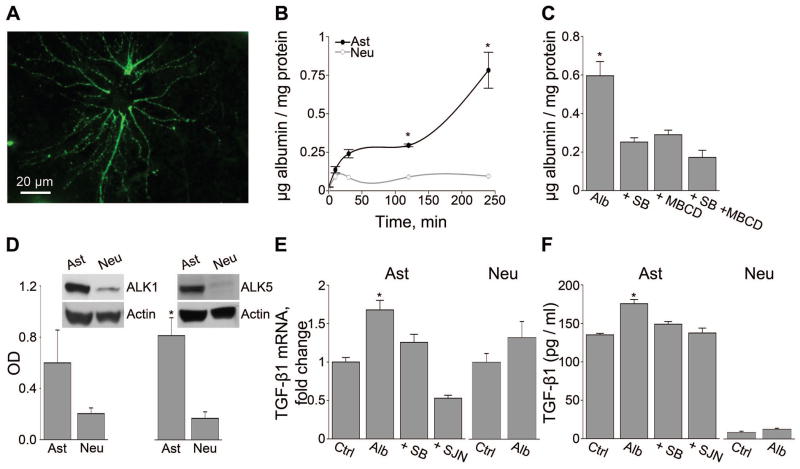
Upon exposure of the secluded brain environment to albumin, it is taken-up into cells6,11,33. We first set out to characterize albumin endocytosis in primary dissociated cultures of astrocytes or neurons. Albumin accumulation in live cultured astrocytes was visualized using microscopic imaging of fluorescently tagged bovine serum albumin (alexa488-BSA) within 5 minutes of incubation (Fig 1A). Using a biochemical assay of fluorescence intensity, we confirmed that albumin is rapidly taken-up into astrocytes (but not into neurons), and continues to accumulate within 4 hours of exposure (Fig 1B). Next, we studied the mechanisms underlying albumin uptake into astrocytes. Since albumin binds and activates TGF-βR12, we tested whether albumin uptake is dependent on TGF-βR activation. Here we show that in the presence of the TGF-βRI ALK4, 5 and 7 selective inhibitor, SB43154234, albumin uptake was significantly, but only partially blocked (42.4%). A similar effect on albumin uptake (48.7%) was found in the presence of the caveolae-mediated endocytosis inhibitor, methyl-β-cyclodextrin (3mM). Incubation with both blockers yielded an increased, yet not full blockage of albumin uptake (28.91%, Fig 1C). This suggests that albumin endocytosis into astrocytes involves both TGF-βR activation and caveolae-mediated endocytosis, as well as additional, yet undefined, pathways.
Figure 1.
Albumin uptake into astrocytes is associated with TGF-β1 up-regulation. (A) Fluorescent imaging demonstrating albumin uptake into a cultured astrocyte. (B) Albumin uptake dynamics shows significant uptake by cultured astrocytes (Ast, filled circles) and not by neurons (Neu, open circles). (C) Uptake of albumin (Alb) (24 hours) by astrocytes is significantly blocked in the presence of the specific TGF-βR blocker, SB431542 (SB) or the caveolae-mediated endocytosis blocker methyl-β-cyclodextran (MBCD). (D) Western blot analysis revealing significantly higher levels of ALK5 in astrocytic cultures compared to neurons. (E) Real-time PCR and (F) ELISA, showing albumin-induced up-regulation of TGF-β1 mRNA and protein secretion from astrocytes, but not from neurons. Albumin-induced TGF-β1 up-regulation was blocked by the TGF-βRs antagonists SB431542 (SB) or SJN2511 (SJN). *p≤0.05.
Since TGF-β1 regulates cell function in the brain via ALK1 and ALK5, we tested their expression profiles in neurons and astrocytes. Primary astrocytic cultures express significantly higher levels of ALK5 compared to primary neuronal cultures (P=0.05, Fig 1D). Since activation of TGF-β signaling can up-regulate the expression of TGF-β1 and its receptors in a positive feedback loop, we measured the expression levels of TGF-β receptors and the endogenous ligand in response to albumin. Exposure of either astrocytic or neuronal cultures to albumin (0.2mM, 50% of serum concentration, 24 hours) did not induce a measurable change in the levels of ALK1 or ALK5 (data not shown). In contrast, albumin induced a significant increase in both TGF-β1 mRNA and secreted protein in astrocytes, but not in neurons. Importantly, baseline levels of TGF-β1 mRNA and protein were about a hundred-fold higher in astrocytes compared to neurons. Albumin-induced TGF-β1 up-regulation in the astrocytes was prevented by pre-treatment with SB431542 or the ALK5 selective blocker SJN2511 (Fig 1E–F).
Albumin elicits cell specific changes in Smad phosphorylation in vitro
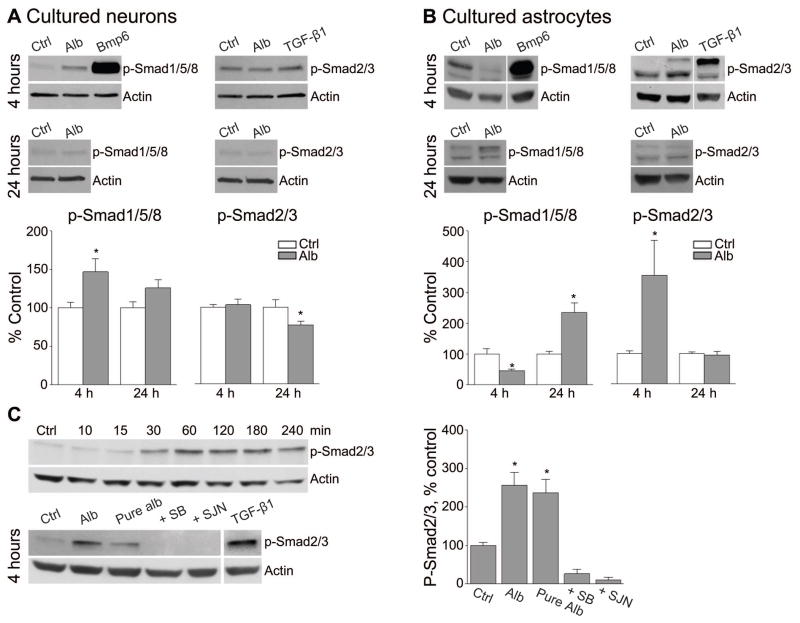
Depending on which type I TGF-βR-dependent kinase (ALK1 or ALK5) is activated, either Smad1/5/8 or Smad2/3 are phosphorylated 14,17,35, respectively. We thus studied the downstream effects of albumin or the ALK1 and ALK5 specific agonists, Bmp6 (50ng/ml) and TGF-β1 (10ng/ml), on the levels of phosphorylated Smads. In cultured neurons, 4 hour exposure to albumin induced an increase in p-Smad1/5/8 (46.84±17.24% change, p=0.026), and 24 hour exposure resulted in a moderated decrease in p-Smad2/3 (22.99±5.02% change, p=0.046), with respect to control values (Fig 2A). A more prominent effect was noted in cultured astrocytes; a 4 hour exposure to albumin induced an increase in p-Smad2/3 (254.1±114% change, p=0.008) and a decrease in p-Smad1/5/8 (54.7±6.0% change, p=0.04), while a 24 hour exposure increased p-Smad1/5/8 levels (135.3±31.1% change, p=0.001), with no difference in the levels of p-Smad2/3 (Fig 2B). A time course experiment in cultured astrocytes showed a gradual increase in the levels of p-Smad2/3 starting 30 minutes after exposure to albumin, with persisting high levels at 1–4 hours. Repeating the experiments (4 hour exposure) in the presence of the SB43154234, or SJN2511 (there are no commercially available ALK1 blockers) prevented albumin-induced increase in p-Smad2/3 (P=0.001, Fig 2C), confirming that albumin rapidly and preferentially activates ALK5 signaling in astrocytes via Smad2/3 phosphorylation.
Figure 2.
Exposure of astrocytes to albumin is associated with Smad2/3 phosphorylation. Analysis of p-Smad1/5/8 and p-Smad2/3 levels 4 and 24 hours post exposure to albumin (Alb) in neurons (A) and astrocytes (B), reveals a robust increase in astrocytic p-Smad2/3 4 hours after treatment. The ALK1 and ALK5 receptor agonists, Bmp6 and TGF-β1, served as positive controls. (C) A time course experiment demonstrating a gradual increase in p-Smad2/3 levels following exposure to albumin prevented using the TGF-βR antagonists, SB431542 (SB) or SJN2511 (SJN). Note that fatty acid free albumin (Pure alb) still elicited an increase in p-Smad2/3 levels. *p≤0.05.
Local application of losartan reduces albumin-induced TGF-β signaling in vivo and blocks epileptogenesis
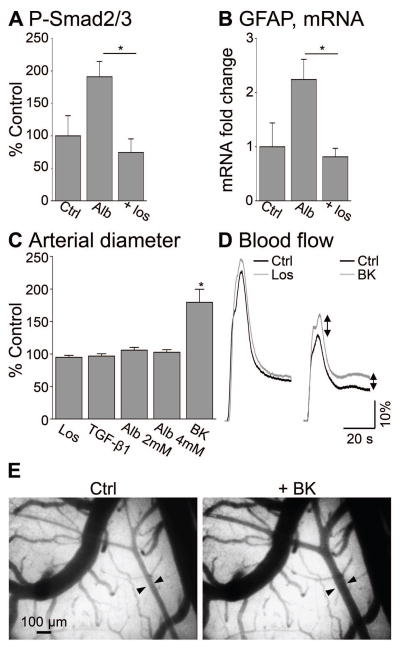
Blocking TGF-β signaling abolished albumin-induced ALK5-dependent phosphorylation in cultured astrocytes (Fig 2C) and albumin-induced transcriptional changes12. These findings suggest TGF-β signaling as a promising therapeutic target for the prevention of acquired epilepsy under dysfunctional BBB. Since the blockers used (SB431542 and SJN2511) are not applicable in clinical settings, we next set out to identify a clinically relevant TGF-β inhibitor. Losartan (Cozaar), an FDA-approved angiotensin II type 1 receptor (AT1) antagonist is a widely used anti-hypertension drug, shown to inhibit TGF-β signaling in peripheral tissues36–40. Thus, we tested whether the administration of losartan may prevent the albumin-induced activation of TGF-β signaling in the brain, and consequently the activation of glia and the development of epilepsy. Serum albumin (0.2mM, n=4)11,32 in the presence or absence of losartan (10μM, n=4) was perfused over the somato-sensory motor cortex through a craniotomy window. 48 hours following cortical exposure to albumin, an increase in p-Smad2/3 levels (190.8±23.9% of the levels in sham controls) was measured, which was significantly blocked in the presence of losartan (74.3±21%, p=0.03, Fig 3A). The glial fibrillary acidic protein (GFAP), a marker of astrocytic activation previously shown to be up-regulated in response to albumin11,12,18,32, confirmed increased astrogliosis in albumin-exposed cortex (n=4), compared to sham controls (n=4, 2.24±0.37 fold increase in GFAP mRNA levels) 7 days following treatment. Local exposure to losartan (n=4), however, prevented the albumin-induced GFAP mRNA up-regulation (0.81±0.16 fold compared to sham controls, p=0.034, Fig 3B). Since losartan is an AT1 antagonist, we measured whether it affects vessel diameter by in vivo time lapse imaging of cortical surface vessels (1Hz) in response to losartan (n=4), TGF-β1 (n=4), albumin (n=8) or the potent vasodilator, bradykinin (n=5), as a positive control. As expected, bradykinin demonstrated a robust vasodilation of cortical surface vessels, whereas losratan had no significant effect on vessels’ diameter (Fig 3C). Additionally, fluorescent angiography31 demonstrated no change in regional blood flow in response to local losartan administration (Fig 3D).
Figure 3.
Losartan prevents albumin-induced brain TGF-β signaling in vivo. (A) Losartan (Los) significantly blocks albumin-induced (Alb) increase in p-Smad2/3 levels in vivo (48 hours) and (B) prevents the increase in GFAP mRNA levels, commonly considered as a marker for astrogliosis, 7 days after treatment. (C) In vivo surface imaging shows no effect of losartan or albumin on arterial diameter, while Bradykinin (BK) induced significant vasodilation compared to control (ACSF). (D) Blood flow curves, calculated from fluorescent angiography, showing no effect of losartan (Los) on regional cerebral blood flow compared to ACSF (Ctrl). (E) Cortical vessels under ACSF (Ctrl) and increased arterial diameter (arrows) upon perfusion with Bradykinin (BK). *p≤0.05.
We next tested whether losartan prevents the development of epilepsy following cortical exposure to albumin (for study design, see Fig 4A and methods). Under these experimental conditions, the BBB remains intact10 and albumin is perfused over the cortical tissue and taken-up into cells (Fig 4B). ECoG was continuously recorded for up to 110 days to confirm the epileptogenic effect of albumin in vivo, reflected by the occurrence of delayed spontaneous electrographic seizures. ECoG was analyzed using a novel ANN-based algorithm, designed for automatic detection of electrographic seizures. Seizure were defined an electrographic even lasting over 5 sec (see Methods). Sham operated rats were randomly selected from the same animal cohort and served as controls. ECoG analysis revealed the appearance of ≥2 spontaneous seizures in 85% of the albumin-treated rats (n=13, hence defined as “epileptic”) and in 29% of the sham-operated controls (n=7, p=0.012, Fig 4C–E). Seizures were observed from day 2 after exposure throughout the remainder of the recordings, with an average rate of 6.08 seizures per week (0.45–21.29 seizures vs. 0.4–0.78 in controls) and duration of 13.62±2.1 seconds (range 5–40, vs. 5–21 in controls). Notably, most electrographic seizures in sham-operated animals lasted less than 10 seconds (except for one animal, with two seizures lasting 18 and 21 seconds). To test the efficacy of losartan in preventing the development of epilepsy, a single dose of losartan was co-perfused with albumin. Losartan significantly reduced the percent of epileptic animals; from 85 to 25% (n=8, p=0.006), and the number of seizures in the animals that did develop epilepsy (6.08 vs. 0.23 seizures per week in albumin- vs. losartan-treated, p=0.003). The duration of seizures was also reduced in losartan-treated epileptic rats (13.69 vs. 5.4±0.4 seconds, in albumin- vs. losartan-treated, p=0.006, Fig 4E). Hence, we conclude that a single cortical co-application of losartan, at the time of exposure to albumin, significantly reduced the number of epileptic animals, and decreased the severity of symptoms in the animals that did develop epilepsy.
Systemic oral losartan application prevents epileptogenesis
To better simulate clinical settings, we next employed a rat model of vascular injury, induced by focal cortical exposure to DOC under a craniotomy window. Exposure to DOC induced an evident extravasation of the Evans blue-albumin complexes into cortical tissue as early as 1 hour after treatment (Fig 5B and10). T2-weighted sequence MRI 24 hours following exposure to DOC revealed local vasogenic edema, and a T1 sequence revealed significant focal increase in signal enhancement following IV injection of the BBB-impermeable contrast agent gadolinium-DTPA (Fig 5C), consistent with focal vascular injury10–12,18,32. ECoG recordings acquired for two weeks, from day 28 after exposure to DOC (n=5), confirmed that all treated rats presented ≥2 spontaneous seizures (range 4–47) (Fig 5D, E).
Figure 5.
Losartan prevents epilepsy following BBB dysfunction. (A) Treatment protocol; animals were treated with local application of DOC in the presence or absence of losartan. A single dose of losartan was injected (100mg/kg) at the end of the surgical procedure, followed by 3 weeks of oral administration (2gr/L in the drinking water). (B) In-vivo imaging of cortical vessels during local application of DOC shows BBB opening and leakage of Evans blue-albumin complexes. (C) T2 sequence brain MRI 24 hours following DOC shows edema in the treated area (arrow). A T1 image shows areas of significantly increased signal following gadolinium-DTPA injection (color bar represents percentage of contrast enhancement). (D) Color coded table showing the number of seizures per day is presented for each rat in DOC- compared to ACSF- (Ctrl) and losartan- (Los) treated animals (see scale bar). (E) Bar graphs represent the percent of epileptic animals, number of seizures per week and the duration of seizures. Overall, compared to the DOC group, losartan treatment significantly lowered the percent of epileptic animals and the number of seizures among animals that did develop epilepsy *p ≤ 0.05.
To test the efficacy of systemic losartan administration in the prevention of epilepsy, losartan was injected (IP, 100mg/kg) 40 minutes after treatment, and thereafter was continuously administered at therapeutic levels through drinking water (2gr/L) for 21 days (n=5) 41,42. This time-course was chosen in order to ensure the presence of the drug throughout the entire duration of BBB dysfunction10,32. To allow drug clearance, ECoG recordings were initiated 7 days after losartan withdrawal for two weeks. While all DOC-treated rats developed epilepsy, losartan treatment significantly reduced the percent of epileptic rats (from 100 to 40%, p=0.038) and decreased the average number of seizures in the epileptic animals from 8 (range 2–23.5, DOC-treatment) to 2.25 seizures per week (2–2.5, p=0.042) (Fig 5D, E). No significant difference was found in the duration of seizures between the groups (Fig 5E). Importantly, albumin- or DOC- rats treated with losartan were not significantly different from sham controls, with respect to the percent of epileptic animals and frequency of seizures (Fig 5). Hence, we conclude that systemic application of losartan following vascular injury prevents epileptogenesis in the majority of the animals and further decreases epilepsy severity in animals that did become epileptic.
Discussion
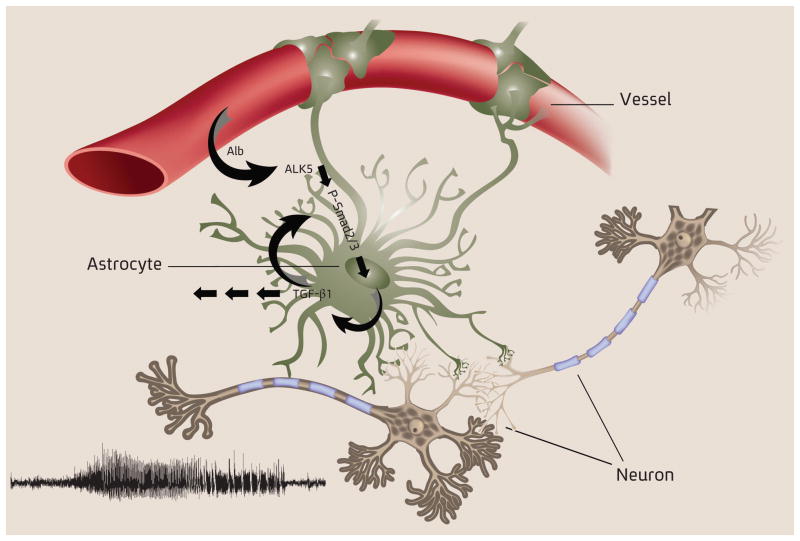
Structural lesional epilepsy is one of the devastating consequences of TBI, occurring in 20–50% of survivors (the range correlates with injury severity)43. Currently there are no biomarkers that reliably predict who will develop epilepsy after injury, nor interventions able to prevent epileptogenesis during the latent period (i.e. after the injury and before the development of epilepsy). Recent animal data from several laboratories suggest a role of BBB dysfunction, TGF-β signaling and activation of brain immune response in the development of epilepsy6,10–12,18,32,44,45. Here we record, for the first time, spontaneous seizures in a rat model of vascular injury and BBB breakdown, and show that local exposure of the cortex to albumin is sufficient to induce seizures. We identified a cell specific signaling pathway, mediated by serum albumin and astrocytic TGF-β signaling, that leads to epileptogenesis and may be blocked by the FDA-approved drug, losartan (Fig 6).
Figure 6.
A schematic diagram illustrating that under dysfunctional BBB the extravasation of albumin into the brain’s extracellular space leads to its interaction with astrocytic TGF-βRs, activation of TGF-β signaling and secretion of TGF-β1, followed by neuronal hyperexcitability and un-provoked epileptic seizures.
We report that serum albumin increases p-Smad2/3 levels predominantly in astrocytes, and induces a mild increase in p-Smad1/5/8 levels in both neurons and astrocytes. Previous studies in neural stem cells have reported that increase in Stat3 is associated with up-regulation of the Smad1 activator, Bmp2. Activated Smad1 forms a complex with the co-activator p300 and Stat3 to induce transcription of GFAP, which leads to the generation of astrocytes46,47. We have previously shown up-regulation of both Stat3 and Bmp2 24 hours after cortical perfusion of albumin in vivo12. Together with the increase in p-Smad1/5/8 levels and the associated up regulation of GFAP, these results suggest that a similar mechanism may underlie reactive astrogliosis in the adult brain, and that albumin induces up-regulation of GFAP via TGF-β signaling and the phosphorylation of Smad1/5/8.
In light of the compelling evidence for the active involvement of the TGF-β pathway in epileptogenesis, we next searched for an FDA-approved drug known to block TGF-β signaling. Losartan, a commonly used anti-hypertensive AT1 antagonist, was previously shown to antagonize TGF-βR signaling in animal models of chronic renal insufficiency, cardiomyopathy and Marfan’s syndrome 38–40. Losartan was also shown to have a neuroprotective effect in an in vitro oxygen–glucose depletion model of ischemic injury48. We found losartan to be extremely effective in blocking the development of seizures post exposure to albumin and following vascular injury corresponding to sham-control characteristics (Fig 4, 5). This can be attributed to the blocking of TGF-β signaling, AT1 signaling or a combination of both. In this respect, it is notable that angiotensin II signaling via AT1 in astrocytes was shown to regulate leukocyte infiltration to the CNS in response to a traumatic injury49. Furthermore, while angiotensin II has been shown to increase TGF-β1 levels in the CNS50, losartan was shown to decrease seizure severity or enhance the efficacy of anti-epileptic drugs in two rodent models of epilepsy51,52. While we cannot rule out AT1-mediated effects of losartan, we show that brain exposure to losartan indeed blocked TGF-β signaling, and reduced albumin-induced increase in p-Smad2/3 levels as well as astrocytic activation. As TGF-β1 was also shown to induce the activation of vascular endothelial growth factor (VEGF) signaling53,54, losartan may also prevent angiogenesis and BBB dysfunction- both known to contribute to the epileptogenic process55,56. These findings, along with reports that losartan decreases BBB permeability in hypertensive rats57,58, raise the possibility of a direct effect of losartan on endothelial cells. However, under our experimental conditions, losartan had no effect on arterial diameter and local blood flow. Further studies are required to determine the mechanisms through which losartan prevents TGF-β signaling in the brain, and the differential effects of losartan on specific cell populations within the neurovascular unit.
Our findings suggest that losartan may become the first available treatment for the prevention of epilepsy development following brain insults, however testing losartan in other models, for example traumatic brain injury (TBI), is of high interest, as well as the need for the development of new clinically approved ALK5 antagonists. The persistence of BBB injury in patients with pharmacoresistant epilepsy33,59 suggests that the process of epileptogenesis may continue after the appearance of seizures and play a role in the natural course of the disease. Since vascular injury and astroglial activation is observed following epileptic seizures, status epilepticus, stroke and brain inflammation33,60–63, the relevance of our findings may extend to many more clinical scenarios beyond TBI.
Acknowledgments
Funding: The research leading to these results has received funding from the European Union’s Seventh Framework Program (FP7/2007–2013) under grant agreement n°602102 (EPITARGET, AF), the Israel Science Foundation (713/11, AF), the United States-Israel Binational Science Foundation (2007185, AF, DK) and the National Institute of Health (RO1/NINDS NS066005 DK, AF).
Footnotes
Author contributions
GBK and LPC conceived and performed the experiments. AF and DK designed, conducted and supervised the experiments. LK, OP, IW, KS, PC, SYK and LW preformed experiments, GBK, LPC, UH, DK and AF wrote the manuscript, and all authors discussed the results and commented on the manuscript.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Friedman A, Kaufer D, Heinemann U. Blood-brain barrier breakdown-inducing astrocytic transformation: novel targets for the prevention of epilepsy. [Internet] Epilepsy Res. 2009;85(2–3):142–149. doi: 10.1016/j.eplepsyres.2009.03.005. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19362806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herman ST. Epilepsy after brain insult: targeting epileptogenesis [Internet] Neurology. 2002;59(9 Suppl 5):S21–S26. doi: 10.1212/wnl.59.9_suppl_5.s21. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12428028. [DOI] [PubMed] [Google Scholar]
- 3.Shinnar S, Berg AT, O’Dell C, et al. Predictors of multiple seizures in a cohort of children prospectively followed from the time of their first unprovoked seizure. [Internet] Ann Neurol. 2000;48(2):122–123. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11261451. [PubMed] [Google Scholar]
- 4.Tomkins O, Kaufer D, Korn A, et al. Frequent blood-brain barrier disruption in the human cerebral cortex. [Internet] Cell Mol Neurobiol. 2001;21(6):675–691. doi: 10.1023/A:1015147920283. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12043841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neuwelt Ea. Mechanisms of Disease: The Blood-Brain Barrier [Internet] Neurosurgery. 2004;54(1):131–142. doi: 10.1227/01.neu.0000097715.11966.8e. [cited 2013 Mar 1] Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006123-200401000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Oby E, Janigro D. The blood-brain barrier and epilepsy [Internet] Epilepsia. 2006;47(11):1761–1774. doi: 10.1111/j.1528-1167.2006.00817.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17116015. [DOI] [PubMed] [Google Scholar]
- 7.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. [Internet] Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16371949. [DOI] [PubMed] [Google Scholar]
- 8.Abbott NJ, Patabendige AAK, Dolman DEM, et al. Structure and function of the blood-brain barrier. [Internet] Neurobiol Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19664713. [DOI] [PubMed] [Google Scholar]
- 9.Abbott NJ, Friedman A. Overview and introduction: the blood-brain barrier in health and disease [Internet] Epilepsia. 2012;53 (Suppl 6):1–6. doi: 10.1111/j.1528-1167.2012.03696.x. [cited 2013 Mar 2] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23134489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seiffert E, Dreier JP, Ivens S, et al. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. [Internet] J Neurosci. 2004;24(36):7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15356194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivens S, Kaufer D, Flores LP, et al. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. [Internet] Brain A J Neurol. 2007;130(Pt 2):535–547. doi: 10.1093/brain/awl317. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17121744. [DOI] [PubMed] [Google Scholar]
- 12.Cacheaux LP, Ivens S, David Y, et al. Transcriptome profiling reveals TGF-beta signaling involvement in epileptogenesis. [Internet] J Neurosci. 2009;29(28):8927–8935. doi: 10.1523/JNEUROSCI.0430-09.2009. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2875073&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massagué J. How cells read TGF-β signals [Internet] Nat Rev Mol cell Biol. 2000;1(3):169–78. doi: 10.1038/35043051. Available from: http://www.nature.com/nrm/journal/v1/n3/abs/nrm1200_169a.html. [DOI] [PubMed] [Google Scholar]
- 14.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus [Internet] Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12809600. [DOI] [PubMed] [Google Scholar]
- 15.König H-G, Kögel D, Rami A, Prehn JHM. TGF-beta1 activates two distinct type I receptors in neurons: implications for neuronal NF-kappaB signaling. [Internet] J Cell Biol. 2005;168(7):1077–1086. doi: 10.1083/jcb.200407027. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2171851&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horbelt D, Denkis A, Knaus P. A portrait of Transforming Growth Factor β superfamily signalling: Background matters. Int J Biochem Cell Biol. 2012;44(3):469–474. doi: 10.1016/j.biocel.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Massague J. TGFβ signalling in context. [Internet] Nat Rev Mol cell Biol. 2012 doi: 10.1038/nrm3434. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=22992590&retmode=ref&cmd=prlinks. [DOI] [PMC free article] [PubMed]
- 18.David Y, Cacheaux LP, Ivens S, et al. Astrocytic dysfunction in epileptogenesis: consequence of altered potassium and glutamate homeostasis? [Internet] J Neurosci. 2009;29(34):10588–10599. doi: 10.1523/JNEUROSCI.2323-09.2009. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2875068&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufer D, Ogle WO, Pincus ZS, et al. Restructuring the neuronal stress response with anti-glucocorticoid gene delivery. [Internet] Nat Neurosci. 2004;7(9):947–953. doi: 10.1038/nn1296. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15300253. [DOI] [PubMed] [Google Scholar]
- 20.Yumoto R, Nishikawa H, Okamoto M, et al. Clathrin-mediated endocytosis of FITC-albumin in alveolar type II epithelial cell line RLE-6TN. [Internet] Am J Physiol Lung Cell Mol Physiol. 2006;290(5):L946–55. doi: 10.1152/ajplung.00173.2005. [cited 2013 Apr 2] Available from: http://www.ncbi.nlm.nih.gov/pubmed/16361359. [DOI] [PubMed] [Google Scholar]
- 21.Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. [Internet] J Comput Biol a J Comput Mol cell Biol. 2005;12(8):1047–1064. doi: 10.1089/cmb.2005.12.1047. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2716216&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bastlund JF, Jennum P, Mohapel P, et al. Measurement of cortical and hippocampal epileptiform activity in freely moving rats by means of implantable radiotelemetry. [Internet] J Neurosci Methods. 2004;138(1–2):65–72. doi: 10.1016/j.jneumeth.2004.03.004. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15325113. [DOI] [PubMed] [Google Scholar]
- 23.Timofeeva OA, Gordon CJ. Changes in EEG power spectra and behavioral states in rats exposed to the acetylcholinesterase inhibitor chlorpyrifos and muscarinic agonist oxotremorine. [Internet] Brain Res. 2001;893(1–2):165–177. doi: 10.1016/s0006-8993(00)03309-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11223004. [DOI] [PubMed] [Google Scholar]
- 24.Levi H, Schoknecht K, Prager O, et al. Stimulation of the sphenopalatine ganglion induces reperfusion and blood-brain barrier protection in the photothrombotic stroke model [Internet] PLoS One. 2012;7(6):e39636. doi: 10.1371/journal.pone.0039636. [cited 2013 Apr 1] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3382129&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ketzef M. Compensatory network alterations upon onset of epilepsy in synapsin triple knock-out mice. Neuroscience. 2011;189:108– 122. doi: 10.1016/j.neuroscience.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 26.Becker AJ, Pitsch J, Sochivko D, et al. Transcriptional upregulation of Cav3.2 mediates epileptogenesis in the pilocarpine model of epilepsy. J Neurosci. 2008;28(49):13341–53. doi: 10.1523/JNEUROSCI.1421-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weissberg I, Reichert A, Heinemann U, Friedman A. Blood-Brain Barrier Dysfunction in Epileptogenesis of the Temporal Lobe. Epilepsy Res Treat. 2011;2011:1–10. doi: 10.1155/2011/143908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitney AW. A direct method of nonparametric measurement selection. IEEE Trans Comput. 1971;100(9):1100–1103. [Google Scholar]
- 29.Rumelhart DE, Hinton GE, Williams RJ. Learning representations by back-propagating errors. Nature. 1986;323(6088):533–536. [Google Scholar]
- 30.Powell MJD. Restart procedures for the conjugate gradient method. Math Program. 1977;12(1):241–254. [Google Scholar]
- 31.Prager O, Chassidim Y, Klein C, et al. Dynamic in vivo imaging of cerebral blood flow and blood-brain barrier permeability [Internet] Neuroimage. 2010;49(1):337–344. doi: 10.1016/j.neuroimage.2009.08.009. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19682584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomkins O, Friedman O, Ivens S, et al. Blood-brain barrier disruption results in delayed functional and structural alterations in the rat neocortex. [Internet] Neurobiol Dis. 2007;25(2):367–77. doi: 10.1016/j.nbd.2006.10.006. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17188501. [DOI] [PubMed] [Google Scholar]
- 33.Van Vliet Ea, da Costa Araújo S, Redeker S, et al. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. [Internet] Brain. 2007;130(Pt 2):521–34. doi: 10.1093/brain/awl318. [cited 2013 Apr 1] Available from: http://www.ncbi.nlm.nih.gov/pubmed/17124188. [DOI] [PubMed] [Google Scholar]
- 34.Matsuyama S, Iwadate M, Kondo M, et al. SB-431542 and Gleevec Inhibit Transforming Growth Factor- β-Induced Proliferation of Human Osteosarcoma Cells SB-431542 and Gleevec Inhibit Transforming Growth Factor- β -Induced Proliferation of Human Osteosarcoma Cells. 2003:7791–7798. [PubMed] [Google Scholar]
- 35.Heldin CH, Miyazono K, Ten Dijke P. TGF-beta signaling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390(390):465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 36.Campistol JM, Iñigo P, Jimenez W, et al. Losartan decreases plasma levels of TGF-beta1 in transplant patients with chronic allograft nephropathy [Internet] 1999 doi: 10.1046/j.1523-1755.1999.00597.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10432413. [DOI] [PubMed]
- 37.Khalil A, Tullus K, Bakhiet M, et al. Angiotensin II type 1 receptor antagonist (losartan) down-regulates transforming growth factor-beta in experimental acute pyelonephritis. [Internet] J Urol. 2000;164(1):186–191. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10840457. [PubMed] [Google Scholar]
- 38.Lim D-S, Lutucuta S, Bachireddy P, et al. Angiotensin II Blockade Reverses Myocardial Fibrosis in a Transgenic Mouse Model of Human Hypertrophic Cardiomyopathy [Internet] Circulation. 2001;103(6):789–791. doi: 10.1161/01.cir.103.6.789. [cited 2013 Mar 4] Available from: http://circ.ahajournals.org/cgi/doi/10.1161/01.CIR.103.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lavoie P, Robitaille G, Agharazii M, et al. Neutralization of transforming growth factor-beta attenuates hypertension and prevents renal injury in uremic rats. J Hypertens. 2005;23(10):1895–1903. doi: 10.1097/01.hjh.0000182521.44440.c5. [DOI] [PubMed] [Google Scholar]
- 40.Cohn RD, Erp C, Van Habashi JP, et al. Angiotensin II type 1 receptor blockade attenuates TGF-[beta]-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13(2):204–210. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaur K, Sharma AK, Singal PK. Significance of changes in TNF-alpha and IL-10 levels in the progression of heart failure subsequent to myocardial infarction. [Internet] Am J Physiol Hear Circ Physiol. 2006;291(1):H106–H113. doi: 10.1152/ajpheart.01327.2005. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16461369. [DOI] [PubMed] [Google Scholar]
- 42.Huang BS, Ahmad M, Tan J, Leenen FHH. Chronic central versus systemic blockade of AT(1) receptors and cardiac dysfunction in rats post-myocardial infarction. [Internet] Am J Physiol Hear Circ Physiol. 2009;297(3):H968–H975. doi: 10.1152/ajpheart.00317.2009. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19617416. [DOI] [PubMed] [Google Scholar]
- 43.Löscher W, Klitgaard H, Twyman RE, Schmidt D. New avenues for anti-epileptic drug discovery and development. [Internet] Nat Rev Drug Discov. 2013;12(10):757–776. doi: 10.1038/nrd4126. [cited 2013 Sep 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/24052047. [DOI] [PubMed] [Google Scholar]
- 44.Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. [Internet] Nat Rev Neurol. 2010;6(7):393–403. doi: 10.1038/nrneurol.2010.74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20551947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7(1):31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakashima K, Yanagisawa M, Arakawa H, et al. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. [Internet] Science (80- ) 1999;284(5413):479–482. doi: 10.1126/science.284.5413.479. Available from: http://www.sciencemag.org/cgi/doi/10.1126/science.284.5413.479. [DOI] [PubMed] [Google Scholar]
- 47.Fukuda S, Abematsu M, Mori H, et al. Potentiation of astrogliogenesis by STAT3-mediated activation of bone morphogenetic protein-Smad signaling in neural stem cells. [Internet] Mol Cell Biol. 2007;27(13):4931–4937. doi: 10.1128/MCB.02435-06. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1951480&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu X, Kihara T, Hongo H, et al. Angiotensin receptor type 1 antagonists protect against neuronal injury induced by oxygen–glucose depletion [Internet] Br J Pharmacol. 2010;161(1):33–50. doi: 10.1111/j.1476-5381.2010.00840.x. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2962815&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Füchtbauer L, Groth-Rasmussen M, Holm TH, et al. Angiotensin II Type 1 receptor (AT1) signaling in astrocytes regulates synaptic degeneration-induced leukocyte entry to the central nervous system. Brain Behav Immun. 2011;25(5):897–904. doi: 10.1016/j.bbi.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 50.Lanz TV, Ding Z, Ho PP, et al. Angiotensin II sustains brain inflammation in mice via TGF [Internet] J Clin Invest. 2010;120(8):2782–2794. doi: 10.1172/JCI41709. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2912186/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pereira MGAG, Becari C, Oliveira JAC, et al. Inhibition of the renin-angiotensin system prevents seizures in a rat model of epilepsy. [Internet] Clin Sci London Engl 1979. 2010;119(11):477–482. doi: 10.1042/CS20100053. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20533906. [DOI] [PubMed] [Google Scholar]
- 52.Łukawski K, Janowska A, Jakubus T, et al. Angiotensin AT1 receptor antagonists enhance the anticonvulsant action of valproate in the mouse model of maximal electroshock. [Internet] Eur J Pharmacol. 2010;640(1–3):172–177. doi: 10.1016/j.ejphar.2010.04.053. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20465998. [DOI] [PubMed] [Google Scholar]
- 53.Ferrari G, Cook BD, Terushkin V, et al. Transforming growth factor-beta 1 (TGF-beta1) induces angiogenesis through vascular endothelial growth factor (VEGF)-mediated apoptosis. [Internet] J Cell Physiol. 2009;219(2):449–458. doi: 10.1002/jcp.21706. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2749291&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki Y, Ito Y, Mizuno M, et al. Transforming growth factor-β induces vascular endothelial growth factor-C expression leading to lymphangiogenesis in rat unilateral ureteral obstruction. [Internet] Kidney Int. 2012;81(9):1–15. doi: 10.1038/ki.2011.464. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22258325. [DOI] [PubMed] [Google Scholar]
- 55.Argaw A, Asp L, Zhang J, et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest. 2012;122(7):2454–2468. doi: 10.1172/JCI60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferrari G, Terushkin V, Wolff MJ, et al. TGF-β1 Induces Endothelial Cell Apoptosis by Shifting VEGF Activation of p38MAPK from the Prosurvival p38β to Proapoptotic p38 [Internet] Mol cancer Res MCR. 2012 doi: 10.1158/1541-7786.MCR-11-0507. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22522454. [DOI] [PMC free article] [PubMed]
- 57.Kucuk M, Kaya M, Kalayci R, et al. Effects of losartan on the blood-brain barrier permeability in long-term nitric oxide blockade-induced hypertensive rats. [Internet] Life Sci. 2002;71(8):937–946. doi: 10.1016/s0024-3205(02)01772-1. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12084390. [DOI] [PubMed] [Google Scholar]
- 58.Kaya M, Kalayci R, Küçük M, et al. Effect of losartan on the blood-brain barrier permeability in diabetic hypertensive rats. [Internet] Life Sci. 2003;73(25):3235–3244. doi: 10.1016/j.lfs.2003.06.014. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0024320503008075. [DOI] [PubMed] [Google Scholar]
- 59.Marchi N, Granata T, Alexopoulos A, Janigro D. The blood-brain barrier hypothesis in drug resistant epilepsy [Internet] Brain. 2012;135(Pt 4):e211. doi: 10.1093/brain/awr343. [cited 2013 Oct 17] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3326249&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Librizzi L, Noè F, Vezzani A, et al. Seizure-induced brain-borne inflammation sustains seizure recurrence and blood-brain barrier damage. [Internet] Ann Neurol. 2012;72(1):82–90. doi: 10.1002/ana.23567. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22829270. [DOI] [PubMed] [Google Scholar]
- 61.Friedman A. Blood-brain barrier dysfunction, status epilepticus, seizures, and epilepsy: a puzzle of a chicken and egg? [Internet] Epilepsia. 2011;52 (Suppl 8):19–20. doi: 10.1111/j.1528-1167.2011.03227.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21967353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Janigro D. Are you in or out? Leukocyte, ion, and neurotransmitter permeability across the epileptic blood-brain barrier [Internet] Epilepsia. 2012;53 (Suppl 1):26–34. doi: 10.1111/j.1528-1167.2012.03472.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22612806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ding G, Jiang Q, Li L, et al. Detection of BBB disruption and hemorrhage by Gd-DTPA enhanced MRI after embolic stroke in rat. [Internet] Brain Res. 2006;1114(1):195–203. doi: 10.1016/j.brainres.2006.07.116. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16950236. [DOI] [PubMed] [Google Scholar]