Abstract
Objective
The role of innate immunity in pathogenesis of cryptococcal meningitis (CM) is unclear. We hypothesised that NK cell and monocyte responses are central nervous system (CNS) compartmentalised, and altered by anti-fungal therapy and combination antiretroviral therapy (cART) during CM/HIV co-infection.
Design
Sub-study of a prospective cohort study of adults with CM/HIV co-infection in Durban, South Africa.
Methods
We used multi-parametric flow cytometry to study compartmentalisation of subsets, activation (CD69pos), CXCR3 and CX3CR1 expression and cytokine secretion of NK cells and monocytes in freshly collected blood and cerebrospinal fluid (CSF) at diagnosis (n=23), completion of anti-fungal therapy induction (n=19) and after a further 4 weeks of cART (n=9).
Results
Relative to blood, CSF was enriched with CD56bright (immunoregulatory) NK cells (p=0.0004). At enrolment, CXCR3 expression was more frequent amongst blood CD56bright than either blood CD56dim (p<0.0001) or CSF CD56bright (p=0.0002) NK cells. Anti-fungal therapy diminished blood (p<0.05) but not CSF CXCR3pos NK cell proportions nor CX3CR1pos NK cell proportions. CD56bright and CD56dim NK cells were more activated in CSF than blood (p<0.0001). Anti-fungal therapy induction reduced CD56dim NK cell activation in CSF (p=0.02). Activation of blood CD56bright and CD56dim NK cells was diminished following cART commencement (p<0.0001, p=0.03). Immunoregulatory NK cells in CSF tended to secrete higher levels of CXCL10 (p=0.06) and lower levels of TNF-α (p=0.06) than blood immunoregulatory NK cells. CSF was enriched with non-classical monocytes (p=0.001), but anti-fungal therapy restored proportions of classical monocytes (p=0.007).
Conclusions
These results highlight CNS activation, trafficking and function of NK cells and monocytes in CM/HIV and implicate immunoregulatory NK cells and pro-inflammatory monocytes as potential modulators of CM pathogenesis during HIV co-infection.
Keywords: Cryptococcal meningitis, cerebrospinal fluid, Natural Killer cells, Monocytes, HIV-1
Introduction
Cryptococcal meningitis (CM) is a major cause of morbidity and mortality in patients with HIV and AIDS. Annually, approximately 957,900 cases of CM occur, resulting in 624,700 deaths by three-months after infection, with sub-Saharan Africa bearing the largest burden of disease [1]. The underlying mechanisms causing death and disability include development of persistently high intracranial pressures, vasculopathies, and local brain inflammation with bystander neuronal damage. Both innate and adaptive immune responses contribute to the immunopathogenesis of CM but the regulation and timing of their development remain poorly understood.
Natural Killer (NK) cells are key effectors of innate immunity that are able to mediate pathogen elimination by directly killing or modulating innate and adaptive immune responses through secretion of cytokines. In humans, expression of CD56, but a lack of CD3, CD14, and CD19, defines their phenotype. Functionally, they can be further subdivided into subsets with primarily cytokine-secretion capabilities (CD56brightCD16neg) or cytolytic capabilities (CD56dimCD16pos) [2]. Typically, CD56bright NK cells are more prevalent at extravascular sites than CD56dim NK cells [2]. During HIV disease a third subset, CD56negCD16pos, increases disproportionately in blood, but this subset is deficient in both cytokine production and cytolysis [3]. In vivo mouse and in vitro human studies suggest that NK cells are able to directly kill cryptococci by perforin-mediated cytotoxicity [4, 5], or indirectly by the potentiation of macrophage anti-fungal activity [6]. NK cells are able to enter the central nervous system (CNS) during inflammatory disease such as multiple sclerosis (MS) [7]; indeed they have been shown to play a major role in a variety of CNS infections [8]. Therefore, it is plausible that in vivo, NK cells may traffic to the site of cryptococcal infection and exert anti-fungal activity. Alternatively, NK cells may secrete immunoregulatory cytokines that affect recruitment and function of other innate and adaptive immune cells. The phenotype, function and mechanisms of NK cell infiltration/trafficking into the CNS are not well described in CM/HIV co-infection, and have only recently been examined in HIV mono-infection [9]. Thus delineating the profiles of NK cells in the CSF during treated CM may allow identification of parameters that play a role in CM/HIV pathogenesis.
Monocytes/macrophages are a second innate immune leukocyte subset that plays a role in the pathogenesis of some inflammatory CNS diseases, and with which NK cells have substantial crosstalk. NK cells are required for monocyte differentiation into dendritic cells in several inflammatory disorders [10]. Conversely, monocytes/macrophages are able to activate NK cells through their secretion of pro-inflammatory cytokines, IL-12 and IL-18 [11]. Monocytes can be divided into three functionally distinct subsets based on their relative expression of CD16 and CD14 (i.e., classical: CD14++CD16−; intermediate: CD14++CD16+; and non-classical: CD14+CD16++) [12]. Among these subsets the non-classical monocytes have the greatest capacity for secreting pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) [13], intermediate monoctes have superior reactive-oxygen species production and classical monocytes appear to have superior phagocytic function [12]. The role of monocytes in CM pathogenesis is unresolved; some reports suggest that monocytes may act as a ‘trojan horse’ allowing entry of intracellular cryptococci into the CNS [14]; others suggest that monocytes may mediate cryptococcal elimination [15]. Similar to other infections by intracellular pathogens disorders, in CM monocytes are likely required for pathogen elimination, but also to harbor pathogens intracellularly and impose clinically relevant immunopathology with their activity in CM [16].
Here we aimed to identify changes in the innate immune response in blood and CSF in patients with CM and HIV in South Africa. We prospectively characterised blood and CSF NK cell phenotypes, monocyte subsets and, to a lesser extent NK cell function in patients with HIV/CM co-infection at admission for care, after induction of anti-fungal therapy, and after a further 4 weeks following commencement of combination antiretroviral therapy (cART) in some patients.
Methods
This study was conducted as a sub-study of the Cryptococcal Immune Restoration Disease (IRD) study, which has been described previously [17]. We prospectively enrolled consenting cART-naïve, HIV-infected adults with a first-episode of microbiologically-confirmed CM at the King Edward VIII Hospital in Durban, South Africa. Briefly, whole blood and CSF were obtained at enrolment (median 2 days after diagnosis, range 0–8 days) from 23 participants. Amphotericin B was commenced immediately on diagnosis for a protocolled time of 14 days. About half of all patients had persistent cryptococcal growth after Amphotericin B therapy [17]. Following this induction period of anti-fungal therapy, 19 patients were re-sampled for blood and CSF (median 14 days after diagnosis range 10–15 days) and were commenced on cART as per contemporary guidelines [18] and continued on oral fluconazole. After 4 weeks of cART a final whole blood specimen was obtained from a subset of 9 patients based on their availability. Serial therapeutic lumbar punctures where conducted as required for therapeutic purposes whilst continuing anti-fungal therapy.
At enrolment, the mean age of participants in this sub-study was 34.7 years (range 21–55 years), and 43% were female; similar to the overall cohort [17]. The median baseline CD4+ T-cell count was 22 cells/mm3 (IQR 6.5–43), and the median plasma HIV viral load was 3.18 Log10 copies/ml (IQR 1.14–5.95). After 4 weeks of cART, amongst 9 participants from whom blood samples were available, the median CD4+ T-cell count was 74 cells/mm3 (IQR 49–153), and the median plasma HIV viral load was 2.56 Log10 copies/ml (IQR 2.31–2.91).
This study was approved by the University of KwaZulu-Natal Biomedical Research Ethics Committee, the Monash University ethics committee and the University of Western Australia ethics committee.
Flow Cytometry analyses
The cellular profile of CSF can be taken as a measure of cells in an intermediate compartment between blood and CNS parenchyma. We used healthy and HIV-infected patients to characterise NK cells and monocytes in the CNS [19–21]. Peripheral blood and CSF leucocytes were simultaneously stained with a panel of fluorophore-conjugated antibodies and subjected to multiparametric flow cytometry using conventional methods. Briefly, for whole blood staining, 150μl undiluted whole blood collected in tubes containing ethylenediaminotetraacetic acid (EDTA) was incubated with the following antibodies for 20 minutes at 4°C: anti-CD3 APC, anti-CD8 Qdot 655, anti-CD14 Pacific Orange (PO), anti-CD16 Pacific Blue (PB), anti-CD45 AlexaFluor700 (AF700), anti-CD56 PC7 (Beckman Coulter, Pasadena, USA) anti-CD69 FITC, anti-CX3CR1 PE and anti-CXCR3 PerCP-Cy5.5. All antibodies were from Becton Dickinson (Franklin Lakes, USA) unless otherwise indicated. Red blood cells were lysed with VersaLyse (Beckman Coulter) as per the manufacturer’s directions, pelleted by centrifugation and fixed with a paraformaldehyde-containing fixative (Reagent A, Life Technologies, Carlsbad, USA). For CSF cell staining, the total volume of CSF obtained from the patient (ranging from 3–30 ml), was centrifuged at 750 x g for 10 minutes, resuspended in 1ml R10 (RPMI 1640 containing penicillin/streptomycin, 10% fetal calf serum, supplemented with 1.0 mg/ml L-glutamine) and live nucleated cells were enumerated with a nucleocounter (Chemometec, Allerod, Denmark). The range of the nuclecounter was 5×103–2×106 cells/ml and since several samples were outside this range we were unable to convert proportions to absolute numbers in this study. One third of the total number of nucleated cells were aliquoted into FACSTubes, washed with Dulbeco’s phosphate buffered saline (DPBS) and stained for 20 minutes with the same panel of antibodies listed above. Cells were washed and fixed as above but the lysis step was omitted.
For intracellular cytokine staining experiments: the primary stain included anti-CD3 APC (Beckman Coulter), anti-CD56 PC7 (Beckman Coulter) and anti-CD16 PB. Following fixation, cells were incubated for 15 minutes, washed, and then permeabilised and stained with anti-cytokine antibodies by adding Reagent B (Life Technologies), anti-CXCL10 PE and anti-TNFα AF700. After a further 15 minutes cells were washed with DPBS.
Flow cytometry data were collected on a BD LSRII and analyzed using FlowJo v10.0.6 (Treestar, Ashland, USA). At least 5,000 CD45+ leucocytes were collected for each CSF sample, and 3×106 events were collected for each whole blood specimen. Fluorescence minus one gating strategies were used to determine gating boundaries as described [22]. The gating strategy is shown in Supplementary Figure 1.
Statistical analyses
For comparisons between paired specimens, from the same individual at different time-points, or at the same time-point but from blood and CSF, a non-parametric matched-pairs Wilcoxon signed rank test was performed. This method ignores data points where the pair is incomplete and thus is robust to missing data for the 4 individuals for whom samples were unavailable at completion of anti-fungal therapy induction. Statistical analyses were conducted in GraphPad Prism v5 (GraphPad, La Jolla, California).
Results
Proportions of immunoregulatory NK cells (CD56bright) are expanded in the CSF of patients with CM and HIV
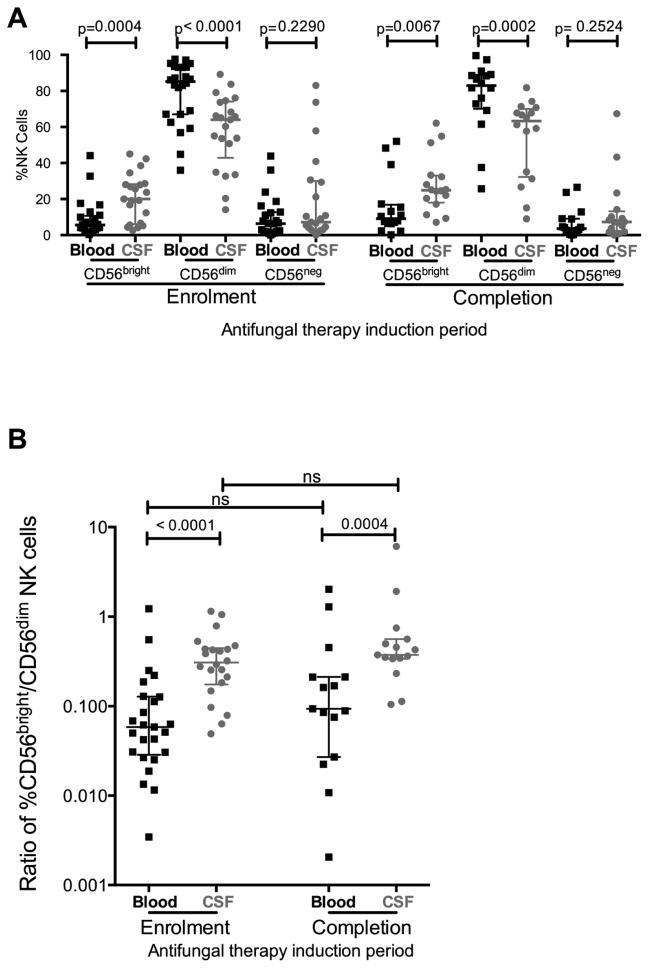
Cytolytic and cytokine-secretory roles are performed by different NK cell subsets that partially overlap in function: low expression of CD56 (CD56dim) demarcates cytolytic NK cells and high expression of CD56 (CD56bright) identifies a cytokine-secreting subset that is thought to be less mature [23]. At both enrolment and after completion of anti-fungal therapy, the CSF was enriched with CD56bright immunoregulatory NK cells compared to blood (at enrolment median 20% vs. 5.4%, median change Δ=13.32%, p=0.0004, Figure 1A), and had fewer CD56dim NK cells (at enrolment median 64% vs. 86.1%, median Δ =20.6%, p<0.0001). The ratio of CD56bright/CD56dim NK cells was significantly higher in the CSF compared to blood (Figure 1B). Neither the absolute proportions nor the ratio of CD56bright and CD56dim NK cells was significantly modified following 14 days of anti-fungal therapy (Figure 1B).
Figure 1. At enrolment and after completion of anti-fungal therapy induction, CSF was enriched with CD56bright NK cells, had fewer CD56dim and similar frequencies of CD56neg NK cells relative to blood (a) and hence the ratio of %CD56bright (immunoregulatory) NK cells to %CD56dim (cytotoxic) NK cells was higher in CSF than blood at enrolment and after completion of anti-fungal therapy induction in HIV patients with CM(a).
Medians (horizontal lines) and interquartile ranges (whiskers) are shown in each graph. Measurements in blood denoted by black squares (■) and in CSF denoted by grey circles (
 ).
).
Expansion of an anergic subset of NK cells with low/absent CD56 expression (CD56neg) has been observed in the blood of patients with advanced HIV disease [24]. Notwithstanding the use of classical methods as opposed more recently described methods that enhance specificity of NK cell gating [25], we did not observe differences in the proportion of CD56neg NK cells in the CSF relative to blood (Supplementary Figure 2). In both blood and CSF the median proportion of CD56neg NK cells was 6–7%.
In both blood and CSF, immunoregulatory (CD56bright) NK cells and cytolytic (CD56dim) NK cells differed in their expression of CXCR3
To investigate whether immunoregulatory NK cells differed from cytotoxic NK cells in CSF, we compared their expression of chemokine receptors. Eisenhardt and colleagues recently demonstrated that CXCR3 expression in extravascular tissues demarcated specific NK cell subsets that play a role in infection [26]. Moreover, CXCR3 is the receptor for pro-inflammatory chemokines: CXCL9 (MIG), CXCL-10 (IP-10) and CXCL-11 (I-TAC) [27]. Based on our prior discovery of an increasing gradient of CXCL-10 from blood to CSF in the participants of this study [28], we speculated that this chemokine could mediate chemotaxis of CXCR3-expressing NK cells into the CNS.
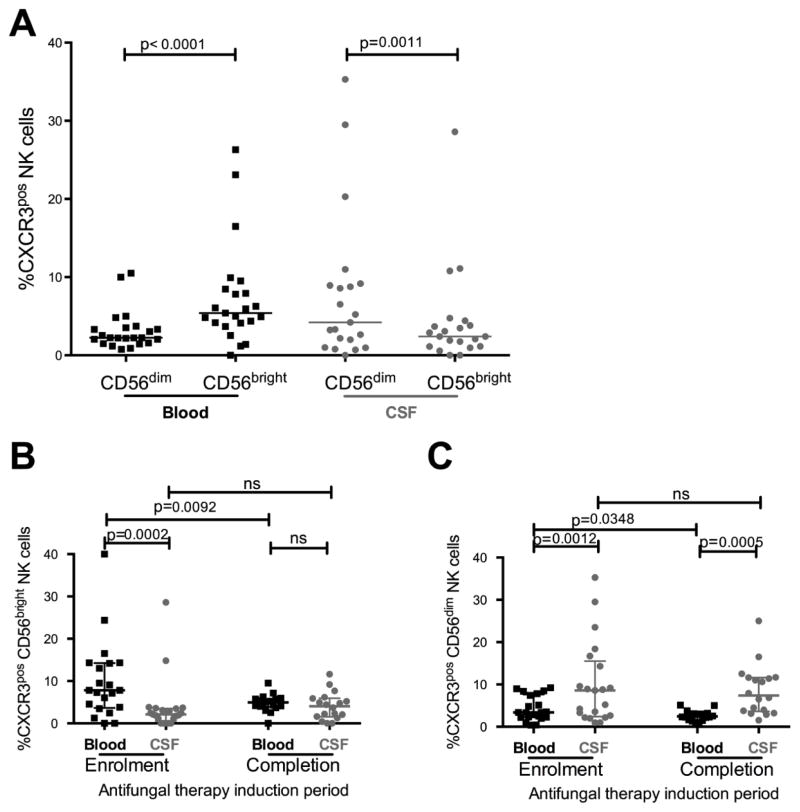
At enrolment in blood we found a greater proportion of CD56bright NK cells expressing CXCR3 than CD56dim NK cells (median 5.4% vs. 2.2%, median Δ=3.2%, p<0.0001; Figure 2A). In contrast, in CSF a significantly greater proportion of CD56dim NK cells expressed CXCR3 than CD56bright NK cells (median 4.2% vs. 2.4%, median Δ=1.5%, p=0.0011). Furthermore, we found that differential CXCR3 expression on CD56bright and CD56dim NK cells extended to comparisons between blood and CSF compartments (Figures 2B and 2C). Amongst CD56bright NK cells, the proportion expressing CXCR3 was significantly greater in blood than CSF at enrolment (median 7.8% vs. 2.1%, median Δ=4.6%, p=0.0002), but after 14 days of anti-fungal therapy the proportion of CXCR3pos CD56bright NK cells in blood declined (median 7.8% vs. 4.8%, median Δ =2.8%, p=0.009). By completion of anti-fungal therapy induction there was no difference between blood and CSF in the proportion of CD56bright NK cells expressing CXCR3 (Figure 2B). The proportion of CXCR3pos CD56bright NK cells in CSF did not change over the period of anti- fungal therapy induction.
Figure 2. CXCR3 expression differed by NK cell subset (CD56bright, CD56dim) and compartment.
In blood, at enrolment the proportion of NK cells expressing CXCR3 was higher among CD56bright NK cells than among CD56dim NK cells, but the opposite was observed in CSF (a). The proportion of CD56bright NK cells expressing CXCR3 was significantly higher in blood at enrolment but declined following anti-fungal therapy. By completion of anti-fungal therapy the proportion in blood was similar to CSF (b). In contrast, the proportion of CD56dim NK cells expressing CXCR3 was significantly higher in CSF than blood at enrolment and completion of anti-fungal therapy (c). Medians (horizontal lines) and interquartile ranges (whiskers) are shown in each graph. Measurements in blood denoted by black squares (■) and in CSF denoted by grey circles (
 ).
).
In contrast, amongst CD56dim NK cells, the proportion expressing CXCR3 was significantly greater in CSF than blood at both enrolment and after completion of anti-fungal therapy (median 8.6% vs. 3.4%, median Δ=3.2% at enrolment, p=0.001; median 7.9% vs. 2.5%, median Δ=5.36% at completion of anti-fungal therapy, p=0.0005; Figure 2C). This difference in CXCR3 expression was maintained in CSF over the period of anti-fungal therapy induction. However, in blood the proportion of CD56dim NK cells expressing CXCR3 declined (median 3.4% at enrolment vs. 2.3% at completion of anti-fungal therapy, median Δ=1.2%, p=0.03).
The proportion of CXCR3pos CD56bright and CD56dim NK cells in blood following 4 weeks of combined antiretroviral therapy (cART) did not differ from that at completion of anti-fungal therapy (Supplementary Figure 3A and B).
We also examined expression of CX3CR1 on the various NK cell subsets, as CX3CR1 expressing NK cells have been reported to be involved in modifying autoimmune CNS disease pathogenesis [29]. At enrolment, in CSF, there was a higher proportion of CD56dim NK cells expressing CX3CR1 than CD56bright NK cells (median 10.6% vs. 2.9%, median Δ=4.8%, p=0.0003) but there was no difference observed in blood. Both at enrolment and at completion of anti-fungal therapy induction, a larger proportion of CD56bright NK cells in blood expressed CX3CR1 than those in CSF (median 15.2% vs. 2.8%, median Δ=14.3%, p=0.001; and median 10.4% vs. 1.4%, median Δ=6.5%, p=0.009, respectively, Figure 2B). The proportion of CX3CR1 expressing CD56dim NK cells did not differ between blood and CSF regardless of timepoint or anti-fungal therapy. In summary, these data demonstrate that NK cells differ in expression of CXCR3 and to a lesser extent CX3CR1 chemokine receptors according to compartment and subset.
Cytotoxic and immunoregulatory NK cells in CSF were more activated than NK cells in blood
Activation is a necessary precursor of both CD56dim and CD56bright NK cell activity. To gain insight into the role of these NK cells in CM pathogenesis we examined the proportion of activated cells in each subset by measuring cell-surface expression of CD69, an early marker of lymphocyte activation. CD56bright and CD56dim NK cells in CSF were more activated than blood NK cells (Figure 3) at enrolment (median 48.1% vs. 14.3%, median Δ=37.7%, p<0.0001; and median 54.2% vs. 19.7%, median Δ=33.3%, p<0.0001 respectively) and after completion of anti-fungal therapy induction (median 52.9% vs. 7.9%, median Δ=43.2%, p=0.0001; and median 46.5% vs. 9.95, median Δ=31.6%, p=0.0003 respectively). Although anti-fungal therapy did not significantly reduce the proportion of activated NK cells in blood, or the proportion of activated CD56bright NK cells in CSF, after completion of anti-fungal therapy induction the proportion of activated CD56dim NK cells in CSF was significantly reduced (Figure 3). After completion of anti-fungal therapy induction, the proportion of activated blood NK cells was positively associated with the plasma HIV VL (r=0.65, p=0.007). Consistent with previous reports [30, 31] cART commencement was associated with a significant decline in the proportions of CD69pos NK cells in both CD56bright (median 16.1% vs. 5.7%, p<0.0001) and CD56dim blood NK-cell subsets (median 10.3% vs. 6.7%, p=0.03, Supplementary Figure 2C and 2D).
Figure 3. CD56bright (a) and CD56dim (b) NK cells were more activated in CSF than in blood at enrolment and completion of anti-fungal therapy, and activation was only partially reduced by anti-fungal therapy induction.
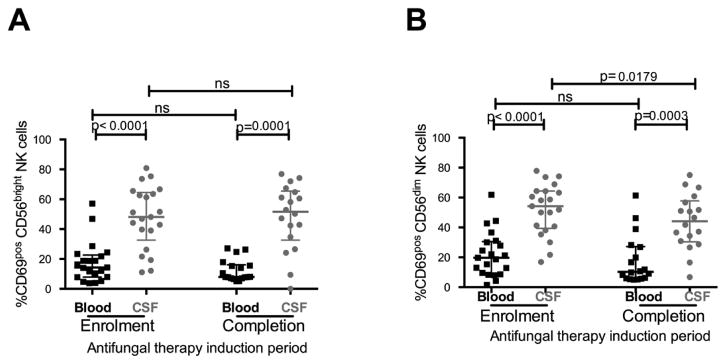
Medians (horizontal lines) and interquartile ranges (whiskers) are shown in each graph. Measurements in blood denoted by black squares (■) and in CSF denoted by grey circles (
 ).
).
Immunoregulatory NK cells in CSF expressed higher levels of CXCL10 and lower levels of TNF-α than NK cells in blood prior to commencing anti-fungal therapy
Next, we examined whether the chemokine and cytokine secretion profiles of CD56bright NK cells in CSF differed from those in blood. CSF levels of the chemokine CXCL-10 (IP-10) levels in CSF correlate directly with neuronal injury in CNS HIV disease [32]. Similarly, the amounts of pro- inflammatory chemokines and cytokines, including CXCL-10 and tumor necrosis factor-alpha (TNF-α), correlate with clinical outcomes in CM and HIV co-infection prior to starting cART [33]. Thus, to quantify differences in functional responses during HIV and CM co-infection we compared intracellular cytokine profiles of CXCL-10 and TNF-α in NK cells. We obtained paired blood and CSF samples at enrolment from five participants and performed intracellular cytokine staining for CXCL-10 and TNF-α. The proportion of CD56bright NK cells in CSF expressing CXCL-10 tended to be higher than in blood (median 57.6% vs. 35.7% median Δ=19.8%, p=0.06, Figure 4). Conversely, the proportion of CD56bright NK cells in CSF expressing TNF-α tended to be lower than in blood (median 64.2 vs. 42.3%, median Δ =19.84%, p=0.06, Figure 4).
Figure 4. At enrolment the proportion of CD56bright NK cells producing CXCL-10 was higher in CSF than in blood, but the proportion producing TNF-α was lower in CSF than in blood (n=5).
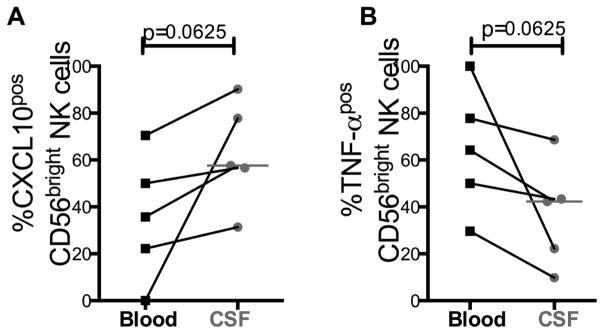
Results are expressed as the percentage of cytokine producing cells. Measurements in blood denoted by black squares (■) and in CSF denoted by grey circles (
 ).
).
CSF was enriched for non-classical monocytes in CM prior to anti-fungal therapy
NK cells engage in a bi-directional communication with other innate and adaptive immune cells. During neuroinflammation, monocytes are a major cell type that is recruited to the CNS [34], unlike other tissues that recruit neutrophils. Therefore we also evaluated our flow cytometric data to quantify monocyte subsets in CSF and blood.
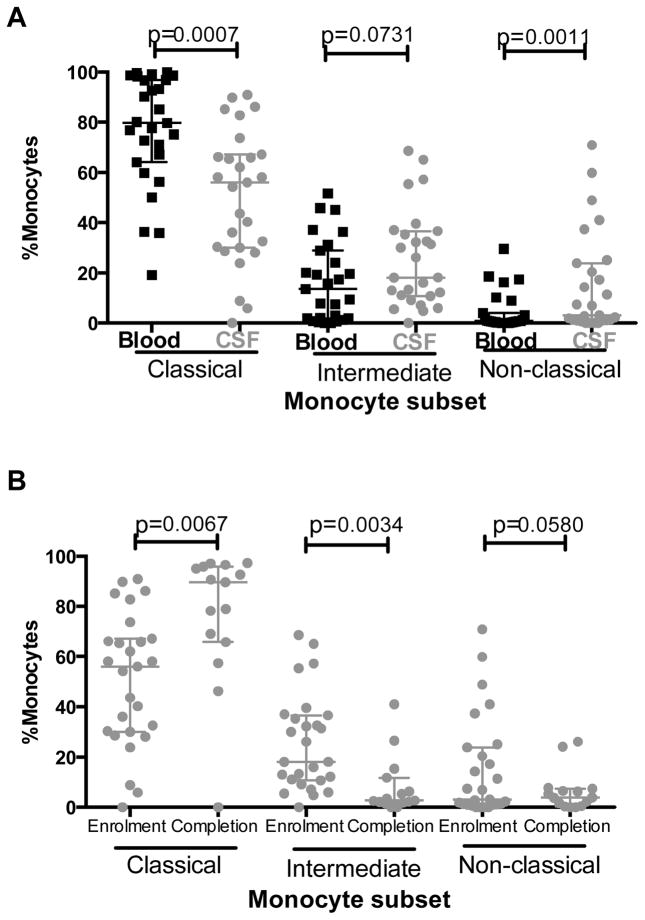
Relative to blood, CSF was enriched for non-classical ‘pro- inflammatory’ monocytes at enrolment (median 3.12% vs. 0.78%, median Δ=1.83%, p=0.001, Figure 5A). Correspondingly, the proportion of classical monocytes was lower in CSF than blood (median 30% vs. 64%, median Δ=59.8%, p=0.0007). There was also a trend towards a greater proportion of intermediate monocytes in CSF than blood (median 18.1% vs. 13.5%, median Δ =6.5%, p=0.07).
Figure 5. The proportions of non-classical and intermediate monocytes in CSF declined following anti-fungal therapy induction, whereas the proportion of classical monocytes increased.
At enrolmentCSF was enriched with non-classical monocytes (CD14loCD16hi) and intermediate monocytes (CD14hiCD16lo), but had significantly lower proportions of classical monocytes (CD14hiCD16neg) (a). After completion of anti-fungal therapy induction the proportions of classical monocyte in CSF were increased and the proportions of intermediate and non-classical monocytes in CSF were reduced (b). Results are expressed as the percentage of total monocytes. Medians (horizontal lines) and interquartile ranges (whiskers) are shown in each graph. Measurements in blood denoted by black squares (■) and in CSF denoted by grey circles (
 ).
).
Anti-fungal therapy restores proportions of classical monocytes in CSF
Comparing the proportions of the three major monocytes subsets in CSF at enrolment and after completion of anti-fungal therapy demonstrated that the proportion of classical monocytes significantly increased over time (median 36.1% to 89.5%, median Δ=23.4%, p=0.006, Figure 5B). In contrast, the proportion of intermediate monocytes significantly decreased (median 13% vs. 2.69%, median Δ=8.4%, p=0.003), while the proportion of non-classical monocytes also declined but the difference did not achieve statistical significance (median 2.78% vs. 3.85%, median Δ=2.5%, p=0.06). In comparison, there were no significant differences between proportions of monocyte subsets in blood at enrolment and after 14 days of anti-fungal therapy (data not shown).
Discussion
Here we assessed NK cells and monocytes in CSF and blood in patients with HIV-CM prior to and following anti-fungal therapy induction and cART. We found that markers of activation and/or function expressed by NK cells and monocytes were compartmentalised in the CNS relative to blood. These findings suggest that immunoregulatory NK cells and non-classical monocytes may play a role in CM pathogenesis. Such changes might contribute to adverse outcomes after commencing cART, such as cryptococcosis-associated immune reconstitution inflammatory syndrome (C-IRIS).
Consistent with previous reports of the phenotype of NK cells in extravascular tissues [2] and during CNS infections [35], we found a higher proportion of immunoregulatory (CD56bright) NK cells in CSF than in blood. Homing of plasmablasts and T-cells to the CNS compartment have been shown to be mediated by CXCR3 [36, 37], a receptor for CXCL10. We previously reported that there was a higher concentration of CXCL-10 in CSF than in blood in this cohort [28]. CX3CL1 has also been reported to mediate migration of NK cells to the CNS during experimental allergic encephalomyelitis [29]. We observed differences between blood and CSF in the proportions of both CXCR3pos and CX3CR1pos NK cells. We therefore speculate that the enhanced CXCR3 and/or CX3CR1 expression on CD56bright NK cells in blood that we observed may have equipped these cells to enter the CNS compartment in response to a CXCL-10 or CX3CL1 gradient. Tracking chemokine expression on particular cells to test this hypothesis was beyond the scope of our work, and remains to be tested.
It is notable that the proportion of CXCR3 expressing NK cells in the CSF was not affected by antifungal therapy induction. This subset has been reported to have impaired cytotoxic and cytokine-secretory capacity in Hepatitis C virus infection [26]. The maintenance of this population of NK cells in CSF may have implications in the development of adverse clinical sequelae such as C-IRIS. Understanding of role of this subset in CM infection will benefit from further study.
We extended previous reports of NK cells in the CNS by assessing their cytokine profiles and activation status over time. As predicted, our findings were consistent with previous reports of decreased activation of NK cells in blood of HIV-infected adults following cART initiation and the reduction of HIV burden [30, 31]. However, we observed a partial decline in NK cell activation only in the CD56dim subset in the CNS following two weeks of anti- fungal therapy. We attributed the maintenance of NK cell activation in the CNS to either residual pathogen burden in the CNS [17] or an intrinsic high threshold for deactivation.
Our preliminary discovery that immunoregulatory NK cells secreted more CXCL-10 in CSF than in blood, suggested that they were preferentially promoting a pro-inflammatory environment in the CNS compartment. The observation that the immunoregulatory NK cells in CSF produced less TNF than in blood may be a result of interaction with dendritic cells or with cryptococci [38]. Taking into consideration that NK cells also generate strong IFN-γ responses to C. neoformans [5] and that IFN-γ levels in CSF are one of the leading predictors of clinical outcomes in CM pathogenesis [33], they could be candidates for immune modulation to improve clinical outcomes in this disease. However, we examined only a small number of patients and further studies are needed.
In contrast to NK cells, we observed a rebalancing of monocyte subsets following anti-fungal therapy induction. After 14 days of anti-fungal therapy the proportions of intermediate monocyte subsets in CSF declined, whereas the proportion of classical monocytes increased. We attribute this change to differences in functional roles during clearance of Cryptococcus. Unlike classical monocytes, non-classical and intermediate monocytes secrete large amounts of TNF-α and IL-1β, are expanded during many infectious diseases [39–41] including HIV [42], and are preferentially recruited to sites of inflammation [16]. Restoration of monocyte subset distribution with anti-fungal therapy suggests that reducing antigen burden is sufficient to restore monocyte homeostasis in the CNS compartment.
Although we discovered novel changes in NK cells and monocytes phenotypes in the CNS compartment during treated CM disease, our findings have limitations. Because we were unable to quantify absolute numbers of cells in either CSF or in blood, we cannot infer whether absolute numbers of specific subsets were altered. With the exception of anti-fungal therapy induction, we were unable to examine the association between innate immunological events in CSF or blood and clinical outcomes. Nor can we definitely demonstrate whether these observations are specific to HIV/CM or may be observed in other forms of meningoencephalitis with or without HIV infection. We examined the effect of cART in only 9 patients. Larger studies are required to establish the clinical relevance of our findings. Nevertheless, our data provided new insights into regulation of compartmentalised immune responses during treated CM disease in adults with advanced HIV.
In summary, our findings suggest that NK cell and monocyte responses to cryptococci are compartmentalised in patients with CM and HIV co-infection. Furthermore, they highlight a potential role of immunoregulatory NK cells and different monocyte subsets in CM pathogenesis. Prospective studies of CNS-resident NK cells and monocytes, and their association with clinical outcomes, such as C-IRIS, are warranted.
Supplementary Material
Acknowledgments
Sources of Funding: This study was supported by the South African HIV/AIDS Research Platform (SHARP), the REACH initiative grant 2007 and US National Institutes for Health FIC K01-TW007793. VN was supported by LIFELab and the Columbia University-South Africa Fogarty AIDS International Training and Research Program (AITRP, grant #D43 TW000231). CCC was supported by an Australian Postgraduate Award 2009, Australian National Health and Medical Research Council (NHMRC) Postgraduate Scholarship 2010–2012. SRL is a NHMRC Practitioner Fellow. TN holds the South African Research Chair in Systems Biology of HIV/AIDS and is a Howard Hughes Medical Institute International Early Career Scientist. Additional training was supported by the South African National Research Foundation KISC Award.
We would like to thank the staff of the HIV Pathogenesis Programme (HPP) at the University of KwaZulu-Natal (Durban, South Africa) for their assistance in processing clinical samples.
Footnotes
Author Contributions
VN and CC conceived and conducted the experiments described in this study. RD, SO, and AL provided technical support for experiments. MYS, JHE, TN, SR, MAF and WHC provided overall oversight of the clinical cohort accrual, follow-up and experimental procedures. MAF and WHC provided critical advice throughout the conduct of the study. All the authors read, commented and approved the final version of this manuscript.
References
- 1.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 3.Mavilio D, Lombardo G, Benjamin J, Kim D, Follman D, Marcenaro E, et al. Characterization of CD56−/CD16+ natural killer (NK) cells: A highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci U S A. 2005;102:2886–2891. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hidore MR, Nabavi N, Sonleitner F, Murphy JW. Murine natural killer cells are fungicidal to Cryptococcus neoformans. Infect Immun. 1991;59:1747–1754. doi: 10.1128/iai.59.5.1747-1754.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marr KJ, Jones GJ, Zheng C, Huston SM, Timm-McCann M, Islam A, et al. Cryptococcus neoformans directly stimulates perforin production and rearms NK cells for enhanced anticryptococcal microbicidal activity. Infect Immun. 2009;77:2436–2446. doi: 10.1128/IAI.01232-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawakami K, Koguchi Y, Qureshi MH, Yara S, Kinjo Y, Uezu K, et al. NK cells eliminate Cryptococcus neoformans by potentiating the fungicidal activity of macrophages rather than by directly killing them upon stimulation with IL-12 and IL-18. Microbiol Immunol. 2000;44:1043–1050. doi: 10.1111/j.1348-0421.2000.tb02601.x. [DOI] [PubMed] [Google Scholar]
- 7.Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 8.Poli A, Kmiecik J, Domingues O, Hentges F, Blery M, Chekenya M, et al. NK cells in central nervous system disorders. J Immunol. 2013;190:5355–5362. doi: 10.4049/jimmunol.1203401. [DOI] [PubMed] [Google Scholar]
- 9.Ho EL, Ronquillo R, Altmeppen H, Spudich SS, Price RW, Sinclair E. Cellular Composition of Cerebrospinal Fluid in HIV-1 Infected and Uninfected Subjects. PLoS One. 2013;8:e66188. doi: 10.1371/journal.pone.0066188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang AL, Colmenero P, Purath U, Teixeira de Matos C, Hueber W, Klareskog L, et al. Natural killer cells trigger differentiation of monocytes into dendritic cells. Blood. 2007;110:2484–2493. doi: 10.1182/blood-2007-02-076364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michel T, Hentges F, Zimmer J. Consequences of the crosstalk between monocytes/macrophages and natural killer cells. Front Immunol. 2012;3:403. doi: 10.3389/fimmu.2012.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM, Wong SC. The three human monocyte subsets: implications for health and disease. Immunol Res. 2012;53:41–57. doi: 10.1007/s12026-012-8297-3. [DOI] [PubMed] [Google Scholar]
- 13.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 14.Charlier C, Nielsen K, Daou S, Brigitte M, Chretien F, Dromer F. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun. 2009;77:120–127. doi: 10.1128/IAI.01065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tascini C, Vecchiarelli A, Preziosi R, Francisci D, Bistoni F, Baldelli F. Granulocyte-macrophage colony-stimulating factor and fluconazole enhance anti-cryptococcal activity of monocytes from AIDS patients. AIDS. 1999;13:49–55. doi: 10.1097/00002030-199901140-00007. [DOI] [PubMed] [Google Scholar]
- 16.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang CC, Dorasamy AA, Gosnell BI, Elliott JH, Spelman T, Omarjee S, et al. Clinical and mycological predictors of cryptococcosis-associated Immune reconstitution inflammatory syndrome (C-IRIS) AIDS. 2013 doi: 10.1097/QAD.0b013e3283614a8d. [DOI] [PubMed] [Google Scholar]
- 18.Republic of South Africa Department of Health. South African National Antiretroviral Treatment Guidelines. 2010. [Google Scholar]
- 19.Svenningsson A, Andersen O, Edsbagge M, Stemme S. Lymphocyte phenotype and subset distribution in normal cerebrospinal fluid. J Neuroimmunol. 1995;63:39–46. doi: 10.1016/0165-5728(95)00126-3. [DOI] [PubMed] [Google Scholar]
- 20.Neuenburg JK, Cho TA, Nilsson A, Bredt BM, Hebert SJ, Grant RM, et al. T-cell activation and memory phenotypes in cerebrospinal fluid during HIV infection. J Acquir Immune Defic Syndr. 2005;39:16–22. doi: 10.1097/01.qai.0000155036.03004.a0. [DOI] [PubMed] [Google Scholar]
- 21.Margolick JB, McArthur JC, Scott ER, McArthur JH, Cohn S, Farzadegan H, et al. Flow cytometric quantitation of T cell phenotypes in cerebrospinal fluid and peripheral blood of homosexual men with and without antibodies to human immunodeficiency virus, type I. J Neuroimmunol. 1988;20:73–81. doi: 10.1016/0165-5728(88)90116-6. [DOI] [PubMed] [Google Scholar]
- 22.Herzenberg LA, Tung J, Moore WA, Herzenberg LA, Parks DR. Interpreting flow cytometry data: a guide for the perplexed. Nat Immunol. 2006;7:681–685. doi: 10.1038/ni0706-681. [DOI] [PubMed] [Google Scholar]
- 23.Moretta L. Dissecting CD56dim human NK cells. Blood. 2010;116:3689–3691. doi: 10.1182/blood-2010-09-303057. [DOI] [PubMed] [Google Scholar]
- 24.Brunetta E, Hudspeth KL, Mavilio D. Pathologic natural killer cell subset redistribution in HIV-1 infection: new insights in pathophysiology and clinical outcomes. J Leukoc Biol. 2010;88:1119–1130. doi: 10.1189/jlb.0410225. [DOI] [PubMed] [Google Scholar]
- 25.Milush JM, Long BR, Snyder-Cappione JE, Cappione AJ, 3rd, York VA, Ndhlovu LC, et al. Functionally distinct subsets of human NK cells and monocyte/DC-like cells identified by coexpression of CD56, CD7, and CD4. Blood. 2009;114:4823–4831. doi: 10.1182/blood-2009-04-216374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenhardt M, Glassner A, Kramer B, Korner C, Sibbing B, Kokordelis P, et al. The CXCR3(+)CD56Bright phenotype characterizes a distinct NK cell subset with anti-fibrotic potential that shows dys-regulated activity in hepatitis C. PLoS One. 2012;7:e38846. doi: 10.1371/journal.pone.0038846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Billottet C, Quemener C, Bikfalvi A. CXCR3, a double-edged sword in tumor progression and angiogenesis. Biochim Biophys Acta. 2013;1836:287–295. doi: 10.1016/j.bbcan.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Chang CC, Omarjee S, Lim A, Spelman T, Gosnell BI, Carr WH, et al. Chemokine levels and chemokine receptor expression in blood and the CSF of HIV-infected patients with cryptococcal meningitis and C-IRIS. J Infect Dis. 2013 doi: 10.1093/infdis/jit388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang D, Shi FD, Jung S, Pien GC, Wang J, Salazar-Mather TP, et al. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J. 2006;20:896–905. doi: 10.1096/fj.05-5465com. [DOI] [PubMed] [Google Scholar]
- 30.Le Guillou-Guillemette H, Renier G, Vielle B, Abgueguen P, Chennebault JM, Lunel F, et al. Immune restoration under HAART in patients chronically infected with HIV-1: diversity of T, B, and NK immune responses. Viral Immunol. 2006;19:267–276. doi: 10.1089/vim.2006.19.267. [DOI] [PubMed] [Google Scholar]
- 31.Alter G, Teigen N, Ahern R, Streeck H, Meier A, Rosenberg ES, et al. Evolution of innate and adaptive effector cell functions during acute HIV-1 infection. J Infect Dis. 2007;195:1452–1460. doi: 10.1086/513878. [DOI] [PubMed] [Google Scholar]
- 32.Letendre SL, Zheng JC, Kaul M, Yiannoutsos CT, Ellis RJ, Taylor MJ, et al. Chemokines in cerebrospinal fluid correlate with cerebral metabolite patterns in HIV-infected individuals. J Neurovirol. 2011;17:63–69. doi: 10.1007/s13365-010-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boulware DR, Bonham SC, Meya DB, Wiesner DL, Park GS, Kambugu A, et al. Paucity of initial cerebrospinal fluid inflammation in cryptococcal meningitis is associated with subsequent immune reconstitution inflammatory syndrome. J Infect Dis. 2010;202:962–970. doi: 10.1086/655785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terry RL, Getts DR, Deffrasnes C, van Vreden C, Campbell IL, King NJ. Inflammatory monocytes and the pathogenesis of viral encephalitis. J Neuroinflammation. 2012;9:270. doi: 10.1186/1742-2094-9-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamann I, Dorr J, Glumm R, Chanvillard C, Janssen A, Millward JM, et al. Characterization of natural killer cells in paired CSF and blood samples during neuroinflammation. J Neuroimmunol. 2013;254:165–169. doi: 10.1016/j.jneuroim.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Marques CP, Kapil P, Hinton DR, Hindinger C, Nutt SL, Ransohoff RM, et al. CXCR3-dependent plasma blast migration to the central nervous system during viral encephalomyelitis. J Virol. 2011;85:6136–6147. doi: 10.1128/JVI.00202-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stiles LN, Hosking MP, Edwards RA, Strieter RM, Lane TE. Differential roles for CXCR3 in CD4+ and CD8+ T cell trafficking following viral infection of the CNS. Eur J Immunol. 2006;36:613–622. doi: 10.1002/eji.200535509. [DOI] [PubMed] [Google Scholar]
- 38.Murphy JW, Zhou A, Wong SC. Direct interactions of human natural killer cells with Cryptococcus neoformans inhibit granulocyte-macrophage colony-stimulating factor and tumor necrosis factor alpha production. Infect Immun. 1997;65:4564–4571. doi: 10.1128/iai.65.11.4564-4571.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castano D, Garcia LF, Rojas M. Increased frequency and cell death of CD16+ monocytes with Mycobacterium tuberculosis infection. Tuberculosis (Edinb) 2011;91:348–360. doi: 10.1016/j.tube.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Fingerle G, Pforte A, Passlick B, Blumenstein M, Strobel M, Ziegler- Heitbrock HW. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82:3170–3176. [PubMed] [Google Scholar]
- 41.Soares G, Barral A, Costa JM, Barral-Netto M, Van Weyenbergh J. CD16+ monocytes in human cutaneous leishmaniasis: increased ex vivo levels and correlation with clinical data. J Leukoc Biol. 2006;79:36–39. doi: 10.1189/jlb.0105040. [DOI] [PubMed] [Google Scholar]
- 42.Han J, Wang B, Han N, Zhao Y, Song C, Feng X, et al. CD14(high)CD16(+) rather than CD14(low)CD16(+) monocytes correlate with disease progression in chronic HIV-infected patients. J Acquir Immune Defic Syndr. 2009;52:553–559. doi: 10.1097/qai.0b013e3181c1d4fe. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.