Abstract
Effective biomedical and structural HIV prevention approaches are being implemented throughout sub-Saharan Africa. A “lifecycle approach” to HIV prevention recognizes the interconnectedness of the health of women, children and adolescents, and prioritizes interventions that have benefits across these populations. We review new biomedical prevention strategies for women, adolescents and children, structural prevention approaches, and new modalities for eliminating infant HIV infection, and discuss the implications of a lifecycle approach for the success of these methods. Some examples of the lifecycle approach include evaluating education and HIV prevention strategies among adolescent girls not only for their role in reducing risk of HIV infection and early pregnancy, but also to promote healthy adolescents who will have healthier future children. Similarly, early childhood interventions such as exclusive breastfeeding not only prevent HIV, but also contribute to better child and adolescent health outcomes.. The most ambitious biomedical infant HIV prevention effort, Option B+, also represents a lifecycle approach by leveraging the prevention benefits of optimal HIV treatment for mothers; maternal survival benefits from Option B+ may have ultimately more health impact on children than the prevention of infant HIV in isolation. The potential for synergistic and additive benefits of lifecycle interventions should be considered when scaling up HIV prevention efforts in sub-Saharan Africa.
Keywords: HIV prevention, maternal health, child health, adolescent health, millennium development goals, PMTCT, mother-to-child transmission of HIV, global epidemic, HIV
Introduction
HIV/AIDS disproportionately afflicts women in Africa. Thus, HIV prevention efforts which focus on women are critical to decreasing the AIDS epidemic in this region. Further, the health of women has a substantial impact on household and child health, making women’s health essential for improving the health of populations. The past several years have yielded major advances in preventing HIV among women and children. Here, we review these advances and then explore how an integrated “lifecycle” approach to HIV prevention and treatment has potential to limit the HIV epidemic and provide broader benefits for global health.
The Lifecycle Approach: New Opportunities in HIV Prevention/Treatment for Women and Children
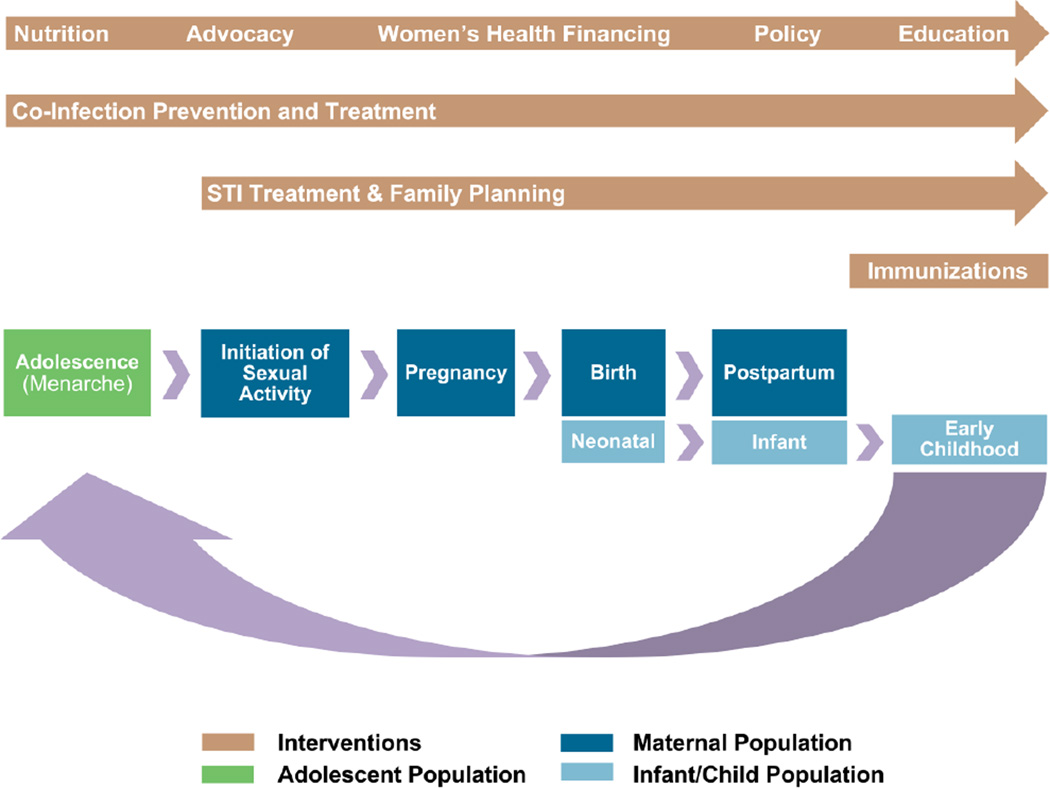
Conventional approaches to medical care and research have viewed women, children, and adolescents as three distinct populations; formal specialties and sub-specialties exist to treat these three patient populations in separate silos with little overlap between disciplines. However, infant health is inextricably tied to maternal health, and childhood and adolescent health have long-term implications for future mothers. In this way, these three stages of a woman’s life can be seen as part of an interconnected “lifecycle” (Figure 1). Health risks at any one of these stages have broader effects on the lifecycle as a whole; similarly each stage represents a unique opportunity to introduce interventions that have broader benefits across these three stages. A “lifecycle approach” thus recognizes the interconnectedness of women, children and adolescents, and prioritizes interventions that have benefits across these populations. Activities which integrate multiple health interventions, such as breastfeeding and family planning, have especially far-reaching benefits across the lifecycle and the community. The lifecycle approach presents new opportunities to engage women and children in coordinated care that integrates HIV/AIDS prevention and care into optimal and proven health interventions at established entry points to the health care system.
Figure 1.
The maternal, infant and adolescent lifecycle and associated health interventions.
Using the lifecycle approach, we have identified several key interventions at different time points for not only preventing HIV but also enhancing the basic health of women, adolescents and children. It is possible to work synergistically with HIV prevention and treatment programs to create broader health benefits for women and children (Table 1). Most of these lifecycle interventions also address Millennium Development Goals, demonstrating the high impact of these interventions [1].
Table 1.
Lifecycle interventions and anticipated benefits for women, adolescent and child health.
| Lifecycle intervention | Outcomes associated with this lifecycle intervention and relationship to HIV prevention and treatment |
Millennium Development Goal (MDG) addressed |
|---|---|---|
| Improve childhood nutrition |
|
MDG 1, 4, 6 |
| Infant immunizations and health screenings |
|
MDG 4 |
| Education |
|
MDG 1, 2, 3 |
| Adolescent health promotion |
|
MDG 1, 2, 3, 4, 5 |
| Family planning |
|
MDG 3, 4, 5, 6 |
| Sexually transmitted infection care and treatment |
|
MDG 3, 6 |
| Co-infection prevention and treatment (including malaria, parasitic infections, enteric infections) |
|
MDG 4, 5, 6 |
| Antenatal care |
|
MDG 4, 5, 6 |
| Facility delivery, safe motherhood strategies |
|
MDG 4, 5, 6 |
| Postpartum care |
|
MDG 1, 4, 5, 6 |
| Breastfeeding promotion |
|
MDG 1, 4, 6 |
| Chronic disease screening/treatment |
|
MDG 2, 3, 7 |
MTCT = mother to child transmission. HIV = human immunodeficiency virus. MDG = millennium development goal.
HIV Prevention Strategies for Women and Adolescents are Evolving
Multiple randomized controlled trials have provided evidence for new effective antiretroviral prevention methods among sexually active women. The HPTN 052 randomized controlled trial showed that in heterosexual discordant couples, treatment of one partner with antiretroviral therapy (ART) reduced risk of HIV transmission within the couple by 96%, although partners remained at risk of acquiring HIV from outside their relationship [2]. Pre-exposure prophylaxis with daily tenofovir or tenofovir-emtricitabine in HIV-discordant couples resulted in a 60 and 75% reduction in HIV acquisition by the HIV-negative partner, and was shown to be effective at protecting both men and women in serodiscordant relationships [3]. The TDF2 study in Botswana demonstrated 62% effectiveness of tenofovir-emtricitabine at protecting individuals at risk of heterosexual acquisition of HIV [4]. The CAPRISA 004 study showed that pericoital application of 1% tenofovir vaginal gel by African women resulted in a 39% reduction in HIV-1 acquisition [5], although these results were not replicated by the VOICE trial (described below) [6]. These diverse antiretroviral interventions for HIV prevention represent a major advance in biomedical strategies to protect women.
Despite these successes in preventing HIV transmission among older, married women, the crisis among adolescent females in Africa remains sobering, with adolescent females (ages 15–24) infected with HIV at twice the rates of adolescent males. Staggeringly high HIV-1 incidence rates of 8.8% per year were noted in unmarried women <25 years of age participating in the VOICE study [6]; in the CAPRISA study, a site in South Africa noted HIV prevalence among girls ages 14–17 of 27.6% [7]. Effective methods to target adolescent women are urgently needed, and this demographic disparity drives the epidemic in both women and children. Unfortunately, in two trials focusing on women-controlled methods, pre-exposure prophylaxis was ineffective. In the FEM-PrEP trial, pre-exposure prophylaxis with oral tenofovir failed to protect young, single women [8], with poor adherence the primary cause for the lack of effectiveness seen. Similarly, in the VOICE study, among a population of young, high-risk women in sub-Saharan Africa, oral tenofovir and tenofovir vaginal gel were ineffective [6]. The failure of these trials underscores challenges of prevention methods that require persistent adherence by users. New women-controlled topical prevention methods under investigation, including the dapivirine ring, and development of combination products that can prevent HIV, STIs and pregnancy may circumvent these adherence challenges, which are particularly critical for adolescent populations [9].
Male circumcision is an effective HIV prevention intervention for adult and adolescent males [10–12] and contributes to population-level reductions in HIV prevalence [13•]. Demand for services are high among young men and boys, and in one country (Kenya) this demand has led to a minimum age for circumcision [14]. Some countries are still struggling to promote circumcision among adult men, but in the long-term, circumcision is an HIV prevention intervention which can be specifically targeted to male adolescents. Finding more effective ways to reach male and female adolescent populations in the lifecycle will be a major advance in HIV prevention.
Structural Interventions for HIV Prevention in Women and Adolescents May Complement Biomedical Advances
Given the complexity and differential benefit of biomedical prevention methods, sociobehavioral and structural interventions have been studied including education and conditional cash transfers (CCT) [15–18•] as ways to prevent HIV among young women. Higher educational attainment is associated with better health outcomes for women both short-term and long-term, and results in better child health. A pilot intervention that focused on keeping adolescent women in school by paying school fees successfully delayed sexual debut and reduced HIV infection in a low-prevalence setting, resulting in 3% infected in the control arm and 1.2% infected in the intervention arm [16]. A randomized controlled trial of CCT to reduce adolescent vulnerability also showed modest reductions in HIV seroconversion among adolescents [19]. The results from these trials show that broader poverty reduction strategies may have beneficial impacts on adolescent and women’s HIV risk [20].
A recent article reviewed diverse efforts to address the social and structural drivers of the HIV epidemic, including transforming gender norms, reducing violence, transforming legal norms, promoting education, promoting employment, and reducing stigma and discrimination [21]. Many of these efforts are effective or promising at preventing HIV among women and girls. Structural interventions across the lifecycle should be implemented in conjunction with biomedical strategies to reduce HIV infection.
Prevention of HIV in Infants Yields Concurrent Impact on Mothers with HIV
World Health Organization (WHO) recommendations for the prevention and treatment of HIV infection in women and infants recognize and incorporate a lifecycle approach in their most recent iteration [22••]. Recommendations to promote Option B (triple antiretroviral therapy during pregnancy and breastfeeding) or Option B+ (triple antiretroviral therapy started in pregnancy and continued for life) optimize risk reduction for the infant while simultaneously addressing the health needs of the mother. Shifting to Option B/B+ helps address significant health systems bottlenecks, including difficulties deploying CD4 counts in rural areas and assuring timely receipt of CD4 count results [23, 24•, 25]. These recommendations also incorporate the preponderance of evidence indicating that HIV positive women in resource-limited settings should breastfeed for optimal infant health and survival [26–29]. In addition, most women currently receiving short-course ART would soon become eligible for ART within a few years of pregnancy [30], and Option B+ also protects women and infants during later intended or unintended pregnancies. The widespread implementation of ART starting in pregnancy in Malawi resulted in an over 7-fold increase in women initiating ART over one year [31]. This approach is being implemented in Africa but is not yet widespread. A recent review attributed 24% of maternal mortality in Africa to HIV [32]. Option B+ offers operational advantages, and may yield improvements in women’s health [33], reductions in maternal mortality, as well as reduction in transmission to uninfected male partners.
Momentum in Elimination of MTCT Offers New Opportunities to Enhance Health of Women, Adolescents and Children
Elimination of pediatric HIV has taken center stage as a multipronged public health strategy. The four pillars of the pediatric HIV elimination strategy are: prevention of maternal HIV infection, family planning to prevented unwanted pregnancies, antiretroviral therapy in pregnancy, delivery and breastfeeding, and provision of care and treatment to all HIV-infected women, children and families [34]. All of these strategies rely on an integrated, lifecycle approach to family health.
Through expansion of maternal HIV testing and short-course antiretroviral regimens, pediatric HIV infections have been reduced by 50% since 2001. In 2012 it was estimated that 260,000 children acquired HIV. However, the 2013 UNAIDS report outlines disparity in access to the most basic PMTCT services. In Africa, 13 countries reach fewer than 50% of women with PMTCT services, including Nigeria, the most populous country in Africa, and a further 10 countries reach fewer than 75% of women with even basic PMTCT services [35]. Antiretroviral coverage for breastfeeding women lags further behind, with only 49% of women protecting breastfeeding infants by using antiretroviral therapy or prophylaxis. Consequently, the incidence of HIV acquired during breastfeeding is beginning to account for a larger proportion of pediatric HIV infections. To achieve a target of elimination of pediatric HIV, these missed opportunities need to be comprehensively addressed. Integrating HIV prevention for children into the events of a child’s lifecycle, such as immunization and pediatric nutrition, could be a way to ensure that pediatric HIV prevention strategies are fully implemented.
For optimal infant survival, early infant diagnosis is a key intervention for HIV-exposed children, but significant barriers remain to diagnosing infants early in their lives. The CHER study showed that early infant diagnosis and prompt initiation of ART resulted in a fourfold decrease in mortality [36]. Further, HIV-exposed infants who are followed have improved growth and survival [37]. However, despite that fact that African infants receive immunizations and are therefore accessing health care, a majority of HIV-exposed infants are not followed after mothers deliver, and do not get the full benefit of early diagnosis and referral to treatment [24,38]. Quality improvement projects aimed at linking mothers from PMTCT programs with early infant diagnosis services have been piloted [39], but most programs remain far from the goal of testing all HIV-exposed infants [40]. Stronger integration of PMTCT and maternal, newborn and child health services are needed to fully address this gap [41,42].
In 2013, UNAIDS estimates that there are 17.8 million orphan children who have lost one or both parents in 2013 [43], and research suggests that orphans remain incredibly vulnerable to morbidity, mortality, exploitation, and acquisition of HIV [44,45]. This information underscores the links between maternal survival and infant health, and lends urgency to efforts to eliminate mother-to-child transmission and also to implement Option B+ to optimize maternal survival and health. In addition, a lifecycle approach which improves overall child health may mitigate some of the additional vulnerability among orphans.
HIV Prevention for Women and Children during Pregnancy, Lactation, and Contraception Must be Enhanced
HIV prevention methods in women, and improvement in access and integration of family planning services in HIV care are part of the pediatric HIV elimination agenda, however neither is well-integrated into traditional programs for PMTCT. Addressing these time points in a woman’s lifecycle with comprehensive HIV prevention services would augment efforts to eliminate pediatric HIV.
Primary prevention of HIV in women, including prevention methods reviewed above (biomedical prevention such as ART in partners, pre-exposure prophylaxis, topical ART, adolescent HIV prevention, male circumcision) all have beneficial downstream effects for a woman and her children, and are an important and often overlooked pathway to eliminate pediatric HIV. Increasing attention has been focused on pregnancy as a high-risk time for HIV acquisition [46–48] and subsequent transmission to infants; HIV acquisition during the two years of lactation also places an infant at high risk [49]. Current PMTCT programs offer little to HIV negative women after initial screening during antenatal care and may not be adequately reaching women at risk for HIV infection during pregnancy and lactation. As PMTCT programs expand, the proportion of HIV infected infants who acquire their infection due to acute maternal infection will increase. HIV preventive interventions such as PrEP have not been evaluated for use in pregnant or lactating women, in part due to ethical concerns about fetal and infant exposure. Because fertility rates are high in Africa, many HIV uninfected women have substantial periods in which they are either pregnant or breastfeeding, and it will be critical to identify HIV preventive options for these periods.
Family planning access has been improving since 1990: worldwide contraceptive prevalence from 1990 to 2010 increased from 55% to 64% of married women having ever used family planning, and “unmet need” for family planning decreased from 15% to 12% [50]. However, in sub-Saharan Africa, where HIV infection is highly prevalent among women, contraceptive use is lower. Use among married women in Southern Africa is estimated at 60%, about 35% for Eastern Africa, but lower than 20% for both Central and West Africa. Using methods that include all fecund women, Darroch et al estimated that in 2012 there were 59 million women in Africa with unmet need for modern contraception and that a majority (53%) of women wanting to use contraception had no access to modern methods [51•]. Recent attention to possible risks of enhanced HIV acquisition and transmission among users of depotmedroxyprogesterone acetate (DMPA) [56–58] are concerning, but the controversy surrounding DMPA use has highlighted both the reproductive health imperatives of access to safe contraception, and the limited contraceptive options currently available and acceptable to African women. Among HIV-positive African women, unmet need for contraception remains high, even among women who are accessing HIV-related services [52–55]. Studies of integration of family planning clinics into HIV services have shown that integration may improve uptake among HIV positive women [60–62]. Further progress in expanding access to diversified family planning methods remains an unfulfilled opportunity to improve health of all women, and represents an opportunity to make further progress on the elimination of MTCT agenda [59].
Challenges to Implementation of the Lifecycle Approach
Integration of HIV prevention into health care delivery services is a goal of 90% of countries surveyed by UNAIDS in 2012 [35]. To have maximum impact, the package of highly effective HIV prevention methods reviewed in this article will need to be integrated into every aspect of the health sector. However, currently HIV interventions are largely considered separate from other health interventions in the lifecycle. The women-adolescent-child lifecycle acts as a framework for building a program to meet these different health goals, and builds on the history of integration of PMTCT services with maternal and child health care. The additional resources and training for PMTCT efforts have brought needed resources to the larger MCH clinical setting. Similarly, implementing a broader and more comprehensive agenda of lifecycle-based HIV prevention into health services could strengthen the health system.
Some examples of relatively simple lifecycle approaches with large dividends include expanding access to family planning for all women in an integrated fashion, which benefits HIV positive women and promotes elimination of mother-to-child transmission, but also benefits HIV negative women and improves their health status. Integrating male circumcision into child and adolescent health programs will reach more people while also strengthening health services for those age groups. Promotion of exclusive breastfeeding can be implemented as a child health intervention as well as an HIV prevention intervention. More complicated lifecycle approaches could include more diverse public health efforts, including improving water and sanitation, malaria prevention in children and pregnant women, and routine deworming, which both provide immediate health benefits and also synergistic downstream benefits to women and children later in the lifecycle.
In addition, the lifecycle approach can unify sectors beyond health care by outlining the health benefits from different interventions. For example, support for early childhood education, support for domestic violence prevention initiatives, and support for women’s legal aid all can build on a lifecycle framework which demonstrates the benefits of integrating around the goal of improving women’s and children’s health and well-being.
Several recent articles have described the strengths and synergies of combination prevention approaches in HIV. Chang et al discussed the potential benefits of pragmatic “combination implementation.” This involves simultaneous implementation of multiple proven HIV prevention strategies to maximize population level effects to affect the HIV epidemic [63]. Vermund and Hayes reviewed the transmission benefits of synergistically applying behavioral, structural and biomedical prevention approaches to reduce HIV viral load and therefore reduce onward viral transmission, creating a “package” of interventions similar to current multipronged approaches in malaria and tuberculosis control [64]. Similarly, Allsallaq and others modeled the potential benefits of synergistic implementation of multiple prevention interventions in a hyperendemic community in South Africa to arrest the HIV epidemic, finding reductions in HIV incidence from 47–63% within 4 years when interventions overlapped [65]. These strategies are focused on maximizing the impacts of the interventions on the HIV/AIDS epidemic. However, a lifecycle approach would expand the scope of the intervention and would include other health objectives outside of HIV/AIDS. The “diagonal approach” to maternal and child health (MCH) outlined by Gounder et al. [66] describes using a scaffolding of HIV/AIDS and MCH programs to build multiple health intervention programs for women. The lifecycle approach similarly is woman-focused and allows each time point in a woman’s life to implement HIV prevention efforts and other health interventions, with a goal of both preventing HIV and improving woman, adolescent and child health and well-being. In this era of static investment in HIV programming, the lifecycle approach maximizes scarce resources by expanding beneficial health interventions out of the HIV silo.
Conclusions
More than ever before, HIV prevention in women, and elimination of pediatric HIV, are tangible goals that will substantially improve the lives of women and children in countries highly affected by HIV. A lifecycle approach provides added efficiency in HIV prevention, and may additionally confer broader benefits across populations. Lifecycle interventions may thus provide an attractive framework for implementation of HIV prevention methods at scale to improve health and well-being of communities. Investments in a lifecycle approach will be a lasting contribution to health care infrastructure beyond the HIV pandemic.
Acknowledgements
This paper benefitted greatly from discussions held as part of the multidisciplinary University of Washington Center for the Integrated Health of Women, Adolescents and Children (Global WACh), a joint effort led by the Departments of Global Health, Pediatrics, and Obstetrics and Gynecology.
Funding: ACR: is supported by K23 HD071788-01A1 from the National Institute of Child Health and Development (NICHD), and by a New Investigator Award from the University of Washington (UW) Center for AIDS Research (CFAR), an NIH funded program (P30 AI027757), which is supported by the following NIH Institutes and Centers (NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA). JAU: is supported by K12 HD09026 (NICHD) and by UW CFAR. JAS: is supported by K01 AI087369 from the National Institute of Allergy and Infectious Diseases (NIAID), the Royalty Research Fund, and a New Investigator Award from the UW CFAR. JK: is supported by P01 AI082976-01 and R01-HD075108 (NIAID), and 1R24TW008907 (NIH). GJS: is supported by K24 HD054314 and R01 HD023412-21 (NICHD), and P01 AI082976-01 (NIAID). JLW: is supported by U19 AI 090882 (NIAID) and by awards from the Bill & Melinda Gates Foundation.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Alison C. Roxby, Jennifer A. Unger, Jennifer A. Slyker, John Kinuthia, Andrew Lewis, Grace John-Stewart, and Judd L. Walson declare that they have no conflict of interest
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Alison C. Roxby, Email: aroxby@u.washington.edu, Department of Medicine, University of Washington, 325 9th Avenue, Box 359909, Seattle, WA USA 98104, Tel: 206-543-4278, Fax: 206-543-4818.
Jennifer A. Unger, Email: junger@u.washington.edu, Department of Obstetrics and Gynecology, University of Washington, 325 9th Ave, Box 356460, Seattle, WA 98104 USA.
Jennifer A. Slyker, Email: jslyker@u.washington.edu, Department of Global Health, University of Washington, 325 9th Ave, Box 359909, Seattle, WA 98104 USA.
John Kinuthia, Email: kinuthia@u.washington.edu, Department of Reproductive Health, Kenyatta National Hospital, Department of Research & Programs, Kenyatta National Hospital, Ngong Road, Box 2590-00202, Nairobi, KENYA.
Andrew Lewis, Email: lewisar@u.washington.edu, Department of Global Health, University of Washington, 325 9th Ave, Box 359909, Seattle, WA 98104 USA.
Grace John-Stewart, Email: gjohn@u.washington.edu, Departments of Medicine, Pediatrics, Epidemiology and Global Health, University of Washington, 325 9th Ave, Box 359909, Seattle, WA 98104 USA.
Judd L. Walson, Email: walson@u.washington.edu, Departments of Global Health, Medicine, Pediatrics and Epidemiology, University of Washington, 325 9th Ave, Box 359909, Seattle, WA 98104 USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.United Nations. New York, NY: 2010. United Nations Millenium Development Goals Report 2010. [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 5.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marrazzo JM, Ramjee G, Nair G, Palanee T, Mkhize B, et al. 2013 Conference on Retroviruses and Opportunistic Infections. Atlanta, GA: 2013. Pre-exposure prophylaxis for HIV in women: Daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE study (MTN-003) [Google Scholar]
- 7.Abdool Karim Q, Kharsany AB, Frohlich JA, Werner L, Mlotshwa M, et al. HIV incidence in young girls in KwaZulu-Natal, South Africa--public health imperative for their inclusion in HIV biomedical intervention trials. AIDS Behav. 2012;16:1870–1876. doi: 10.1007/s10461-012-0209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friend DR, Kiser PF. Assessment of topical microbicides to prevent HIV-1 transmission: concepts, testing, lessons learned. Antiviral Res. 2013;99:391–400. doi: 10.1016/j.antiviral.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, et al. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey RC, Moses S, Parker CB, Agot K, Maclean I, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 12.Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 13. Auvert B, Taljaard D, Rech D, Lissouba P, Singh B, et al. Association of the ANRS-12126 male circumcision project with HIV levels among men in a South African township: evaluation of effectiveness using cross-sectional surveys. PLoS Med. 2013;10:e1001509. doi: 10.1371/journal.pmed.1001509. Using population surveys in an area where circumcision was recently introduced, this study showed durable reductions in HIV prevalence among circumcised men and 19% lower HIV prevalence than would have been expected without the voluntary male circumcision program.
- 14.Centers for Disease Control and Prevention. Progress in voluntary medical male circumcision service provision - Kenya, 2008–2011. MMWR Morb Mortal Wkly Rep. 2012;61:957–961. [PubMed] [Google Scholar]
- 15.Kohler HP, Thornton R. Conditional Cash Transfers and HIV/AIDS Prevention: Unconditionally Promising? World Bank Econ Rev. 2012;26:165–190. doi: 10.1093/wber/lhr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baird SJ, Garfein RS, McIntosh CT, Ozler B. Effect of a cash transfer programme for schooling on prevalence of HIV and herpes simplex type 2 in Malawi: a cluster randomised trial. Lancet. 2012;379:1320–1329. doi: 10.1016/S0140-6736(11)61709-1. [DOI] [PubMed] [Google Scholar]
- 17.Pettifor A, MacPhail C, Nguyen N, Rosenberg M. Can money prevent the spread of HIV? A review of cash payments for HIV prevention. AIDS Behav. 2012;16:1729–1738. doi: 10.1007/s10461-012-0240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heise L, Lutz B, Ranganathan M, Watts C. Cash transfers for HIV prevention: considering their potential. J Int AIDS Soc. 2013;16:18615. doi: 10.7448/IAS.16.1.18615. This paper reviews the available evidence on conditional cash transfers and HIV prevention including recommendations for future research.
- 19.de Walque D, Dow WH, Nathan R, Abdul R, Abilahi F, et al. Incentivising safe sex: a randomised trial of conditional cash transfers for HIV and sexually transmitted infection prevention in rural Tanzania. BMJ Open. 2012;2:e000747. doi: 10.1136/bmjopen-2011-000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowan F, Pettifor A. HIV in adolescents in sub-Saharan Africa. Curr Opin HIV AIDS. 2009;4:288–293. doi: 10.1097/COH.0b013e32832c7d10. [DOI] [PubMed] [Google Scholar]
- 21.Hardee K, Gay J, Croce-Galis M, Peltz A. Strengthening the enabling environment for women and girls: what is the evidence in social and structural approaches in the HIV response? J Int AIDS Soc. 2014;17:18619. doi: 10.7448/IAS.17.1.18619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Health Organization. Programmatic Update: Use of antiretroviral drugs for treating pregnant woman and preventing HIV infection in infants. 2012 This report outlines the rationale for Options B and B+ for prevention of mother-to-child transmission of HIV and describes the benefits to mothers, infants and partners.
- 23.Stringer EM, Ekouevi DK, Coetzee D, Tih PM, Creek TL, et al. Coverage of nevirapine-based services to prevent mother-to-child HIV transmission in 4 African countries. JAMA. 2010;304:293–302. doi: 10.1001/jama.2010.990. [DOI] [PubMed] [Google Scholar]
- 24. Sibanda EL, Weller IV, Hakim JG, Cowan FM. The magnitude of loss to follow-up of HIV-exposed infants along the prevention of mother-to-child HIV transmission continuum of care: a systematic review and meta-analysis. AIDS. 2013;27:2787–2797. doi: 10.1097/QAD.0000000000000027. This review pools data from multiple published studies worldwide and shows that HIV-exposed children are highly unlikely to receive all services; based on their estimates, only 20% of infants are followed to a postnatal test.
- 25.Wettstein C, Mugglin C, Egger M, Blaser N, Vizcaya LS, et al. Missed opportunities to prevent mother-to-child-transmission: systematic review and meta-analysis. AIDS. 2012;26:2361–2373. doi: 10.1097/QAD.0b013e328359ab0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Homsy J, Moore D, Barasa A, Were W, Likicho C, et al. Breastfeeding, mother-to-child HIV transmission, and mortality among infants born to HIV-Infected women on highly active antiretroviral therapy in rural Uganda. J Acquir Immune Defic Syndr. 2010;53:28–35. doi: 10.1097/QAI.0b013e3181bdf65a. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362:2282–2294. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mbori-Ngacha D, Nduati R, John G, Reilly M, Richardson B, et al. Morbidity and mortality in breastfed and formula-fed infants of HIV-1-infected women: A randomized clinical trial. JAMA. 2001;286:2413–2420. doi: 10.1001/jama.286.19.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas TK, Masaba R, Borkowf CB, Ndivo R, Zeh C, et al. Triple-antiretroviral prophylaxis to prevent mother-to-child HIV transmission through breastfeeding--the Kisumu Breastfeeding Study, Kenya: a clinical trial. PLoS Med. 2011;8:e1001015. doi: 10.1371/journal.pmed.1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watts DH, Brown ER, Maldonado Y, Herron C, Chipato T, et al. HIV disease progression in the first year after delivery among African women followed in the HPTN 046 clinical trial. J Acquir Immune Defic Syndr. 2013;64:299–306. doi: 10.1097/QAI.0b013e3182a2123a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Impact of an innovative approach to prevent mother-to-child transmission of HIV--Malawi, July 2011–September 2012. MMWR Morb Mortal Wkly Rep. 2013;62:148–151. [PMC free article] [PubMed] [Google Scholar]
- 32. Zaba B, Calvert C, Marston M, Isingo R, Nakiyingi-Miiro J, et al. Effect of HIV infection on pregnancy-related mortality in sub-Saharan Africa: secondary analyses of pooled community-based data from the network for Analysing Longitudinal Population-based HIV/AIDS data on Africa (ALPHA) Lancet. 2013;381:1763–1771. doi: 10.1016/S0140-6736(13)60803-X. Using pooled data from East and Southern Africa, this paper concluded that pregnant and postpartum HIV-infected women experience 8 times the mortality of uninfected women, and predicted that 24% of maternal death in Africa is attributable to HIV.
- 33.Moodley J, Pattinson RC, Baxter C, Sibeko S, Abdool Karim Q. Strengthening HIV services for pregnant women: an opportunity to reduce maternal mortality rates in Southern Africa/sub-Saharan Africa. BJOG. 2011;118:219–225. doi: 10.1111/j.1471-0528.2010.02726.x. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. 2010 Version. Geneva: 2010. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: recommendations for a public health approach. [PubMed] [Google Scholar]
- 35.UNAIDS. UNAIDS Report on the Global HIV Epidemic. 2013
- 36.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zunza M, Mercer GD, Thabane L, Esser M, Cotton MF. Effects of postnatal interventions for the reduction of vertical HIV transmission on infant growth and non-HIV infections: a systematic review. J Int AIDS Soc. 2013;16:18865. doi: 10.7448/IAS.16.1.18865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braun M, Kabue MM, McCollum ED, Ahmed S, Kim M, et al. Inadequate coordination of maternal and infant HIV services detrimentally affects early infant diagnosis outcomes in Lilongwe, Malawi. J Acquir Immune Defic Syndr. 2011;56:e122–e128. doi: 10.1097/QAI.0b013e31820a7f2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciampa PJ, Burlison JR, Blevins M, Sidat M, Moon TD, et al. Improving retention in the early infant diagnosis of HIV program in rural Mozambique by better service integration. J Acquir Immune Defic Syndr. 2011;58:115–119. doi: 10.1097/QAI.0b013e31822149bf. [DOI] [PubMed] [Google Scholar]
- 40.Adebimpe WO. Challenges facing early infant diagnosis of HIV among infants in resource poor settings. Afr J Reprod Health. 2013;17:122–129. [PubMed] [Google Scholar]
- 41.Chi BH, Bolton-Moore C, Holmes CB. Prevention of mother-to-child HIV transmission within the continuum of maternal, newborn, and child health services. Curr Opin HIV AIDS. 2013;8:498–503. doi: 10.1097/COH.0b013e3283637f7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghadrshenas A, Ben Amor Y, Chang J, Dale H, Sherman G, et al. Improved access to early infant diagnosis is a critical part of a child-centric prevention of mother-to-child transmission agenda. AIDS. 2013;27(Suppl 2):S197–S205. doi: 10.1097/QAD.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 43.UNAIDS. AIDS by the numbers. 2013 [Google Scholar]
- 44.Nyberg BJ, Yates DD, Lovich R, Coulibaly-Traore D, Sherr L, et al. Saving lives for a lifetime: supporting orphans and vulnerable children impacted by HIV/AIDS. J Acquir Immune Defic Syndr. 2012;60(Suppl 3):S127–S135. doi: 10.1097/QAI.0b013e31825da836. [DOI] [PubMed] [Google Scholar]
- 45.Amoako Johnson F, Padmadas SS, Smith PW. Orphanhood and vulnerability: a conduit to poor child health outcomes in Rwanda. AIDS Care. 2010;22:314–323. doi: 10.1080/09540120903193682. [DOI] [PubMed] [Google Scholar]
- 46.Kinuthia J, Kiarie JN, Farquhar C, Richardson B, Nduati R, et al. Cofactors for HIV-1 incidence during pregnancy and postpartum period. Curr HIV Res. 2010;8:510–514. doi: 10.2174/157016210793499213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moodley D, Esterhuizen T, Reddy L, Moodley P, Singh B, et al. Incident HIV infection in pregnant and lactating women and its effect on mother-to-child transmission in South Africa. J Infect Dis. 2011;203:1231–1234. doi: 10.1093/infdis/jir017. [DOI] [PubMed] [Google Scholar]
- 48.Theron GB, Shapiro DE, Van Dyke R, Cababasay MP, Louw J, et al. Rapid intrapartum or postpartum HIV testing at a midwife obstetric unit and a district hospital in South Africa. Int J Gynaecol Obstet. 2011;113:44–49. doi: 10.1016/j.ijgo.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Humphrey JH, Marinda E, Mutasa K, Moulton LH, Iliff PJ, et al. Mother to child transmission of HIV among Zimbabwean women who seroconverted postnatally: prospective cohort study. BMJ. 2010;341:c6580. doi: 10.1136/bmj.c6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alkema L, Kantorova V, Menozzi C, Biddlecom A. National, regional, and global rates and trends in contraceptive prevalence and unmet need for family planning between 1990 and 2015: a systematic and comprehensive analysis. Lancet. 2013;381:1642–1652. doi: 10.1016/S0140-6736(12)62204-1. [DOI] [PubMed] [Google Scholar]
- 51. Darroch JE, Singh S. Trends in contraceptive need and use in developing countries in 2003, 2008, and 2012: an analysis of national surveys. Lancet. 2013;381:1756–1762. doi: 10.1016/S0140-6736(13)60597-8. This review analyzes national data on contraceptive use in 2003, 2008 and 2012 including both married and unmarried women, and concludes ongoing high unmet need for contraception, especially in sub-Saharan Africa.
- 52.Stuart GS. Fourteen million women with limited options: HIV/AIDS and highly effective reversible contraception in sub-Saharan Africa. Contraception. 2009;80:412–416. doi: 10.1016/j.contraception.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 53.Peltzer K, Chao LW, Dana P. Family planning among HIV positive and negative prevention of mother to child transmission (PMTCT) clients in a resource poor setting in South Africa. AIDS Behav. 2009;13:973–979. doi: 10.1007/s10461-008-9365-5. [DOI] [PubMed] [Google Scholar]
- 54.Jhangri GS, Heys J, Alibhai A, Rubaale T, Kipp W. Unmet need for effective family planning in HIV-infected individuals: results from a survey in rural Uganda. J Fam Plann Reprod Health Care. 2012;38:23–29. doi: 10.1136/jfprhc-2011-0108. [DOI] [PubMed] [Google Scholar]
- 55.Leslie JA, Munyambanza E, Adamchak SE, Janowitz B, Grey TW, et al. Without strong integration of family planning into PMTCT services in Rwanda, clients remain with a high unmet need for effective family planning. Afr J Reprod Health. 2010;14:144–146. [PubMed] [Google Scholar]
- 56.Heffron R, Donnell D, Rees H, Celum C, Mugo N, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2011 doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Polis CB, Phillips SJ, Curtis KM. Hormonal contraceptive use and female-to-male HIV transmission: a systematic review of the epidemiologic evidence. AIDS. 2013;27:493–505. doi: 10.1097/QAD.0b013e32835ad539. [DOI] [PubMed] [Google Scholar]
- 58.Polis CB, Curtis KM. Use of hormonal contraceptives and HIV acquisition in women: a systematic review of the epidemiological evidence. Lancet Infect Dis. 2013;13:797–808. doi: 10.1016/S1473-3099(13)70155-5. [DOI] [PubMed] [Google Scholar]
- 59.Wilcher R, Petruney T, Cates W. The role of family planning in elimination of new pediatric HIV infection. Curr Opin HIV AIDS. 2013;8:490–497. doi: 10.1097/COH.0b013e3283632bd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harrington EK, Newmann SJ, Onono M, Schwartz KD, Bukusi EA, et al. Fertility intentions and interest in integrated family planning services among women living with HIV in Nyanza Province, Kenya: a qualitative study. Infect Dis Obstet Gynecol. 2012;2012:809682. doi: 10.1155/2012/809682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwartz SR, Rees H, Mehta S, Venter WD, Taha TE, et al. High incidence of unplanned pregnancy after antiretroviral therapy initiation: findings from a prospective cohort study in South Africa. PLoS One. 2012;7:e36039. doi: 10.1371/journal.pone.0036039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilcher R, Hoke T, Adamchak SE, Cates W., Jr Integration of family planning into HIV services: a synthesis of recent evidence. AIDS. 2013;27(Suppl 1):S65–S75. doi: 10.1097/QAD.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 63.Chang LW, Serwadda D, Quinn TC, Wawer MJ, Gray RH, et al. Combination implementation for HIV prevention: moving from clinical trial evidence to population-level effects. Lancet Infect Dis. 2013;13:65–76. doi: 10.1016/S1473-3099(12)70273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vermund SH, Hayes RJ. Combination prevention: new hope for stopping the epidemic. Curr HIV/AIDS Rep. 2013;10:169–186. doi: 10.1007/s11904-013-0155-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alsallaq RA, Baeten JM, Celum CL, Hughes JP, Abu-Raddad LJ, et al. Understanding the potential impact of a combination HIV prevention intervention in a hyper-endemic community. PLoS One. 2013;8:e54575. doi: 10.1371/journal.pone.0054575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gounder CR, Chaisson RE. A diagonal approach to building primary healthcare systems in resource-limited settings: women-centred integration of HIV/AIDS, tuberculosis, malaria, MCH and NCD initiatives. Trop Med Int Health. 2012;17:1426–1431. doi: 10.1111/j.1365-3156.2012.03100.x. [DOI] [PubMed] [Google Scholar]