Abstract
Objectives
We sought to determine: 1) if early weight regain between one and two years after RYGB is associated with worsened hepatic and peripheral insulin sensitivity, and 2) if preoperative levels of ghrelin and leptin are associated with early weight regain after RYGB.
Design and Methods
Hepatic and peripheral insulin sensitivity and ghrelin and leptin plasma levels were assessed longitudinally in 45 subjects before RYGB and at one month, six months, one year, and two years post operatively. Weight regain was defined as ≥ 5% increase in body weight between one and two years after RYGB.
Results
Weight regain occurred in 33% of subjects, with an average increase in body weight of 10 ± 5 % (8.5 ± 3.3 kg). Weight regain was not associated with worsening of peripheral or hepatic insulin sensitivity. Subjects with weight regain after RYGB had higher preoperative and postoperative levels of ghrelin compared to those who maintained or lost weight during this time. Conversely, the trajectories of leptin levels corresponded with the trajectories of fat mass in both groups.
Conclusions
Early weight regain after RYGB is not associated with a reversal of improvements in insulin sensitivity. Higher preoperative ghrelin levels might identify patients that are more susceptible to weight regain after RYGB.
Keywords: gastric bypass surgery, weight regain, insulin sensitivity, ghrelin
Introduction
Bariatric surgery is the most effective weight loss approach for obesity. Patients undergoing Roux-en-Y gastric bypass (RYGB) surgery lose approximately 60% of excess body weight in two years (1). This amount of weight loss is not typically achieved or sustained with lifestyle or pharmacological interventions. RYGB is also associated with increased recovery and decreased mortality from numerous chronic obesity-related comorbidities (2, 3). Recent randomized trials demonstrate that bariatric surgery is more effective than conventional medical therapy for achieving glycemic control in patients with type 2 diabetes over the course of a year (4, 5). RYGB also improves the hepatic and peripheral insulin resistance (6, 7) which develops with weight gain and predisposes individuals to type 2 diabetes (8).
Weight regain and surgical weight-loss “failure” have been reported to occur in a subset of patients five to ten years after RYGB (9, 10, 11, 12, 13). It is unknown if weight regain is detrimental to the substantial metabolic improvements that occur after RYGB. Weight regain after RYGB may be benign, given that some of the beneficial metabolic effects of RYGB are considered to be weight-loss independent. The severe caloric restriction that occurs immediately after RYGB has beneficial effects on hepatic insulin sensitivity (14, 15, 16); however, weight regain could imply an increased caloric intake and, as a result, lead to worsened hepatic insulin sensitivity. On the other hand, the improvement in peripheral insulin sensitivity after RYGB is weight-loss dependent (17) and, therefore, might also be negatively affected by weight regain after RYGB. Impaired insulin action occurs early in the natural history of diabetes (8); thus, worsened insulin sensitivity with weight regain after RYGB could be a significant contributor to relapse of type 2 diabetes.
It would be of great clinical value to determine which patients are at risk to regain weight early after RYGB in order to focus more intensive follow-up treatment and counseling in this patient population. Relatively few studies have addressed this issue. Analysis of 11 obesity-related genes failed to find a relationship with weight regain after bariatric surgery (18), but the potential involvement of other genes in the durability of surgical weight loss remains to be determined. Alterations in peptides that regulate energy balance, such as leptin and ghrelin, have been shown to predict weight regain after diet-induced weight loss (19). Decreased plasma levels of the orexigenic hormone ghrelin have been proposed to contribute to the weight-loss effects of RYGB (20).
The objectives of the present study were to determine: 1) if early weight regain between one and two years after RYGB is detrimental to hepatic and peripheral insulin sensitivity, and 2) if preoperative levels of ghrelin and leptin are associated with early weight regain after RYGB.
Methods
Participants
Written informed consent was obtained from all participants prior to the study, which was approved by the Vanderbilt University Institutional Review Board. Participants were recruited from the Vanderbilt Center for Surgical Weight Loss after approval for RYGB. This study was initiated as a randomized trial to determine the effect of surgical removal of the omentum at the time of RYGB surgery on insulin sensitivity. After we reported that omentectomy provided no additional benefit to RYGB at one year after surgery (6, 7), enrollment was terminated, and we continued to follow participants to explore the long-term effects of RYGB on insulin sensitivity. Participants with both a one year and two year postoperative study visit (n = 45) were included in the analyses of weight regain.
Study Procedures
All study visits (preoperative and at one month, six months, one year and two years post-RYGB) were conducted at the Vanderbilt Clinical Research Center from March 2005 to October 2011. Oral anti-diabetic agents and long-acting insulin were discontinued for five days prior to each study visit. Measurements of body composition by dual x-ray absorptiometry and steady-state glucose turnover by hyperinsulinemic-euglycemic clamp with glucose isotope infusion were obtained at each study visit as previously described (7, 21). Participants were admitted the afternoon before each study visit, given a standardized meal, and then fasted for 10-12 hours. The next morning, blood was collected in chilled EDTA tubes and immediately centrifuged at 4°C to separate plasma, which was immediately stored at -80°C until measurement of insulin, total ghrelin (acyl and desacyl), and leptin by radioimmunoassay (Millipore) by the Vanderbilt Assay & Analytical Services Core. Then, a primed (33μCi), continuous (0.14 μCi/min) infusion of [3-3H] glucose tracer was maintained for 2.5 hours (basal period), followed by a primed, continuous insulin infusion for 2 hours. Insulin infusion rates were empirically increased postoperatively to obviate the post-surgical increases in insulin clearance and achieve comparable plasma insulin levels at preoperative and postoperative study visits. This would allow the evaluation of the effect of surgery on insulin sensitivity independent of changes in plasma insulin levels. Insulin infusion rates at each study visit were as follows: pre-RYGB, 2.3 μU/ml; one month, 2.5 μU/ml; six months, 2.9 μU/ml; one year, 3.3 μU/ml; and two years, 3.3 μU/ml. Glycemia was maintained at 90-100 mg/dl during the insulin infusion period by a variable infusion of 20% dextrose. Blood samples were obtained during the last 30 minutes of the basal and insulin infusion periods to determine steady-state plasma glucose specific activity and insulin concentrations.
Calculations
Excess body weight was calculated using an ideal body weight derived from a BMI of 25 kg/m2 (22). The hepatic insulin sensitivity index (HISI) was calculated as the inverse product of basal endogenous glucose production (mg/min) and fasting insulin levels (μU/ml) (23). Peripheral (primarily skeletal muscle) insulin sensitivity (M/I) was calculated as the steady-state glucose infusion rate (M; mg/kg.min) needed to maintain euglycemia under hyperinsulinemia divided by the steady-state plasma insulin levels (I; mU/ml). Weight regain was defined as ≥ 5% increase in body weight between postoperative years one and two; the remainder of the participants were classified as having maintained/lost weight. This threshold was selected to define weight regain in our cohort because a weight loss of 5 to 10% is recommended to improve obesity-related metabolic comorbidities (24, 25).
Statistical Analyses
Study data were managed using REDCap electronic data capture tools hosted at Vanderbilt. Exploratory analyses were conducted to examine frequency distributions of key measures. Independent sample t-tests and chi-squared tests were used to compare baseline measures between weight groups (regain or maintain/lose). The effect of time on weight and metabolic outcome measures was tested using linear mixed effects models with Bonferroni adjustments for multiple comparisons. Separate mixed effects models, each including two main effects (time and diabetes or time and weight regain group) and an interaction effect (time by diabetes or time by weight regain group) were used to determine whether weight regain (regain or maintain/lose) and preoperative diabetes (yes or no) were associated with differing trajectories over time of fat mass, lean mass, peripheral and hepatic insulin sensitivity, leptin and ghrelin. If an interaction effect was statistically significant, parameter estimates for individual interaction contrasts were interpreted to identify specific time points between which trajectories differed. If an overall interaction effect was not statistically significant, time point - specific trajectories were not examined further. Each mixed effects model included all available data for each time point. Secondary mixed effects models that held percent change in weight between years one and two as a continuous covariate were tested to evaluate findings in relation to the primary models (which treated weight change as a dichotomous factor - regain or maintain/lose). This approach preserved maximum statistical power while systematically examining the association of weight change between years one to two with the complete temporal course of body composition and metabolic measures. All analyses were performed using IBM SPSS Statistics software.
Results
Longitudinal assessment of weight loss and insulin sensitivity within two years after RYGB
Over the two-year follow-up period, RYGB resulted in significant weight loss (Table 1). Hyperinsulinemic-euglycemic clamps with glucose isotope infusion were performed preoperatively, and one month, 6 months, one year, and 2 years post-RYGB. Steady- state plasma insulin levels achieved during the clamp decreased over time (pre-RYGB, 362 ± 155 μU/ml; one month, 338 ± 124 μU/ml; six months, 305 ± 125 μU/ml; one year, 303 ± 126 μU/ml; and two years, 293 ± 108 μU/ml; P = 0.029). Both peripheral and hepatic insulin sensitivity improved significantly during the initial two years after RYGB, but with different temporal trajectories (Table 1). Peripheral insulin sensitivity (M/I) did not change at one-month post-RYGB, increased up to one year, and stabilized thereafter. Hepatic insulin sensitivity (HISI) improved within the first month after surgery, further increased at six months, and then remained stable. A diagnosis of type 2 diabetes by a primary care physician was present in 49% (22/45) of subjects before surgery. Anti-diabetes therapy for these subjects included (number of participants): diet (1); insulin only (1); one oral medication (9); two oral medications (6); or three oral medications (5). At two years after RYGB, only one subject remained on diabetes therapy (insulin + metformin). Postoperative improvements in weight, BMI, HISI, or M/I over the first two years following RYGB were not significantly different between participants with and without a preoperative diagnosis of type 2 diabetes (all group by time interaction effects P ≥ 0.672, data not shown).
Table 1.
Anthropometric measures and insulin sensitivity within two years after RYGB
| Measure | Preop | 1 month | 6 months | 1 year | 2 years | P value |
|---|---|---|---|---|---|---|
| BMI (kg/m2) | 48.7 ± 10.1 | 42.4 ± 7.7 | 35.9 ± 8.3 | 32.6 ± 8.0 | 32.8 ± 7.9 | <0.001 |
| Weight (kg) | 135 ± 27 | 118 ± 24 | 99 ± 22 | 90 ± 21 | 91 ± 21 | <0.001 |
| Weight loss (kg) | ---- | 15 ± 5 | 36 ± 8 | 45 ± 11 | 44 ± 14 | <0.001 |
| BWL (%) | ---- | 11 ± 4 | 27 ± 4 | 33 ± 7 | 33 ± 9 | <0.001 |
| EBWL (%) | ---- | 25 ± 10 | 58 ± 14 | 73 ± 20 | 71 ± 21 | <0.001 |
| FPG (mg/dl) | 123 ± 40 | 96 ± 18 | 91 ± 10 | 92 ± 23 | 95 ± 21 | <0.001 |
| FPI (μU/ml) | 24 ± 12 | 15 ± 11 | 8 ± 5 | 8 ± 4 | 9 ± 4 | <0.001 |
| GIR (mg•kg−1•min−1) | 5.1 ± 1.9 | 5.4 ± 1.6 | 7.7 ± 1.6 | 9.6 ± 2.2 | 9.6 ± 2.3 | <0.001 |
| EGP (mg•kg−1•min−1) | 1.0 ± 0.3 | 0.9 ± 0.2 | 1.1 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 | <0.001 |
| M/I | 17 ± 11 | 18 ± 8 | 29 ± 12 | 36 ± 18 | 38 ± 16 | <0.001 |
| HISI | 0.4 ± 0.2 | 1.3 ± 1.0 | 1.6 ± 1.0 | 1.7 ± 1.0 | 1.5 ± 0.5 | <0.001 |
BWL, body weight loss; EBWL, excess body weight loss; FPG, fasting plasma glucose; FPI, fasting plasma insulin; GIR, glucose infusion rate during insulin infusion; EGP, endogenous glucose production; M/I, peripheral insulin sensitivity; HISI, hepatic insulin sensitivity. Data are mean ± SD.
Weight regain occurs between one and two years after RYGB
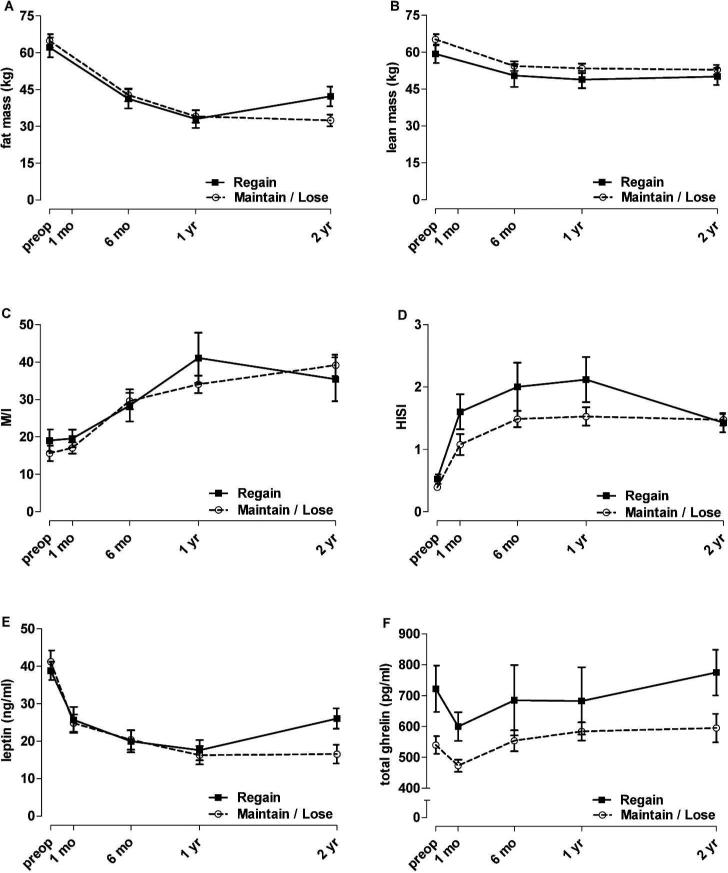
During the first year after RYGB, all participants continued to lose weight or were weight stable. While the mean weight of the participants remained stable between one and two years post-RYGB, there was variability in individual weight trajectories during this period. Values for percent weight change between years one and two were normally distributed and ranged from -17% to 22%. A weight gain of ≥ 5% occurred in 33% of the cohort (the upper tertile) between postoperative years one and two, with an average increase in body weight of 10 ± 5 %. This represents a weight regain of 8.5 ± 3.3 kg and 22 ± 9% of the weight lost during the first postoperative year. The gain in body weight consisted primarily of fat mass and not lean mass (Figure 1A & 1B). There was no significant difference in preoperative age, sex, type 2 diabetes, BMI, or weight between those participants that regained weight and those that maintained or continued to lose weight (Table 2).
Figure 1. Weight regain at two years after RYGB is not associated with worsened insulin sensitivity, but is associated with elevated plasma ghrelin levels.
Weight regain was defined as ≥ 5% weight change between one and two years after RYGB, and occurred in 33% of the cohort. Trajectories of fat (A) and lean (B) mass losses were similar between groups up to one year after RYGB. There was no effect of weight regain on peripheral (C) or hepatic (D) insulin sensitivity (both group by time interaction P ≥ 0.137). Leptin levels (E) increased between one and two years after RYGB in the weight regain group (group by time interaction P = 0.011). Ghrelin levels (F) were higher in the weight regain group at baseline (P = 0.009) and this stratification was maintained over time (group by time interaction P = 0.707). Data are mean ± SEM.
Table 2.
Baseline characteristics of subjects with and without weight regain between one and two years after RYGB
| Regain | Maintain / Lose | P value | |
|---|---|---|---|
| n | 15 | 30 | ---- |
| Age (years) | 43 ± 10 | 44 ± 9 | 0.882 |
| Female (%) | 93 | 87 | 0.651 |
| T2D (%) | 47 | 50 | 0.999 |
| BMI (kg/m2) | 47 ± 9 | 50 ± 11 | 0.346 |
| Weight (kg) | 127 ± 30 | 138 ± 25 | 0.182 |
| Fat Mass (kg) | 62 ± 15 | 65 ± 14 | 0.574 |
| Lean Mass (kg) | 59 ± 13 | 65 ± 11 | 0.137 |
| M/I | 19 ± 10 | 16 ± 11 | 0.354 |
| HISI | 0.5 ± 0.3 | 0.4 ± 0.2 | 0.103 |
| Leptin (ng/ml) | 39 ± 9 | 41 ± 16 | 0.587 |
| Ghrelin (pg/ml) | 722 ± 290 | 540 ± 156 | 0.009 |
T2D, type 2 diabetes; M/I, peripheral insulin sensitivity; HISI, hepatic insulin sensitivity. Data are mean ± SD.
Early weight regain does not affect insulin sensitivity after RYGB
We hypothesized that those participants with weight regain between one and two years post-RYGB would experience a deterioration of insulin sensitivity. While peripheral and hepatic insulin sensitivity increased significantly over time (both P < 0.001), there was no difference between the weight regain and maintain/lose groups in the trajectories of peripheral insulin sensitivity (Figure 1C; group by time interaction P = 0.191) or hepatic insulin sensitivity (Figure 1D; group by time interaction P = 0.137). Secondary analyses, which treated percent weight change between years one and two as a continuous covariate, were completely consistent with these findings.
High preoperative ghrelin levels are associated with early weight regain after RYGB
Ghrelin and leptin are appetite-related hormones with opposing actions; ghrelin is orexigenic and leptin is anorexigenic. Given the role of these hormones in the regulation of food intake, we sought to determine if ghrelin or leptin levels were associated with early weight regain after RYGB. Subjects with ≥ 5% weight regain between postoperative years one and two had significantly higher preoperative levels of total ghrelin than those who maintained or lost weight during this period (Table 2). Figure 1F shows that the weight regain group maintained these higher levels of ghrelin after surgery (main effect of group P = 0.014, group by time interaction effect P = 0.707). Preoperative ghrelin levels were related to the trajectories of weight and BMI after RYGB (preoperative ghrelin by time interaction effects, P ≤ 0.001). Baseline plasma leptin levels did not differ between the weight regain and maintain/lose group (Table 2). Leptin levels were decreased overall after RYGB (P < 0.001), but increased in the weight regain group between one and two years post-RYGB (overall interaction P=0.011, 1 to 2 year interaction contrast P = 0.017) (Figure 1E). The trajectories of leptin levels corresponded with fat mass (Figure 1A). Secondary analyses, which treated percent weight change between years one and two as a continuous covariate, were completely consistent with these findings.
Discussion
Overall, the subjects in our cohort of bariatric surgery patients experienced significant weight loss up to two years after surgery, consisting of losses in both lean and fat tissue. Although, on average, subjects were still obese two years after surgery, they experienced significant and sustained improvements in hepatic and peripheral insulin sensitivity. A strength of our study is the longitudinal measurement of tissue-specific insulin sensitivity using the gold-standard technique, the hyperinsulinemic-eyglycemic clamp, in a relatively large cohort of subjects for this technique. Our assessments demonstrate that hepatic insulin sensitivity improves within the first month after surgery, while peripheral insulin sensitivity does not improve until after the first postoperative month. This suggests that, in the extremely obese, hepatic insulin sensitivity improves with reduced nutrient intake and small amounts of weight loss, while a more significant weight loss may be required to affect skeletal muscle insulin sensitivity. Based on these findings, we anticipated that early weight regain after RYGB may be detrimental to insulin sensitivity, and hepatic insulin sensitivity in particular.
Several studies have indicated that some patients experience weight regain after RYGB (9, 10, 11, 12, 13). It is not uncommon for 20-25% of initial weight loss to be regained by 10 years postoperatively(26). There is no strict definition of weight-loss maintenance, although a weight change of less than 3% has been proposed for diet- induced weight loss (27). A weight increase of 5 kg has been proposed (28) or used (29) to indicate weight regain after bariatric surgery or diet-induced weight loss (30). Because a weight loss of 5 to 10% is recommended to improve obesity-related metabolic comorbidities (24, 25), we selected a weight increase of 5% or more as a threshold to assess the impact of weight regain on insulin sensitivity. In out cohort, one- third of participants experienced a 10 ± 5% weight regain between postoperative years one and two. This represents a regain of approximately 8 kg and 20% of the weight loss that was achieved in the first year after RYGB. Thus, the participants categorized as having weight regain in our study are encompassed by all of the aforementioned criteria for weight regain.
A major question among clinicians and patients alike is whether failure to maintain weight loss after RYGB will lead to recurrence obesity-related comorbidities. Our data indicate that a weight regain of at least 5% incurred between the first and second postoperative years is not associated with reductions in either peripheral or hepatic insulin sensitivity. This is a significant finding because impaired insulin sensitivity is characteristic of conversion to pre-diabetes (8). Several recent studies explored the durability of type 2 diabetes remission after RYGB (31, 32, 33). Arterburn and colleagues, in a recent retrospective cohort study of nearly 4500 RYGB cases, reported that one-third of patients experienced relapse of type 2 diabetes within five years of initial remission (31). Only a limited number of studies have examined the effect of RYGB weight recidivism on the durability of type 2 diabetes remission, and found that weight regain was a significant predictor of type 2 diabetes recurrence (32, 33, 34, 35). The mean follow-up time for these studies ranged from 3-8.8 years. It remains possible that a longer follow-up time in our cohort would reveal a negative relationship between weight regain and insulin sensitivity. While it is important to better understand the long-term durability of metabolic improvements after RYGB, it still remains the most effective means to achieve glycemic control in type 2 diabetes (4, 5).
Ghrelin and leptin have opposing roles in regulation of energy balance: ghrelin increases and leptin decreased food intake (36). Elevated ghrelin levels after diet- induced weight loss have been proposed to explain the difficulty in attaining and maintaining weight loss (20, 37). Conversely, decreased ghrelin levels after RYGB are proposed to underlie some of the effectiveness of this surgical procedure to reduce food intake and induce weight loss (20). We did not find a relationship between preoperative ghrelin levels and overall degree of weight loss at 1 and 2 years after RYGB (data not shown). However, we demonstrate that higher preoperative and postoperative ghrelin levels are associated with early weight regain after RYGB. In contrast, levels of the anorexic hormone leptin followed the groups’ weight trajectories. This suggests that ghrelin does not impact degree of initial weight loss, but rather maintenance of surgical weight loss. It is important to note that we assessed total ghrelin levels, which provides a composite measure of the two circulating forms of ghrelin: acyl ghrelin and des-acyl ghrelin. Acyl ghrelin is predominately considered the form that mediates the effects of ghrelin to increase food intake and adiposity via interaction with the growth hormone secretagogue receptor (38). However, rodent studies suggest that desacyl ghrelin also functions to increase food intake and adiposity (39, 40) . Thus, the relative contributions of acyl and desacyl ghrelin to the regulation of body weight are unclear at present.
We recognize several potential limitations of the present study. The size of the cohort and predominance of females limits the generalization of the findings. Even though the cohort included subjects with and without preoperative type 2 diabetes, both groups experienced comparable improvements in insulin sensitivity after RYGB. Our study lacked information regarding whether post-prandial metabolic responses (e.g. insulin secretion, the incretin effect, ghrelin suppression) are influenced by weight regain. Since this study was designed to evaluate the metabolic responses to RYGB, we were unable to assess the influence of genetic and psychosocial factors on weight loss outcomes after RYGB. Regardless, the present findings indicate that early weight regain does not adversely affect insulin sensitivity after RYGB. Importantly, we report that preoperative ghrelin levels might identify those patients more susceptible to weight regain after RYGB and should receive more intense post-surgical follow-up to prevent post-RYGB weight recidivism. Additional studies in larger cohorts with longer postoperative follow up are necessary to fully appreciate the impact of weight regain on the durability of metabolic improvements after RYGB.
What is already known about this subject:
Regain of lost weight can occur after RYGB
Type 2 diabetes can re-occur after RYGB, and is associated with weight regain
Worsened insulin sensitivity occurs early in the natural history of type 2 diabetes
What this study adds:
Weight regain between RYGB postoperative years one and two is not associated with worsened insulin sensitivity
High preoperative and postoperative plasma levels of ghrelin are associated with early weight regain after RYGB
ACKNOWLDEGEMENTS
R.A.T. acquired, analyzed and interpreted data, performed statistical analyses, and wrote the manuscript; I.B. analyzed and interpreted data; P.A.M-S. acquired data and provided administrative support; K.J. acquired data; W.M. acquired data; B.W. acquired data; R.H.C. acquired data; I.D.F. performed and interpreted statistical analyses and edited the manuscript; N.N.A. designed and supervised the experiments, interpreted data, and edited the manuscript. All authors critically revised and approved the manuscript. N.N.A. is the guarantor of the study.
We appreciate participants who volunteered for this study and thank the following Vanderbilt colleagues for invaluable assistance in conducting these studies: Phil Williams, Erik N. Hansen, James M. Isbell, Jabbar Saliba, and the CRC nurses.
This study was supported by the following grants from the National Institutes of Health: DK070860 and DK091748 to N.N.A.; UL1TR000445 (Vanderbilt Clinical and Translational Science Award); DK020593 and DK059637 (Vanderbilt Diabetes Research and Training Center); DK058404 (Vanderbilt Digestive Disease Research Center).
Footnotes
Clinical Trial Registry: NCT00212160, clinicaltrials.gov
CONFLICTS OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. Jama. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 2.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 3.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 4.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 5.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn JP, Abumrad NN, Breitman I, Marks-Shulman PA, Flynn CR, Jabbour K, et al. Hepatic and peripheral insulin sensitivity and diabetes remission at 1 month after Roux-en-Y gastric bypass surgery in patients randomized to omentectomy. Diabetes Care. 2012;35:137–142. doi: 10.2337/dc11-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabbrini E, Tamboli RA, Magkos F, Marks-Shulman PA, Eckhauser AW, Richards WO, et al. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology. 2010;139:448–455. doi: 10.1053/j.gastro.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freire RH, Borges MC, Alvarez-Leite JI, Toulson Davisson Correia MI. Food quality, physical activity, and nutritional follow-up as determinant of weight regain after Roux-en-Y gastric bypass. Nutrition. 2012;28:53–58. doi: 10.1016/j.nut.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Topart P, Becouarn G, Ritz P. Weight loss is more sustained after biliopancreatic diversion with duodenal switch than Roux-en-Y gastric bypass in superobese patients. Surg Obes Relat Dis. 2012 doi: 10.1016/j.soard.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Barhouch AS, Zardo M, Padoin AV, Colossi FG, Casagrande DS, Chatkin R, et al. Excess weight loss variation in late postoperative period of gastric bypass. Obes Surg. 2010;20:1479–1483. doi: 10.1007/s11695-010-0202-3. [DOI] [PubMed] [Google Scholar]
- 12.Christou NV, Look D, Maclean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244:734–740. doi: 10.1097/01.sla.0000217592.04061.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magro DO, Geloneze B, Delfini R, Pareja BC, Callejas F, Pareja JC. Long-term weight regain after gastric bypass: a 5-year prospective study. Obes Surg. 2008;18:648–651. doi: 10.1007/s11695-007-9265-1. [DOI] [PubMed] [Google Scholar]
- 14.Jazet IM, Pijl H, Frolich M, Romijn JA, Meinders AE. Two days of a very low calorie diet reduces endogenous glucose production in obese type 2 diabetic patients despite the withdrawal of blood glucose-lowering therapies including insulin. Metabolism. 2005;54:705–712. doi: 10.1016/j.metabol.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology. 2009;136:1552–1560. doi: 10.1053/j.gastro.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christiansen MP, Linfoot PA, Neese RA, Hellerstein MK. Effect of dietary energy restriction on glucose production and substrate utilization in type 2 diabetes. Diabetes. 2000;49:1691–1699. doi: 10.2337/diabetes.49.10.1691. [DOI] [PubMed] [Google Scholar]
- 17.Campos GM, Rabl C, Peeva S, Ciovica R, Rao M, Schwarz JM, et al. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 2010;14:15–23. doi: 10.1007/s11605-009-1060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarzynski MA, Jacobson P, Rankinen T, Carlsson B, Sjostrom L, Bouchard C, et al. Associations of markers in 11 obesity candidate genes with maximal weight loss and weight regain in the SOS bariatric surgery cases. Int J Obes (Lond) 2011;35:676–683. doi: 10.1038/ijo.2010.166. [DOI] [PubMed] [Google Scholar]
- 19.Crujeiras AB, Goyenechea E, Abete I, Lage M, Carreira MC, Martinez JA, et al. Weight regain after a diet-induced loss is predicted by higher baseline leptin and lower ghrelin plasma levels. J Clin Endocrinol Metab. 2010;95:5037–5044. doi: 10.1210/jc.2009-2566. [DOI] [PubMed] [Google Scholar]
- 20.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 21.Tamboli RA, Hossain HA, Marks PA, Eckhauser AW, Rathmacher JA, Phillips SE, et al. Body composition and energy metabolism following Roux-en-Y gastric bypass surgery. Obesity (Silver Spring. 2010;18:1718–1724. doi: 10.1038/oby.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatoum IJ, Kaplan LM. Advantages of percent weight loss as a method of reporting weight loss after Roux-en-Y gastric bypass. Obesity (Silver Spring) 2013;21:1519–1525. doi: 10.1002/oby.20186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 24.Klein S. Outcome success in obesity. Obes Res. 2001;9(Suppl 4):354S–358S. doi: 10.1038/oby.2001.142. [DOI] [PubMed] [Google Scholar]
- 25.Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heber D, Greenway FL, Kaplan LM, Livingston E, Salvador J, Still C. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2010;95:4823–4843. doi: 10.1210/jc.2009-2128. [DOI] [PubMed] [Google Scholar]
- 27.Stevens J, Truesdale KP, McClain JE, Cai J. The definition of weight maintenance. Int J Obes (Lond) 2006;30:391–399. doi: 10.1038/sj.ijo.0803175. [DOI] [PubMed] [Google Scholar]
- 28.Johnson Stoklossa C, Atwal S. Nutrition Care for Patients with Weight Regain after Bariatric Surgery. Gastroenterol Res Pract. 2013;2013:256145. doi: 10.1155/2013/256145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bond DS, Phelan S, Leahey TM, Hill JO, Wing RR. Weight-loss maintenance in successful weight losers: surgical vs non-surgical methods. Int J Obes (Lond) 2009;33:173–180. doi: 10.1038/ijo.2008.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camps SG, Verhoef SP, Westerterp KR. Weight loss, weight maintenance, and adaptive thermogenesis. Am J Clin Nutr. 2013;97:990–994. doi: 10.3945/ajcn.112.050310. [DOI] [PubMed] [Google Scholar]
- 31.Arterburn DE, Bogart A, Sherwood NE, Sidney S, Coleman KJ, Haneuse S, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg. 2013;23:93–102. doi: 10.1007/s11695-012-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chikunguwo SM, Wolfe LG, Dodson P, Meador JG, Baugh N, Clore JN, et al. Analysis of factors associated with durable remission of diabetes after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2010;6:254–259. doi: 10.1016/j.soard.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 33.DiGiorgi M, Rosen DJ, Choi JJ, Milone L, Schrope B, Olivero-Rivera L, et al. Re-emergence of diabetes after gastric bypass in patients with mid- to long-term follow-up. Surg Obes Relat Dis. 2010;6:249–253. doi: 10.1016/j.soard.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Jimenez A, Casamitjana R, Flores L, Viaplana J, Corcelles R, Lacy A, et al. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg. 2012;256:1023–1029. doi: 10.1097/SLA.0b013e318262ee6b. [DOI] [PubMed] [Google Scholar]
- 35.Laurino Neto RM, Herbella FA, Tauil RM, Silva FS, de Lima SE., Jr. Comorbidities remission after Roux-en-Y Gastric Bypass for morbid obesity is sustained in a long-term follow-up and correlates with weight regain. Obes Surg. 2012;22:1580–1585. doi: 10.1007/s11695-012-0731-z. [DOI] [PubMed] [Google Scholar]
- 36.Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 2007;8:21–34. doi: 10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 37.Hansen TK, Dall R, Hosoda H, Kojima M, Kangawa K, Christiansen JS, et al. Weight loss increases circulating levels of ghrelin in human obesity. Clin Endocrinol (Oxf) 2002;56:203–206. doi: 10.1046/j.0300-0664.2001.01456.x. [DOI] [PubMed] [Google Scholar]
- 38.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 39.Heppner KM, Piechowski CL, Muller A, Ottaway N, Sisley S, Smiley DL, et al. Both acyl and des-acyl ghrelin regulate adiposity and glucose metabolism via CNS ghrelin receptors. Diabetes. 2013 doi: 10.2337/db13-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toshinai K, Yamaguchi H, Sun Y, Smith RG, Yamanaka A, Sakurai T, et al. Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology. 2006;147:2306–2314. doi: 10.1210/en.2005-1357. [DOI] [PubMed] [Google Scholar]