Abstract
Valvular interstitial cells (VICs) respond to 3D matrix interactions in a complex manner, but better understanding these effects on VIC function is important for applications ranging from valve tissue engineering to studying valve disease. Here, we encapsulated VICs in poly(ethylene glycol) (PEG) hydrogels modified with three different adhesive ligands derived from fibronectin (RGDS), elastin (VGVAPG), and collagen-1 (P15). By day 14, VICs became significantly more elongated in RGDS containing gels compared to VGVAPG or P15. This difference in cell morphology appeared to correlate with global matrix metalloproteinase (MMP) activity, as VICs encapsulated in RGDS-functionalized hydrogels secreted higher levels of active MMP at day 2. VIC activation to a myofibroblast phenotype was also characterized by staining for α-smooth muscle actin (αSMA) at day 14. The percentage of αSMA+ VICs in the VGVAPG gels was the highest (56%) compared to RGDS (33%) or P15 (38%) gels. Matrix deposition and composition were also characterized at day 14 and 42 and found to depend on the initial hydrogel composition. All gel formulations had similar levels of collagen, elastin, and chondroitin sulfate deposited as the porcine aortic valve. However, the composition of collagen deposited by VICs in VGVAPG functionalized gels had a significantly higher collagen-X to collagen-1 ratio, which is associated with stenotic valves. Taken together, these data suggest that peptide functionalized PEG hydrogels are a useful system to culture VICs in 3D, and with the ability to systematically alter biochemical and biophysical properties, this platform may prove useful in manipulating VIC function for valve regeneration.
Keywords: Hydrogels, VICs, ECM Deposition, Matricellular Interactions, 3D, αSMA
1. Introduction
Over the past two decades, there has been much progress towards the regeneration of heart valves using tissue engineering strategies (Syedain & Transquillo, 2009; Long & Tranquillo, 2003; Durst, et al., 2011; Shah, et al., 2008; Ramaswamy, et al., 2010; Engelmayr, et al., 2005; Flanagan, et al., 2006). Despite this progress, clinical treatment of valve disease primarily relies on synthetic valve replacements or allografts (Clarke, et al., 1993), yet a “living” tissue engineered equivalent would provide many benefits, especially for younger patients who currently require multiple surgeries (Henaine, et al., 2012). Part of the limitation of tissue engineering strategies relates to the lack of more robust and tailorable scaffolds, as well as a better understanding of which cues to incorporate in those scaffolds to regulate desired properties of the seeded cells, typically aortic valvular interstitial cells (VICs). Here, we sought to investigate a poly(ethylene glycol) (PEG) platform for the three-dimensional culture of VICs, and one that would allow systematic variations in the scaffold biophysical and biochemical properties to better understand how initial scaffold properties can influence VICs and their secretion of extracellular matrix (ECM) molecules.
The field’s current understanding of VICs has shown that they are able to differentiate, (Chester & Taylor, 2007) and secrete matrix metalloproteinases (MMPs) (Adelow, et al., 2008; Chester & Taylor, 2007; Chester, et al., 2008; Cushing, et al., 2005) and ECM molecules in both 2 and 3D (Adelow, et al., 2008; Chester & Taylor, 2007; Chester, et al., 2008; Cushing, et al., 2005; Hinz, 2007; Masters, et al., 2005). More recently, there has been a growing appreciation that VIC activation and secretory properties depend on the context of their microenvironment, including both biochemical (Gu & Masters, 2010; Masters, et al., 2005; Cushing, et al., 2005) and biophysical cues (Kloxin, et al., 2009; Yip, et al., 2009). However, less is known about how VICs may respond differently to these signals in 3D culture, where transport of growth factors and matricellular signaling can be quite complex.
Biomaterials for VIC culture have integrated biochemical signals, such as integrin-binding peptides or delivery of cytokines like transforming growth factor-β1 (TGF-β1), to study VIC response to these cues. In synthetic biomaterials, RGD is the most widely incorporated peptide and used to promote cell-material interactions (LeBaron & Athanasiou, 2000), as RGD is known to bind to several integrin α/β complexes (Ruoslahti, 1996). However, very little is known about how other potentially important cell-matrix interactions with ECM molecules also found in the native aortic valve, like elastin and collagen, affect VIC phenotype and function. Furthermore, there may also be synergies between these cell-matrix interactions and a physiologically relevant culture platform (i.e., elasticity and 3D context) that may be lost in 2D experiments completed on tissue culture polystyrene (TCPS). This potential synergy between the signal and the manner in which it is presented is a very complex aspect of VIC biology as VICs actively remodel their initial microenvironments. Utilizing a PEG hydrogel culture platform may aid in elucidating how various signals in the initial VIC microenvironment, like cell-matrix interactions, elasticity, and in a three-dimensional context, affect VIC phenotype and function for the development of tissue-engineered replacements of stenotic valves.
Here, we exploited a PEG hydrogel system first developed by Fairbanks et al. that utilizes a thiol-ene photochemical reaction to create peptide functionalized gels with varying biophysical properties and degradability (Fairbanks, et al., 2009); one formulation containing RGDS and an MMP-cleavable linker peptide (KCGGPQGI↓WGQGCK) was demonstrated to be compatible for the encapsulation and long-term three-dimensional culture of VICs (Benton, et al., 2009). Here, we endeavored to study VICs cultured in similar gels, but systematically incorporated three different integrin-binding peptides (RGDS, VGVAPG, and P15). We hypothesized that varying cell-matrix interactions would induce differences in VIC phenotype, as well as newly deposited tissue. VIC viability, metabolic activity, elongation, MMP activity/regulation, and deposition of ECM molecules found in native aortic valves (collagen-1, collagen-X, elastin, and chondroitin sulfate) were followed as a function of culture time to gain a better understanding of the effects of these initial cell-matrix interactions on the secretory properties of VICs and their ECM deposition. Such information may prove useful to better understand how initial cell-matrix interactions might be engineered to regenerate valves for tissue engineering applications or in vitro models for studying valve disease progression.
2. Materials and Methods
2.1. Materials
Eight-armed poly(ethylene glycol) (Mn: 40 000 g/mol) was purchased from JenChem. All amino acids and resin for solid phase peptide synthesis (SPPS) were purchased from Anaspec or ChemImpex and Novabiochem, respectively. Porcine hearts for VIC isolation were obtained from Hormel Inc. M199 media, fetal bovine serum (FBS), penicillin/streptomyocin, fungizone, alamar blue, and LIVE/DEAD assay kit were purchased from Life Technologies. All other chemicals were purchased from Sigma-Aldrich, unless otherwise specified.
2.2. PEG-Norbornene and Peptide Preparation
Eight-armed PEG-norbornene (PEG-N, Figure 1b) (Mn: 40 000 g/mol) was synthesized as previously described by Fairbanks et al. (2009). Briefly, the reaction was carried out under anhydrous conditions in dichloromethane (DCM), where a PEG solution was added drop-wise to a stirred solution of N,N’-dicyclohexylcarbodiimide (DCC) and norbornene acid, and allowed to react overnight. The norbornene functionalized PEG in this solution was then precipitated in ice-cold ethyl ether, filtered, and re-dissolved in chloroform. This chloroform PEG solution was then washed with a glycine buffer and brine solution before precipitating in ice-cold ethyl ether. The product was then filtered again, and excess ethyl ether was removed via a vacuum chamber. The percent functionalization of PEG arms with norbornene end-groups was determined using 1H-NMR by comparing the hydrogen peaks associated with the carbon adjacent to the ester linkage (~ 4.2 ppm) to the hydrogen peaks associated with the PEG molecule (~ 3.6 ppm). Only products with greater than 95% functionalization were used.
Figure 1.
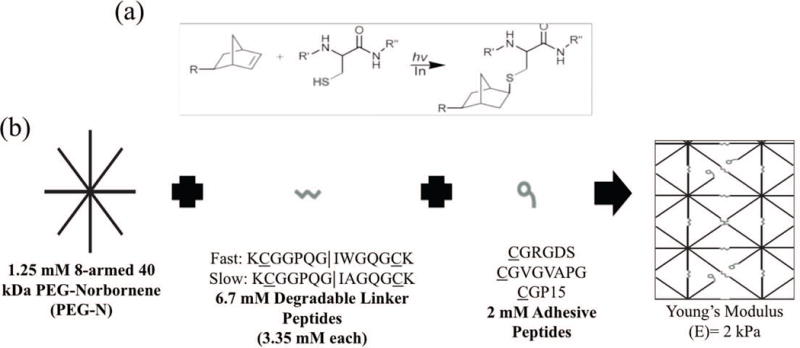
(a) Demonstrates the thiol-ene reaction between a norbornene functionality on the PEG arm (R) and the thiol side group of a cysteine amino acid in the presence of light and a photo initiator to form a covalent bond. (Bowman & Kloxin, 2008) (b) Monomers and concentrations used in gel formulation, which react to form the final idealized gel network structure shown.
Three integrin-binding small peptides were synthesized with a thiol containing cysteine (C) amino acid to facilitate the thiol-ene reaction and a glycine (G) spacer between the reactive thiol and the cell recognition site and included in the thiol-ene formulation (Figure 1). These included one derived from fibronectin (CGRGDS) (Pierschbacher & Ruoslahti, 1984), one from elastin (CGVGVAPG) (Gobin & West, 2003), and one from collagen-1 (CGGTPGPQGIAGQRGVV) (i.e., CGP15) (Hennessy, 2009). Additionally, two MMP cleavable peptides derived from collagen-1 (KCGGPQG↓IWGQGCK (faster degradation, tryptophan variant) and KCGGPQG↓IAGQGCK (slower degradation, alanine variant)) were also synthesized where the sequence was engineered to include the thiol containing cysteine (C) amino acids on both sides to facilitate crosslinking of the gel during the thiol-ene reaction. These sequences were selected based on previous work with 3D VIC cultures (Benton, et al., 2009; Fairbanks, et al., 2009), as well as characterization of their susceptibility to MMP cleavage (Patterson & Hubbell, 2010).
All peptides were synthesized using solid phase peptide synthesis (SPPS) on a Protein Technologies Tribute peptide synthesizer. After a 5 wt% phenol trifluoroacetic acid (TFA) cleavage and ice cold ethyl ether precipitation, if the purity was found to be less than 95% via high pressure liquid chromatography (HPLC), then large scale HPLC purification was performed. The correct eluate fraction, based on molecular weight, was determined through matrix assisted laser desorption ionization (MALDI). The HPLC aqueous, acetonitrile buffer was removed from the peptide via lyophilization.
2.3. VIC Isolation
Primary porcine aortic valvular interstitial cells (VICs) were isolated via a sequential collagenase digestion as previously described by Filip et al. (1986), from aortic valve leaflets, which were excised from fresh porcine hearts acquired from Hormel Inc. within 24 hours of slaughter. Briefly, the leaflets were incubated at 37°C in Earle’s balanced salt solution containing 250 U/mL collagenase (Worthington) for 30 minutes to remove the endothelial cell lining, followed by a 60 minute incubation in fresh collagenase solution to remove the VICs. The VICs were pelleted and resuspended in growth media (M199 media supplemented with 15 v/v% FBS, 2 v/v% penicillin/streptomyocin, and 0.4 v/v% fungizone); plated on TCPS dishes; and cultured to 50% confluency at 37°C and 5% CO2. The VICs were then trypsinized, pelleted, and resuspended in 45 v/v% growth media, 50 v/v% FBS, and 5 v/v% dimethyl-sulfoxide (DMSO) to 1 000 000 cells/mL. This suspension was transferred to cryovials, which were stored in a liquid nitrogen cooled tank until needed, at which point they were thawed and expanded on TCPS in growth media in an incubator set to 37°C and 5% CO2. Only VICs of the third passage (i.e., 3 doublings) were used for experiments.
2.4. Encapsulation and Culture of VICs in Peptide-Functionalized Hydrogels
Eight-armed PEG-norbornene (Mn: 40 000 g/mol) was dissolved in sterile PBS at 2.5 mM, and this stock solution was then sterile filtered using a 0.2 μm porous filter. In a tissue culture hood, the stock solution was then added to thiol containing peptide solutions and FBS free M199 media supplemented with 2 v/v% penicillin/streptomyocin and 0.4 v/v% fungizone. The final monomer concentrations are summarized in Figure 1. Pelleted VICs were resuspended in the desired monomer solution to yield a final concentration of 15 million cells/mL. 40 μL of the monomer and VIC suspension was then injected into sterile syringes and polymerized with 0.2 mM 4-(2-hydroxyethoxy)phenyl-(2-hydroxy-2-propyl)ketone (Irgacure-2959, Ciba) photoinitiator and 365 nm UV light at 8.5 mW/cm2 for 10 minutes (Fairbanks, et al., 2009). Figure 1 illustrates the thiol-ene reaction and resulting covalent bond, an idealized final network structure, and a summary of the resulting material properties, which were measured in shear using a parallel plate rheometer and converted to Young’s modulus assuming a Poisson’s ratio of 0.5 (Fairbanks, et al., 2009). The experimentally measured modulus values for the selected gel formation were very similar to theoretical predictions for the modulus of thiol-ene gels at the concentrations of crosslinking functionalities used in these experiments (Gould, et al., 2012). The resulting cell-laden gels were placed in ultra low adhesion culture dishes (Corning) in M199 media supplemented with 10 v/v% FBS, 2 v/v% penicillin/streptomyocin, and 0.4 v/v% fungizone, and placed in an incubator at 37°C and 5% CO2. Media was changed every 3 days.
2.5. VIC Viability in 3D Hydrogels
A membrane integrity assay and metabolic activity assay were used as measures of VIC survival in the various hydrogel formulations. After 14 days of culture, media from VIC-laden gels was removed and replaced with a LIVE/DEAD solution containing 2 μM calcein AM and 4 μM EthD-1. The gels were incubated at room temperature for 30 minutes with the LIVE/DEAD solution and then imaged on an inverted fluorescent scope (Nikon Eclipse 300). Images in triplicate per sample were analyzed for the percentage of live cells, which were stained only green.
For the metabolic activity assay, VIC-laden gels were cultured for 2 or 14 days and transferred into a fresh plate containing sterile 10 v/v% alamar blue solution in 5% FBS M199 media. The hydrogels were incubated in the alamar blue solution at 37°C, shaking, for 4 hours and then transferred to a black 96-well plate. Fluorescence was measured at 535/590 nm in triplicate.
2.6. VIC–Matrix Interactions
VIC-matrix interactions were characterized by following both morphological changes and measuring their global MMP activity. Bright field images of VIC morphology were collected on days 2 and 14, and ImageJ software was used to measure and calculate the elongation factor (cell length/cell width) for each peptide condition of at least 20 cells per image. As an indirect measure of VIC remodeling of the local gel environment, media was removed from the gels after 2 and 14 days to gauge the initial and long-term alterations in MMP activity caused by VIC interaction with the three different integrin-binding peptides. The total protein concentration was determined using a nano-drop spectrometer absorbance reading at 280 nm, and then the media was frozen and lyophilized. Lyophilized media samples were re-suspended to a total protein concentration of 15 mg/mL in assay buffer (50 mM Tris, 150 mM NaCl, 10 mM CaCl2, 0.05 w/v% Brij-35, pH 7.5). 50 μL of these samples were transferred to a black 96-well plate in triplicate with 50 μL of a 20 μM fluorogenic peptide substrate (R&D Systems) that is cleaved by MMPs 1, 2, 7, 8, 9, 12, 13, 14, 15, 16. The samples were incubated at room temperature for 5 minutes, protected from light, and then the fluorescence was read at 320/405 nm as a measure of total enzyme activity. MMP activity was normalized to that of RGDS for each day separately.
2.7. VIC Activation and Matrix Deposition in 3D hydrogels
Both immunochemistry and histology were used to monitor myofibroblast activation and type of extracellular matrix deposited by encapsulated VICs as a function of the hydrogel chemistry. Samples for immunohistochemistry and histology were taken after 14 and 42 days in culture and fixed in 10% formalin solution overnight at 4°C. The gels were then soaked in a 30 wt% sucrose solution overnight at 4°C and subsequently flash frozen in liquid nitrogen cooled isopentane. Samples were sectioned to 30 μm on a cryostat (Leica CM1850), which were then washed with a 1X PBS solution and stained for collagen-1, collagen-X, elastin, chrondroitin sulfate, α-smooth muscle actin (αSMA) (abcam), and VIC nuclei (DAPI, Life Technologies) with a FITC secondary antibody. Immunostained images were analyzed using ImageJ software for immunofluorescence intensity of the ECM component (i.e., FITC) channel and normalized to number of nuclei per field of view. The fraction of VICs positive for αSMA expression was determined by counting the number of VICs per field of view with αSMA present and normalized to the number of nuclei per field of view. Sections were also stained for total collagen deposition after 42 days via Masson’s Trichrome histology.
Quantitative ECM Deposition Assays
The amount of ECM deposited within the synthetic PEG hydrogel matrices was quantified after 42 days using colorimetric assays for collagen (Sirius Red assay, (MacNeil, et al., 2011; Lee, et al., 1998)), elastin (Fastin elastin assay (Biocolour) (Srokowski, et al., 2001; Gacchina, et al., 2011)), and glycosaminoglycans (GAGs) (DMMB assay, (Le & Fleming, 2008; Wilda, et al., 2007; Holledge, et al., 2008)). Since these are all high molecular weight macromolecules, the synthetic component of the hydrogel was designed to have a peptide that completely degraded during the pepsin or collagenase treatment (degradation conditions described below for each assay) after the gels were mechanically separated into smaller pieces with a pedestal.
To complete the collagen/Sirius Red assay, nine gels from each peptide condition were homogenized in a 0.01 wt% pepsin solution in 0.5 M acetic acid and incubated overnight at 4°C. A standard curve of collagen and samples (0.5 mL) were added to 0.5 mL of Sirius red dye solution (0.01 wt% direct red in 0.2 v/v% Tween 20-PBS) and incubated at room temperature for 30 minutes with shaking. Afterward, all standards and samples were centrifuged at 10 000 g for 10 minutes, and washed with 0.5 M acetic acid three times. Then, 0.25 mL of 1N NaOH was added and incubated at room temperature for 30 minute with shaking. Finally, 125 μL of each sample and standard were transferred to a clear 96-well plate in triplicate, and the solution absorbance read at 540 nm.
To complete the DMMB assay for sulfated GAGs, nine gels from each peptide condition were incubated in a 76 U/mL collagenase solution overnight at 4°C. 10 μL of chondroitin sulfate standards and samples were transferred into a clear 96-well plate in triplicate, and 250 μL of the DMMB solution (16 mg DMMB in 5 mL 100% ethanol added to 3.04 g glycine and 2.37 g NaCl in 1 L diH2O (pH 3)) was added. The absorbance at 530 nm was read immediately.
To complete the Fastin Elastin assay, nine gels from each peptide condition were incubated in a 76 U/mL collagenase solution overnight at 4°C. The assay protocol provided by Biocolour was followed without modification.
2.8. Statistics
Data are presented as mean ± standard error of three images or samples per each of three gel samples completed three times with separate VIC isolations (i.e., biological replicates). Data were compared using a Tukey’s Test and significance was established for p ≤ 0.05.
3. Results
VIC phenotype and matrix secretion can be influenced by the local microenvironment, including properties such as elasticity (Kloxin, et al., 2009; Yip, et al., 2009), as well as biochemical signals that bind to integrins or cell surface receptors (Gould, et al., 2012; Shah, et al., 2008; Rodriquez, et al., 2011; Rodriguez KJ, et al., 2008). While valuable information has been gained from these collective studies, most of these studies have characterized VIC response to these signals in two-dimensions (i.e., VICs cultured on modified TCPS or biomaterials). How VIC response to these cues will translate in a three-dimensional microenvironment is less studied, but is crucial for design of scaffolds for many tissue engineering applications. While this understanding is desired, three-dimensional systems are inherently complex, and interpretation of results can be convoluted as VICs dynamically change their local microenvironment through matrix-interactions, degradation of the surrounding material, and deposition of new ECM molecules. Here, we sought to better understand how initial matrix chemistry, by varying a cell binding epitope, might influence VIC interactions with the surrounding material environment, remodeling of the microenvironment, and long-term matrix deposition. We exploited a peptide-functionalized hydrogel system that is MMP-degradable and has been previously used to encapsulate and culture VICs for up to 4 weeks (Benton, et al., 2009). We began by using a similarly crosslinked hydrogels with mechanical properties that were essentially identical, but varied the adhesive peptide ligand from RGDS to VGVAPG to P15. Before examining VIC phenotypic characteristics and tissue deposition, we first studied VIC survival and metabolic activity as a function of the pendant peptide sequences.
3.1. VICs Survive in Peptide-Functionalized PEG Hydrogels
VICs were encapsulated in an MMP degradable, 2 kPa PEG hydrogel at a density of 15 million cells per mL, and the gels were covalently functionalized with three different adhesive small peptides (RGDS, VGVAPG, or P15) at a concentration of 2 mM (Fig. 1). A membrane integrity assay was used as a measure of VIC survival after 14 days of culture and using quantitative image analysis, we observed at least 95% VIC viability regardless of the adhesive peptide sequence (Fig. 2a). Additionally, VIC metabolic activity was similar in each gel formulation at days 2 and 14, with the exception of VICs in the RGDS-functionalized gels, which had a slightly higher metabolic activity after day 14 (Fig. 2b). Collectively, these results suggest that these peptide-functionalized PEG hydrogels are suitable for encapsulating and culturing VICs in three-dimensions, and each of the peptide epitopes provides interactions that promote survival.
Figure 2.
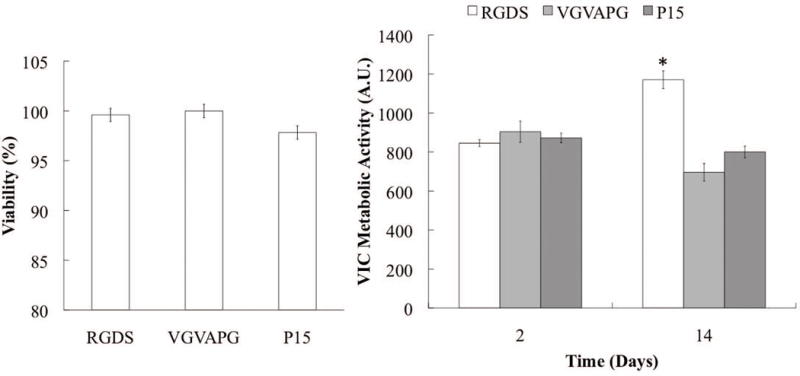
(a) VIC viability after 14 days in culture as a function of cell-matrix binding epitope. (b) VIC metabolic activity for each peptide as a function of culture time.
3.2. VICs Locally Remodel Their Microenvironment in Peptide-Functionalized PEG Hydrogels
While VICs survive in the RGDS, VGVAPG, and P15 functionalized gels, less is known about how these cell-matrix interactions influence local matrix remodeling and degradation of the gel. A simple, but indirect, measure of matrix degradation is to assess VIC morphology as a function of time, as VICs are initially rounded and confined when encapsulated in these covalently crosslinked gels. VIC morphology was imaged in bright field in each of the hydrogel matrices functionalized with RGDS, VGVAPG, or P15 after 2 and 14 days in culture. An elongation factor (cell length / cell width) was calculated from these images and was nearly 1 (i.e., a circular cell shape) at day 2 for all of the peptide compositions. However, by day 14, significant morphological differences were observed, as VICs in RGDS-functionalized gels were significantly more elongated (5.5) than VICs in either VGVAPG or P15-containing gels (1.2 and 1.6, respectively) (Fig. 3 a & b).
Figure 3.
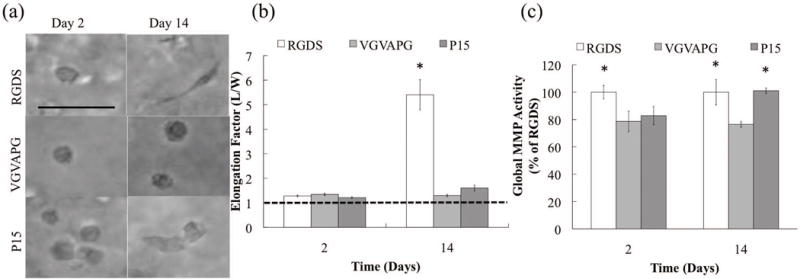
(a) Representative morphological images for each peptide on days 2 and 14. (b) Quantification of VIC elongation for each peptide as a function of culture time. Dotted line indicates a circular cell shape. (c) MMP Activity for each peptide as a function of culture time, which are normalized to the RGDS MMP activity on each day separately.
As these gels are crosslinked with peptide sequences derived from collagen-1 (KCGGPQGI↓WGQGCK & KCGGPQGI↓AGQGCK) that are degraded by cell secreted MMPs (Adelow, et al., 2008; Fairbanks, et al., 2009), we next measured global MMP activity as a function of gel composition. Results in Figure 3c reveal that all of the encapsulated cells had relatively high levels of MMP activity as compared to 2D (Supplemental Figure 1). However, on day 2, VICs encapsulated in RGDS-containing gels had a slightly higher MMP activity, compared to VGVAPG and P15 gels. In general, cell morphology and spreading appeared to correlate with global MMP activity, as some spreading is seen at later times in the P15 gels as well by day 14 when MMP activity is elevated.
3.3. VIC Activation and Matrix Deposition Depend on the Peptide Ligand Composition
Myofibroblasts can have substantially different secretory properties than quiescent fibroblasts, so we examined both VIC activation and matrix deposition in hydrogels of similar elasticity (E=2 kPa), but varying adhesive ligand content. By day 14, we observed between 33 to 56% of the VICs were αSMA positive (Fig. 4), which is higher than that typically found in native leaflets (Wirrig, et al., 2011), but significantly lower than VICs cultured on TCPS (essentially 100%) (Walker, et al., 2004). Interestingly, we observed the highest levels of αSMA positive VICs in the VGVAPG-containing gels (56%).
Figure 4.
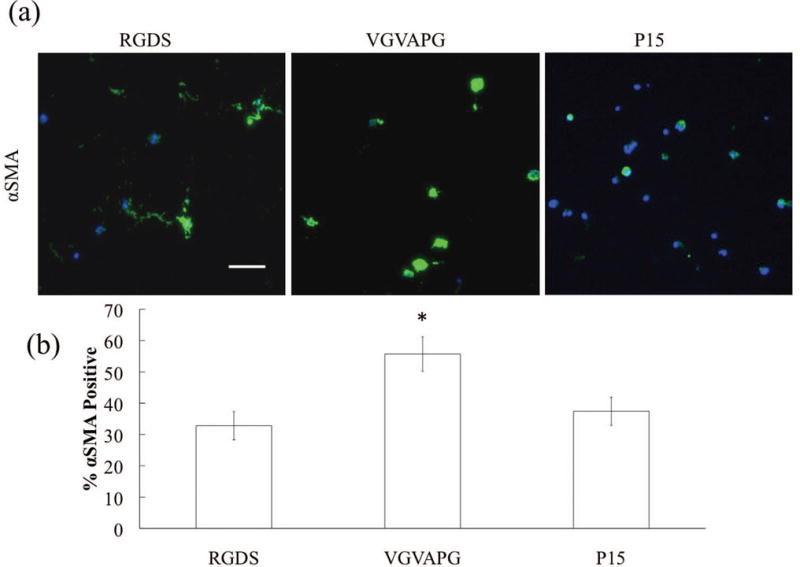
(a) Representative images of αSMA (green) immunostaining of VICs (nuclei in blue) after 14 days in culture. Scale bar = 100 μm. (b) Percentage of αSMA positive VICs as a function of adhesive small peptide after 14 days.
Early stage matrix deposition (14 days) was assessed by cryosectioning hydrogels and immunostaining for ECM molecules of interest (i.e., collagen-1, collagen-X, elastin, and chondroitin sulfate). Figure 5a contains representative images of VICs and their deposition of ECM molecules in response to each of the three small peptides, and Figure 5b summarizes the results from quantification of this type of images. VICs encapsulated in VGVAPG-functionalized gels deposited significantly less collagen-1 and significantly more collagen-X and chondroitin sulfate than those in RGDS-functionalized gels, while elastin deposition did not appear to be a function of peptide identity.
Figure 5.
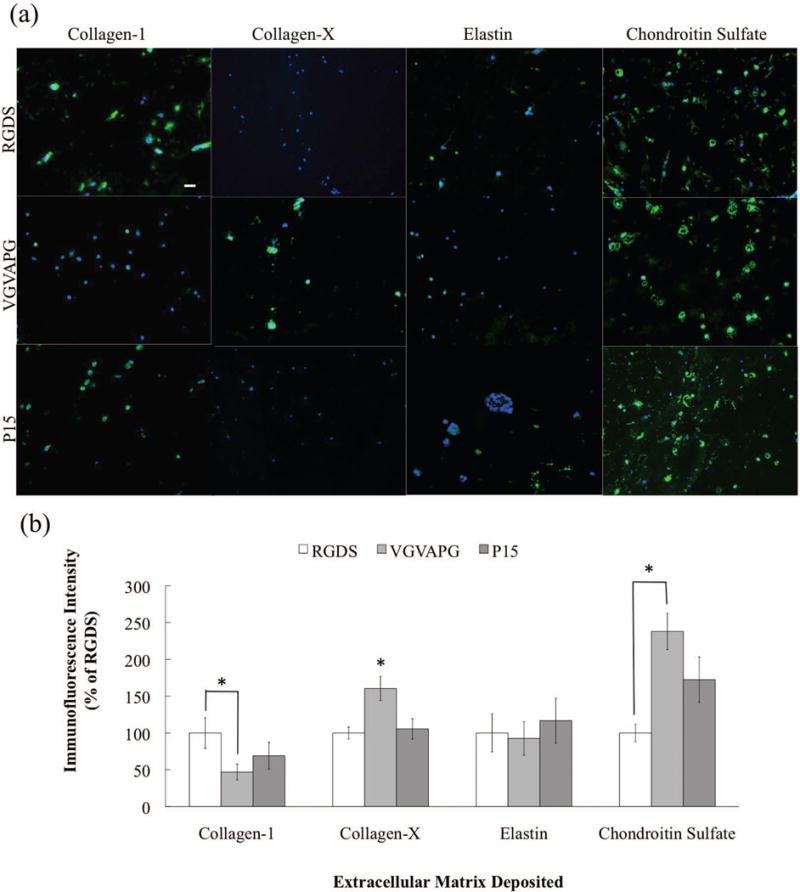
(a) Representative immunostaining images of deposited ECM molecules (green) by VICs (nuclei in blue) after 14 days in culture. Scale bar = 100 μm. (b) Amount of each ECM deposited as a percentage of the RGDS for that ECM molecule for each peptide condition after 14 days.
While this early time point analysis of tissue deposition yields some insight as to how initial cell-matrix interactions influence VIC activation and matrix synthesis, changes in the microenvironment are complex and can vary significantly with time. Since it is difficult to characterize this local remodeling and predict how these early events might influence longer-term matrix deposition in 3D, we extended our culture time to 42 days after which significant tissue is deposited in the hydrogels. Both biochemical and histological measures of matrix deposition were conducted. After 42 days in culture, collagen deposition (in blue) and distribution in RGDS-functionalized hydrogels approached that of the native porcine valve, more so than either VGVAPG or P15 functionalized gels, which only had collagen deposition in the pericellular region (Fig. 6a). No nonspecific staining of the remaining synthetic gel network was observed. When total deposition of each ECM molecule was quantified, all peptide-functionalized gels were very similar to the positive control (native porcine aortic valve); especially with respect to the overall composition of the matrix components (Fig. 6b). However, when collagen types were analyzed via immunostaining, VICs encapsulated in VGVAPG-functionalized gels had a significantly higher ratio of collagen-X to collagen-1 on days 14 and 42 than VICs in the RGDS-functionalized gels (Fig. 6c). Additionally, P15-functionalized gels had a significantly higher collagen-X to collagen-1 ratio than RGDS gels only on day 42. Immunostaining images of collagen-1 and collagen-X after 42days are shown in Supplemental Figure 2.
Figure 6.
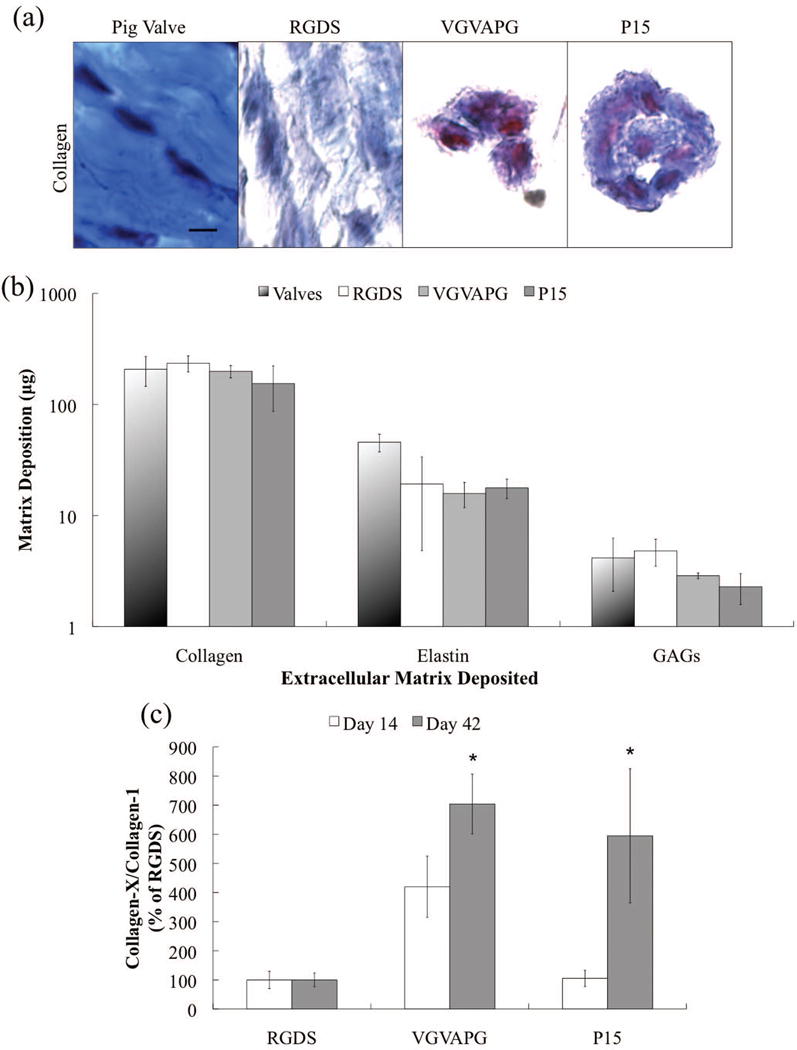
(a) Representative Masson’s Trichrome histology images for each peptide after 42 days and a native porcine aortic valve, with collagen deposition in blue and cytoplasm in red. No nonspecific staining of the remaining PEG hydrogel network was observed. Scale bar = 100 μm. (b) Mass (μg) of each type of ECM molecule deposited for each peptide after 42 days in culture. (c) The ratio of deposited collagen-X to collagen-1 as a percentage of the RGDS condition as a function of adhesive peptide 14 (white bars) and 42 (grey bars) days.
4. Discussion
Much insight to how matrix cues influence VIC function has been gained from two-dimensional culture studies. However, many aspects of these studies are difficult to translate to tissue engineering applications, as VICs are in an elastic, three-dimensional environment in vivo. This work aimed to elucidate how VICs respond to changes in a hydrogel environment, such as cell-matrix interactions, in three dimensions. Specifically, VIC functions like phenotype and tissue deposition were characterized by immunohistochemistry and colorimetric assays. Collectively, these results suggest variation in initial biochemical ligands presented in a gel environment directly impact a cascade of events that influence long term matrix deposition.
A peptide functionalized PEG gel was used as a synthetic ECM mimic where we selected an initial Young’s modulus of 2 kPa to more closely mimic valve tissue (Chen, et al., 2011), but varied the adhesive ligand composition. These peptide-functionalized gels were synthesized using a thiol-ene chemistry and facilitated VIC viability greater than 95% after 14 days of culture and sustained metabolic activity. This is important as a common concern with synthetic gel platforms, especially radical mediated photopolymerizations, is impaired cell viability and function (Lin, et al., 2011; Schmidt, et al., 2006). Further, RGDS-functionalized gels enabled increased VIC metabolic activity, which suggests higher levels of proliferation as compared to gels functionalized with VGVAPG or P15. Additionally, interaction with RGDS-functionalized gels facilitated more spreading and elongation of encapsulated VICs (i.e., a fibroblastic morphology). This variation in VIC elongation when presented with different cell-matrix interactions may be attributed to differences in remodeling of the surrounding synthetic gel environment mediated by the cells responding to the bound cell surface receptors.
We hypothesized that VICs interacting with RGDS were upregulating active MMPs as compared to those interacting with VGVAPG and P15. Indeed, VICs encapsulated in RGDS-functionalized gels had significantly higher MMP activity on both day 2 and 14, while the P15 MMP activity lagged behind and was only elevated on day 14. These differences in MMP activity may correlate with earlier remodeling of and spreading in the surrounding synthetic gel network microenvironment in the RGDS-functionalized gels as compared to P15 and VGVAPG, which both lag behind. While MMP activity is most likely a major player in VIC spreading, the local environment is very complex and other factors are likely at play as well. For example, the binding strength of integrins to these small peptides may also vary as well along with the local modulus, both of which could influence the ability of VICs to spread within these networks. Additionally, this result may be due to varied intracellular signaling caused by binding of different cell surface receptors or integrins, as RGDS is known to interact with multiple integrins and this adhesion sequence is found in many different ECM molecules. For example, the fibronectin variant interacts with the integrin α5β1 and the fibrinogen variant interacts with the integrin αvβ3 (Ruoslahti, 1996). Depending on which integrin VICs use to interact with RGDS, differences in intracellular signaling may occur and potentially translate into differences in gene expression (i.e., MMPs and tissue inhibitors of metalloproteinases (TIMPs)). This line of reasoning can also be applied to differences in binding to all integrins or the 67 kDa affinity binding protein, which is known to interact with elastin and laminin (Hinek, 1996) and is sometimes referred to as the laminin binding protein. The collagen-1 derived P15 and the elastin derived VGVAPG adhesive small peptides are known to bind to the integrin α2β1 (Hennessy, 2009) and the 67 kDa affinity binding protein (Gobin & West, 2003), respectively. Depending on which integrin or receptor is bound on the cell surface, genes associated with VIC phenotype and tissue deposition may be differentially regulated. However, more work needs to be done to isolate which signaling pathways are involved and if there is any crosstalk that may help explain differences in expression of genes like αSMA, which are known to be linked to the SMAD pathway (Li & Gotlieb, 2011). In addition to the type of integrin bound, the resistance behind that binding may also be an important aspect of in vitro VIC culture platform development in the future. For example, collagen is inherently more rigid than elastin, so binding to P15 may be more physiologically relevant in a stiffer matrix and similarly, the binding to VGVAPG a softer one. Here, we kept the initial gel elasticity (i.e., Young’s modulus) constant at 2 kPa, but this stiffness can be systematically increased or decreased via the network connectivity or crosslinking density.
VICs are known to alter their phenotype (i.e., fibroblast versus myofibroblast) in response to varied matrix interaction and matrix elasticity (Chen & Simmons, 2011; Gould, et al., 2012; Gu & Masters, 2010). For example, VIC activation has been observed to increase significantly when cultured on TCPS and in the presence of increasing RGD adsorbed to the TCPS (Ortega-Velazquez, et al., 2003). However, less is known about VIC response to other cell-matrix interactions that are present in the native aortic heart valve, like elastin (VGVAPG) and collagen-1 (P15), and in three-dimensions. Further, the effects of the local biophysical properties (e.g., elasticity) are difficult to predict, as VICs are constantly remodeling their surrounding environment. This remodeling complicates the measurement of the local matrix properties, especially in 3D.
In Figure 4, the percentage of myofibroblasts was quantified by the number of αSMA positive cells, and VIC myofibroblast properties depend on the biochemical composition of the adhesive ligands. However, one should note that VICs cultured in 3D were not observed to form stress fibers (Benton, et al., 2009). Interestingly, VGVAPG-functionalized gels caused a significant increase in the percentage of VICs expressing αSMA, and this result may be related to the lower levels of matrix degradation (i.e., least spread and lowest MMP activity) when VICs are cultured in these gels. Additionally, VGVAPG is known to upregulate expression of calcific aortic valve disease (CAVD)/bone associated genes like osteocalcin and alkaline phosphatase (ALP) (Simionescu, et al., 2005), and our results also demonstrate an increased deposition of the disease-associated collagen-X. These results suggest that VGVAPG may be activating intracellular signaling pathways associated with CAVD progression, and it would be worthwhile to follow up with additional experiments to test this hypothesis. One potential target may be the 67 kDa affinity binding protein that interacts with VGVAPG instead of α/β pair integrins. Very little is known about this affinity binding transmembrane protein and its role in gene expression regulation when bound; however, the 67 kDa binding protein is known to be upregulated in osteogenic-like smooth muscle cells (Simionescu, et al., 2005).
Differences in VIC activation have also been shown to influence secretory properties, including matrix molecules (Chester & Taylor, 2007; Chester, et al., 2008; Cushing, et al., 2005; Hinz, 2007; Masters, et al., 2005). Figure 6b demonstrates that VICs encapsulated in these peptide-functionalized PEG hydrogels are able to deposit levels of total collagen, elastin, and GAGs very similar to that of the porcine valve control. This result suggests that these hydrogels may be a good platform for manipulating VIC matrix deposition for valve regeneration research. Interestingly, when the composition of the deposited collagen was analyzed (Fig. 5b & 6c), VGVAPG-functionalized gels caused significantly higher deposition of collagen-X as compared to RGDS-functionalized gels at early and late time points. Collagen-X is only found in stenotic aortic valves (Wirrig, et al., 2011). These results are especially interesting because it suggests that initial interactions of VICs with the varying adhesive small peptides covalently tethered within the synthetic hydrogel platform, which are most likely masked by VIC tissue deposition within just a few days. These results also suggest that the initial biophysical and matricellular cues may be able to set VICs down a cascade of pathways and early stage tissue deposition that continue to influence their phenotype and matrix deposition even after 42 days in culture. Additionally, given that tissue deposition in RGDS- and P15-functionalized gels were generally not significantly different from one another, there may be more substantial differences in intracellular signaling between bound integrins and the 67kDa affinity binding protein than between different integrin α/β pairs (e.g., αvβ3/RGDS and α2β1/P15).
The influence of cell-matrix interactions on VIC matrix deposition is especially important in regenerating valve tissue because tissue distribution and composition both play critical roles in valve function (Sacks, et al., 2009). Unfortunately, less is known about cues that affect VIC tissue deposition, especially in three-dimensional studies that more accurately mimic the native environment. Much work still remains to study how various gel properties (e.g., elasticity, degradability, etc.) and biochemical signals like TGF-β, potentially used in conjunction with biomechanical stimulation, could be tuned to control VIC deposition of de novo matrix molecules to regenerate valve tissue for the treatment of CAVD.
5. Conclusions
Peptide-functionalized, PEG hydrogels provide a useful for platform for studying VICs in a 3D context, allowing for the long-term culture of VICs by permitting high levels of viability and metabolic activity. RGDS-functionalized gels were observed to promote a more fibroblastic (i.e., elongated) VIC morphology and tissue distribution and composition very similar to that of the native aortic valve. In contrast, VGVAPG-functionalized gels led to a significantly higher myofibroblast population and increased deposition of collagen-X, which is primarily associated with diseased valves, after 14 and 42 days. With this knowledge of how cell-matrix interactions influence VIC phenotype and tissue deposition in three-dimensions, we can begin to design more physiologically relevant synthetic culture platforms to promote or suppress diseased VIC function and understand how matrix chemistry can be manipulated to influence valve regeneration.
Supplementary Material
Supplemental Figure 1: Normalized MMP Activity in 3D RGDS functionalized gels and on TCPS after 2 days in culture.
Supplemental Figure 2: Immunostaining images for collagen-1 (top) and collagen-X (bottom) deposition in green by VICs (blue, nuclei) encapsulated at 15 million cells/mL in gels functionalized with RGDS, VGVAPG, or P15 after 42 days in culture. Scale bar = 100 μm.
Acknowledgments
The authors would like to thank Dr. Jennifer Leight and Dr. Nikki Farnsworth for experimental set-up and assay discussions, as well as acknowledge funding from HHMI and NIH (R01 HL089260).
Footnotes
Author Disclosure Statement: No competing financial interests exist.
References
- Adelow C, Segura T, Hubbell JA, Frey P. The Effect of Enzymatically Degradable Poly(Ethylene Glycol) Hydrogels on Smooth Muscle Cell Phenotype. Biomaterials. 2008;29:314–326. doi: 10.1016/j.biomaterials.2007.09.036. [DOI] [PubMed] [Google Scholar]
- Benton JA, Fairbanks BD, Anseth KS. Characterization of Valvular Interstitial Cell Function in Three Dimensional Matrix Metalloproteinase Degradable PEG Hydrogels. Biomaterials. 2009;30:6593–6603. doi: 10.1016/j.biomaterials.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman CN, Kloxin CJ. Toward and Enhanced Understanding and Implementation of Photopolymerization Reactions. AIChE Journal. 2008;54:2775–2795. [Google Scholar]
- Chen JH, Simmons CA. Cell-matrix interactions in the pathobiology of calcific aortic valve disease. Circ Res. 2011;108:1510–1524. doi: 10.1161/CIRCRESAHA.110.234237. [DOI] [PubMed] [Google Scholar]
- Chen JH, Chen WLK, Sider KL, Yip CYY, Simmons CA. β-Catenin Mediates Mechanically Regulated, Transforming Growth Factor-β 1–Induced Myofibroblast Differentiation of Aortic Valve Interstitial Cells. Arterioscler Thromb Vasc Biol. 2011;31:590–597. doi: 10.1161/ATVBAHA.110.220061. [DOI] [PubMed] [Google Scholar]
- Chester AH, Taylor PM. Molecular and Functional Characteristics of Heart-Valve Interstitial Cells. Phil Trans R Soc B. 2007;362:1437–1443. doi: 10.1098/rstb.2007.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester AH, Latif N, Yacoub MH, Taylor PM. Vascular Complications in Human Disease, Chapter 18: Aortic Valve: From Function to Tissue Engineering. London, England: Springer London; 2008. [Google Scholar]
- Clarke DR, Campbell DN, Hayward AR, Bishop DA. Degeneration of aortic valve allografts in young recipients. J Thorac Cardiovasc Surg. 1993;105:934–941. [PubMed] [Google Scholar]
- Cushing MC, Jaeggli MP, Masters KS, Leinwand LA, Anseth KS. Serum Deprivation Improves Seeding and Repopulation of Acellular Matrices with Valvular Interstitial Cells. J Biomed Mater Res A. 2005;75:232–241. doi: 10.1002/jbm.a.30412. [DOI] [PubMed] [Google Scholar]
- Cushing MC, Liao JT, Anseth KS. Activation of Valvular Interstitial Cells is Mediated by Transforming Growth Factor-Beta1 Interactions with Matrix Molecules. Matrix Bio. 2005;24:428–437. doi: 10.1016/j.matbio.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Durst CA, Cuchiara MP, Mansfield EG, West JL, Grande-Allen KJ. Flexural characterization of cell encapsulated PEGDA hydrogels with applications for tissue engineered heart valves. Acta Biomater. 2011;7:2467–2476. doi: 10.1016/j.actbio.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmayr GC, Rabkin E, Sutherland FW, Schoen FJ, Mayer JE, Sacks MS. The independent role of cyclic flexure in the early in vitro development of an engineered heart valve tissue. Biomaterials. 2005;26:175–187. doi: 10.1016/j.biomaterials.2004.02.035. [DOI] [PubMed] [Google Scholar]
- Fairbanks BD, Schwartz MP, Halevi AE, Nuttleman CR, Bowman CN, Anseth KS. A Versatile Synthetic Extracellular Matrix Mimic Via Thiol-ene Photopolymerization. Adv Mater. 2009;21:5005–5010. doi: 10.1002/adma.200901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip DA, Radu A, Simionescu M. Interstitial Cells of the Heart Valves Possess Characteristics Similar to Smooth Muscle Cells. Circ Res. 1986;59:310–320. doi: 10.1161/01.res.59.3.310. [DOI] [PubMed] [Google Scholar]
- Flanagan TC, Wilkins B, Black A, Jockenhoevel S, Smith TJ, Pandit AS. A collagen-glycosaminoglycan co-culture model for heart valve tissue engineering applications. Biomaterials. 2006;27:2233–2246. doi: 10.1016/j.biomaterials.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Gacchina CE, Deb PP, Barth J, Ramamurthi A. Elastogenic Inductability of Smooth Muscle Cells from a Rat Model of Late-Stage Abdominal Aortic Aneurysms. Tissue Eng Part A. 2011;17:1699–1711. doi: 10.1089/ten.tea.2010.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobin AS, West JL. Val-ala-pro-gly, an Elastin-derived Non-integrin Ligand: Smooth Muscle Cell Adhesion and Specificity. J Biomed Mater Res A. 2003;67A:255–259. doi: 10.1002/jbm.a.10110. [DOI] [PubMed] [Google Scholar]
- Gould ST, Darling NJ, Anseth KS. Small Peptide Functionalized Thiol-Ene Hydrogels as Culture Substrates for Understanding Valvular Interstitial Cell Activation and de novo Tissue Deposition. Acta Biomater. 2012;8:3201–3209. doi: 10.1016/j.actbio.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Masters KS. Regulation of Valvular Interstitial Cell Calcification by Adhesive Peptide Sequences. J Biomed Mater Res A. 2010;93A:1620–1630. doi: 10.1002/jbm.a.32660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henaine R, Roubertie F, Vergnat M, Ninet J. Valve replacement in children: A challenge for a whole life. Arch Cardiovasc Dis. 2012;105:517–528. doi: 10.1016/j.acvd.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Hennessy K. The effect of collagen I mimetic peptides on mesenchymal stem cell adhesion and differentiation, and on bone formation at hydroxyapatite surfaces. Biomaterials. 2009;30:1898–1909. doi: 10.1016/j.biomaterials.2008.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A. Biological roles of the non-integrin elastin/laminin receptor. Biol Chem. 1996;377:471–480. [PubMed] [Google Scholar]
- Hinz B. Formation and Function of the Myofibroblast During Tissue Repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- Holledge MM, Millward-Sadler SJ, Nuki G. Mechanical regulation of proteoglycan synthesis in normal and osteoarthritic human articular chondrocytes - roles for alpha 5 and alpha V beta 5 integrins. Biorheology. 2008;45:275–288. [PubMed] [Google Scholar]
- Kloxin AM, Benton J, Anseth KS. In situ Elasticity Modulation with Dynamic Substrates to Direct Cell Phenotype. Biomaterials. 2009;31:1–8. doi: 10.1016/j.biomaterials.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le NT, Fleming BC. Measuring fixed charge density of goat articular cartilage using indentation methods and biochemical analysis. J Biomech. 2008;41:715–720. doi: 10.1016/j.jbiomech.2007.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBaron RG, Athanasiou KA. Extracellular matrix cell adhesion peptides: functional applications in orthopedic materials. Tissue Eng. 2000;6:85–103. doi: 10.1089/107632700320720. [DOI] [PubMed] [Google Scholar]
- Lee DA, Assoku E, Doyle V. A specific quantitatie assay for collagen synthesis by cells seeded in collagen-based biomaterials using sirius red F3B precipitation. J Mater Sci Mater Med. 1998;9:47–51. doi: 10.1023/a:1008882628142. [DOI] [PubMed] [Google Scholar]
- Li C, Gotlieb AI. Transforming Growth Factor-Beta Regulates the Growth of Valve Interstitial Cells in Vitro. Am J Pathol. 2011;179:1746–1755. doi: 10.1016/j.ajpath.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CC, Raza A, Shih H. PEG hydrogels formed by thiol-ene photo-click chemistry and their effect on the formation and recovery of insulin-secreting cell spheroids. Biomaterials. 2011;32:9685–9695. doi: 10.1016/j.biomaterials.2011.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JL, Tranquillo RT. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol. 2003;22:339–350. doi: 10.1016/s0945-053x(03)00052-0. [DOI] [PubMed] [Google Scholar]
- MacNeil S, Bains S, Johson C. Gadolinium Contrast Agent Associated Stimulation of Human Fibroblast Collagen Production. Invest Radio. 2011;46:711–717. doi: 10.1097/RLI.0b013e31822b1f38. [DOI] [PubMed] [Google Scholar]
- Mann BK, Tsai AT, Scott-Burden T, West JL. Modification of surfaces with cell adhesion peptides alters extracellular matrix deposition. Biomaterials. 1999;20:2281–2286. doi: 10.1016/s0142-9612(99)00158-1. [DOI] [PubMed] [Google Scholar]
- Masters KS, Shah DN, Leinwand LA, Anseth KS. Crosslinked Hyaluronan Scaffolds as a Biologically Active Carrier for Valvular Interstitial Cells. Biomaterials. 2005;26:2517–2525. doi: 10.1016/j.biomaterials.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Ortega-Velazquez R, Diez-Marques ML, Ruiz-Torres MP, Gonzalez-Rubio M, Rodriquez-Puyol M, Rodriquez-Puyol D. Arg-Gly-Asp-Ser Peptide Stimulates Transforming Growth Facotr-Beta1 Transcription and Secretion Through Integrin Activation. FASEB Journal. 2003;17:1529–1531. doi: 10.1096/fj.02-0785fje. [DOI] [PubMed] [Google Scholar]
- Patterson J, Hubbell JA. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 2010;31:7836–7845. doi: 10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- Pierschbacher MD, Ruoslahti E. Cell Attachment Activity of Fibronectin can be Duplicated by Small Synthetic Fragments of the Molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Gottlieb D, Engelmayr GC, Aikawa E, Schmidt DE, Gaitan-Leon DM. The role of organ level conditioning on the promotion of engineered heart valve tissue development in-vitro using mesenchymal stem cells. Biomaterials. 2010;31:1114–1125. doi: 10.1016/j.biomaterials.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez MK, Rodriguez KJ, Masters KS. Regulation of Valvular Interstitial Cell Calcification by Components of the Extracellular Matrix. J Biomed Mater Res A. 2008;90A:1043–1053. doi: 10.1002/jbm.a.32187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriquez KJ, Piechura LM, Masters KS. Regulation of valvular interstitial cell phenotype and function by hyaluronic acid in 2-D and 3-D culture. Matrix Biol. 2011;30:70–82. doi: 10.1016/j.matbio.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- Sacks MS, Merryman WD, Schmidt DE. On the Biomechanics of Heart Valve Function. J Biomech. 2009;42:1804–1824. doi: 10.1016/j.jbiomech.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt O, Mizrahi J, Elisseeff J, Seliktar D. Immobilized Fibrinogen in PEG Hydrogels Does not Improve Chondroctye-Mediated Matrix Depositionin Response to Mechanical Stimulation. Biotechnol Bioeng. 2006;95:1061–1069. doi: 10.1002/bit.21072. [DOI] [PubMed] [Google Scholar]
- Shah DN, Recktenwall-Work SM, Anseth KS. The effect of bioactive hydrogels on the secretion of extracellular matrix molecules by valvular interstitial cells. Biomaterials. 2008;29:2060–2072. doi: 10.1016/j.biomaterials.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu A, Philips K, Vyavahare N. Elastin-derived peptides and TGF-beta1 induce osteogenic responses in smooth muscle cells. Biochem Biophys Res Commun. 2005;334:524–532. doi: 10.1016/j.bbrc.2005.06.119. [DOI] [PubMed] [Google Scholar]
- Srokowski EM, Blit PH, McClung WG, Brash JL, Woodhouse KA. Platlet adhesion and fibrinogen accretion on a family of elastin-like polypeptides. J Biomater Sci, Polymer Ed. 2001;22:41–57. doi: 10.1163/092050609X12578498935594. [DOI] [PubMed] [Google Scholar]
- Syedain ZH, Transquillo RT. Controlled cyclic stretch bioreactor for tissue-engineered heart valves. Biomaterials. 2009;30:4078–4084. doi: 10.1016/j.biomaterials.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Throm A, Hinds H, Billiar K. Valvular interstitial cell mechanobiology: effects of substrate stiffness. J Biomech. 2006;39:S621. [Google Scholar]
- Walker GA, Masters KS, Shah DN, Anseth KS. Valvular Myofibroblast Activation by Transforming Growth Factor-Beta: Implications for Pathological Extracellular Matrix Remodeling in Heart Valve Disease. Circ Res. 2004;95:253–260. doi: 10.1161/01.RES.0000136520.07995.aa. [DOI] [PubMed] [Google Scholar]
- Wilda H, Merry CL, Blaker JJ. Three-dimensional culture of annulus fibrosus cells within PDLLA/Bioglass (R) composite foam scaffolds: Assessment of cell attachment, proliferation and extracellular matrix production. Biomaterials. 2007;28:2010–2020. doi: 10.1016/j.biomaterials.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Wirrig EE, Hinton RB, Yutzey KE. Differential Expression of Cartilage and Bone-Related Proteins in Pediatric and Adult Diseased Aortic Valves. J Mol Cell Cardiol. 2011;50:561–569. doi: 10.1016/j.yjmcc.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip CY, Chen JH, Zhao R, Simmons CA. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler Thromb Vasc Biol. 2009;29:936–942. doi: 10.1161/ATVBAHA.108.182394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Normalized MMP Activity in 3D RGDS functionalized gels and on TCPS after 2 days in culture.
Supplemental Figure 2: Immunostaining images for collagen-1 (top) and collagen-X (bottom) deposition in green by VICs (blue, nuclei) encapsulated at 15 million cells/mL in gels functionalized with RGDS, VGVAPG, or P15 after 42 days in culture. Scale bar = 100 μm.


