Abstract
Background
Treatment guidance for chronic hepatitis C (CHC) released by the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) offer two options for interferon-ineligible/intolerant individuals with genotype 1 infection: sofosbuvir/ribavirin (SOF/RBV) for 24 weeks, or sofosbuvir/simeprevir (SOF/SMV) for 12 weeks. A 24-week course of SOF/RBV costs approximately US$169,000, with sustained virologic response (SVR) rates ranging from 52–84%; 12 weeks of SOF/SMV costs approximately $150,000, with SVR between 89% and 100%. Because SOF/SMV is currently used off-label, debate exists among physicians and payers about whether it should be prescribed and covered. This paper presents a cost-effectiveness analysis of these two treatment regimens accounting for costs of drugs, treatment-related medical care, re-treatment for individuals who do not achieve SVR, and natural history of continued HCV infection after failed re-treatment. The model uses a lifetime horizon and a societal perspective.
Results
In the base case scenario, SOF/SMV dominated SOF/RBV in a modeled 50-year-old cohort of treatment-naïve and treatment-experienced subjects, excluding those who failed prior therapy with telaprevir or boceprevir. SOF/SMV yielded lower costs and more quality-adjusted life years (QALYs) for the average subject compared to SOF/RBV ($165,336 and 14.69 QALYs vs. $243,586 and 14.45 QALYs, respectively). In base case cost-analysis, the SOF/SMV treatment strategy saved $91,590 per SVR compared to SOF/RBV. Under all one-way sensitivity scenarios, SOF/SMV remained dominant and resulted in cost savings.
Conclusions
These results suggest that a 12-week course of SOF/SMV is a more cost-effective treatment for genotype 1 CHC than 24 weeks of SOF/RBV among interferon-ineligible/intolerant individuals, supporting the AASLD/IDSA guidance and offering implications for both clinical and regulatory decision-making as well as pharmaceutical pricing.
Keywords: Olysio, Sovaldi, direct-acting antiviral agent, interferon-free
Newly approved direct-acting antiviral agents for chronic hepatitis C (CHC) have increased rates of sustained virologic response (SVR) to unprecedented levels, curing more than 90% of infections in many subgroups without the need for interferon (1). However, individuals with genotype 1 infection remain more difficult to treat, and both clinical guidelines and Food and Drug Administration approvals call for continued use of interferon alongside direct-acting antivirals sofosbuvir or simeprevir for this group (2–4). In some populations, up to 56% of those with CHC are ineligible to receive interferon-based treatment due to medical or psychological comorbidities, substance abuse, or prior treatment failure (5, 6). For these people, there are currently two interferon-free treatment options available in some regions: sofosbuvir plus ribavirin (SOF/RBV) for 24 weeks, which yields SVR rates between 52% and 84% at a cost of approximately US$169,000 (7–12), and sofosbuvir plus simeprevir (SOF/SMV) for 12 weeks, with SVR rates ranging from 89–100% at a cost of $150,000 (13, 14). Although treatment with SOF/SMV is more effective, less costly, and of shorter duration compared to SOF/RBV, it has not yet been specifically approved for clinical use by the US Food and Drug Administration, resulting in debate among both physicians and payers about whether it should be prescribed and reimbursed. This analysis compares the long-term cost-effectiveness of these two treatment strategies.
Methods
Markov Model
Using TreeAge Pro 2013 software (TreeAge Software, Inc., Williamstown, MA, USA), we constructed a decision-analytic Markov model to simulate the progression of a 50-year-old genotype 1 cohort through CHC natural history and treatment with either SOF/RBV or SOF/SMV, using a societal perspective over a lifetime horizon (Figure 1). Cohort age was chosen based on estimates that US hepatitis C virus (HCV) seroprevalence peaks in the 50–59 age group (15). The model cohort reflects both treatment-naïve and treatment-experienced subjects, with the exception of those who have failed prior telaprevir/boceprevir-based treatment and may harbor HCV variants resistant to HCV protease inhibitors including simeprevir.
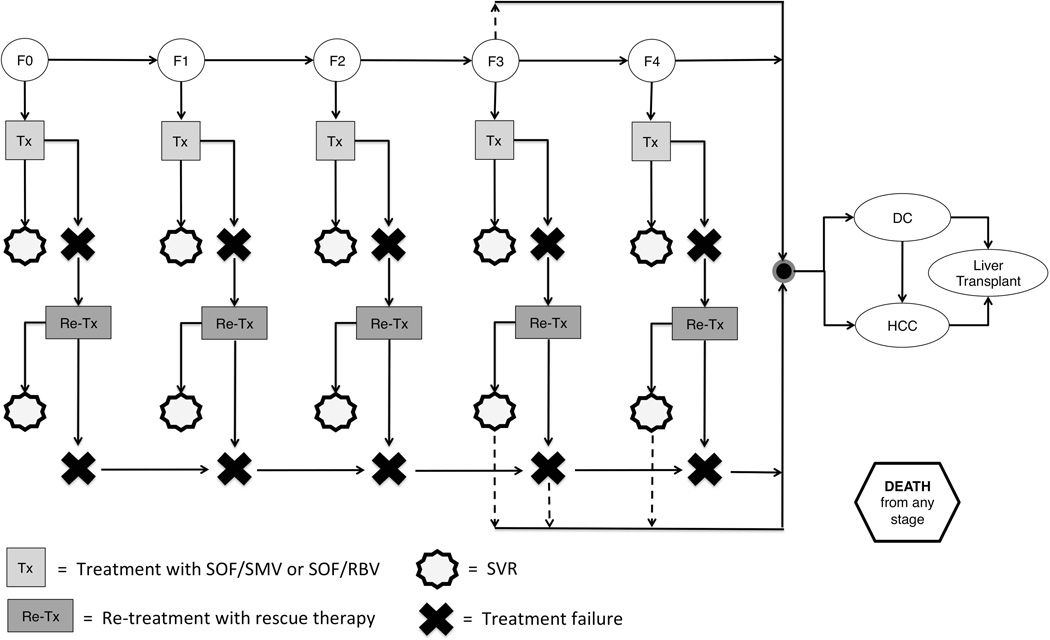
Figure 1. Simplified Markov Model.
HCV+ model subjects progress through fibrosis stages F0-F4, DC, and HCC based on annual probabilities. Those who fail treatment can be re-treated with rescue therapy; those who fail re-treatment continue progressing through CHC natural history. Further fibrosis progression after SVR is possible for subjects in stages F3 and F4, and F3 subjects can progress directly to DC or HCC, bypassing F4 (dotted lines). CHC = chronic hepatitis C; DC = decompensated cirrhosis; F0-F4 = Metavir fibrosis stages; HCC = hepatocellular carcinoma; HCV = hepatitis C virus; Re-tx = re-treatment; SVR = sustained virologic response; Tx = treatment.
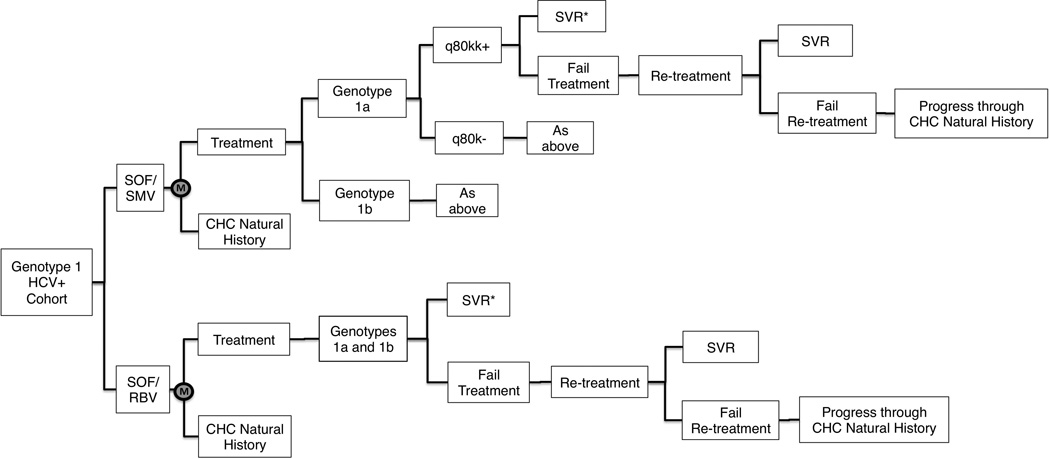
The decision tree that underlies the Markov model is presented in Figure 2. Parameters representing CHC natural history, treatment, treatment outcomes, and re-treatment determined subjects’ pathways through the decision tree. Base case values for all model parameters and ranges used in sensitivity analyses are listed in Tables 2–6.
Figure 2. Decision tree excerpt.
A selection from the decision tree underlying the Markov model, where M represents the starting point for each annual model cycle in the two treatment arms. Subjects who fail treatment and re-treatment continue progressing through CHC natural history in the subsequent model year. *SVR rates vary by fibrosis stage (not depicted). HCV = hepatitis C virus; CHC = chronic hepatitis C; SOF/RBV = 24-week sofosbuvir + ribavirin treatment regimen; SOF/SMV = 12-week sofosbuvir + simeprevir treatment regimen.
Table 2.
Model Cohort Characteristics
| Model Parameter | Base Case Value (Range) | References |
|---|---|---|
| Average Life Expectancy (years) | ||
| 50-year-old cohort | 81 | (17) |
| Viral Genotype and Q80K Distribution | ||
| Genotype 1a | 0.60 | (31) |
| Q80K+ | 0.40 (0.23–0.48) | (19, 20, 32, 33) |
| Genotype 1b | 0.40 | (31) |
| Initial Fibrosis Distribution | ||
| F0 | 0.17 (0.15–0.19) | (28, 34) |
| F1 | 0.35 (0.32-.39) | (28, 34) |
| F2 | 0.22 (0.20–0.24) | (28, 34) |
| F3 | 0.14 (0.13–0.15) | (28, 34) |
| F4 | 0.12 (0.11–0.13) | (28, 34) |
| Mortality Rates (compared to non-CHC population) | ||
| All-cause mortality before SVR | 2.37 | (16) |
| All-cause mortality after SVR | 1.4 | (18) |
Abbreviations: CHC = chronic hepatitis C; F0-F4 = Metavir fibrosis stages; SVR = sustained virologic response
Values in tables have been rounded. For full decimal places, consult cited sources.
Table 6.
Costs of CHC Treatment and Medical Care
| Model Parameter | Base Case Value (Range) | References |
|---|---|---|
| Annual Costs of Non Treatment-related CHC Care (in 2013 US Dollars) | ||
| CHC F0-F3 | 187 (89–547) | (28, 38, 47) |
| CHC F4 | 1,245 (728–1,761) | (28, 38, 47) |
| Decompensated cirrhosis | 15,958 (12,767– 39,444) | (28, 38, 48) |
| HCC | 49,954 (24,977–74,930) | (28, 38) |
| Liver transplant (year 1) | 305,136 (244,109–366,163) | (28, 49) |
| Liver transplant (years 2+) | 46,245 (36,996- 55,493) | (28, 50) |
| Costs of Treatment (in 2013 US Dollars) | ||
| SOF/RBV regimen | ||
| SOF (24 weeks) | 168,000 | (11) |
| RBV (24 weeks) | 1,038 | (12) |
| Treatment-associated medical care | 2,100 (1,890–2,310) | Calculated value |
| SOF/SMV regimen | ||
| SOF (12 weeks) | 84,000 | (11) |
| SMV (12 weeks) | 66,000 | (14) |
| Treatment-associated medical care | 2,096 (1,886–2,306) | Calculated value |
| SOF/LDV regimen | ||
| Drugs only (combination) | 252,000 (0–336,000) | Calculated value |
| Treatment-associated medical care | 1,160 (1,044–1,276) | Calculated value |
Abbreviations: CHC = chronic hepatitis C; F0-F4 = Metavir fibrosis stages; HCC = hepatocellular carcinoma; LDV = ledipasvir; RBV = ribavirin; SMV = simeprevir; SOF = sofosbuvir
Values in tables have been rounded. For full decimal places, consult cited sources.
At the end of each model year (stage), subjects accrued the costs and quality-adjusted life years (QALYs) associated with their disease state and treatment status during that year. Death was possible from any model stage. Subjects alive at the end of a given stage continued progressing through the model as determined by their disease state or treatment outcome in the preceding year, and they continued accumulating costs and QALYs each year until death. Analysis terminated when the cohort reached its average life expectancy. Cumulatively, the costs and QALYs accrued by the subjects in each treatment branch were used to calculate the incremental cost-effectiveness ratio (ICER). Cumulative costs were used to calculate the cost per SVR under each treatment strategy. Costs considered in this analysis include the direct costs of treatment-related drugs, medical care, and adverse events, as well as continued CHC-related medical care for subjects who failed treatment.
Measures
To assess cost-effectiveness, we calculated the ICER, which measures the incremental cost of the more effective treatment strategy per QALY it adds to the average subject’s lifespan, compared to the less effective treatment strategy. We also calculated cost savings per SVR under the dominant treatment strategy. We conducted one-way sensitivity analyses to determine whether variation in key model parameters impacted the ICER or the cost per SVR, including sub-analyses exploring differences by viral genotype (1a vs. 1b) and Q80K status.
Background Mortality Rates
Age-specific background mortality rates were applied throughout the model. Before SVR, they were set at 2.37 times the mortality rates for individuals without CHC (16, 17). After SVR, subjects were assigned lower mortality rates, set at 1.4 times non-CHC rates, based on evidence that viral clearance improves overall health outcomes (Table 2) (18). Subjects with end-stage liver disease were assigned higher mortality rates based on published literature (Table 3).
Table 3.
CHC Natural History Parameters
| Model Parameter | Base Case Value (Range) | References |
|---|---|---|
| CHC Natural History and Fibrosis Progression (annual rates) | ||
| F0 to F1 | 0.12 (0.09–0.14) | (28, 34) |
| F1 to F2 | 0.09 (0.07–0.10) | (28, 34) |
| F2 to F3 | 0.12 (0.10–0.14) | (28, 34) |
| F3 to F4 | 0.12(0.09–0.14) | (28, 34) |
| F3 to decompensated cirrhosis | 0.012 (0.010–0.014) | (28, 35) |
| F3 to HCC | 0.011 (0.009–0.013) | (28, 36) |
| F4 to decompensated cirrhosis | 0.04 (0.03–0.05) | (28, 29, 35, 37) |
| F4 to HCC | 0.03 (0.02–0.04) | (28, 36) |
| Decompensated cirrhosis to HCC | 0.014 (0.011–0.017) | (28, 37) |
| Decompensated cirrhosis to liver transplant | 0.031 (0.025–0.037) | (28, 38) |
| Decompensated cirrhosis to death | 0.13 (0.10–0.16) | (28, 37) |
| HCC to liver transplant | 0.031 (0.025–0.037) | (28, 38) |
| HCC to death | 0.43 (0.34–0.51) | (28, 37) |
| Liver transplant to death (year 1) | 0.14 (0.11–0.16) | (28, 39) |
| Liver transplant to death (years 2+) | 0.030 (0.027–0.057) | (28, 39, 40) |
Abbreviations: CHC = chronic hepatitis C; F0-F4 = Metavir fibrosis stages; HCC = hepatocellular carcinoma; SVR = sustained virologic response
Values in tables have been rounded. For full decimal places, consult cited sources.
Genotype 1a vs. 1b and Q80K Polymorphism
Q80K is a naturally occurring polymorphism found in up to 47% of those infected with HCV genotype 1/subtype A, and its presence has been associated with lower SVR rates among individuals treated with simeprevir in combination with other drugs (19, 20). In the SOF/SMV treatment arm of this model, genotype 1a outcomes were stratified by Q80K status to reflect lower SVR rates among Q80K+ subjects, and Q80K screening was included in treatment costs associated with SOF/SMV treatment. Variation in the proportion of Q80K+ individuals was explored in sensitivity analyses. In the SOF/RBV arm, genotypes 1a and 1b were collapsed, Q80K status was not incorporated into the model structure, and Q80K testing was not included in treatment-associated costs.
Fibrosis Stages
Probability of SVR was dependent on fibrosis stage, defined by METAVIR score (F0 = no fibrosis; F1 = portal fibrosis without septa; F2 = portal fibrosis with few septa; F3 = numerous septa without cirrhosis; F4 = compensated cirrhosis) (21). Cost of non-invasive fibrosis staging was included in the analysis for both treatment arms. Initial distribution of subjects across fibrosis stages and annual progression probabilities to later stages are listed in Tables 2–3. The model did not include subjects with decompensated cirrhosis due to uncertainty regarding treatment tolerance. To reflect the possibility that individuals with early stage fibrosis may elect to postpone treatment, sensitivity analyses include a scenario under which only subjects with advanced fibrosis (stages F3-F4) are treated.
To account for evidence of liver regeneration after viral eradication, the model allowed post-SVR fibrosis regression according to probabilities from published literature (Table 4) (22–26). When a disease state transition occurred (e.g., progression from F0 to F1 before SVR, or from F3 to F2 after SVR), subjects accrued the costs and QALYs associated with the disease state in which they began the year; they accrued the costs and QALYs associated with their new health state in the following year.
Table 4.
CHC Treatment Parameters
| Model Parameter | Base Case Value (Range) | References |
|---|---|---|
| SVR Rates | ||
| SOF/RBV | ||
| F0-F4 | 0.70 (0.52–0.84) | (7–10) |
| SOF/SMV | ||
| Q80K+ | ||
| F0-F2 | 0.89 (0.80–0.98) | (13) |
| F3-F4 | 0.91 (0.82–0.98) | (13) |
| Q80K- | ||
| F0-F4 | 0.98 (0.88–0.98) | (13) |
| SOF/LDV | ||
| F0-F4 | 0.96 (0.90–0.98) | (27) |
| Re-treatment | ||
| Proportion re-treated after initial treatment failure | 0.90 (0.80–1.0) | Estimation |
| Disease Progression after SVR (annual rates) | ||
| F3 SVR to decompensated cirrhosis | 0.0010 (0.0008–0.0012) | (28, 36) |
| F3 SVR to HCC | 0.007 (0.006–0.008) | (28, 36) |
| F4 SVR to decompensated cirrhosis | 0.003 (0.002–0.004) | (28, 36) |
| F4 SVR to HCC | 0.019 (0.015–0.023) | (28, 36) |
| Fibrosis Regression after SVR (proportions) | ||
| F3 to F2 | 0.50 (0.25–0.82) | (22–24, 26) |
| F4 to F2 | 0.08 (0.06–0.10) | (25) |
| F4 to F3 | 0.34 (0.19–0.49) | (22, 24, 26) |
Abbreviations: CHC = chronic hepatitis C; F0-F4 = Metavir fibrosis stages; HCC = hepatocellular carcinoma; LDV = ledipasvir; RBV = ribavirin; SMV = simeprevir; SOF = sofosbuvir; SVR = sustained virologic response
Values in tables have been rounded. For full decimal places, consult cited sources.
Re-treatment
To reflect clinical expectations, most subjects not cured with initial treatment (SOF/SMV or SOF/RBV) were re-treated with rescue therapy. The percentage re-treated was assumed to be 90% for base case analysis and was varied in sensitivity analyses (Table 4). Sofosbuvir plus ledipasvir for 24 weeks (SOF/LDV) was chosen as the rescue therapy drug regimen for this model, based on its estimated availability in 2014 and its very high SVR rates in clinical trials (95–100%) (27) Further, since LDV is an inhibitor of HCV NS5A, this agent is expected to be effective in individuals who failed prior treatment that included HCV NS3/4A protease inhibitors such as SMV. The base case cost of SOF/LDV was set at 1.5 times the cost of sofosbuvir alone for 24 weeks, with a range extending from $0 to 2 times the cost of sofosbuvir (Table 6). QALYs associated with SOF/LDV rescue therapy were set equal to QALY estimates for SOF/SMV treatment due to the absence of ribavirin in both regimens. The rate of possible re-infection after viral clearance was assumed equal for both treatment arms and was not included in the model structure.
Treatment-related Medical Care
Both SOF/RBV and SOF/SMV treatment strategies included the costs of initial HCV screening, a new patient visit, genotype assay, and non-invasive fibrosis staging (estimated based on the mean of FibroScan and FibroSURE prices through the US Department of Veterans Affairs National Acquisition Center) (12). The SOF/SMV arm included the cost of a Q80K test, also based on Department of Veterans Affairs pricing.
Medical monitoring was included in the costs associated with all treatment regimens. In the SOF/RBV treatment arm (24 weeks), monitoring included office visits at weeks 0, 4, 8, 12, 16, 20, 24, and post-treatment week 12 (8 total visits). Metabolic panels and complete blood counts were included in all 8 visits, and quantitative PCR checks to monitor viral load were included in 4 visits (weeks 0, 12, 24, and post-treatment week 12).
In the SOF/SMV treatment arm (12 weeks), monitoring included office visits at weeks 0, 4, 8, 12, and post-treatment week 12 (5 total visits). Metabolic panels were included in all 5 visits, and quantitative PCR checks were included in 4 visits (weeks 0, 4, 12, and post-treatment week 12). Treatment with SOF/SMV has not been shown to induce anemia (due to absence of ribavirin); therefore, a complete blood count test was only included in the week 0 baseline visit.
Medical monitoring was also included in the cost of re-treatment with SOF/LDV (24 weeks). Monitoring included office visits at weeks 0, 4, 8, 12, 16, 20, 24, and post-treatment week 12 (8 total visits). Metabolic panels were included in all 8 visits, and quantitative PCR checks were included in 4 visits (weeks 0,12, 24, and post-treatment week 12). A complete blood count test was included in the week 0 baseline visit.
The model also includes the cost of treatment-related adverse events, identified from published prescribing information and clinical trial data for the relevant drug regimens. Costs of adverse events were weighted based on estimated incidence of each event (3, 4, 27).
Costs and QALYs
Most medical costs and QALYs were derived from two recent cost-effectiveness studies that provide comprehensive reviews of these parameters from a variety of published sources (Tables 5–6) (28, 29). Costs to manage treatment-related adverse events were obtained from the Department of Veterans Affairs National Acquisition Center database (12). Costs were adjusted to 2013 US dollars, and both costs and QALYs were discounted 3% per year.
Table 5.
QALY Estimates
| Model Parameter | Base Case Value (Range) | References |
|---|---|---|
| QALYs Associated with Disease Stages | ||
| CHC F0-F2 | 0.85 (0.83–0.87) | (28, 41) |
| CHC F3 | 0.79 (0.77–0.81) | (28, 41) |
| CHC F4 | 0.76 (0.70–0.79) | (28, 41) |
| Decompensated cirrhosis | 0.69 (0.44–0.69) | (28, 41) |
| HCC | 0.67 (0.60–0.72) | (28, 41) |
| Liver transplant (year 1) | 0.50 (0.40–0.69) | (28, 38, 42, 43) |
| Liver transplant (years 2+) | 0.77 (0.57–0.77) | (28, 41, 44–46) |
| QALYs after SVR | ||
| SVR after F0-F2 | 0.92 (0.90–0.94) | (28) |
| SVR after F3 | 0.86 (0.84–0.88) | (28, 41) |
| SVR after F4 | 0.83 (0.81–0.85) | (28) |
| QALYs during Treatment | ||
| SOF/RBV | ||
| F0-F2 | 0.80 (0.72–0.88) | (30) |
| F3 | 0.74 (0.67–0.81) | (30) |
| F4 | 0.71 (0.64–0.78) | (30) |
| SOF/SMV | ||
| F0-F2 | 0.85 (0.77–0.93) | Calculated value |
| F3 | 0.79 (0.72–0.86) | Calculated value |
| F4 | 0.76 (0.69–0.83) | Calculated value |
| SOF/LDV | ||
| F0-F2 | 0.85 (0.77–0.93) | Calculated value |
| F3 | 0.79 (0.72–0.86) | Calculated value |
| F4 | 0.76 (0.69–0.83) | Calculated value |
Abbreviations: CHC = chronic hepatitis C; F0-F4 = Metavir fibrosis stages; HCC = hepatocellular carcinoma; LDV = ledipasvir; QALY = quality-adjusted life year; RBV = ribavirin; SMV = simeprevir; SOF = sofosbuvir; SVR = sustained virologic response
Values in tables have been rounded. For full decimal places, consult cited sources.
QALY values associated with interferon-free treatments have not yet been published due to the short amount of time since US Food and Drug Administration approval. For these parameters, we used treatment-related QALY values generated in a previous cost-effectiveness study based on expert opinion (30). Due to the uncertainty of these values, sensitivity analyses involving QALY parameters incorporated ranges of ±10%.
Because these QALY estimates refer to an all-oral regimen that included ribavirin, we increased them slightly in the SOF/SMV and SOF/LDV regimens in the present analysis to reflect a reduction in adverse events without ribavirin (Table 5). This decision is supported by placebo-controlled trials of SOF/RBV, in which subjects in the active treatment group experienced greater incidence of hemolytic anemia, insomnia, fatigue, and cough compared to the placebo group (4). Documented photosensitivity and increased bilirubin associated with simeprevir were not deemed severe enough to counteract the absence of ribavirin in terms of quality of life measures during treatment with SOF/SMV. Likelihood of adverse events was doubled in sensitivity analyses.
Drug interactions between sofosbuvir and simeprevir are not expected due to differing modes of metabolism (renal vs. hepatic), and published prescribing information for these drugs indicates minimal interactions with other medications (3, 4). Therefore, drug-drug interactions were not included in related quality of life measures or treatment costs, though we expect that on an individual level, clinicians will consider concurrent medications in decisions regarding eligibility for treatment with SOF/SMV or SOF/RBV.
Results
Base Case Analysis
In the base case scenario, SOF/SMV dominated SOF/RBV. Over the duration of the model, the SOF/SMV regimen yielded lower costs and more quality-adjusted life years (QALYs) for the average subject compared to the SOF/RBV regimen ($165,336 and 14.69 QALYs vs. $243,586 and 14.45 QALYs respectively), resulting in a negative incremental cost-effectiveness ratio (ICER). Because of the clear dominance of SOF/SMV in cost-effectiveness analysis, we also conducted a cost-analysis to calculate cost savings per SVR. The SOF/SMV strategy resulted in cost savings of $91,590 per SVR compared to SOF/RBV in base case analysis (Table 1).
Table 1.
Cost savings per SVR with SOF/SMV: Base case and sensitivity analyses
| Scenario | Cost per SVR (SOF/RBV) |
Cost per SVR (SOF/SMV) |
Cost savings per SVR with SOF/SMV |
|---|---|---|---|
| Base case | $262,046 | $170,456 | $91,590 |
| Sensitivity Analyses | |||
| Disease characteristics | |||
| Advanced fibrosis (Metavir stage F3/F4) only | $284,407 | $186,968 | $97,439 |
| Genotype 1/subtype A with Q80K polymorphism only (all fibrosis stages) | $262,046 | $188,856 | $73,190 |
| SVR rates* | |||
| Low SOF/SMV; high SOF/RBV | $221,156 | $175,450 | $45,706 |
| High SOF/SMV; low SOF/RBV | $312,160 | $162,404 | $149,756 |
| High SOF/SMV; base case SOF/RBV | $262,046 | $162,404 | $99,642 |
| Low SOF/LDV | $265,425 | $170,494 | $94,931 |
| Tolerability | |||
| Doubled likelihood of adverse events during treatment | $262,171 | $170,463 | $91,708 |
| Retreatment | |||
| Cost of SOF/LDV rescue therapy set at $0 per treatment | $194,347 | $165,432 | $28,915 |
| Decreased proportion retreated after initial failure* | $261,081 | $169,965 | $91,116 |
See Table 4 for low and high SVR thresholds for each drug regimen, and for low threshold for proportion retreated after initial failure.
F3/F4 = Metavir fibrosis stages 3 and 4; LDV = ledipasvir; RBV = ribavirin; SMV = simeprevir; SOF = sofosbuvir; SVR = sustained virologic response; Q80K = a naturally occurring polymorphism found in up to 47% of HCV genotype 1/subtype A infected individuals and less commonly in those with HCV genotype 1/subtype B; the presence of this polymorphism has been associated with lower SVR rates in subjects treated with simeprevir in combination with other drugs.
Sensitivity Analyses
We conducted sensitivity analyses among subgroups that have experienced lower SVR rates with SOF/SMV treatment in clinical trials (genotype 1a, Q80K+ subjects only), as well as those who have shown greater incidence of adverse events with both regimens (F3 and F4 subjects only). Additional parameters that were varied in sensitivity analyses included the proportion of subjects in all fibrosis stages experiencing treatment-related adverse events, cost of SOF/LDV rescue therapy for those who fail to achieve SVR with initial treatment with SOF/RBV or SOF/SMV, SVR rates for SOF/SMV, SOF/RBV, and SOF/LDV regimens, and proportion of subjects re-treated with SOF/LDV rescue therapy after initial treatment failure. Under all scenarios tested, the SOF/SMV treatment strategy remained dominant and resulted in cost savings per SVR compared to SOF/RBV (Table 1).
Discussion
The initial approval of sofosbuvir was limited to its use with ribavirin, with or without peginterferon, and the approval of simeprevir was limited to a combination with both ribavirin and peginterferon. As a result, some clinicians face reimbursement barriers when prescribing SOF/SMV together because it is not a specifically approved combination of antiviral agents. However, clinical guidance recently released by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America recommends SOF/SMV as the preferred treatment modality for genotype 1, interferon-ineligible or intolerant individuals as well as those who are non-responders to treatment with peginterferon and ribavirin (2). The present analysis provides economic support for this guidance, demonstrating that a 12-week course of SOF/SMV is not only more effective against genotype 1 CHC but also results in cost savings compared to 24 weeks of SOF/RBV, both in base case analysis as well as multiple sensitivity analyses in which clinically relevant individual and treatment-related characteristics were varied.
This study has some limitations. Primary efficacy measurements for these drug regimens are based on data from clinical trials, some of which have not yet been completed. Specifically, SVR12 data for cohort 2 in the COSMOS trial are not yet available, necessitating the use of SVR4 data for F3-F4 individuals treated with SOF/SMV (13). Further, the sample sizes in some cited trials are relatively small, contributing to parameter uncertainty with respect to the expected SVR rates with these regimens (e.g., n = 167 in the COSMOS study for SOF/SMV, and n = 114 and n = 60 in the PHOTON-1 and SPARE studies for SOF/RBV, respectively) (9, 10, 13). CHC natural history after treatment with these specific drug regimens is also uncertain, as is the specific distribution of fibrosis stages among genotype 1 interferon ineligible/intolerant individuals (assumed in this analysis to mirror the fibrosis distribution in the general population with CHC). Sensitivity analyses were included to model these uncertainties in SVR and natural history parameters; in each of these models, treatment with SOF/SMV remained dominant.
The cost of a SOF/LDV regimen is not yet known and is modeled under a wide range in sensitivity analyses. QALY estimates for treatment with interferon-free regimens are not available in published literature, thus quality of life studies specific to these treatment regimens are warranted. In addition, this model does not account for the possibility of reinfection after SVR, nor for the population benefit of preventing incident HCV infections through reductions in the pool of infected individuals. This analysis considers direct medical costs only and does not reflect indirect costs such as productivity losses attributable to CHC. Some of these limitations could result in over-estimation of cost savings (i.e., high SVR rates and absence of re-infection probabilities), while others could contribute to estimates that are more conservative (i.e., absence of indirect costs and the prevention benefit of infections cured).
While these potential limitations warrant careful consideration, this analysis offers evidence that the ability of SOF/SMV to cure a greater number of individuals and to reduce the need for re-treatment can be expected to result in lower immediate and long-term costs to payers as well as better outcomes for individuals compared to SOF/RBV. Combined with its potential for improved tolerability in the absence of ribavirin, shorter treatment duration, and higher resulting adherence to treatment, the economic dominance of SOF/SMV can contribute to the debate regarding its reimbursement.
In this analysis, SOF/SMV continued to yield cost savings per SVR when the cost of SOF/LDV rescue therapy was set to zero. Thus, even if the cost of re-treatment for those who fail to achieve SVR with SOF/RBV were to be covered at no additional cost to the payer, SOF/SMV remains a less costly option overall for payers, patients, and providers. In addition to the direct costs considered here, individuals failing SOF/RBV and re-treated with SOF/LDV would require a total of 48 weeks of therapy instead of 12, resulting in higher exposure to drugs, greater risk of complications, and added pressure on providers amidst high treatment demand. Prospective payers should consider this larger context when determining whether to include SOF/SMV in their drug formularies.
Acknowledgments
Financial support
This work was supported in part by NIH grants P30-AI-050409 (to RFS) and K24-DA-034621 (to MSS) and the Department of Veterans Affairs (to RFS).
Abbreviations
- CHC
chronic hepatitis C
- SVR
sustained virologic response
- SOF/RBV
sofosbuvir plus ribavirin for 24 weeks
- SOF/SMV
sofosbuvir plus simeprevir for 12 weeks
- HCV
hepatitis C virus
- SOF/LDV
sofosbuvir plus ledipasvir for 24 weeks
Contributor Information
Liesl M. Hagan, Email: lhagan2@emory.edu.
Mark S. Sulkowski, Email: msulkowski@jhmi.edu.
Raymond F. Schinazi, Email: rschina@emory.edu.
References
- 1.Schinazi R, Halfon P, Marcellin P, Asselah T. HCV direct-acting antiviral agents: the best interferon-free combinations. Liver Int. 2014;34(Suppl 1):69–78. doi: 10.1111/liv.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Association for the Study of Liver Diseases and Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. 2014 doi: 10.1002/hep.31060. Retrieved from: http://www.hcvguidelines.org/full-report-view. [DOI] [PMC free article] [PubMed]
- 3.US Food and Drug Administration. OLYSIO. 2013 Retrieved from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/205123s001lbl.pdf.
- 4.US Food and Drug Administration. SOVALDI. 2013 Retrieved from: http://www.accessdata.fda.gov/spl/data/b0de1fcd-6d03-4a91-a7df-72a14c8bc7d0/b0de1fcd-6d03-4a91-a7df-72a14c8bc7d0.xml-section-12.
- 5.Bini EJ, Brau N, Currie S, Shen H, Anand BS, Hu KQ, et al. Prospective multicenter study of eligibility for antiviral therapy among 4,084 U.S. veterans with chronic hepatitis C virus infection. Am J Gastroenterol. 2005;100:1772–1779. doi: 10.1111/j.1572-0241.2005.41860.x. [DOI] [PubMed] [Google Scholar]
- 6.Cawthorne CH, Rudat KR, Burton MS, Brown KE, Luxon BA, Janney CG, et al. Limited success of HCV antiviral therapy in United States veterans. Am J Gastroenterol. 2002;97:149–155. doi: 10.1111/j.1572-0241.2002.05439.x. [DOI] [PubMed] [Google Scholar]
- 7.Gane EJ, Stedman CA, Hyland RH, Ding X, Pang PS, Symonds WT, et al. 48th Annual Meeting of the European Association for the Study of the Liver. Amsterdam: The Netherlands; 2013. Apr 24–28, ELECTRON: All-oral sofosbuvir-based 12-week regimens for the treatment of chronic HCV genotype 1 infection. [Google Scholar]
- 8.Lalezari JP, Nelson DR, Hyland RH, Lin M, Rossi SJ, Symonds WT, et al. Once daily sofosbuvir plus ribavirin for 12 and 24 weeks in treatment-naive patients with HCV infection: the QUANTUM study. J Hepatol. 2013;58:S346. [Google Scholar]
- 9.Osinusi A, Meissner EG, Lee YJ, Bon D, Heytens L, Nelson A, et al. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA. 2013;310:804–811. doi: 10.1001/jama.2013.109309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sulkowski MS, Rodriguez-Torres M, Lalezari J, Fessel WJ, Mounzer K, Shuhart M, et al. The Liver Meeting, American Association for the Study of Liver Diseases. Washington, DC, USA: 2013. Nov 1–4, All-oral therapy with sofosbuvir plus ribavirin for the treatment of HCV genotype 1, 2, and 3 infection in patients co-infected with HIV (PHOTON-1) [Google Scholar]
- 11.Gilead Sciences. Press release: US Food and Drug Administration approves Gilead’s Sovaldi (sofosbuvir) for the treatment of chronic hepatitis C. 2013 Retrieved from: http://www.gilead.com/news/press-releases/2013/12/us-food-and-drug-administration-approves-gileads-sovaldi-sofosbuvir-for-the-treatment-of-chronic-hepatitis-c.
- 12.US Department of Veterans Affairs. National Acquisition Center Contract Catalog Search Tool. 2014 Retrieved from http://www.va.gov/nac/index.cfm?template=Search_Pharmaceutical_Catalog.
- 13.Jacobson JM, Ghalib RM, Rodriguez-Torres M, Younossi Z, Corregidor A, Sulkowski MS, et al. 48th Annual Meeting of the European Association for the Study of the Liver. Amsterdam: The Netherlands; 2013. Apr 24–28, SVR results of a once-daily regimen of simeprevir (TMC435) plus sofosbuvir (GS-7977) with or without ribavirin in cirrhotic and non-cirrhotic HCV genotype 1 treatment-naive and prior null responder patients: the COSMOS study. [Google Scholar]
- 14.WebMD. New oral hepatitis C drugs: FAQ. 2013 Retrieved from: http://www.webmd.com/hepatitis/news/20131126/new-hepatitis-c-drugs?page=2.
- 15.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 16.El-Kamary SS, Jhaveri R, Shardell MD. All-cause, liver-related, and non-liver-related mortality among HCV-infected individuals in the general US population. Clin Infect Dis. 2011;53:150–157. doi: 10.1093/cid/cir306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Compressed Mortality File 1999–2007. CDC WONDER On-line Database; Compiled from Compressed Mortality File 1999–2007 Series 20 No. 2M; 2010. Centers for Disease Control and Prevention National Center for Health Statistics. [Google Scholar]
- 18.Veldt BJ, Saracco G, Boyer N, Camma C, Bellobuono A, Hopf U, et al. Long term clinical outcome of chronic hepatitis C patients with sustained virological response to interferon monotherapy. Gut. 2004;53:1504–1508. doi: 10.1136/gut.2003.038257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bae A, Sun SC, Qi X, Chen X, Ku K, Worth A, et al. Susceptibility of treatment-naive hepatitis C virus (HCV) clinical isolates to HCV protease inhibitors. Antimicrob Agents Chemother. 2010;54:5288–5297. doi: 10.1128/AAC.00777-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobson I, Dore GJ, Foster GR, Fried MW, Radu M, Rafalskiy VV, et al. Simeprevir (TMC435) with peginterferon/ribavirin for chronic HCV genotype 1 infection in treatment-naive patients: results from QUEST-1, a phase III trial. J Hepatol. 2013b;58:S574. [Google Scholar]
- 21.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 22.Mallet V, Gilgenkrantz H, Serpaggi J, Verkarre V, Vallet-Pichard A, Fontaine H, et al. Brief communication: the relationship of regression of cirrhosis to outcome in chronic hepatitis C. Ann Intern Med. 2008;149:399–403. doi: 10.7326/0003-4819-149-6-200809160-00006. [DOI] [PubMed] [Google Scholar]
- 23.Maylin S, Martinot-Peignoux M, Moucari R, Boyer N, Ripault MP, Cazals-Hatem D, et al. Eradication of hepatitis C virus in patients successfully treated for chronic hepatitis C. Gastroenterology. 2008;135:821–829. doi: 10.1053/j.gastro.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 24.Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis. 2011;52:889–900. doi: 10.1093/cid/cir076. [DOI] [PubMed] [Google Scholar]
- 25.Pol S, Carnot F, Nalpas B, Lagneau JL, Fontaine H, Serpaggi J, et al. Reversibility of hepatitis C virus-related cirrhosis. Hum Pathol. 2004;35:107–112. doi: 10.1016/j.humpath.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303–1313. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- 27.Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2013;383:515–523. doi: 10.1016/S0140-6736(13)62121-2. [DOI] [PubMed] [Google Scholar]
- 28.Coffin PO, Scott JD, Golden MR, Sullivan SD. Cost-effectiveness and population outcomes of general population screening for hepatitis C. Clin Infect Dis. 2012;54:1259–1271. doi: 10.1093/cid/cis011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, Cipriano LE, Holodniy M, Owens DK, Goldhaber-Fiebert JD. New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis. Ann Intern Med. 2012;156:279–290. doi: 10.1059/0003-4819-156-4-201202210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagan LM, Yang Z, Ehteshami M, Schinazi RF. All-oral, interferon-free treatment for chronic hepatitis C: cost-effectiveness analyses. J Viral Hepat. 2013;20:847–857. doi: 10.1111/jvh.12111. [DOI] [PubMed] [Google Scholar]
- 31.Manos MM, Shvachko VA, Murphy RC, Arduino JM, Shire NJ. Distribution of hepatitis C virus genotypes in a diverse US integrated health care population. J Med Virol. 2012;84:1744–1750. doi: 10.1002/jmv.23399. [DOI] [PubMed] [Google Scholar]
- 32.Berger KL, Triki I, Cartier M, Marquis M, Massariol MJ, Bocher WO, et al. Baseline hepatitis C virus (HCV) NS3 polymorphisms and their impact on treatment response in clinical studies of the HCV NS3 protease inhibitor faldaprevir. Antimicrob Agents Chemother. 2014;58:698–705. doi: 10.1128/AAC.01976-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarrazin C, Berger KL, Ferenci P, Jensen DM, Buynak R, Dufour J-F, et al. HEP DART: Frontiers in Drug Development for Viral Hepatitis. The Big Island, Hawaii, USA: 2013. Dec 8–12, NS3/4A baseline polymorphisms and treatment-emergent variants in HCV genotype-1 infected, treatment-naive patients from the Phase III STARTVerso1 and 2 clinical studies of faldaprevir plus pegylated interferon a-2A and ribavirin. [Google Scholar]
- 34.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418–431. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 35.Dienstag JL, Ghany MG, Morgan TR, Di Bisceglie AM, Bonkovsky HL, Kim HY, et al. A prospective study of the rate of progression in compensated, histologically advanced chronic hepatitis C. Hepatology. 2011;54:396–405. doi: 10.1002/hep.24370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruno S, Zuin M, Crosignani A, Rossi S, Zadra F, Roffi L, et al. Predicting mortality risk in patients with compensated HCV-induced cirrhosis: a long-term prospective study. Am J Gastroenterol. 2009;104:1147–1158. doi: 10.1038/ajg.2009.31. [DOI] [PubMed] [Google Scholar]
- 37.Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–472. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 38.Bennett WG, Inoue Y, Beck JR, Wong JB, Pauker SG, Davis GL. Estimates of the cost-effectiveness of a single course of interferon-alpha 2b in patients with histologically mild chronic hepatitis C. Ann Intern Med. 1997;127:855–865. doi: 10.7326/0003-4819-127-10-199711150-00001. [DOI] [PubMed] [Google Scholar]
- 39.Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999–2008. Am J Transplant. 2010;10:1003–1019. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 40.Ascher NL, Lake JR, Emond J, Roberts J. Liver transplantation for hepatitis C virus-related cirrhosis. Hepatology. 1994;20:24S–27S. doi: 10.1016/0270-9139(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 41.Thein HH, Krahn M, Kaldor JM, Dore GJ. Estimation of utilities for chronic hepatitis C from SF-36 scores. Am J Gastroenterol. 2005;100:643–651. doi: 10.1111/j.1572-0241.2005.40976.x. [DOI] [PubMed] [Google Scholar]
- 42.Ratcliffe J, Longworth L, Young T, Bryan S, Burroughs A, Buxton M. Assessing health-related quality of life pre- and post-liver transplantation: a prospective multicenter study. Liver Transpl. 2002;8:263–270. doi: 10.1053/jlts.2002.31345. [DOI] [PubMed] [Google Scholar]
- 43.Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Jackson S, et al. Surveillance of cirrhosis for hepatocellular carcinoma: a cost-utility analysis. Br J Cancer. 2008;98:1166–1175. doi: 10.1038/sj.bjc.6604301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bownik H, Saab S. The effects of hepatitis C recurrence on health-related quality of life in liver transplant recipients. Liver Int. 2010;30:19–30. doi: 10.1111/j.1478-3231.2009.02152.x. [DOI] [PubMed] [Google Scholar]
- 45.McLernon DJ, Dillon J, Donnan PT. Health-state utilities in liver disease: a systematic review. Med Decis Making. 2008;28:582–592. doi: 10.1177/0272989X08315240. [DOI] [PubMed] [Google Scholar]
- 46.Saab S, Hunt DR, Stone MA, McClune A, Tong MJ. Timing of hepatitis C antiviral therapy in patients with advanced liver disease: a decision analysis model. Liver Transpl. 2010;16:748–759. doi: 10.1002/lt.22072. [DOI] [PubMed] [Google Scholar]
- 47.Younossi ZM, Singer ME, McHutchison JG, Shermock KM. Cost effectiveness of interferon alpha2b combined with ribavirin for the treatment of chronic hepatitis C. Hepatology. 1999;30:1318–1324. doi: 10.1002/hep.510300518. [DOI] [PubMed] [Google Scholar]
- 48.Brown DM, Everhart J. Cost of digestive diseases in the United States. Bethesda, MD: Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 1994. [Google Scholar]
- 49.Naugler WE, Sonnenberg A. Survival and cost-effectiveness analysis of competing strategies in the management of small hepatocellular carcinoma. Liver Transpl. 2010;16:1186–1194. doi: 10.1002/lt.22129. [DOI] [PubMed] [Google Scholar]
- 50.Showstack J, Katz PP, Lake JR, Brown RS, Jr., Dudley RA, Belle S, et al. Resource utilization in liver transplantation: effects of patient characteristics and clinical practice. NIDDK Liver Transplantation Database Group. JAMA. 1999;281:1381–1386. doi: 10.1001/jama.281.15.1381. [DOI] [PubMed] [Google Scholar]