Abstract
Background
Torpedo formation and Purkinje cell (PC) loss represent standard and inter-related cerebellar responses to injury. Surprisingly, the nature of their relationship has not been carefully characterized across a range of normal and disease states. Are brains with more torpedoes expected to have fewer PCs? We quantified torpedoes and PCs in four groups: essential tremor (ET), spinocerebellar ataxia (SCA), multiple system atrophy-cerebellar (MSA-C), and controls.
Methods
Brains from 100 individuals (58 ET, 27 controls, 7 SCA, 8 MSA-C) were available at the New York Brain Bank. After complete neuropathological assessment, a standard parasagittal neocerebellar block was harvested, a 7-μm thick section was stained with Luxol Fast Blue/Hematoxylin and Eosin, and torpedoes and PCs were quantified.
Results
For a given PC count, SCA and MSA-C cases often had higher torpedo counts than ET cases or controls. Furthermore, the relationship between torpedo and PC counts was complex. The correlation between torpedo and PC counts was negative in ET cases (i.e., individuals with more torpedoes had fewer PCs [i.e., more PC loss]) whereas the relationship was positive in MSA-C cases (i.e., individuals with fewer PCs [i.e., more PC loss] had fewer torpedoes). Patients with SCA showed both patterns. When all diagnostic groups were combined, the correlation was best fit by a quadratic (i.e. parabolic) model rather than a simple linear model; this model incorporated data on the negative correlation in ET cases, the mixed results in SCA cases, and the positive correlation in MSA-C cases (r = 0.636).
Conclusions
The relationship between torpedo and PC counts was complex and heterogeneous across a range of cerebellar disease states, and was best characterized by a quadratic rather than a simple model. With more severe cerebellar disease, torpedoes can be quite numerous, and are likely a common feature of surviving PCs, but eventually, dramatic loss of PC leads to a paradoxical reduction in observable torpedoes.
Keywords: Cerebellum, Purkinje cells, torpedoes, essential tremor, multiple system atrophy, spinocerebellar ataxia, pathophysiology, neurodegeneration
Introduction
Torpedoes are ovoid swellings of the proximal portion of the Purkinje cell (PC) axon [1-3]. They are found in small numbers in the normal human cerebellum [4, 5], and have been observed to a similar extent across a wide range of ages (i.e., they do not accumulate with age) [5].
Increased torpedo formation seems to occur as a standard cerebellar response to injury, and may represent a cellular compensatory mechanism in the setting of PC stress [1, 6]. We have previously shown in essential tremor (ET), a common neurological disorder clinically linked to the cerebellum [7-10], that the number of torpedoes is increased several-fold compared with control brains [3]. These swellings occur in other disorders of the cerebellum as well [1, 6].
Another marker of cerebellar injury is PC loss. This being said, PCs are an unusual central nervous system neuron because they are particularly resistant to degeneration after axotomy [11-13]; in such settings, the response of PCs to stress is a partly degenerative and partly compensatory response [14]. This process involves the formation of torpedoes, as well as other structural changes in the axon (e.g., recurrent collateral formation, axonal branching, terminal axonal sprouting) [15].
The extent to which one sees a loss of PCs and an increase in torpedoes differs across the spectrum of cerebellar diseases. For example, in many of the spinocerebellar ataxias (SCAs), there is marked PC loss [16-22], whereas in ET, PC loss is more subtle and difficult to detect [23, 24], leading us to hypothesize that the primary problem in ET is one that is a stressor to PCs but not to a degree that always overwhelms this type of neuron. To our knowledge, although torpedoes have been observed, they have not been systematically quantified in the SCAs.
While the numbers of PCs and torpedoes differ from disease to disease, of perhaps greater mechanistic interest is the relationship between them, about which surprisingly little has been documented. Is there a positive correlation or a negative (i.e., inverse correlation)? Is the correlation the same across all disease states? Is it the same within disease states? Is the relationship simple and linear? An understanding of this relationship is likely to provide elementary insights into the pathobiological processes that are operative in basic cerebellar responses to injury across a range of disease states.
We quantified PCs and torpedoes in a large number of ET cases prospectively collected over 10 years, as well as a group of cases with two other more virulent cerebellar diseases (SCA and multiple system atrophy-cerebellar [MSA-C]), and controls. These data allowed us to examine the relationship between PC and torpedo counts across a spectrum of conditions from normal, to a generally mild cerebellopathy (ET), to more severe forms of cerebellar degeneration (SCA and MSA-C). To our knowledge, this is the only study that has quantitatively addressed the relationship between PC counts and torpedo number, and in patients within a spectrum of diseases of the cerebellum, including ET as well as MSA-C and SCA.
Methods
Cases and Controls
This study was conducted at the New York Brain Bank (NYBB), Columbia University Medical Center (CUMC). All ET cases were diagnosed by their treating neurologist, and the ET diagnosis was confirmed using Essential Tremor Centralized Brain Bank Criteria by a second neurologist specializing in movement disorders (E.D.L.) [3]. Control brains were normal elderly control subjects from the NYBB, derived from the Alzheimer’s Disease Research Center and the Washington Heights Inwood Columbia Aging Project. They were free of clinical diagnoses of Alzheimer’s disease (AD), ET, or Parkinson’s disease (PD) and without neuropathological diagnoses of neurodegenerative disease [3]. MSA-C cases were diagnosed during life using published clinical criteria [25]. SCA cases were diagnosed during life based on clinical features (i.e., ataxia and other cerebellar signs) and confirmed by quantification of CAG repeat expansions. The NYBB operates under approval of the Institutional Review Board of CUMC.
At the NYBB, complete data were available on 27 controls, and these were age-matched (1:2) to 58 ET cases that had been prospectively collected over the past 10 years (2003- 2013). In addition, we identified 8 available cases with clinical and postmortem diagnoses of MSA-C and 7 cases with clinical and postmortem diagnoses of SCA as well as genotype data. These included 1 SCA-7 and 6 SCA-1 (these six were from Albany - see acknowledgements), and they were chosen based on the availability of tissue.
Demographic and clinical data were prospectively collected [3]. In ET cases, data on lifetime exposure to medications known to cause cerebellar damage (e.g., lithium, diphenylhydantoin, chemotherapeutic agents) were collected. The amount of beer, wine, and liquor were carefully quantified (i.e. the average number of daily drinks of each during adult lifetime). Heavy ethanol use was defined previously as consumption of an average of four or more standard drinks (15 ml of absolute ethanol) per day for a man, or three or more per day for a woman, at any point in their lives [3, 26]. Data on medical conditions, clinical diagnoses and terminal events were collected. ET cases also underwent a standardized, videotaped neurological examination, which included an assessment of postural tremor (sustained arm extension), five tests of kinetic tremor (pouring, drinking, using spoon, finger-nose-finger maneuver, and drawing spirals), and head and voice tremors [27].
Standard Neuropathological Assessment
As previously described, all brains that were sent to the NYBB underwent a complete neuropathological assessment by a senior neuropathologist (J.P.G.V.) [3]. Each brain had a standardized measurement of brain weight (grams), postmortem interval (PMI, hours between death and placement of brain in a cold room or upon ice), Braak and Braak AD staging for neurofibrillary tangles [28, 29], Braak PD staging [30], and Consortium to Establish a Registry for AD (CERAD) ratings for neuritic plaques [31]. The number of microscopic infarcts was also quantified.
Quantification of Postmortem Changes in Cerebellum
As described, a standard 3 × 20 × 25 mm parasagittal, formalin-fixed, tissue block was harvested from the neocerebellum [3, 32]; the block included the cerebellar cortex, white matter and dentate nucleus. One senior neuropathologist (P.L.F.), who was blinded to all clinical information, counted torpedoes throughout one entire LH&E section [32] and counted and averaged PCs in fifteen 100× fields (LH&E). For analyses in paraffin sections, we have previously shown that examination of a single, standard section provides an adequate representation of the pathology within that sample block. Using a systematic uniform random (SUR) sampling approach, one of every five sections obtained from a series of 40 collected paraffin sections from the standard cerebellar block was stained with LH&E. We determined that there was little variation in torpedo and PC counts among sampled sections within this block in 11 ET and 9 control brains. The agreement between these counts was very high (for torpedo counts, intraclass correlation coefficient = 0.96, p < 0.001; for PC counts, intraclass correlation coefficient = 0.94, p < 0.001). We also conducted a reliability study in which two senior neuropathologists (P.L. F. and J.P.G.V.) independently quantified torpedoes and PCs in a sample of 10 ET cases with a broad range of findings (i.e., as per rater 1, PC counts ranged from 2.9 to 11.8, and torpedo counts ranged from 3 to 31); the inter-rater agreement was high (for PC counts, intraclass correlation coefficient = 0.93, p < 0.001; for torpedo counts, intraclass correlation coefficient = 0.94, p < 0.001).
Statistical Analyses
Analyses were performed in SPSS (version 21.0). Clinical and postmortem findings were compared across the four diagnostic groups (controls, ET cases, SCA, MSA-C) using analysis of variance (ANOVA), chi-square tests (χ2) and, for variables that were not normally distributed, Kruskal-Wallis tests. If a group difference was detected, then group by group post-hoc tests were performed. Correlations were Pearson’s correlation coefficients (r), although a non-parametric test (Spearman’s r) was also used when necessary. The torpedo count was not normally distributed (Kolmogorov-Smirnov test z = 2.74, p < 0.001) when all four diagnostic groups were combined, but was normally distributed in each diagnostic group when considered separately.
Results
There were 100 brains (27 controls, 58 ET, 7 SCA, 8 MSA-C). ET cases and controls were similar in age; SCA and MSA-C cases were younger than both groups (both Tukey’s tests p< 0.001, Table 1). The groups did not differ in terms of gender, brain weight, CERAD plaque score, or Braak AD stage. PMI was shorter in ET cases vs. controls (Mann Whitney test p < 0.001). Lifetime exposure to medications known to cause cerebellar damage and heavy ethanol use were not observed. Two controls and one ET case had rare, incidental Lewy bodies (Braak PD stage = 1 and 2).
Table 1. Clinical Characteristics of ET Cases and Controls.
| Controls N = 27 |
ET Cases N = 58 |
SCA N = 7 |
MSA-C N = 8 |
Significance a | |
|---|---|---|---|---|---|
| Age (years) Range |
82.5 ± 7.7 67 - 95 |
85.1 ± 7.0 67 - 97 |
58.8 ± 7.2 47 - 66 |
62.8 ± 11.2 39 - 74 |
p<0.001 (ANOVA) |
| Female gender | 11 (40.7) | 34 (58.6) | 2 (33.3) | 6 (75.0) | p = 0.18 (χ2) |
| PMI (median hours) |
4.9 | 2.8 | Not available |
4.6 | p < 0.001 (Kruskal- Wallis) |
| Brain weight (grams) |
1194 ± 153 | 1200 ± 165 | Not available |
1156 ± 144 | p = 0.77 (ANOVA) |
| CERAD plaque scored |
p = 0.29 (χ2) | ||||
| 0, A, or B C |
24 (100) 0 (0) |
47 (92.2) 4 (7.8) |
Not available |
6 (100) 0 (0) |
|
| Braak AD stage d |
p = 0.76 (χ2) | ||||
| 0 - 4 5 or 6 |
22 (95.7) 1 (4.3) |
48 (98.0) 1 (2.0) |
Not available |
4 (100) 0 (0) |
|
| Torpedo count (LH&E) |
2.9 ± 2.1, Median = 3.0 |
15.2 ± 12.1, Median = 12.5 |
77.7 ± 62.8, Median = 42.0 |
91.3 ± 67.7, Median = 82.0 |
p < 0.001 (Kruskal- Wallis) b |
| Purkinje cell count (LH&E) e |
10.8 ± 1.6 | 8.5 ± 1.8 | 5.7 ± 1.9 | 4.5 ± 2.1 | p < 0.001 (ANOVA) c |
comparing all four groups.
Mann Whitney test p < 0.001 for ET vs. controls, Mann Whitney test p < 0.001 for SCA vs. controls, Mann Whitney test p < 0.001 for MSA-C vs. controls.
Tukey test p = 0.001 for ET vs. controls, Tukey test p < 0.001 for MSA-C vs. controls, Tukey test p <0.001 for SCA vs. controls.
sample size < 100; therefore, for some brains, data were not available.
number of PCs/100× microscopic field averaged over 15 fields.
The torpedo count differed across the four diagnostic groups (p <0.001), as did the PC count (p < 0.001, Table 1, Figure 1). The data showed the mild but significant loss of PCs in ET brains vs. controls (p = 0.001) and the more profound loss of PCs in MSA-C and SCA brains vs. controls (both p values < 0.001)(Figure 1), although all ranges overlapped. In a multivariate linear regression model, diagnostic group (controls, ET, SCA, MSA-C) was associated with PC count (p < 0.001), even after adjusting for the possible confounders (age and PMI).
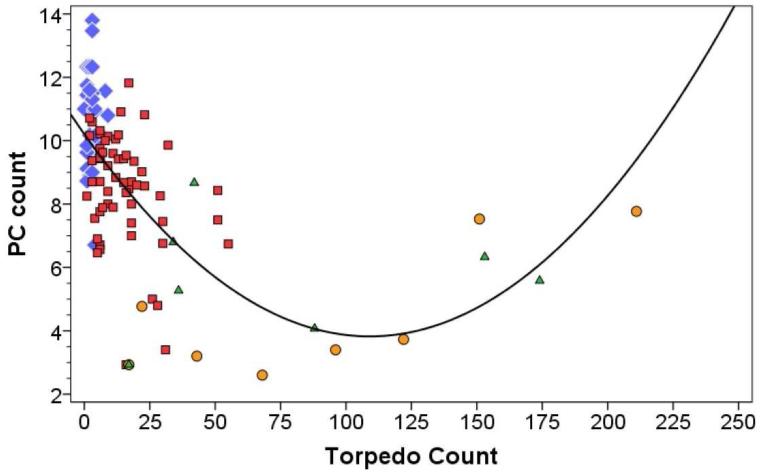
Figure 1.
Purkinje cell count (number/100× microscopic field) (Y axis) by torpedo count (X axis) in ET cases (red square markers), normal controls (blue diamond markers), MSA-C cases (orange circle markers) and SCA cases (green diamond markers). The fit line is quadratic, with r = 0.636 (i.e., y = 10.21 ± 0.12× + 0.000537×2).
The torpedo count was often quite high in MSA-C and SCA cases, and far greater than that seen in ET brains having similar PC counts. There was a significant negative correlation between torpedo count and PC count in ET cases (Pearson’s r = −0.31, p = 0.016) (i.e., ET cerebella with more torpedoes also had fewer PCs); there was no association in controls (Pearson’s r = −0.04, p = 0.86) (Figure 1). By contrast, in the MSA-C sample, there was a strong positive correlation between the torpedo and PC counts (Pearson’s r = 0.77, p = 0.026) so that brains with fewer PCs had dramatically fewer torpedoes (Figure 1). The pattern in the SCA group was mixed, with some cases having data points that were intermixed with the ET cases and others having data points that were intermixed with the MSA-C cases (Pearson’s r = 0.07, p = 0.88). In the entire group, the correlation was complex and was best fit by a quadratic (i.e., parabolic) rather than a simple linear model; this quadratic model incorporated features of the negative correlation in ET cases and the positive correlation in MSA-C cases (r = 0.636, Figure 1).
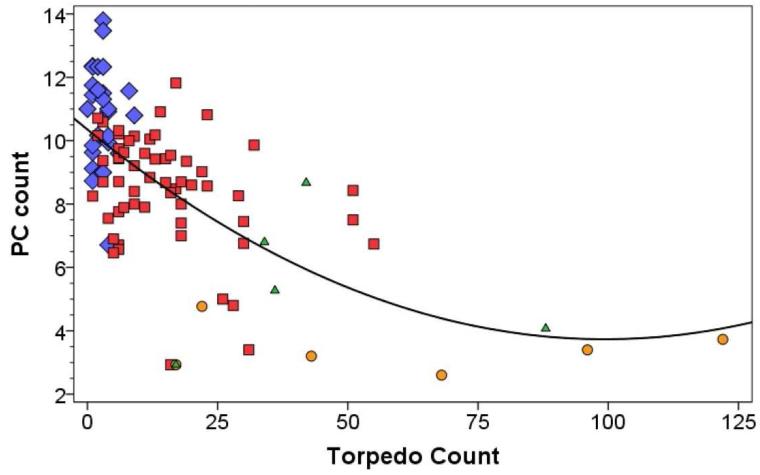
There were four data points (two SCA cases and two MSA-C cases) in which torpedo counts were very high (>130) and it is conceivable that they were unduly influencing the nature of the model. In a sensitivity analysis, we removed these four data points; even in this situation, the data were best fit by a quadratic rather than linear model (r = 0.643, Figure 2).
Figure 2.
Purkinje cell count (number/100× microscopic field) (Y axis) by torpedo count (X axis) in ET cases (red square markers), normal controls (blue diamond markers), MSA-C cases (orange circle markers) and SCA cases (green diamond markers). Four data points have been removed. The fit line is quadratic, with r = 0.643.
We carefully quantified the amount of beer, wine, and liquor used during adult lifetime. There was no association between this measure and torpedo count (Spearman’s r = −0.14, p = 0.34) or between this measure and PC count (Spearman’s r = −0.19, p = 0.19). The number of microscopic infarcts was also quantified during the standard neuropathological assessment, and this did not correlate with the PC counts (Spearman’s r = −0.16, p = 0.20). We compared the 20 ET cases with medical conditions or terminal events that could potentially have resulted in cerebral hypoxia ischemia (e.g., asthma, bronchitis, emphysema, chronic obstructive pulmonary disease, pulmonary edema, cardiac bypass surgery, cardiac arrest) to the remaining cases who did not have such conditions, and the PC counts did not differ (t = 1.03, p = 0.31).
In ET cases (disease duration = 43.1 ± 26.6 years), there was no correlation between disease duration and PC count (Pearson’s r = 0.22, p = 0.11) or torpedo count (r = −0.11, p = 0.41).
Discussion
Though PC death and the formation of torpedoes are two commonly observed cerebellar cortical responses to injury, basic features about the relationship between these responses have not been established, nor has the configuration of this relationship been assessed systematically across different disease states. We found that there was a clear correlation between these two responses, and that the nature and the direction (positive vs. negative) of the correlation was very much dependent on the diagnosis, and when combined across different cerebellar disease states, the correlation was not simple and linear but was more appropriately modeled using a quadratic function.
Though the models that fit the individual diagnostic groups were often linear, and either positive or negative, the more complex model, which best accounted for the data points across all samples, was a quadratic (i.e., parabolic or U-shaped) model. Quadratic models are often found to best explain biological phenomena. For example, U-shaped models are often used to describe the relationship between ethanol intake and risk of myocardial infarction (i.e., lower risk of myocardial infarction in individuals with light to moderate consumption) [33] and the relationship between cholesterol intake and mortality (both low and high cholesterol are associated with increased risk of mortality) [34].
Aside from their empirical value, the observed data are consistent with the following biological model of the diseases under study:
The underlying primary molecular/metabolic problem in ET is one that places stress on the PC economy; however, it often does not overwhelm it. Therefore, signs of PC stress (torpedoes and other axonal changes) rather than PC loss predominates. This model is consistent with recently published studies on the pathology of ET, which have identified several structural changes within the cerebellum of ET patients, with these changes centered on or around the PC [3, 35-38]. Prominent among these changes is a substantial increase in the number of torpedoes [3]. Electron microscopic studies have revealed that these axonal swellings contain an excess and disorganization of neurofilaments [2, 39]. These accumulations, in general, can either be a cause or result of damaged axonal transport [40, 41]. In contrast to ET, in MSA-C and SCA, the underlying molecular defect(s) more often overwhelm the PC economy, leading to extensive PC death. Therefore, PC loss is more evident in these disorders and PC loss is more of a prominent feature of MSA-C and SCA than ET (Figure 1).
One observes greater levels of PC loss from controls to ET to SCA to MSA (Table 1, and see greater right-ward shift of the diagnostic distributions in Figure 1). We hypothesize that there is no underlying molecular/metabolic problem in normal controls, a mild problem that occasionally overwhelms the PCs in ET, and a more severe molecular/metabolic problem with greater loss of PCs in MSA/SCA.
In the relationship between torpedoes and PC loss, there is a point of “genuflection” (i.e., bottom of U shaped curve in Figure 1). To the left of this point (i.e., as seen in ET cases), there is an inverse association between the number of torpedoes and the number of PCs: cases with more torpedoes also have fewer PCs, reflecting a greater level of PC stress/injury in those cases. To the right of this point (i.e., as seen in MSA-C and many SCA cases), there is a reversal of this association to a positive association. With more severe cerebellar disease, torpedoes can be quite numerous, but eventually, dramatic loss of PC leads to a paradoxical reduction in observable torpedoes and cerebella with fewer PCs have fewer torpedoes. One needs to have a surviving PC to observe a torpedo. When there are no remaining PCs, there will be no torpedoes either.
The strong positive correlation between torpedo number and PC count in MSA-C (Figure 1) is distinctive, and despite a quite significant loss of PCs in these patients, the number of torpedoes observed in many of the MSA-C brains was several fold higher than seen in ET brains. This suggests that a much greater majority of the surviving PCs in MSA-C brains have torpedoes at the time of autopsy evaluation. As we have observed multiple torpedoes on one PC in ET brains [15], it is also possible that this occurs to a greater extent in MSA-C brains. The relationship between torpedoes and other remodeling of the PC axon in the cerebellar cortex in ET, SCA and MSA-C requires further study.
The other issue is that (1) torpedo formation with axonal remodeling and PC preservation, and (2) torpedo formation with axonal remodeling and PC loss, are likely to be occurring at the same time across various areas of the cerebellum. Furthermore, torpedo formation, axonal remodeling, PC loss, and eventual loss of torpedoes, are expected to be occurring simultaneously in a given region of the cerebellar cortex. This mixed array of processes is likely to make simple models of torpedo count by disease duration unrevealing. Furthermore, models of PC count by disease duration do not assess loss of PCs per se. The number of PCs that individuals have at baseline is likely to vary considerably and simple counts of PCs do not take this change from baseline to measurement point into consideration; therefore, models of PC count by disease duration may be equally unrevealing.
This study had several limitations. First, we did not use a stereological approach for the quantification of PCs. A small pilot study showed that the method we used correlated highly with stereologically-based PC counts (r = 0.781, p < 0.05). Despite this potential lack of precision in PC counts using this method, we were still able to observe robust associations between PC counts and torpedo counts. Second, as disease duration was unknown for most of the SCA and MSA-C cases, we were not able to correlate our findings with disease duration in those groups. Third, SCA tissue is rare, and our SCA cases were limited to two types of SCA; it would be of interest in the future to explore the Purkinje cell-torpedo relationship in a larger number of SCAs. Fourth, we recognize that immunohistochemical preparations (e.g., calbindin) provide another means of assessing torpedoes and changes in the PC population, and could add additional insights about the relationship between PCs and torpedoes. Fifth, we recognize that the sample size in several of our case groups was small. An issue with small sample size is whether some data points are unduly influencing the results. To address this concern, we performed additional analyses. Specifically, there were four data points (two SCA cases and two MSA-C cases) in which torpedo counts were very high, and it is conceivable that they were unduly influencing the nature of the model. In a sensitivity analysis, we removed these four data points; even in this situation, the data were best fit by a quadratic rather than linear model (Figure 2), providing some reassurance that the model was not an artifact. This being said, we recognize that the addition of more cases would be a worthwhile goal of future studies. Nonetheless, this is the only study to our knowledge that has quantitatively addressed the relationship between PC counts and torpedo number, and in patients within a spectrum of diseases of the cerebellum, including ET as well as MSA-C and SCA.
In summary, the relationship between torpedo and PC counts was complex and heterogeneous across a range of cerebellar disease states, and was best characterized by a quadratic rather than a simple model. With more severe cerebellar disease, torpedoes can be quite numerous, and are likely a common feature of surviving PCs, but eventually, dramatic loss of PC leads to a paradoxical reduction in observable torpedoes. This study provides elementary insights into a pathobiological process that operates variably, non-uniformly and heterogeneously, across a range of cerebellar disease states. Further understanding the relationship between torpedo formation and PC loss will provide additional insights into the cerebellum and its basic responses to injury.
Acknowledgements
Tissue samples from six SCA-1 cerebellum were provided by Dr. Arnulf H. Koeppen, Albany, N.Y., on behalf of the National Ataxia Foundation. This work was funded by NIH R01NS042859.
Footnotes
Conflict of Interest: There are no conflicts of interest or competing financial interests.
References
- 1.Mizushima S. An ultrastructural observation of torpedoes in the human degenerative cerebellum. J Clinical Electron Microscopy. 1976;9:672–3. [Google Scholar]
- 2.Mann DM, Stamp JE, Yates PO, Bannister CM. The fine structure of the axonal torpedo in Purkinje cells of the human cerebellum. Neurol Res. 1980;1:369–78. doi: 10.1080/01616412.1980.11739567. [DOI] [PubMed] [Google Scholar]
- 3.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 4.Louis ED, Faust PL, Vonsattel JP, et al. Torpedoes in Parkinson’s disease, Alzheimer’s disease, essential tremor, and control brains. Mov Disord. 2009;24:1600–5. doi: 10.1002/mds.22567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louis ED, Faust PL, Vonsattel JP, Erickson-Davis C. Purkinje cell axonal torpedoes are unrelated to advanced aging and likely reflect cerebellar injury. Acta Neuropathol. 2009;117:719–21. doi: 10.1007/s00401-009-0534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi N, Iwatsubo T, Nakano I, Machinami R. Focal appearance of cerebellar torpedoes associated with discrete lesions in the cerebellar white matter. Acta Neuropathol. 1992;84(2):153–6. doi: 10.1007/BF00311388. [DOI] [PubMed] [Google Scholar]
- 7.Grimaldi G, Manto M. Is essential tremor a Purkinjopathy? The role of the cerebellar cortex in its pathogenesis. Mov Disord. 2013;28:1759–61. doi: 10.1002/mds.25645. [DOI] [PubMed] [Google Scholar]
- 8.Bares M, Lungu OV, Husarova I, Gescheidt T. Predictive motor timing performance dissociates between early diseases of the cerebellum and Parkinson’s disease. Cerebellum. 2010;9:124–35. doi: 10.1007/s12311-009-0133-5. [DOI] [PubMed] [Google Scholar]
- 9.Cerasa A, Messina D, Nicoletti G, et al. Cerebellar atrophy in essential tremor using an automated segmentation method. Am J Neuroradiol. 2009;30(6):1240–3. doi: 10.3174/ajnr.A1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benito-Leon J. Essential Tremor: One of the most common neurodegenerative diseases? Neuroepidemiology. 2011;36:77–8. doi: 10.1159/000323572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossi F, Jankovski A, Sotelo C. Differential regenerative response of Purkinje cell and inferior olivary axons confronted with embryonic grafts: environmental cues versus intrinsic neuronal determinants. J Comp Neurol. 1995;359:663–77. doi: 10.1002/cne.903590412. [DOI] [PubMed] [Google Scholar]
- 12.Bravin M, Savio T, Strata P, Rossi F. Olivocerebellar axon regeneration and target reinnervation following dissociated Schwann cell grafts in surgically injured cerebella of adult rats. Eur J Neurosci. 1997;9:2634–49. doi: 10.1111/j.1460-9568.1997.tb01693.x. [DOI] [PubMed] [Google Scholar]
- 13.Dusart I, Sotelo C. Lack of Purkinje cell loss in adult rat cerebellum following protracted axotomy: degenerative changes and regenerative attempts of the severed axons. J Comp Neurol. 1994;347:211–32. doi: 10.1002/cne.903470206. [DOI] [PubMed] [Google Scholar]
- 14.Rossi F, Jankovski A, Sotelo C. Target neuron controls the integrity of afferent axon phenotype: a study on the Purkinje cell-climbing fiber system in cerebellar mutant mice. J Neurosci. 1995;15:2040–56. doi: 10.1523/JNEUROSCI.15-03-02040.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babij R, Lee M, Cortes E, Vonsattel JP, Faust PL, Louis ED. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain. 2013;136:3051–61. doi: 10.1093/brain/awt238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scherzed W, Brunt ER, Heinsen H, et al. Pathoanatomy of cerebellar degeneration in spinocerebellar ataxia type 2 (SCA2) and type 3 (SCA3) Cerebellum. 2012;1:749–60. doi: 10.1007/s12311-011-0340-8. [DOI] [PubMed] [Google Scholar]
- 17.Auburger GW. Spinocerebellar ataxia type 2. Handb Clin Neurol. 2012;103:423–36. doi: 10.1016/B978-0-444-51892-7.00026-7. [DOI] [PubMed] [Google Scholar]
- 18.Hellenbroich Y, Gierga K, Reusche E, et al. Spinocerebellar ataxia type 4 (SCA4): Initial pathoanatomical study reveals widespread cerebellar and brainstem degeneration. J Neural Transm. 2006;113:829–43. doi: 10.1007/s00702-005-0362-9. [DOI] [PubMed] [Google Scholar]
- 19.Seidel K, Siswanto S, Brunt ER, den Dunnen W, Korf HW, Rub U. Brain pathology of spinocerebellar ataxias. Acta Neuropathol. 2012;124:1–21. doi: 10.1007/s00401-012-1000-x. [DOI] [PubMed] [Google Scholar]
- 20.Donato SD, Mariotti C, Taroni F. Spinocerebellar ataxia type 1. Handb Clin Neurol. 2012;103:399–421. doi: 10.1016/B978-0-444-51892-7.00025-5. [DOI] [PubMed] [Google Scholar]
- 21.Hoche F, Baliko L, den Dunnen W, et al. Spinocerebellar ataxia type 2 (SCA2): identification of early brain degeneration in one monozygous twin in the initial disease stage. Cerebellum. 2011;10:245–53. doi: 10.1007/s12311-010-0239-9. [DOI] [PubMed] [Google Scholar]
- 22.Solodkin A, Gomez CM. Spinocerebellar ataxia type 6. Handb Clin Neurol. 2012;103:461–73. doi: 10.1016/B978-0-444-51892-7.00029-2. [DOI] [PubMed] [Google Scholar]
- 23.Louis ED, Faust PL, Vonsattel JP. Purkinje cell loss is a characteristic of essential tremor: Towards a more mature understanding of pathogenesis. Parkinsonism Relat Disord. 2012;18:1003–4. doi: 10.1016/j.parkreldis.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Axelrad JE, Louis ED, Honig LS, et al. Reduced Purkinje cell number in essential tremor: a postmortem study. Arch Neurol. 2008;65:101–7. doi: 10.1001/archneurol.2007.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–6. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harasymiw JW, Bean P. Identification of heavy drinkers by using the early detection of alcohol consumption score. Alcohol Clin Exp Res. 2001;25:228–35. [PubMed] [Google Scholar]
- 27.Louis ED, Zheng W, Applegate L, Shi L, Factor-Litvak P. Blood harmane concentrations and dietary protein consumption in essential tremor. Neurology. 2005;65:391–6. doi: 10.1212/01.wnl.0000172352.88359.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braak H, Braak E. Diagnostic criteria for neuropathologic assessment of Alzheimer’s disease. Neurobiol Aging. 1997;18:S85–8. doi: 10.1016/s0197-4580(97)00062-6. [DOI] [PubMed] [Google Scholar]
- 29.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 31.Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer’s disease: a commentary. Neurobiol Aging. 1997;18:S91–4. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- 32.Louis ED, Vonsattel JP, Honig LS, Ross GW, Lyons KE, Pahwa R. Neuropathologic findings in essential tremor. Neurology. 2006;66:1756–9. doi: 10.1212/01.wnl.0000218162.80315.b9. [DOI] [PubMed] [Google Scholar]
- 33.Miyamae M, Kaneda K, Domae N, Figueredo VM. Cardioprotection by regular ethanol consumption: potential mechanisms and clinical application. Curr Drug Abuse Rev. 2010;3:39–48. doi: 10.2174/1874473711003010039. [DOI] [PubMed] [Google Scholar]
- 34.Wesley D, Cox HF. Modeling total cholesterol as predictor of mortality: the low-cholesterol paradox. J Insur Med. 2011;42:62–75. [PubMed] [Google Scholar]
- 35.Louis ED. Essential tremor: evolving clinicopathological concepts in an era of intensive post-mortem enquiry. Lancet Neurol. 2010;9:613–22. doi: 10.1016/S1474-4422(10)70090-9. [DOI] [PubMed] [Google Scholar]
- 36.Erickson-Davis CRFP, Vonsattel JGV, Gupta S, Honig LS, Louis ED. “Hairy baskets” associated with degenerative Purkinje cell changes in essential tremor. J Neuropathol Exp Neurol. 2010;69:262–71. doi: 10.1097/NEN.0b013e3181d1ad04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu M, Ma K, Faust PL, et al. Increased number of Purkinje cell dendritic swellings in essential tremor. Eur J Neurol. 2012;19:625–30. doi: 10.1111/j.1468-1331.2011.03598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuo SH, Erickson-Davis C, Gillman A, Faust PL, Vonsattel JP, Louis ED. Increased number of heterotopic Purkinje cells in essential tremor. J Neurol Neurosurg Psychiatry. 2011;82:1038–40. doi: 10.1136/jnnp.2010.213330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Louis ED, Yi H, Erickson-Davis C, Vonsattel JP, Faust PL. Structural study of Purkinje cell axonal torpedoes in essential tremor. Neurosci Lett. 2009;450:287–91. doi: 10.1016/j.neulet.2008.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beaulieu JM, Nguyen MD, Julien JP. Late onset of motor neurons in mice overexpressing wild-type peripherin. J Cell Biol. 1999;147:531–44. doi: 10.1083/jcb.147.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liem RK, Leung CL. Neuronal intermediate filament overexpression and neurodegeneration in transgenic mice. Exp Neurol. 2003;184:3–8. doi: 10.1016/s0014-4886(03)00291-7. [DOI] [PubMed] [Google Scholar]