Abstract
Environmental pollution is increasing worldwide, and there is evidence that exposure to halogenated persistent organic pollutants (POPs) such as polychlorinated biphenyls can contribute to the pathology of inflammatory diseases such as atherosclerosis, diabetes, and cancer. Pollutant removal from contaminated sites and subsequent pollutant degradation are critical for reducing the long-term health risks associated with exposure. However, complete remediation of a toxicant from the environment is very difficult and cost-prohibitive. Furthermore, remediation technologies often result in the generation of secondary toxicants. Considering these circumstances, environmentally-friendly and sustainable remediation technologies and biomedical solutions to reduce vulnerability to environmental chemical insults need to be explored to reduce the overall health risks associate with exposure to environmental pollutants. We propose that positive lifestyle changes such as healthful nutrition and consumption of diets rich in fruits and vegetables or bioactive nutrients with antioxidant and/or anti-inflammatory properties will reduce the body’s vulnerability to environmental stressors and thus reduce toxicant-mediated disease pathologies. Interestingly, emerging evidence now implicates the incorporation of bioactive nutrients, such as plant-derived polyphenols, in technologies focused on the capture, sensing and remediation of halogenated POPs. We propose that human nutritional intervention in concert with the use of natural polyphenol sensing and remediation platforms may provide a sensible means to develop primary and long-term prevention strategies of diseases associated with many environmental toxic insults including halogenated POPs.
Keywords: POP toxicity, oxidative stress, polyphenol, antioxidant response, nutrition, sustainable remediation
Introduction
1. Combining environmental science and biomedical strategies to better protect against POP toxicity
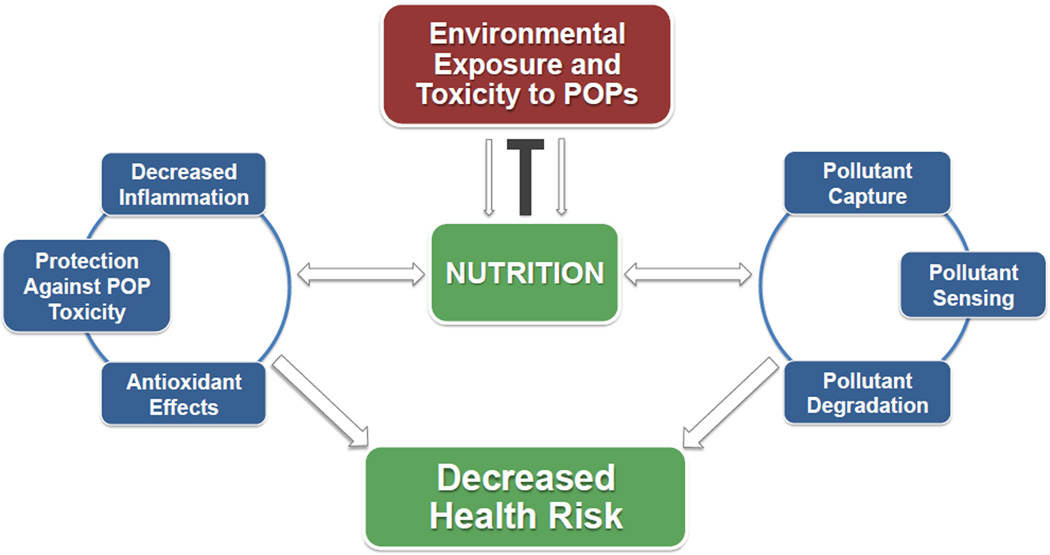
Contaminant remediation and biological modulation of environmentally persistent pollutants are two crucial means of reducing the human health risks of Superfund chemicals/toxicants and related hazardous materials. The Superfund program, designed to clean up sites contaminated with a variety of hazardous substances, was formed as the United States’ response to this growing environmental and public health concern (EPA, 2013), but these pollutants are of worldwide concern. Although complete remediation of a hazardous site may continue to be an ultimate goal of human exposure risk reduction, it is important to design and implement other biologically relevant means of buffering against toxicant exposure before, during, and after remediation activities. New data now implicate the importance of an individual’s nutritional status and the use of protective bioactive food components to decrease the overall toxicity of environmental pollutants to biological systems (Petriello et al., 2013). Nutrition is being substantiated as an important modulator of inflammatory and antioxidant pathways, especially with regard to environmental insults. Interestingly, novel research now shows that bioactive food components that are environmentally friendly can also be integrated into remediation technologies, which in turn allow for more sustainable, inexpensive, and effective pollutant removal and detoxification (Newsome et al., 2014). Implementing bioactive molecules in both biomedical and environmental science settings, together, will allow for decreased overall body burden and human toxicity of a multitude of pollutants (see Figure 1 for overview). In this review article, we discuss the relevant literature concerning this novel paradigm and point to the possible future uses of bioactive food components in healthful nutrition as well as their emerging uses in environmentally friendly remediation strategies for ultimately reducing human risks to persistent environmental pollutants.
Figure 1.
Illustration of nutrition as 1) a modulator of health risks associated with environmental exposure and toxicity of persistent organic pollutants (POPs) and as 2) an environmentally sustainable tool to capture, sense and remediate POPs. We propose that nutrition, and in particular diet-derived bioactive compounds such as polyphenols, can protect against POP toxicity through their antioxidant and anti-inflammatory properties. Polyphenols also can participate in a remediation platform that includes their roles in POP capture and sensing technologies, thus allowing for environmentally friendly and sustainable remediation of POPs.
Thus, this review is subdivided into 1) topics related to healthful nutrition (e.g., increased consumption of protective bioactive food components) as a means of reducing the disease vulnerability associated with exposure to environmental pollutants, and in particular pollutants found at Superfund sites, and 2) topics relevant to the emerging field pertaining to the utilization of environmentally friendly bioactive food components (e.g., polyphenols) to increase the efficiency and effectiveness for pollutant sensing, binding, and remediation efforts.
2. Exposure to POPs, risks for non-communicable diseases, and mechanisms of toxicity
Halogenated persistent organic pollutants (POPs), including polychlorinated biphenyls (PCBs) and trichloroethylene, are highly prevalent in the environment in soil, air, and water (Petriello et al., 2013). For example, a major source of human exposure to PCBs is through dietary intake of contaminated foods and through inhalation of airborne pollutants (Crinnion, 2011). Because most halogenated POPs, including PCBs, are lipid soluble, they easily accumulate in human tissues, leading to a perpetually increasing disease risk throughout a life span, especially in overweight populations (Kim et al., 2011). Exposure to halogenated POPs, and in particular pollutants that are ligands of the aryl hydrocarbon receptor (AhR), can lead to inflammatory events tied to diseases such as atherosclerosis, diabetes, obesity, cancer, etc. (Petriello et al., 2013). The pathology of many inflammatory diseases, especially of non-communicable or chronic diseases, develops over a long period of time and thus can be easily modulated by environmental exposures, specifically to persistent organic pollutants.
Mechanistically, an increase in cellular oxidative stress often precedes an inflammatory response (Schulze and Lee, 2005). PCBs, and in particular coplanar PCBs, have been shown to cause oxidative stress primarily through a cytochrome P450 (CYP1A1)-mediated uncoupling mechanism (Schlezinger et al., 2006). CYP1A1 induction allows for the detoxification of multiple xenobiotics, but when in the presence of PCB, can become inefficient and leaky (i.e., uncoupled) and produce detrimental reactive oxygen species (Schlezinger et al., 2006). A hallmark of the pathology of vascular diseases, including atherosclerosis, includes a change in the cellular redox status and a resultant increase in oxidative stress, which favors chronic and low level inflammation (Libby, 2012). Such changes in redox status and oxidative stress levels can be driven in part by pro-oxidative and pro-inflammatory environmental pollutants that are persistent and which can be easily stored in adipose tissue. Thus, an increased body burden of persistent environmental pollutants is a particular risk factor during obesity, a disease characterized by excessive adipose tissue (Kim et al., 2011). There is sufficient evidence that POPs contribute to inflammation by activating oxidative stress-sensitive transcription factors such as nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) (Hennig et al., 2002). For example, our studies suggest that PCBs, and in particular coplanar PCBs, can increase cellular oxidative stress and induce inflammatory parameters such as inflammatory cytokines, chemokines, and adhesion molecules in the vascular endothelium, which are metabolic events that foster an inflammatory response and atherosclerosis (Eske et al., 2013; Hennig et al., 2002; Lim et al., 2007; Majkova et al., 2009). Through these pro-inflammatory mechanisms, PCBs and related environmental toxicants have been correlated with increased risk of multiple human chronic disease phenotypes including diabetes and heart disease (Carpenter, 2011; Goncharov et al., 2008; Silverstone et al., 2012; Uemura, 2012). Since many populations susceptible to toxicant-induced disease are often also afflicted by diet-induced diseases, future human studies and integrated risk assessments should better investigate the interaction between nutrition and toxicology (Hennig et al., 2012).
Nutrition as a modulator of environmental chemical insults
1. Sub-optimal or unhealthy nutrition modulates the toxicity of environmental pollutants
POPs and subprime nutritional status share mechanisms of disease development including the induction of pro-inflammatory pathways. Interestingly, a growing body of evidence implicates an exacerbated toxic effect of PCBs and other environmental pollutants when combined with an additive pro-inflammatory dietary environment (Petriello et al., 2013). For example, our group has shown that, when combined, pro-inflammatory PCBs and omega-6 fatty acids create an exacerbated toxicological response that is not simply the sum of their individual inflammatory responses (Wang et al., 2008). Importantly, animal fats such as those found in beef and chicken are significant sources of pro-inflammatory medium and long-chain omega-6 fatty acids as well as PCBs and other related POPs (NCI, 2013). Also, other groups have investigated a linkage between high saturated fat diets and increased toxicity of POPs such as polycyclic aromatic hydrocarbons and have shown an increased risk for adenomas and cancer (Harris et al., 2009). In addition, other environmental contaminants such as heavy metals may work in concert with sub-optimal nutrition to promote chronic inflammation and disease, but studies that investigate the impacts of mixing multiple types of exposures, (e.g. chlorinated pollutants and heavy metals) in combination with the added variable of an unhealthy diet are severely lacking (Houston, 2011; Jomova and Valko, 2011). These types of comprehensive integrated toxicity studies will better mirror real world exposure conditions especially for people residing in close proximity to Superfund and other hazardous waste sites. Although obesogen research (e.g. toxicants promoting obesity) is becoming a more developed area of study, work is still needed to elucidate interactions between nutrients, nutritional status, environmental toxicants and human health.
2. Healthful nutrition decreases the toxicity of pro-inflammatory pollutants
Polychlorinated biphenyls and related Superfund POPs induce chronic oxidative stress and disregulated inflammatory responses, but anti-inflammatory nutritional antioxidants may buffer and protect against toxicant-induced disease through multiple cell signaling mechanisms (Petriello et al., 2013). Polyphenols and omega-3 polyunsaturated fatty acids have been shown to decrease toxicant-induced maladies including liver diseases, tumor formation and growth and endothelial cell activation (Petriello et al., 2013; Slim et al., 1999; Watkins et al., 2007). Our work has shown that plant-derived flavonoids such as epigallocatechin-3-gallate (EGCG), and long-chain omega-3 fatty acids such as docosahexaenoic acid (DHA) can protect cellular systems by decreasing pro-inflammatory lipid raft signaling domains called caveolae and by simultaneously upregulating antioxidant defenses through increased nuclear factor (erythroid-derived 2)-like 2 (Nrf2) activation (Majkova et al., 2011; Zheng et al., 2012). Most recently we have elucidated novel cross-talk mechanisms between caveolae and Nrf2 pathways and have shown that nutrients and/or bioactive food compounds may protect against vascular dysfunction, oxidative stress and inflammation by downregualting caveolae and simultaneously upregulating Nrf2 target antioxidant genes (Petriello et al., 2013). Other groups have shown that bioactive food compounds such as those found in broccoli (sulphoraphane) and red wine (resveratrol) can activate Nrf2 through multiple mechanisms and decrease oxidative stress levels in cells and in animals (Baur and Sinclair, 2006; Higgins and Hayes, 2011; Kang et al., 2012; Nadtochiy and Redman, 2011; Wu et al., 2001; Zakkar et al., 2009). Polyunsaturated fatty acids, and especially omega-3 fatty acids, as well as their anti-inflammatory breakdown metabolites can protect against PCB-induced disease by activating Nrf2 but interestingly also by disrupting functional caveolae (Majkova et al., 2011). Although caveolae and Nrf2 pathways appear to be prime candidates for bioactive food components to work through, more research is necessary to better elucidate other novel pathways that can be targeted both in vitro and in vivo.
3. Healthful nutrition can decrease body burden of environmental toxicants
An emerging paradigm implicates healthful nutrition as an effective protective modulator of environmental toxicant-induced inflammation and human disease. Multiple laboratories have investigated the decreased toxicities of environmental pollutants due to bioactive nutrients such as flavonoids and omega-3 polyunsaturated fatty acids, but many of these studies rely on in vitro assays that lack the complexity of a whole body organismal approach. Importantly, emerging classes of bioactive food components such as polyphenols also have been shown to modulate the pro-inflammatory effects of environmental toxicants. Our laboratory and others have shown that a wide array of phenolic compounds such as EGCG, curcumin and quercetin can decrease toxicant-induced oxidative stress and inflammation in multiple cell types, tissues and animal species (Choi et al., 2010; Ciftci et al., 2012; Morita et al., 1997; Sciullo et al., 2010; Slim et al., 1999; Zheng et al., 2012). Although human studies are lacking, strong evidence in animal models implicates a protective role for flavonoids and other polyphenols perhaps through the induction of antioxidant enzyme pathways and increased fecal excretion rates (Morita et al., 1997; Newsome et al., 2013). Since the protective effects of healthful nutrition against biological insults induced by exposure to POPs has been reviewed recently (Petriello et al., 2013), the remainder of this review will focus on what is known concerning the overall decrease in body burden of POPs, with a special emphasis on PCBs and related compounds, by nutrients such as polyphenols. In other words, plant-derived polyphenols are being found to not only protect against POP-mediated oxidative stress, inflammation, and toxicity but also to bind to POPs and thus contribute to a decrease in body burden. In fact, if has been proposed that green tea, containing high levels of polyphenols including EGCG, can inhibit the intestinal absorption of lipids and highly lipophilic organic compounds and accelerate excretion of PCBs (Kim et al., 2012; Koo and Noh, 2007; Morita et al., 1997).
Certain foods and polluted air can be major sources of toxicant exposures (Crinnion, 2011). Thus, altering nutritional choices may prove to effectively modulate risks associated with exposure to POPs. Making informed dietary decisions such as substituting lower fat versions of protein sources (e.g. legumes, nuts, or lean meats and dairies) may have multiple health benefits including decreased exposure to detrimental pollutants such as dioxins and PCBs (Yaktine et al., 2006). For many populations it may be difficult or cost-prohibitive to change major dietary protein sources; thus, increasing dietary intake of other bioactive nutrients may buffer already exposed individuals against the negative ramifications of pollutant exposures. For example, several laboratories have shown that diets high in fiber can alter the absorption and excretion rates of pollutants such as PCBs (Aozasa et al., 2001; De Vos and De Schrijver, 2005; Sera et al., 2005). Mechanistically, multiple dietary fibers have been shown to effectively bind pollutants such as dioxins, which may help to explain increased fecal excretion rates, decrease in body burden, and observed protection (Aozasa et al., 2001). Increasing excretion of lipophilic toxicants through other nutritional compounds such as fat substitutes may also effectively decrease risk. For example, much emphasis including human clinical trials has been placed on the interactions between the non-absorbable fat substitute olestra and environmental toxicants (Arguin et al., 2010; Geusau et al., 2002; Geusau et al., 1999; Jandacek et al., 2010; Moser and McLachlan, 1999). Researchers determined that low levels of olestra supplementation (25 grams/day) increased the excretion of multiple PCBs and related contaminants upwards of 11 fold compared to normal diet (Moser and McLachlan, 1999). Interestingly, in a human case study, an olestra-supplemented diet completely eliminated excessive POP concentrations in adipose tissue and reversed POP-mediated clinical conditions such as diabetes and hyperlipidemia (Redgrave et al., 2005). Diet-derived bioactive compounds such as EGCG are attractive modulators of toxic exposure because they may both help to prime the physiological system prior to a toxic insult by upregulating protective detoxifying enzymes as well as protect therapeutically after exposure by increasing the rate of excretion and lowering overall body burden (Newsome et al., 2013). Altering diets to increase fiber and bioactive nutrients from fruits and vegetables is an effective and cost-efficient means of modulating POP toxicity, but interestingly an emerging and growing body of knowledge also implicates the use of bioactive nutrients such as quercetin and curcumin as effective components of novel environmentally friendly remediation technologies.
Environmental remediation not only relies on efficient pollutant degradation but initially on effective pollutant sensing and capture/removal from contaminated settings. Emerging research suggests that the utilization of polyphenols for capture and sensing of POPs may provide a technologically efficient and cost effective remediation platform that results in minimal environmental and health impacts. This work adds to the paradigm that nutrients or diet-derived bioactive compounds can broadly impact health concerns associated with POP exposure (see Figure 1).
Utilizing polyphenols for pollutant sensing, binding, and remediation efforts
Persistent organics, and especially PCBs, have proven difficult to remove from contaminated sources and subsequently remediate due to their poor aqueous solubility and low volatility, which makes extraction from soil and water especially challenging (Gomes et al., 2013). Further, sensing of these pollutants is both difficult and cost prohibitive, relying heavily on GC-MS methods and complicated extraction techniques (Avino et al., 2011; Awawdeh and Harmon, 2005; Oshita et al., 2010). POP remediation to date often involves dredging and subsequent deposition in landfills or further pollutant degradation through incineration or various chemical dehalogenation techniques (NCR, 2001). Studies have shown, though, that dredging and deposition results in substantial leaching into the surrounding environment, incineration can produce even more harmful byproducts if insufficient temperatures are reached, and organic solvents used for chemical dehalogenation are often as toxic as the pollutants being remediated (Ham et al., 2008; Hutzinger et al., 1985). There is a clear need for more effective pollutant remediation involving inexpensive, environmentally friendly, stable systems for the capture/sensing of PCBs and other POPs, as well as environmentally-conscious pollutant degradation. In fact, as of 2011, the Ecologic Institute of the European Union’s Screening methods for the Water Framework Directive (SWIFT-WFD) has continued to focus on this need for discovery and translation of low-cost screening tools for environmental contaminants (Castro-Jimenez and Gonzalez, 2011).
As a result, much research has been performed to determine novel technologies and methods to capture, sense, and remediate POPs. One such example includes engineered antibodies that contain binding domains that can specifically associate with PCBs and other POPs, which can be utilized for specific binding and sensing applications (Pellequer et al., 2005). Due to inherent cost barriers and stability issues of these antibodies, though, other low-cost, stable engineered materials have been sought as an alternative platform to mimic this specific binding interaction; growing data are implicating the use of nutrient polyphenols as effective components of engineered POP capture/sensing platforms both due to their fluorescent detection characteristics and affinity for chlorinated organics (Newsome et al., 2013). It has been reported that the binding domains of PCB-specific antibodies (e.g., the monoclonal antibody S2B1) form sterically constrained, highly aromatic pockets, which allow for pi-pi bond stacking interactions (Pellequer et al., 2005). Researchers have successfully mimicked these interactions in the development of synthetic materials, including those composed of polyphenolic moieties, for capture and sensing applications (Haupt and Mosbach, 2000; Hilt, 2004; Hilt and Byrne, 2004; Hilt et al., 2006). For instance, by exploiting their biomimetic binding properties, researchers have developed porphyrin-containing compounds (highly aromatic compounds with similar molecular geometries to polymeric polyphenols) for the detection of environmental contaminants (Awawdeh and Harmon, 2005; Johnson et al., 2010).
There is emerging evidence that biomimetic PCB binding domains can be synthesized by incorporating phenolic and related moieties into polymeric coatings, which will greatly enhance the ability to detect and remove/remediate PCB contamination (Newsome et al., 2013). Polyphenols such as quercetin and curcumin are of special interest not only because of their beneficial health effects and environmentally benign nature but also because of their highly aromatic structures, which may enable effective and/or specific binding and sensing of aromatic pollutants (Ramadass et al., 2003). Recently, we developed a novel strategy to synthesize polymeric networks that incorporate these polyphenols as both stable coating materials and biodegradable hydrogels for use as a pollutant binding platform and an antioxidant release platform to combat localized cellular oxidative stress, respectively (Biswal et al., 2011; Wattamwar et al., 2012; Wattamwar et al., 2010b). These materials can have enhanced biocompatibility over their free compound form (Wattamwar et al., 2010a) and in preliminary studies, we have shown that PCB binding is increased through the incorporation of phenolic moieties in a polymeric matrix (Newsome et al., 2014). Additionally, the characteristic fluorescence peaks associated with these flavonoids effectively have been used preliminarily to sense PCB congeners in a concentration-dependent fashion (Newsome et al., 2014).
Although, biomimetic polymeric networks have been utilized for rapid binding of relevant pollutants (Zhao et al., 2011), these pollutant-bound materials must be easily removed from contaminated environments in order to be effective remediation systems. Combining polyphenolic polymers with magnetic nanoparticles in nanocomposite microparticle systems, then, allows for rapid binding of aromatic pollutants followed by high-throughput magnetic separation from contaminated samples. Magnetic nanoparticles (MNPs) have found widespread applications in magnetic fluids, catalysis, and bioseparation, and are being utilized increasingly in environmental remediation, either as uncoated particles or polymer-functionalized particles for increased pollutant adsorption, sensing and degradation (Qu et al., 2013; Tang and Lo, 2013; Zhao et al., 2011; Zhu et al., 2013). Although nanoparticles in general have been studied for some time to elucidate their potential toxicity, magnetic iron oxide nanoparticles have shown minimal cytotoxicity and additionally have been used successfully in biomedical applications for many years in areas such as magnetic resonance imaging, targeted drug delivery, and hyperthermia (Mahmoudi et al., 2012; Frimpong et al., 2010; Frimpong and Hilt, 2010; Gao et al., 2009; Mornet et al., 2004)). Further, recent studies in our group have shown that polyphenolic polymers can enhance the biocompatibility of metal nanoparticles like iron oxide (Cochran et al., 2013), thus forming the basis for a safe, environmentally-sound remediation platform. MNPs have found great utility in biomedical applications due to their superparamagnetic properties (conferred by their nanoscale size), which allow for easy manipulation with a magnetic field (e.g., for effective magnetic separation of captured molecules or for transport and immobilization at target sites) and heat generation in response to an alternating magnetic field (AMF) for local heating to modify properties (e.g. reaction rates, binding properties, thermal treatment) (Meenach et al., 2010). By utilizing diet-derived polymeric polyphenol coatings of defined thickness and matrix structure, our work has shown that pollutants can be very quickly removed from contaminated water samples (Newsome et al., 2014). Further, heating of the material can result in thermal destabilization of the polymer matrix and subsequent release of bound pollutants for potential reuse. This combined system, incorporating the binding potential of nutrient polyphenols with the separation and remote heating potential of magnetic nanoparticles potentially could be used for rapid removal of pollutants from environmental samples. The addition and implementation of bioactive nutrients into engineered polymers and nanoparticles allows for efficient remediation of contaminated sites and is worthy of increased investigation due to the high efficiency and specificity potential as well as the environmentally responsible makeup of the technology.
Summary and Conclusions
Nutrition is being further substantiated as an important modulator of inflammatory and antioxidant pathways, especially associated with environmental insult, and is also emerging as a tool to address exposure toxicity of persistent organic pollutants as both a sensing and remediation platform (Figure 1). Evidence now shows that a person’s nutritional status can play a key role in determining the severity of environmental toxicant-induced pathologies such as diabetes and cardiovascular diseases. People with subprime nutrition may be more susceptible to the toxicity of environmentally persistent pollutants whereas individuals that make healthy nutritional decisions and emphasize the importance of foods high in bioactive nutrients such as flavonoids may be less vulnerable to environmental insults by being able to buffer against the toxicity of pro-inflammatory pollutants. Although augmenting the nutritional profiles of at-risk populations may prove to be an effective modulator of toxicant-induced disease in the long term, it is critical to discover and design novel sensing and remediation technologies that are effective, sustainable and environmentally friendly to address immediate needs. Therefore, the novel and emerging evidence that bioactive nutrients may be incorporated into sensing and remediation components may lead to viable technologies that circumvent many of the issues concerning current-day decontamination platforms. Ultimately, further research into nutrient polyphenols, such as EGCG, quercetin, and curcumin, will highlight their potential as sustainable, inexpensive components both that can decrease health concerns associated with environmental pollutants in the short term and remediate these concerns in the long term.
Highlights.
Nutrition modulates vulnerability to disease risks associated with exposure to environmental pollutants.
Healthful nutrient polyphenols are protective by upregulating antioxidant and anti-inflammatory pathways.
Polyphenols can be used in sensing, capture, and remediation technologies.
Healthy nutrition may provide a cost-effective and environmentally friendly means of modulating environmental toxicity.
Acknowledgments
Funding: This work was supported by the National Institute of Environmental Health Sciences at the National Institutes of Health [P42ES007380] and the University of Kentucky Agricultural Experiment Station.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aozasa O, Ohta S, Nakao T, Miyata H, Nomura T. Enhancement in fecal excretion of dioxin isomer in mice by several dietary fibers. Chemosphere. 2001;45:195–200. doi: 10.1016/s0045-6535(00)00557-9. [DOI] [PubMed] [Google Scholar]
- Arguin H, Sanchez M, Bray GA, Lovejoy JC, Peters JC, Jandacek RJ, et al. Impact of adopting a vegan diet or an olestra supplementation on plasma organochlorine concentrations: results from two pilot studies. Br J Nutr. 2010;103:1433–1441. doi: 10.1017/S000711450999331X. [DOI] [PubMed] [Google Scholar]
- Avino P, Cinelli G, Notardonato I, Russo MV. Evaluation of different adsorbents for large-volume pre-concentration for analyzing atmospheric persistent organic pollutants at trace levels. Anal Bioanal Chem. 2011;400:3561–3571. doi: 10.1007/s00216-011-5039-7. [DOI] [PubMed] [Google Scholar]
- Awawdeh AM, Harmon HJ. Spectrophotometric detection of pentachlorophenol (PCP) in water using immobilized and water-soluble porphyrins. Biosens Bioelectron. 2005;20:1595–1601. doi: 10.1016/j.bios.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Biswal D, Wattamwar PR, Dziubla TD, Hilt JZ. A single-step polymerization method for poly(beta-amino ester) biodegradable hydrogels. Polymer. 2011;52:5985–5992. [Google Scholar]
- Carpenter D. Exposure to Polychlorinated Biphenyls Is Associated With an Increased Risk of Hypertension and Cardiovascular Disease. Epidemiology. 2011;22:S147–S147. [Google Scholar]
- Castro-Jimenez J, Gonzalez C. Immunoassay-based screening of polychlorinated biphenyls (PCB) in sediments: requirements for a new generation of test kits. Journal of Environmental Monitoring. 2011;13:894–900. doi: 10.1039/c0em00569j. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Arzuaga X, Kluemper CT, Caraballo A, Toborek M, Hennig B. Quercetin blocks caveolae-dependent pro-inflammatory responses induced by co-planar PCBs. Environ Int. 2010;36:931–934. doi: 10.1016/j.envint.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciftci O, Aydin M, Ozdemir I, Vardi N. Quercetin prevents 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced testicular damage in rats. Andrologia. 2012;44:164–173. doi: 10.1111/j.1439-0272.2010.01126.x. [DOI] [PubMed] [Google Scholar]
- Cochran DB, Wattamwar PP, Wydra R, Hilt JZ, Anderson KW, Eitel RE, et al. Suppressing iron oxide nanoparticle toxicity by vascular targeted antioxidant polymer nanoparticles. Biomaterials. 2013;34:9615–9622. doi: 10.1016/j.biomaterials.2013.08.025. [DOI] [PubMed] [Google Scholar]
- Crinnion WJ. Polychlorinated biphenyls: persistent pollutants with immunological, neurological, and endocrinological consequences. Altern Med Rev. 2011;16:5–13. [PubMed] [Google Scholar]
- De Vos S, De Schrijver R. Polychlorinated biphenyl distribution and faecal excretion in rats fed wheat bran. Chemosphere. 2005;61:374–382. doi: 10.1016/j.chemosphere.2005.02.086. [DOI] [PubMed] [Google Scholar]
- EPA United States Environmental Protection Agency. Cleaning up the Nation’s Hazardous Wastes Sites. [11/13/2013]; < http://www.epa.gov/superfund/>.
- Eske K, Newsome B, Han SG, Murphy M, Bhattacharyya D, Hennig B. PCB 77 dechlorination products modulate pro-inflammatory events in vascular endothelial cells. Environ Sci Pollut Res Int. 2013 doi: 10.1007/s11356-013-1591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frimpong RA, Dou J, Pechan M, Hilt JZ. Enhancing remote controlled heating characteristics in hydrophilic magnetite nanoparticles via facile co-precipitation. Journal of Magnetism and Magnetic Materials. 2010;322:326–331. [Google Scholar]
- Frimpong RA, Hilt JZ. Magnetic nanoparticles in biomedicine: synthesis, functionalization and applications. Nanomedicine. 2010;5:1401–1414. doi: 10.2217/nnm.10.114. [DOI] [PubMed] [Google Scholar]
- Gao JH, Gu HW, Xu B. Multifunctional Magnetic Nanoparticles: Design, Synthesis, and Biomedical Applications. Accounts of Chemical Research. 2009;42:1097–1107. doi: 10.1021/ar9000026. [DOI] [PubMed] [Google Scholar]
- Geusau A, Schmaldienst S, Derfler K, Papke O, Abraham K. Severe 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) intoxication: kinetics and trials to enhance elimination in two patients. Arch Toxicol. 2002;76:316–325. doi: 10.1007/s00204-002-0345-7. [DOI] [PubMed] [Google Scholar]
- Geusau A, Tschachler E, Meixner M, Sandermann S, Papke O, Wolf C, et al. Olestra increases faecal excretion of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Lancet. 1999;354:1266–1267. doi: 10.1016/S0140-6736(99)04271-3. [DOI] [PubMed] [Google Scholar]
- Gomes HI, Dias-Ferreira C, Ribeiro AB. Overview of in situ and ex situ remediation technologies for PCB-contaminated soils and sediments and obstacles for full-scale application. Sci Total Environ. 2013;445–446:237–260. doi: 10.1016/j.scitotenv.2012.11.098. [DOI] [PubMed] [Google Scholar]
- Goncharov A, Haase RF, Santiago-Rivera A, Morse G, McCaffrey RJ, Rej R, et al. High serum PCBs are associated with elevation of serum lipids and cardiovascular disease in a Native American population. Environ Res. 2008;106:226–239. doi: 10.1016/j.envres.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham SY, Kim YJ, Lee DH. Leaching characteristics of PCDDs/DFs and dioxin-like PCBs from landfills containing municipal solid waste and incineration residues. Chemosphere. 2008;70:1685–1693. doi: 10.1016/j.chemosphere.2007.07.050. [DOI] [PubMed] [Google Scholar]
- Harris DL, Niaz MS, Morrow JD, Mary K, Ramesh A. Diet as a Modifier of Benzo (a) pyrene Metabolism and Benzo (a) pyrene—Induced Colon Tumors in ApcMin mice. Interdisciplinary Studies on Environmental Chemistry—Environmental Research in Asia. 2009;2:227–238. [Google Scholar]
- Haupt K, Mosbach K. Molecularly imprinted polymers and their use in biomimetic sensors. Chemical Reviews. 2000;100:2495–2504. doi: 10.1021/cr990099w. [DOI] [PubMed] [Google Scholar]
- Hennig B, Meerarani P, Slim R, Toborek M, Daugherty A, Silverstone AE, et al. Proinflammatory properties of coplanar PCBs: in vitro and in vivo evidence. Toxicol Appl Pharmacol. 2002;181:174–183. doi: 10.1006/taap.2002.9408. [DOI] [PubMed] [Google Scholar]
- Hennig B, Ormsbee L, McClain CJ, Watkins BA, Blumberg B, Bachas LG, et al. Nutrition can modulate the toxicity of environmental pollutants: implications in risk assessment and human health. Environ Health Perspect. 2012;120:771–774. doi: 10.1289/ehp.1104712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins LG, Hayes JD. Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug Metab Rev. 2011;43:92–137. doi: 10.3109/03602532.2011.567391. [DOI] [PubMed] [Google Scholar]
- Hilt JZ. Nanotechnology and biomimetic methods in therapeutics: molecular scale control with some help from nature. Advanced Drug Delivery Reviews. 2004;56:1533–1536. doi: 10.1016/j.addr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Hilt JZ, Byrne ME. Configurational biomimesis in drug delivery: molecular imprinting of biologically significant molecules. Advanced Drug Delivery Reviews. 2004;56:1599–1620. doi: 10.1016/j.addr.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Hilt JZ, Byrne ME, Peppas NA. Microfabrication of intelligent biomimetic networks for recognition of D-glucose. Chemistry of Materials. 2006;18:5869–5875. [Google Scholar]
- Houston MC. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J Clin Hypertens (Greenwich) 2011;13:621–627. doi: 10.1111/j.1751-7176.2011.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutzinger O, Choudhry GG, Chittim BG, Johnston LE. Formation of polychlorinated dibenzofurans and dioxins during combustion, electrical equipment fires and PCB incineration. Environ Health Perspect. 1985;60:3–9. doi: 10.1289/ehp.85603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandacek RJ, Rider T, Keller ER, Tso P. The effect of olestra on the absorption, excretion and storage of 2,2',5,5' tetrachlorobiphenyl; 3,3',4,4' tetrachlorobiphenyl; perfluorooctanoic acid. Environ Int. 2010;36:880–883. doi: 10.1016/j.envint.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BJ, Melde BJ, Thomas C, Malanoski AP, Leska IA, Charles PT, et al. Fluorescent silicate materials for the detection of paraoxon. Sensors (Basel) 2010;10:2315–2331. doi: 10.3390/s100302315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Hong YB, Kim HJ, Wang A, Bae I. Bioactive food components prevent carcinogenic stress via Nrf2 activation in BRCA1 deficient breast epithelial cells. Toxicol Lett. 2012;209:154–160. doi: 10.1016/j.toxlet.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Koo SI, Noh SK. Green tea extract markedly lowers the lymphatic absorption and increases the biliary secretion of 14C-benzo[a]pyrene in rats. J Nutr Biochem. 2012;23:1007–1111. doi: 10.1016/j.jnutbio.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Marchand P, Henegar C, Antignac JP, Alili R, Poitou C, et al. Fate and complex pathogenic effects of dioxins and polychlorinated biphenyls in obese subjects before and after drastic weight loss. Environ Health Perspect. 2011;119:377–383. doi: 10.1289/ehp.1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo SI, Noh SK. Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect. J Nutr Biochem. 2007;18:179–183. doi: 10.1016/j.jnutbio.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim EJ, Smart EJ, Toborek M, Hennig B. The role of caveolin-1 in PCB77-induced eNOS phosphorylation in human-derived endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H3340–H3347. doi: 10.1152/ajpheart.00921.2007. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, Hofmann H, Rothen-Rutishauser B, Petri-Fink A. Assessing the In Vitro and In Vivo Toxicity of Superparamagnetic Iron Oxide Nanoparticles. Chemical Reviews. 2012;112:2323–2338. doi: 10.1021/cr2002596. [DOI] [PubMed] [Google Scholar]
- Majkova Z, Layne J, Sunkara M, Morris AJ, Toborek M, Hennig B. Omega-3 fatty acid oxidation products prevent vascular endothelial cell activation by coplanar polychlorinated biphenyls. Toxicol Appl Pharmacol. 2011;251:41–49. doi: 10.1016/j.taap.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkova Z, Smart E, Toborek M, Hennig B. Up-regulation of endothelial monocyte chemoattractant protein-1 by coplanar PCB77 is caveolin-1-dependent. Toxicol Appl Pharmacol. 2009;237:1–7. doi: 10.1016/j.taap.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meenach S, Anderson K, Hilt J. Synthesis and Characterization of Thermoresponsive Poly(ethylene glycol)-Based Hydrogels and Their Magnetic Nanocomposites. Journal of polymer science part A: polymer chemistry. 2010;48:3229–3235. [Google Scholar]
- Morita K, Matsueda T, Iida T. [Effect of green tea (matcha) on gastrointestinal tract absorption of polychlorinated biphenyls, polychlorinated dibenzofurans and polychlorinated dibenzo-p-dioxins in rats] Fukuoka Igaku Zasshi. 1997;88:162–168. [PubMed] [Google Scholar]
- Mornet S, Vasseur S, Grasset F, Duguet E. Magnetic nanoparticle design for medical diagnosis and therapy. Journal of Materials Chemistry. 2004;14:2161–2175. [Google Scholar]
- Moser GA, McLachlan MS. A non-absorbable dietary fat substitute enhances elimination of persistent lipophilic contaminants in humans. Chemosphere. 1999;39:1513–1521. doi: 10.1016/s0045-6535(99)00219-2. [DOI] [PubMed] [Google Scholar]
- Nadtochiy SM, Redman EK. Mediterranean diet and cardioprotection: the role of nitrite, polyunsaturated fatty acids, and polyphenols. Nutrition. 2011;27:733–744. doi: 10.1016/j.nut.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (NRC) A Risk Management Strategy for PCB-Contaminated Sediments. Washington, DC: The National Academies Press; 2001. [Google Scholar]
- NCI. Table 2. Food sources of the total omega 6 fatty acids (18:2 + 20:4), listed in descending order by percentages of their contribution to intake, based on data from the national health and nutrition examination survey 2005–2006, NIH, 2013. http://appliedresearch.cancer.gov/diet/foodsources/fatty_acids/table2.html.
- Newsome B, Gutierrez MA, Dziubla T, Hilt J. Biomimetic nanocomposite-mediated removal of chlorinated organic pollutants from contaminated water sources. University of Kentucky; 2014. in process. [Google Scholar]
- Newsome B, Petriello M, Han S, Murphy M, Eske K, Sunkara M, et al. Green tea diet decreases PCB 126-induced oxidative stress in mice by upregulating antioxidant enzymes. Journal of nutritional biochemistry. 2014 Feb;25(2):126–135. doi: 10.1016/j.jnutbio.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome B, Hennig B, Hilt JZ, Dziubla T. Magnetic nanocomposite microparticles for on/off binding of persistent organic pollutants from water sources. 2013 meeting of the Pacific Basin Consortium; Honolulu, HI. 2013. Plenary Presentation. [Google Scholar]
- Oshita K, Takaoka M, Kitade S, Takeda N, Kanda H, Makino H, et al. Extraction of PCBs and water from river sediment using liquefied dimethyl ether as an extractant. Chemosphere. 2010;78:1148–1154. doi: 10.1016/j.chemosphere.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Pellequer JL, Chen SW, Keum YS, Karu AE, Li QX, Roberts VA. Structural basis for preferential binding of non-ortho-substituted polychlorinated biphenyls by the monoclonal antibody S2B1. J Mol Recognit. 2005;18:282–294. doi: 10.1002/jmr.740. [DOI] [PubMed] [Google Scholar]
- Petriello MC, Newsome B, Hennig B. Influence of nutrition in PCB-induced vascular inflammation. Environ Sci Pollut Res Int. 2013 doi: 10.1007/s11356-013-1549-5. PMC3686851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Alvarez PJ, Li Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013;47:3931–3946. doi: 10.1016/j.watres.2012.09.058. [DOI] [PubMed] [Google Scholar]
- Ramadass P, Meerarani P, Toborek M, Robertson LW, Hennig B. Dietary flavonoids modulate PCB-induced oxidative stress, CYP1A1 induction, and AhR-DNA binding activity in vascular endothelial cells. Toxicol Sci. 2003;76:212–219. doi: 10.1093/toxsci/kfg227. [DOI] [PubMed] [Google Scholar]
- Redgrave TG, Wallace P, Jandacek RJ, Tso P. Treatment with a dietary fat substitute decreased Arochlor 1254 contamination in an obese diabetic male. J Nutr Biochem. 2005;16:383–384. doi: 10.1016/j.jnutbio.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Schlezinger JJ, Struntz WD, Goldstone JV, Stegeman JJ. Uncoupling of cytochrome P450 1A and stimulation of reactive oxygen species production by co-planar polychlorinated biphenyl congeners. Aquat Toxicol. 2006;77:422–432. doi: 10.1016/j.aquatox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Schulze PC, Lee RT. Oxidative stress and atherosclerosis. Curr Atheroscler Rep. 2005;7:242–248. doi: 10.1007/s11883-005-0013-5. [DOI] [PubMed] [Google Scholar]
- Sciullo EM, Vogel CF, Wu D, Murakami A, Ohigashi H, Matsumura F. Effects of selected food phytochemicals in reducing the toxic actions of TCDD and p,p'-DDT in U937 macrophages. Arch Toxicol. 2010;84:957–966. doi: 10.1007/s00204-010-0592-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sera N, Morita K, Nagasoe M, Tokieda H, Kitaura T, Tokiwa H. Binding effect of polychlorinated compounds and environmental carcinogens on rice bran fiber. J Nutr Biochem. 2005;16:50–58. doi: 10.1016/j.jnutbio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Silverstone AE, Rosenbaum PF, Weinstock RS, Bartell SM, Foushee HR, Shelton C, et al. Polychlorinated biphenyl (PCB) exposure and diabetes: results from the Anniston Community Health Survey. Environ Health Perspect. 2012;120:727–732. doi: 10.1289/ehp.1104247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slim R, Toborek M, Robertson LW, Hennig B. Antioxidant protection against PCB-mediated endothelial cell activation. Toxicol Sci. 1999;52:232–239. doi: 10.1093/toxsci/52.2.232. [DOI] [PubMed] [Google Scholar]
- Tang SC, Lo IM. Magnetic nanoparticles: essential factors for sustainable environmental applications. Water Res. 2013;47:2613–2632. doi: 10.1016/j.watres.2013.02.039. [DOI] [PubMed] [Google Scholar]
- Uemura H. [Associations of exposure to dioxins and polychlorinated biphenyls with diabetes: based on epidemiological findings] Nihon Eiseigaku Zasshi. 2012;67:363–374. doi: 10.1265/jjh.67.363. [DOI] [PubMed] [Google Scholar]
- Wang L, Lim EJ, Toborek M, Hennig B. The role of fatty acids and caveolin-1 in tumor necrosis factor alpha-induced endothelial cell activation. Metabolism. 2008;57:1328–39. doi: 10.1016/j.metabol.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins BA, Hannon K, Ferruzzi M, Li Y. Dietary PUFA and flavonoids as deterrents for environmental pollutants. J Nutr Biochem. 2007;18:196–205. doi: 10.1016/j.jnutbio.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Wattamwar P, Hardas SS, Butterfield DA, Anderson KW, Dziubla T. Tuning of the Pro-oxidant and Antioxidant Activity of Trolox Through the Controlled Release from Biodegradable Poly(trolox ester) Polymers. Free Radical Biology and Medicine. 2010a;49:S184–S184. doi: 10.1002/jbm.a.33174. [DOI] [PubMed] [Google Scholar]
- Wattamwar PP, Biswal D, Cochran DB, Lyvers AC, Eitel RE, Anderson KW, et al. Synthesis and characterization of poly(antioxidant beta-amino esters) for controlled release of polyphenolic antioxidants. Acta Biomater. 2012;8:2529–2537. doi: 10.1016/j.actbio.2012.03.022. [DOI] [PubMed] [Google Scholar]
- Wattamwar PP, Mo YQ, Wan R, Palli R, Zhang QW, Dziubla TD. Antioxidant Activity of Degradable Polymer Poly(trolox ester) to Suppress Oxidative Stress Injury in the Cells. Advanced Functional Materials. 2010b;20:147–154. [Google Scholar]
- Wu JM, Wang ZR, Hsieh TC, Bruder JL, Zou JG, Huang YZ. Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine (Review) Int J Mol Med. 2001;8:3–17. doi: 10.3892/ijmm.8.1.3. [DOI] [PubMed] [Google Scholar]
- Yaktine AL, Harrison GG, Lawrence RS. Reducing exposure to dioxins and related compounds through foods in the next generation. Nutr Rev. 2006;64:403–409. doi: 10.1111/j.1753-4887.2006.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Zakkar M, Van der Heiden K, Luong le A, Chaudhury H, Cuhlmann S, Hamdulay SS, et al. Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler Thromb Vasc Biol. 2009;29:1851–1857. doi: 10.1161/ATVBAHA.109.193375. [DOI] [PubMed] [Google Scholar]
- Zhao X, Lv L, Pan BC, Zhang WM, Zhang SJ, Zhang QX. Polymer-supported nanocomposites for environmental application: A review. Chemical Engineering Journal. 2011;170:381–394. [Google Scholar]
- Zheng Y, Morris A, Sunkara M, Layne J, Toborek M, Hennig B. Epigallocatechin-gallate stimulates NF-E2-related factor and heme oxygenase-1 via caveolin-1 displacement. J Nutr Biochem. 2012;23:163–168. doi: 10.1016/j.jnutbio.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JH, Wei SY, Chen MJ, Gu HB, Rapole SB, Pallavkar S, et al. Magnetic nanocomposites for environmental remediation. Advanced Powder Technology. 2013;24:459–467. [Google Scholar]