Abstract
Objective
The diagnosis of Sjögren's Syndrome (SS) in routine practice is largely a clinical one and requires a high index of suspicion by the treating physician. This great dependence upon clinical judgment frequently leads to delayed diagnosis or misdiagnosis. Tear protein profiles have been proposed as simple and reliable biomarkers for SS diagnosis. Given that cathepsin S activity is increased in the lacrimal glands and tears of NOD mice (a murine model of SS), we explored the clinical utility of using tear cathepsin S (CTSS) activity as a biomarker for SS.
Methods
A method to measure CTSS activity in tears eluted from Schirmer's strips was developed and validated. Schirmer's tests and CTSS activity measurements were performed on 278 female subjects, including patients with SS (n=73), rheumatoid arthritis (n=79), systemic lupus erythematosus (n=40), blepharitis (n=10), non-specific dry eye (n=31), or other autoimmune diseases (n=12), along with 33 healthy controls.
Results
Median tear CTSS activity in SS patients was 4.1-fold higher than in patients with non-SS autoimmune diseases, 2.1-fold higher than in patients with non-specific dry eye, and 41.1-fold higher than in healthy controls. Tear CTSS levels were equally elevated in primary and secondary SS independent of the Schirmer's strip values or of circulating anti-SSA or anti-SSB autoantibodies.
Conclusion
Markedly high levels of tear CTSS activity are suggestive of SS. CTSS activity in tears can be measured in a simple, quick, economical, and non-invasive fashion and may serve as a novel biomarker and indicator of autoimmune dacryoadenitis during the workup for SS.
Introduction
Sjögren's Syndrome (SS) is an autoimmune disease that affects the lacrimal glands (LG) and salivary glands (SG), leading to the hallmark clinical symptoms of dry eye (keratoconjunctivitis sicca) and dry mouth (xerostomia sicca) (1, 2). Constitutional symptoms include weight loss and fatigue, and dysfunctions of the heart, kidney, liver, lungs, nervous system, brain, and gastrointestinal tract may develop over time (3). SS patients also bear a greatly increased risk of developing lymphoma (4-8). SS is the second most common autoimmune disease in the United States, affecting nearly four million people, of which nine out of ten are women (2, 9).
SS can be classified into primary SS (pSS) and secondary SS (sSS) based on its association with other autoimmune diseases, such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), scleroderma, and polymyositis (10). Despite its prevalence and severity, the diagnosis of SS in clinical practice is often delayed or mis-diagnosed. Although diagnostic criteria for SS have been established (11), some of these criteria, such as salivary scintigraphy or salivary gland biopsy are cumbersome and are only infrequently performed in routine practice. Indeed, salivary gland biopsy, considered by many to be the “gold standard test” for SS, is invasive and can be associated with serious post-operative complications including excessive bleeding, swelling, and numbness in the lower lip (12). Moreover, the hallmark clinical features of SS(dry eye, dry mouth, and positive Schirmer's test) are non-specific (11, 13, 14). Even the SS-associated autoantibodies, such as anti-SSA and anti-SSB, can be found in diseases such as RA, SLE, and polymyositis, even in the absence of any clinical features of SS (15). Therefore, a specific, sensitive, and non-invasive biomarker for SS could aid in the timely diagnosis of SS and, thereby, ultimately reduce the morbidity in SS patients.
In an earlier study with the non-obese diabetic (NOD) mouse, a well-established murine model for SS, we demonstrated increased cathepsin S (Ctss) expression and activity in LG acinar cells that was paralleled by elevated tear Ctss activity as compared to control mice (16). Similarly, Ctss gene expression is up-regulated in the early stages of disease and, in contrast to other cathepsins, remains up-regulated throughout the later stages of disease in the C57BL/6.NOD-Aec1Aec2 mouse model of SS (17). Based on these findings, we hypothesized that tear CTSS activity may also be elevated in patients with SS. In the present study, we investigated the clinical usefulness of tear CTSS activity as a biomarker for SS by studying tears from SS patients and from patients with other autoimmune or ocular surface disorders and healthy volunteers.
Subjects and Methods
Subjects
A total of 245 female patients were recruited at the Doheny Eye Institute, Keck Medical Center of the University of Southern California (USC), and the Los Angeles County + University of Southern California Medical Center (LAC+USC MC). Sample sizes and mean age by patient group are presented in Fig. 1. We studied only female subjects for the study since 1) Nine out of ten SS patients are female and 2) unpublished pilot studies suggested slightly elevated basal CTSS in female versus male patients in an all-comers study, suggesting comparison across female patients was the most rigorous choice. All procedures were approved by the USC Institutional Review Board (IRB) and performed in accordance with the Declaration of Helsinki, and all subjects gave their written informed consent. All recruited patients were evaluated and diagnosed by their physicians with autoimmune or ocular surface diseases; clinical diagnoses were based on medical history, physical examination, laboratory evaluation, and clinical judgment. Dry eye patients seen by an ophthalmologist were clinically diagnosed using the Ocular Surface Disease Index (OSDI), but the etiologies of the dry eyes in these patients were largely unknown and it is probable that some undiagnosed patients with SS were among these patients. All healthy female volunteer control subjects (33 total) were recruited from LAC+USC MC hospital staff and USC medical students who were using no medications, experiencing no dry eye or dry mouth symptoms, and lacking reported autoimmune or ocular surface diseases.
Figure 1.
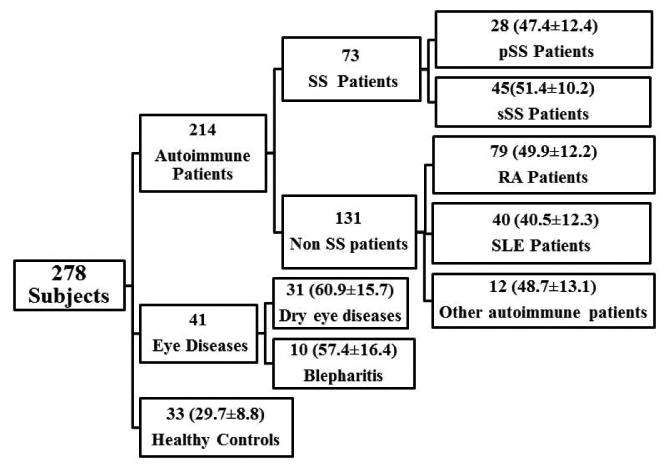
Subjects recruited for the present study. All the disease and Healthy control groups except SS did not manifest any signs of SS. Data are presented as Number of Subjects (mean age ± SD).
Tear Fluid Collection
After local anesthesia (0.5% proparacaine hydrochloride, Bausch & Lomb, Rochester, NY), residual fluid was removed from the conjunctival cul-de-sac, avoiding corneal contact, and Schirmer's strips were gently placed in the inferior fornix of each eye. Use of fluorescein was strictly avoided during tear collection. Schirmer's strips were removed after 5 minutes, and the length of tear saturation was recorded in mm. To elute tear fluids, the strips collected from both eyes were placed into two separate tubes, and 50 μl of CTSS reaction buffer (Biovision, Milpitas, CA) for every 1 mm of tear saturation was added. The tubes were then spun at 10,000 RPM for 10 seconds. Tear samples were placed on ice and analyzed within 4 hr of collection or, alternatively, were stored at -20°C and analyzed within 24 hr. After analysis, the remaining samples were stored at -80°C until measurement of protein concentration.
Blood collection and anti-SSA/SSB determination
Peripheral blood samples were collected after Schirmer's tests were performed and were analyzed for anti-SSA and anti-SSB antibodies by the LAC+USC MC clinical laboratory.
CTSS assay
Tear fluid CTSS activity was measured using a commercial kit (Biovision, Milpitas, CA) following the manufacturer's instructions. In brief, 100 μl of eluted tear sample and 2 μl of the CTSS substrate z-VVR-AFC (the tripeptide valine-valine-arginine conjugated to amino-4-trifluoromethyl coumarin [AFC]) were loaded into a 96-well clear plate in the presence and absence of the supplied CTSS inhibitor, followed by incubation at 37°C for 1 hr. The quantity of free AFC obtained by the reaction of CTSS with substrate was measured by a spectrofluorometer (Spectromax Gemini EM, Molecular devices, Sunnyvale, CA) with excitation at 400 nm and emission at 505 nm. CTSS specific activity was calculated by subtracting CTSS activity obtained in the presence and absence of the inhibitor.
CTSS assay kinetics
In order to evaluate the time and concentration linearity of the CTSS assay, active human CTSS (Biovision, Milpitas CA) was used. To that end, 1.25, 2.5, 5, or 10 ug of protein was dissolved in 100 μl of CTSS reaction buffer and activity measured as described above. Linearity over time for different concentrations was monitored every 5 min for up to 75 min at 37°C. Linearity of the assay for the protein doses chosen was determined after 1 hr of incubation at 37°C.
Determination of CTSS stability
To determine the stability of CTSS in Schirmer's strips, we used 5 strips that had been stored at different temperatures after tear fluid collection. 25 μg of active CTSS was dissolved in 25 μl CTSS reaction buffer, and 5 μl of the solution was added to each Schirmer's strip. The strips were analyzed immediately or were stored in a tube at room temperature (RT), 4°C, -20°C, or -80°C for 24 hr before analysis. Percent CTSS activity was calculated by dividing CTSS activity obtained after incubating at different temperatures for 24 hr with CTSS activity obtained at 0 hr.
To determine the stability of CTSS in tear fluid 0, 2.5, or 25 μg of active CTSS protein were added to either the tear samples or CTSS reaction buffer, and enzyme activity was analyzed as described above.
Measurement of Protein Concentration
Total protein concentration of the tear samples was analyzed with a protein assay kit (Bio-Rad, Hercules, CA). Bovine serum albumin (BSA) was used as standard, and assays followed the manufacturer's instructions.
Statistical Analysis
Mean age was compared between study groups with analysis of variance. As CTSS activity was measured in tears from each eye, each patient contributed two values to the study. To account for the correlated data (CTSS data for each eye within subject), the comparison of mean CTSS activity among study groups used generalized estimating equations for correlated outcomes, specifying an identity link function and an exchangeable correlation structure. CTSS activity was natural log-transformed to achieve normality. Because the ages differed between study groups and tear CTSS activity positively associated with age, group comparisons on tear CTSS activities were adjusted for age.
A receiver operating characteristic (ROC) analysis used the maximum CTSS value (over eyes) for each subject. The ROC area under the curve was computed for: (1) SS (primary and secondary combined, n =73 subjects) vs. other autoimmune disease (RA, SLE, and other autoimmune groups combined, n=131 subjects); (2) SS (primary and secondary, n=73 subjects) vs. other dry eye (blepharitis and dry eye combined, n=41 subjects); (3) SS (n=73 subjects) vs. healthy control (n=33 subjects). ROC analysis used a parametric approach, with the diagnostic biomarker of maximum CTSS categorized into deciles.
Results
Validation of the CTSS assay
To validate the kinetic characteristics of the CTSS assay, we determined its time and concentration linearity. As shown in Fig. 2A, fluorescence produced by 10 μg of CTSS increased proportionally with time up to 75 min, with a coefficient of determination of 0.9876, showing the reaction rate was linear between 5 to 75 min. Using 1.25, 2.5, and 5 μg of CTSS, linearity was observed at all concentrations (data not shown), with a coefficient of determination of 0.97, 0.99, and 0.99, respectively, for each concentration.
Figure 2.
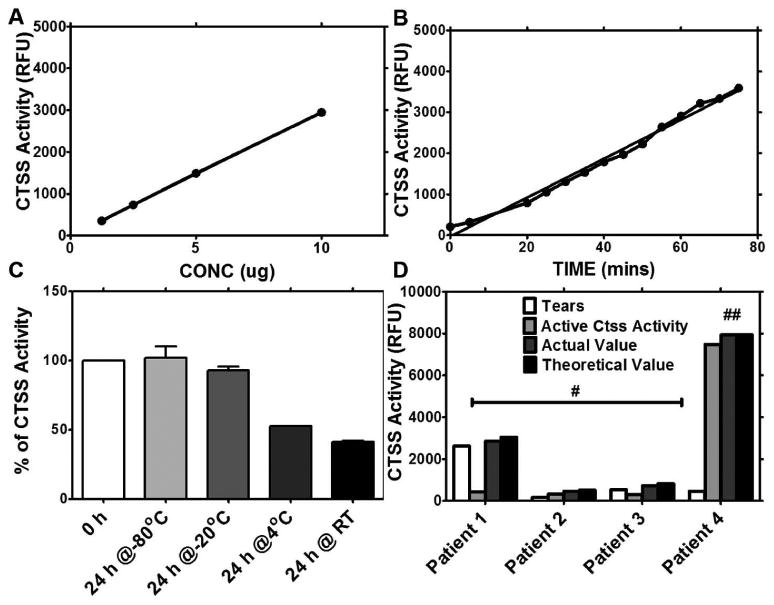
Validation of CTSS activity. (A) Relationship of CTSS activity versus time. (B) Relationship of CTSS activity versus concentration. (C) CTSS activity at different storage temperatures. Data are presented as percentage of CTSS activity analyzed immediately after collection (0 hours). Error bars represent SD. (D) Representative results of CTSS activity in the presence of native proteins present in tears from 4 patients. Actual value is the amount of CTSS activity obtained when a given amount of active CTSS was added to patient tears. The theoretical value is the sum of CTSS activity obtained from tears and active CTSS individually. # represents samples in which 2.5 μg of active CTSS protein was added to patient tears while ## represents samples in which 25 μg of active CTSS to was added to patient' tears.
As shown in Fig. 2B, the concentration of CTSS was directly proportional to its activity, with a coefficient of determination of 0.99. The clear relationship between CTSS concentration and CTSS activity justifies the normalization of CTSS activity to total protein concentration.
We evaluated the effect of different storage conditions on CTSS activity. The enzyme activity was analyzed after purified active CTSS was stored at room temperature (RT), 4°C, -20°C, and -80°C for 24 hr and these activities were compared to CTSS activity analyzed immediately after collection. There was a significant reduction of enzyme activity in the samples stored at RT and 4°C, whereas no decrease was detected in samples stored at -20°C and -80°C (Fig. 2C).
To determine the potential effect of native tear proteins on CTSS activity, 2.5 μg or 25 μg of active CTSS was added to tear samples and incubated at 37°C for 1 h. Actual CTSS activity was analyzed and presented in Fig. 2D, along with the theoretical values that we expected. No significant differences were observed between these two values. These data strongly suggest that the presence of native proteins in tears does not affect CTSS activity.
CTSS activity in tears
Patients were stratified as indicated in Fig. 1, and tears were collected from these patients. Median tear CTSS activities without age adjustment were significantly higher in SS patients (both pSS and sSS) than in tears from all other patient groups. No significant difference (p=0.84) was found in median tear CTSS activities between pSS and sSS patients. Patients from both groups exhibited a significant elevation in median tear CTSS levels relative to patients with other non-SS autoimmune diseases (combining RA, SLE, and other autoimmune disease groups) and healthy controls (Fig. 3A). Median tear CTSS activity in pSS patients was 3.8-fold higher than in non-SS Autoimmune patients (p<0.0001) and 37.8-fold higher than in healthy controls (p<0.0001). Among sSS patients, median tear CTSS activity was 4.2-fold higher than in non-SS Autoimmune patients (p<0.0001) and 42-fold higher than in healthy controls (p<0.0001).
Figure 3.
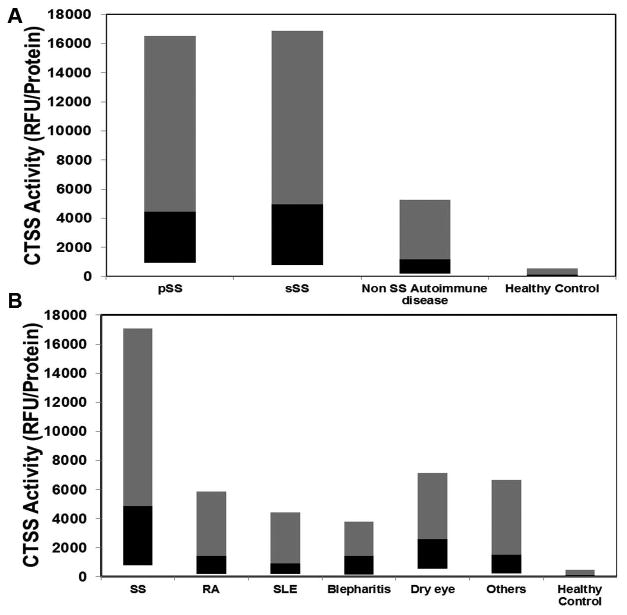
CTSS activity in tears. A) CTSS activity in tears from pSS and sSS patients compared to non-SS autoimmune disease patients and healthy controls. Each patient contributed two Schirmer's strips, one from each eye, thus number of samples in each group is twice the number of patients recruited as shown in Fig 1. The grey bar represents 50th-90th percentile, while the black bar represents the 10th-50th percentile, and the junction represents the median. B) CTSS activity in tears of autoimmune diseases (SS, RA, SLE and Other), Blepharitis, Dry eye and Healthy controls. Each patient contributed two Schirmer's strips, one from each eye, thus number of samples in each group is twice the number of patients recruited as shown in Fig 1. The grey bar represents 50th-90th percentile, while the black bar represents the 10th-50th percentile, and the junction represents the median.
Given the lack of a significant difference between pSS and sSS patients, these groups were pooled for subsequent comparisons. As shown in Fig. 3B, SS median tear CTSS activity levels were increased 3.4-fold over RA (p<0.0001), 5.2-fold over SLE (p<0.0001), 3.4-fold over blepharitis (p<0.0001), 1.9-fold over dry eye (p=0.0005), 3.2-fold over other autoimmune diseases (p=0.004), and 41.1-fold over healthy controls (p<0.0001). Statistical analysis indicated significant differences between groups (p<0.0001). As shown in Fig. 4, the ROC area under the curve (95% confidence interval) was 0.771 (0.703-0.834) for SS compared to other autoimmune disease and 0.686 (0.587-0.785) for SS compared to other dry eye disease. The ROC area under the curve was 0.954 (0.893-0.985) for SS compared to healthy controls (data not shown).
Figure 4.
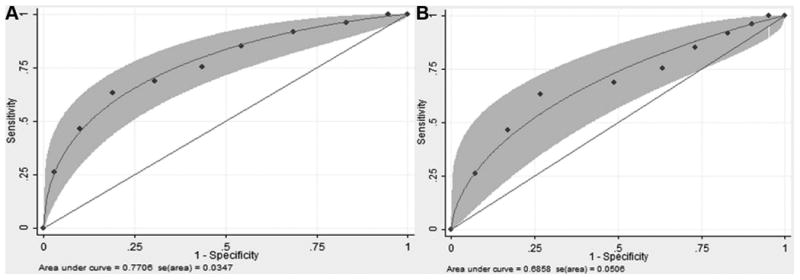
Smooth-fitted receiver operating characteristic (ROC) curve using maximum likelihood estimation for binormal model. A) Comparisons are SS (pSS and sSS) vs. autoimmune (RA, SLE, other autoimmune). B) SS (pSS and sSS) vs. other eye diseases (dry eye, blepharitis). CTSS was categorized into 10 levels based on deciles.
The groups significantly differed on mean age and schirmer's value (overall in both cases p<0.0001). Most notably, the healthy control group was significantly younger than all the patient groups. Among the patient groups, the dry eye patients were significantly older than all groups, with the exception of the blepharitis patients (p<0.05), and the SLE patients were significantly younger than all but the primary SS patients (p<0.05). In the case of Schirmer's values the length of tear saturation was highest in healthy controls and lowest in SS patients. Age and Schirmer's values did not significantly differ among the primary and secondary SS and RA patients. Group comparisons of natural log-transformed mean CTSS activity were calculated with and without age and Schirmer's value adjustment are shown in Table 1.
Table 1.
Mean Ln (CTSS) activity by diagnostic group.
| Group | Unadjusted | Adjusted for age | Unadjusted | Adjusted for Schirmer's value |
|---|---|---|---|---|
| SS* (n=73) | 8.254 (0.138) | 8.199 (0.143) | 8.330 (0.130) | 8.009 (0.113) |
| RA (n=79) | 7.164 (0.140) (<0.0001) | 7.112 (0.139) (<0.0001) | 7.183 (0.140) (<0.0001) | 7.241 (0.115) (<0.0001) |
| SLE (n=40) | 6.932 (0.164) (<0.0001) | 7.076 (0.165) (<0.0001) | 6.932 (0.164) (<0.0001) | 7.064 (0.127) (<0.0001) |
| Blepharitis (n=10) | 6.921 (0.383) (0.0011) | 6.686 (0.321) (<0.0001) | 6.921 (0.383) (0.0005) | 7.207 (0.385) (0.047) |
| Dry eye (n=31) | 7.708 (0.196) (0.023) | 7.390 (0.195) (0.0005) | 7.680 (0.192) (0.005) | 7.583 (0.175) (0.037) |
| Other auto-immune diseases (n=12) | 7.005 (0.436) (0.0064) | 6.977 (0.403) (0.0041) | 7.005 (0.436) (0.0036) | 7.132 (0.305) (0.008) |
| Healthy controls (n=33) | 4.865 (0.216) (<0.0001) | 5.294 (0.231) (<0.0001) | 4.865 (0.216) (<0.0001) | 5.278 (0.177) (<0.0001) |
| p-value between groups | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Primary and Secondary SS combined. n represents number of patients. Numbers in cells are mean Ln (Ctss), SE, and p-value for difference from SS group. P-value between groups was calculated using GEE method.
CTSS activity Vs. Anti-SSA and Anti-SSB antibodies
Although not specific for SS, serum anti-SSA and -SSB autoantibodies are frequently present in patients with SS. Therefore, we also measured serum anti-SSA/SSB levels in a subset of SS patients to explore the correlation with tear CTSS activity. Mean CTSS activity was not significantly different between SS patients who tested positive or negative for anti-SSA or anti-SSB autoantibodies (data not shown).
CTSS activity Vs. Schirmer's values
The CTSS activity in SS patient tears was compared with its activity in the Rest Of Subjects (ROS) over different ranges of Schirmer's values which included 0-5 mm, 5.1-10 mm, 10.1-15 mm and 15.1-35 mm. In each range, CTSS activity was significantly increased in SS patient tears over the activity levels in tears from the ROS. As shown in Fig. 5, SS median tear CTSS activity levels were increased 2.3-fold over ROS in the 0-5 mm range (p=0.0002), 1.6-fold over ROS in the 5.1-10 mm range. (p=0.005), 4.0-fold over ROS in the 10.1-15 mm range (p=0.002), and 2.4-fold over ROS in the 15.1-35 mm range (p=0.002).
Figure 5.
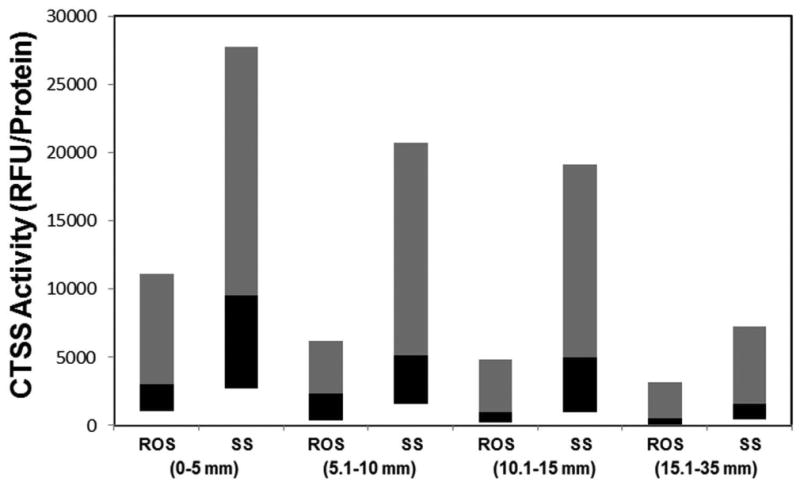
CTSS activity vs. Schirmer's value. Based on the values obtained from Schirmer's strips, the subjects were divided into 4 groups, as shown in the Figure. Data represents tear CTSS activity in SS patients compared with tear CTSS activity of ROS in each group. The grey bar represents 50th-90th percentile, while the black bar represents the 10th-50th percentile, and the junction represents the median.
Discussion
Autoimmune rheumatic diseases at their onset are often difficult to diagnose. Up to 50% of patients do not receive a definitive diagnosis within one year of the onset of symptoms, and many patients remain without a diagnosis even after five years of follow up (18, 19). A recent survey conducted by the Sjögren's Syndrome Foundation (SSF) on patients who were diagnosed with SS determined that it required approximately 4.7 years to make an accurate diagnosis after the initial appearance of clinical signs and symptoms (source: https://www.sjogrens.org/home/about-the-foundation/breakthrough-goal-/1yearupdate). This finding is attributed largely to a dearth of useful diagnostic tests with high sensitivity and specificity. Early symptoms of SS, such as dry eye and dry mouth, are non-specific and are often seen in patients without SS (2, 20, 21). Delayed diagnosis in turn compromises early intervention and can lead to serious consequences for the patients' quality of life, result in an increased socioeconomic burden, and increase the likelihood of life-threatening health sequellae. Recent literature estimates that up to 50% of pSS and 30% of sSS cases remain undiagnosed (2). A simple, fast, and non-invasive diagnostic technique with high sensitivity and specificity is clearly needed in the diagnostic workup of SS.
CTSS is a cysteine endopeptidase belonging to the C1 family (papain family), which is present in abundance in lysosomes and involved in immune responses (22, 23). CTSS has sequence identity with several other cathepsin family members but differs from others by its limited distribution, being highly expressed primarily in antigen-presenting cells (24). CTSS is synthesized as an inactive preproenzyme, which is converted to its active proenzyme form in late endosomes or lysosomes (25). Once activated, these proenzymes are involved in MHC class II-mediated immune responses, which involve the proper maturation and trafficking of MHC II αβ heterodimers with type II membrane glycoprotein invariant chain (li) (26). Signaling motifs present in the cytoplasmic tail of li help the αβli complex move into late endosomal/lysosomal compartments (27), where CTSS sequentially degrades li until class II associated invariant chain peptide (CLIP) is left seated in the peptide groove of the MHC class II molecule (28). Following this processing, antigens can be loaded on to the complex and molecules can move to the cell surface for presentation. Based on the finding that Ctss is up regulated and resistant to regulation by Cystatin-C in the C57BL/6.NOD-Aec1Aec2 mouse lacrimal glands, it has been suggested that the high levels of CTSS in the lysosomes breaks the binding of MHCII and CLIP, thereby preventing proper antigen presentation by macrophages and dendritic cells. This, in turn, permits increased antigen presentation by B cells, thereby increasing the risk for autoimmunity (17, 29).
Although over-expression of CTSS in immune cells has been suggested in autoimmunity (30-33), its potential utility as a diagnostic tool in SS is related directly to our finding that it is over-expressed, not only in the infiltrating lymphocytes of the LG but also in the acinar epithelial cells of the LG early in the onset of autoimmune dacryoadenitis in a mouse model of SS. LG acinar cells are professional secretory cells normally responsible for packaging and release of tear proteins critical to ocular surface function in a highly regulated fashion. In healthy cells, including acinar cells, CTSS reaches lysosomes, its major site of accumulation after being processed in the endoplasmic reticulum (ER) and the Golgi apparatus, via the trans Golgi-network (TGN) and late endosomes (34). However, in LG acinar cells of SS-model mice, overexpressed Ctss is mis-sorted in the TGN into mature secretory vesicles, which have contents discharged at the apical surface into ducts that drain with other tear proteins into tear fluid. Findings of elevated CTSS levels in tears of SS patients suggest a comparable abnormality in the fidelity of sorting of secretory versus lysosomal proteins as a fundamental component of the disease pathway.
In the present study, our data indicate that tear CTSS activity from SS patients is significantly elevated compared to that in healthy controls or in other patients suffering from non-SS related dry eye and other non-SS autoimmune diseases, suggesting it may be used as a novel biomarker for SS. The elevated tear CTSS activity in SS is not a spurious consequence of small tear volumes leading to concentration of tear CTSS, since we observed higher tear CTSS activity in SS patients compared to ROS, regardless of the volume of tears produced as measured by Schirmer's strips. Thus, tear volume levels are not a confounding variable. Since there are no significant differences in tear CTSS activities between pSS and sSS patients, tear CTSS activity may be useful for the diagnosis of either type of SS.
Although significantly elevated in both cases, tear CTSS was statistically more robust in distinguishing SS patients from a general autoimmune disease patient population than from a sicca or non-specific dry eye population. Dry eye patients in our study were diagnosed based on signs and symptoms. Although the patients designated as “non-specific dry eye” in our study did not have a known history of autoimmune disease, it is possible and even likely that there are patients within this group who either currently have undiagnosed SS or who will develop SS over time and are also currently undiagnosed, since up to 50% of pSS and 30% of sSS cases are not diagnosed (2). Our future goals include the longitudinal evaluation of tear CTSS activity and manifestations of autoimmune disease in patients presenting with sicca symptoms, enabling us to determine whether elevated CTSS in a sicca or dry eye patient is an indicator (biomarker) that the patient will develop SS over time.
The present criteria for diagnosing SS are complex, time-consuming, hampered by low specificity, and may require invasive procedures, including salivary gland biopsy. In contrast, tear CTSS activity determination is simple, economical, and noninvasive. Even though detection of anti-SSA and -SSB antibodies is regarded as one of the most important criteria for SS and widely used in clinics, these antibodies are not specific to SS. It has been reported that they are found only in 30-60% of pSS patients, while they are also present in 30% of SLE patients (35). This, finding is supported by our data, which indicates that tear CTSS activities in SS patients are not correlated with the presence of anti-SSA or anti-SSB in blood. While new serum biomarkers for workup of SS have recently been proposed, it is unclear how specific and sensitive they will be (36-40). A subset of newly suggested biomarkers may identify subsets of patients. For example, the antiviral protein MxA correlates well with the IFN signature and features of active disease, and it may be useful in identifying patients that respond to certain types of therapy (41, 42). In addition, FMS-like tyrosine kinase 3 Ligand (Flt3-L) may identify patients with a higher risk of developing lymphoma (43), and higher levels of beta2-microglobulin and free light chains of immunoglobulins are associated with increased systemic disease activity (44). The presence of SSA and other autoantibodies in saliva have been suggested to be more indicative of pSS than serum SSA autoantibodies, Although SSA autoantibodies are also present in saliva in other autoimmune diseases, the levels are higher in pSS patients (45). Other salivary proteins have also been proposed as biomarkers for pSS, such as cathepsin D (46). Development of autoimmune dacryoadenitis does not necessarily parallel sialoadenitis in disease models of SS such as the NOD mouse (47), suggesting the same may be true in SS patients. Reduced tear flow does not correlate well with the presence of SSA and SSB antibodies, but reduced salivary flow does (48), further enhancing the need for a sensitive diagnostic test for patients with primarily ocular symptoms. A reliable diagnostic indicator of sicca disease in the eye, as well as the oral cavity seems warranted given the clinical need to accurately diagnose and treat SS patients earlier to minimize its chronic effects.
The amount and composition of proteins in tears is an excellent indicator of the functional status of LG and the other organs (49), and a number of studies have explored the potential of tear biomarkers for diagnosing diseases, including SS (50, 51).
However, the tear proteome is a highly complex mixture, with hundreds of proteins identified to date. Markers that have been considered in tears include several cytokines, matrix metalloproteinase (MMP)-9, aquaporin 5, SSA/SSB, and α-fodrin autoantibodies (50). A general change in the tear fluid protein pattern has also been suggested to be indicative of SS (52). However, none of these biomarkers have been routinely evaluated in the clinic and they often require high cost immunoassays or complex proteomics approaches for detection.
In conclusion, data presented here strongly support tear CTSS activity as a novel biomarker for a simple, fast, economical, and non-invasive diagnosis of SS, which may prove to be of tremendous benefit to millions of patients who are being evaluated for this debilitating disease.
Acknowledgments
We would like to thank all the patients who participated in the study. This study was supported by an Ideas Empowered Grant from the USC Stevens Institute for Innovation. As well, the SC-CTSI (5UL1RR031986) provided consultation support from PTRS and Bio-statistical Resources as well as an SC-CTSI pilot grant to WS. DB was awarded an AOA Carolyn L. Kuckein student research fellowship from Fight for Sight. We would also like to thank Anuja Raut and Frances Yarber for aiding with tear CTSS activity measurements, Deana Cabrera, Armando Rivera and Sylvia L. Ramos for assistance with sample collection and Dr. Chuanqing Ding for providing expert assistance with manuscript preparation.
Footnotes
Author contributions: All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Hamm-Alvarez had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design: Edman, Hamm-Alvarez, Irvine, Mack, Stohl
Data acquisition: Arkfeld, Bach, Bricel, Christianakis, Frousiakis, Heur, Irvine, Janga, Madrigal, Renduchintala, Shah, Silka, Zhu
Data analysis and interpretation: Edman, Hamm-Alvarez, Janga, Mack, Stohl
Statistical Analysis: Mack
Manuscript Preparation: Edman, Hamm-Alvarez, Heur, Irvine, Janga, Mack, Stohl,
References
- 1.Fox RI, Kang HI. Pathogenesis of Sjogren's syndrome. Rheum Dis Clin North Am. 1992;18(3):517–38. [PubMed] [Google Scholar]
- 2.Peri Y, Agmon-Levin N, Theodor E, Shoenfeld Y. Sjogren's syndrome, the old and the new. Best Pract Res Clin Rheumatol. 2012;26(1):105–17. doi: 10.1016/j.berh.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Voulgarelis M, Tzioufas AG, Moutsopoulos HM. Mortality in Sjogren's syndrome. Clinical Exp Rheumatol. 2008;26(5 Suppl 51):S66–71. [PubMed] [Google Scholar]
- 4.Anderson LG, Talal N. The spectrum of benign to malignant lymphoproliferation in Sjogren's syndrome. Clin Exp Immunol. 1972;10(2):199–221. [PMC free article] [PubMed] [Google Scholar]
- 5.Launay D, Hachulla E, Hatron PY, Jais X, Simonneau G, Humbert M. Pulmonary arterial hypertension: a rare complication of primary Sjogren syndrome: report of 9 new cases and review of the literature. Medicine. 2007;86(5):299–315. doi: 10.1097/MD.0b013e3181579781. [DOI] [PubMed] [Google Scholar]
- 6.Mitsias DI, Kapsogeorgou EK, Moutsopoulos HM. Sjogren's syndrome: why autoimmune epithelitis? Oral Dis. 2006;12(6):523–32. doi: 10.1111/j.1601-0825.2006.01292.x. [DOI] [PubMed] [Google Scholar]
- 7.Parambil JG, Myers JL, Lindell RM, Matteson EL, Ryu JH. Interstitial lung disease in primary Sjogren syndrome. Chest. 2006;130(5):1489–95. doi: 10.1378/chest.130.5.1489. [DOI] [PubMed] [Google Scholar]
- 8.Soy M, Piskin S. Cutaneous findings in patients with primary Sjogren's syndrome. Clin Rheumatol. 2007;26(8):1350–2. doi: 10.1007/s10067-006-0374-3. [DOI] [PubMed] [Google Scholar]
- 9.Porola P, Laine M, Virkki L, Poduval P, Konttinen YT. The influence of sex steroids on Sjogren's syndrome. Ann N Y Acad Sci. 2007;1108:426–32. doi: 10.1196/annals.1422.045. [DOI] [PubMed] [Google Scholar]
- 10.Whitcher JP. Clinical diagnosis of the dry eye. Int Ophthalmol Clin. 1987;27(1):7–24. doi: 10.1097/00004397-198702710-00003. [DOI] [PubMed] [Google Scholar]
- 11.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6):554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richards A, Mutlu S, Scully C, Maddison P. Complications associated with labial salivary gland biopsy in the investigation of connective tissue disorders. Ann Rheum Dis. 1992;51(8):996–7. doi: 10.1136/ard.51.8.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baldini C, Talarico R, Tzioufas AG, Bombardieri S. Classification criteria for Sjogren's syndrome: a critical review. J Autoimmun. 2012;39(1-2):9–14. doi: 10.1016/j.jaut.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Vitali C, Bootsma H, Bowman SJ, Dorner T, Gottenberg JE, Mariette X, et al. Classification criteria for Sjogren's syndrome: we actually need to definitively resolve the long debate on the issue. Ann Rheum Dis. 2013;72(4):476–8. doi: 10.1136/annrheumdis-2012-202565. [DOI] [PubMed] [Google Scholar]
- 15.Burbelo PD, Ching KH, Issa AT, Loftus CM, Li Y, Satoh M, et al. Rapid serological detection of autoantibodies associated with Sjogren's syndrome. J Transl Med. 2009;7:83. doi: 10.1186/1479-5876-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Wu K, Edman M, Schenke-Layland K, MacVeigh-Aloni M, Janga SR, et al. Increased expression of cathepsins and obesity-induced proinflammatory cytokines in lacrimal glands of male NOD mouse. Invest Ophthalmol Vis Sci. 2010;51(10):5019–29. doi: 10.1167/iovs.09-4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen CQ, Sharma A, She JX, McIndoe RA, Peck AB. Differential gene expressions in the lacrimal gland during development and onset of keratoconjunctivitis sicca in Sjogren's syndrome (SJS)-like disease of the C57BL/6.NOD-Aec1Aec2 mouse. Exp Eye Res. 2009;88(3):398–409. doi: 10.1016/j.exer.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldblatt F, O'Neill SG. Clinical aspects of autoimmune rheumatic diseases. Lancet. 2013;382(9894):797–808. doi: 10.1016/S0140-6736(13)61499-3. [DOI] [PubMed] [Google Scholar]
- 19.Alarcon GS, Williams GV, Singer JZ, Steen VD, Clegg DO, Paulus HE, et al. Early undifferentiated connective tissue disease. I. Early clinical manifestation in a large cohort of patients with undifferentiated connective tissue diseases compared with cohorts of well established connective tissue disease. J Rheumatol. 1991;18(9):1332–9. [PubMed] [Google Scholar]
- 20.Hu S, Wang J, Meijer J, Ieong S, Xie Y, Yu T, et al. Salivary proteomic and genomic biomarkers for primary Sjogren's syndrome. Arthritis Rheum. 2007;56(11):3588–600. doi: 10.1002/art.22954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pink R, Simek J, Vondrakova J, Faber E, Michl P, Pazdera J, et al. Saliva as a diagnostic medium. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009;153(2):103–10. doi: 10.5507/bp.2009.017. [DOI] [PubMed] [Google Scholar]
- 22.Shi GP, Munger JS, Meara JP, Rich DH, Chapman HA. Molecular cloning and expression of human alveolar macrophage cathepsin S, an elastinolytic cysteine protease. The Journal of biological chemistry. 1992;267(11):7258–62. [PubMed] [Google Scholar]
- 23.Shi GP, Villadangos JA, Dranoff G, Small C, Gu L, Haley KJ, et al. Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity. 1999;10(2):197–206. doi: 10.1016/s1074-7613(00)80020-5. [DOI] [PubMed] [Google Scholar]
- 24.Shi GP, Webb AC, Foster KE, Knoll JH, Lemere CA, Munger JS, et al. Human cathepsin S: chromosomal localization, gene structure, and tissue distribution. The J Biol Chem. 1994;269(15):11530–6. [PubMed] [Google Scholar]
- 25.Wolters PJ, Chapman HA. Importance of lysosomal cysteine proteases in lung disease. Respir Res. 2000;1(3):170–7. doi: 10.1186/rr29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riese RJ, Wolf PR, Bromme D, Natkin LR, Villadangos JA, Ploegh HL, et al. Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity. 1996;4(4):357–66. doi: 10.1016/s1074-7613(00)80249-6. [DOI] [PubMed] [Google Scholar]
- 27.Lotteau V, Teyton L, Peleraux A, Nilsson T, Karlsson L, Schmid SL, et al. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990;348(6302):600–5. doi: 10.1038/348600a0. [DOI] [PubMed] [Google Scholar]
- 28.Beers C, Burich A, Kleijmeer MJ, Griffith JM, Wong P, Rudensky AY. Cathepsin S controls MHC class II-mediated antigen presentation by epithelial cells in vivo. J Immunol. 2005;174(3):1205–12. doi: 10.4049/jimmunol.174.3.1205. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen CQ, Peck AB. Unraveling the pathophysiology of Sjogren syndrome-associated dry eye disease. The ocular surface. 2009;7(1):11–27. doi: 10.1016/s1542-0124(12)70289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conus S, Simon HU. Cathepsins and their involvement in immune responses. Swiss Med Wkly. 2010;140:w13042. doi: 10.4414/smw.2010.13042. [DOI] [PubMed] [Google Scholar]
- 31.Saegusa K, Ishimaru N, Yanagi K, Arakaki R, Ogawa K, Saito I, et al. Cathepsin S inhibitor prevents autoantigen presentation and autoimmunity. J Clin Invest. 2002;110(3):361–9. doi: 10.1172/JCI14682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy VY, Zhang QY, Weiss SJ. Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages. Proc Natl Acad Sci U S A. 1995;92(9):3849–53. doi: 10.1073/pnas.92.9.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baugh M, Black D, Westwood P, Kinghorn E, McGregor K, Bruin J, et al. Therapeutic dosing of an orally active, selective cathepsin S inhibitor suppresses disease in models of autoimmunity. J Autoimmun. 2011;36(3-4):201–9. doi: 10.1016/j.jaut.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Brix K, Dunkhorst A, Mayer K, Jordans S. Cysteine cathepsins: cellular roadmap to different functions. Biochimie. 2008;90(2):194–207. doi: 10.1016/j.biochi.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 35.Goeb V, Salle V, Duhaut P, Jouen F, Smail A, Ducroix JP, et al. Clinical significance of autoantibodies recognizing Sjogren's syndrome A (SSA), SSB, calpastatin and alpha-fodrin in primary Sjogren's syndrome. Clin Exp Immunol. 2007;148(2):281–7. doi: 10.1111/j.1365-2249.2007.03337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen L, Suresh L, Lindemann M, Xuan J, Kowal P, Malyavantham K, et al. Novel autoantibodies in Sjogren's syndrome. Clin Immunol. 2012;145(3):251–5. doi: 10.1016/j.clim.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Tzioufas AG, Tatouli IP, Moutsopoulos HM. Autoantibodies in Sjogren's syndrome: clinical presentation and regulatory mechanisms. Presse Med. 2012;41(9 Pt 2):e451–60. doi: 10.1016/j.lpm.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 38.Kramer JM, Klimatcheva E, Rothstein TL. CXCL13 is elevated in Sjogren's syndrome in mice and humans and is implicated in disease pathogenesis. J Leukoc Biol. 2013 doi: 10.1189/jlb.0113036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li YN, Guo JP, He J, Liu X, Yin FR, Ding Y, et al. Serum IgA against type 3 muscarinic acetylcholine receptor is a novel marker in diagnosis of Sjogren's syndrome. Chin Med J. 2011;124(16):2490–5. [PubMed] [Google Scholar]
- 40.Kitagawa T, Shibasaki K, Toya S. Clinical significance and diagnostic usefulness of anti-centromere antibody in Sjogren's syndrome. Clin Rheumatol. 2012;31(1):105–12. doi: 10.1007/s10067-011-1789-z. [DOI] [PubMed] [Google Scholar]
- 41.Kroese FG, Bootsma H. Biomarkers: New biomarker for Sjogren's syndrome-time to treat patients. Nat Rev Rheumatol. 2013;9(10):570–2. doi: 10.1038/nrrheum.2013.143. [DOI] [PubMed] [Google Scholar]
- 42.Maria NI, Brkic Z, Waris M, van Helden-Meeuwsen CG, Heezen K, van de Merwe JP, et al. MxA as a clinically applicable biomarker for identifying systemic interferon type I in primary Sjogren's syndrome. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-202552. pii:annrheumdis-2012-202552v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tobon GJ, Saraux A, Gottenberg JE, Quartuccio L, Fabris M, Seror R, et al. The FMS-like tyrosine kinase 3-ligand may be biological marker of lymphoma in primary Sjogren's syndrome. Arthritis Rheum. 2013 doi: 10.1002/art.38129. [DOI] [PubMed] [Google Scholar]
- 44.Gottenberg JE, Seror R, Miceli-Richard C, Benessiano J, Devauchelle-Pensec V, Dieude P, et al. Serum levels of beta2-microglobulin and free light chains of immunoglobulins are associated with systemic disease activity in primary Sjogren's syndrome. Data at enrollment in the prospective ASSESS cohort. PloS one. 2013;8(5):e59868. doi: 10.1371/journal.pone.0059868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu S, Vissink A, Arellano M, Roozendaal C, Zhou H, Kallenberg CG, et al. Identification of autoantibody biomarkers for primary Sjogren's syndrome using protein microarrays. Proteomics. 2011;11(8):1499–507. doi: 10.1002/pmic.201000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu S, Gao K, Pollard R, Arellano-Garcia M, Zhou H, Zhang L, et al. Preclinical validation of salivary biomarkers for primary Sjogren's syndrome. Arthritis Care Res. 2010;62(11):1633–8. doi: 10.1002/acr.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.da Costa SR, Wu K, Veigh MM, Pidgeon M, Ding C, Schechter JE, et al. Male NOD mouse external lacrimal glands exhibit profound changes in the exocytotic pathway early in postnatal development. Exp Eye Res. 2006;82(1):33–45. doi: 10.1016/j.exer.2005.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haga HJ. Clinical and immunological factors associated with low lacrimal and salivary flow rate in patients with primary Sjogren's syndrome. J Rheumatol. 2002;29(2):305–8. [PubMed] [Google Scholar]
- 49.Vissink A, Bootsma H, Kroese FG, Kallenberg CG. How to assess treatment efficacy in Sjogren's syndrome? Curr Opin Rheumatol. 2012;24(3):281–9. doi: 10.1097/BOR.0b013e3283524c37. [DOI] [PubMed] [Google Scholar]
- 50.von Thun Und Hohenstein-Blaul N, Funke S, Grus FH. Tears as a source of biomarkers for ocular and systemic diseases. Exp Eye Res. 2013 doi: 10.1016/j.exer.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 51.Zhou L, Beuerman RW. Tear analysis in ocular surface diseases. Prog Retin Eye Res. 2012;31(6):527–50. doi: 10.1016/j.preteyeres.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Tomosugi N, Kitagawa K, Takahashi N, Sugai S, Ishikawa I. Diagnostic potential of tear proteomic patterns in Sjogren's syndrome. J Proteome Res. 2005;4(3):820–5. doi: 10.1021/pr0497576. [DOI] [PubMed] [Google Scholar]


