Abstract
Significant challenges remain in targeting drugs to diseased vasculature; most important being rapid blood flow with high shear, limited availability of stable targets, and heterogeneity and recycling of cellular markers. We developed nanoparticles (NPs) to target degraded elastic lamina, a consistent pathological feature in vascular diseases. In-vitro organ and cell culture experiments demonstrated that these NPs were not taken up by cells, but instead retained within the extracellular space; NP binding was proportional to the extent of elastic lamina damage. With three well-established rodent models of vascular diseases such as aortic aneurysm (calcium chloride mediated aortic injury in rats), atherosclerosis (fat-fed apoE−/− mice), and vascular calcification (warfarin + vitamin K injections in rats), we show precise NPs spatial targeting to degraded vascular elastic lamina while sparing healthy vasculature when NPs were delivered systemically. Nanoparticle targeting degraded elastic lamina is attractive to deliver therapeutic or imaging agents to the diseased vasculature.
Keywords: Vascular nanomedicine, elastin, nanoparticles, extracellular matrix targeting
Introduction
Targeting drugs to diseased vessel walls is difficult. Due to high shear flow conditions in arteries, oral, parenteral, and intra-arterial administrations of therapeutics have shown limited success and unwanted systemic side effects in treating vascular diseases.1 For example, anticoagulants administered for coronary thrombotic occlusions have severe side-effects of hemorrhage2 and systemic doxycycline delivery for treatment of abdominal aortic aneurysm (AAA) has shown negligible therapeutic benefit and side-effects such as cutaneous photosensitive reactions, tooth discoloration, gastro-intestinal symptoms, and yeast infection.3 To maximize therapeutic benefit and minimize off-target effects, many approaches to target vasculature are focused on cell markers on activated endothelium, vascular smooth muscle cells, or inflammatory cells such as macrophages.4-6 Although abundantly over-expressed in inflammatory conditions, these markers provide limited targeting as they are heterogeneous and transiently expressed in the vasculature and undergo physiological receptor recycling. One of the consistent features of vascular disease is the fragmentation and degradation of elastic lamina. Elastic fibers in a healthy aorta are coated with microfibrillar glycoproteins such as fibrillin and fibulins, among others.7, 8 Elastic lamina degradation is observed in vascular calcification in age-related elastocalcinosis (Monckenberg’s sclerosis)9, 10, diabetes,11 end-stage renal disease12, 13, and aortic aneurysm.14 In addition, vascular proliferative diseases affecting smaller arteries like coronary arterial occlusion and atherosclerotic-mediated stenosis share common pathological features such as elastin degradation15, 16 and accumulation of vascular smooth muscle cells in the intima.17 In such disease conditions, elastic lamina is degraded and the amorphous elastin core protein is exposed (Figure 1B). Taking advantage of this characteristic pathological feature, we developed a novel biodegradable nanoparticle system that can be targeted to the site of vascular degraded-elastic fibers. Successful targeting of degraded elastic lamina provides the advantage of particle retention in the extracellular matrix as opposed to the rapid cellular uptake generally found in cell-targeted therapies thereby enabling delivery of several agents to the extracellular space.
Figure 1.
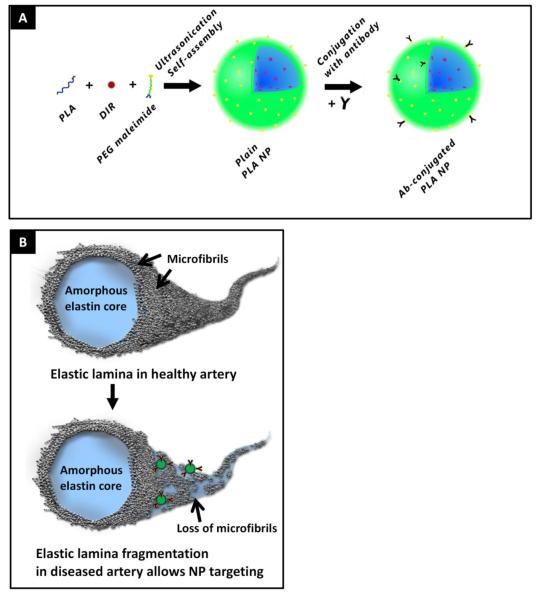
(A) Nanoparticle schematic. Nano-precipitation method was used to create PLA nanoparticles. DIR fluorescent dye (red) was incorporated to track particles. Particle surfaces were coated with PEG maleimide groups (PEG as green, maleimide as yellow) where antibodies were bound with thiol chemistry. (B) Elastic lamina fibers schematic. In healthy elastic lamina, core amorphous elastin is coated on the surface with microfibrills such as fibrillins and fibulins. In the diseased state, the microfibrillar proteins degrade along with amorphous elastin, thus exposing core elastin. Nanoparticles (NPs) coated with antibodies that are specific to core elastin are used to target degraded elastic lamina in diseased artery while sparing healthy vessel with native elastic lamina.
In this paper, we show the feasibility of targeting nanoparticles to degraded elastic lamina when administered intravenously. This minimally invasive technique can augment the concentrations of drug and imaging agents at the site of vascular damage, mitigate the undesirable effects of systemic delivery, and increase efficacy of the drug.
Methods
Nanoparticle preparation: Detailed methods of NP preparation and antibody conjugation are provided in an online supplement
Briefly, poly (D,L-lactide) (PLA) nanoparticles loaded with 1, 1-dioctadecyl-3, 3, 3, 3-tetramethylindotricarbocyanine iodide (DIR) dye were prepared using nanoprecipitation method. Surface maleimide groups were conjugated with thiolated elastin/IgG antibodies to create elastin antibody tethered NPs (EL-NPs) or IgG antibody tethered NPs (IgG-NPs). The extent of antibody conjugation is reported in the results section.
Transmission electron microscopy of immunogold-stained nanoparticles
The IgG antibody tethered nanoparticles (IgG-NPs) (30μg) were incubated with 10 nm gold stained goat-anti-rabbit IgG (5×1011 gold particles) (Sigma Aldrich, St.Louis, MO) overnight in 0.018M tris buffered saline (TBS), pH=8, with 0.9% bovine serum albumin and 17% glycerol. Following incubation, the particles were washed twice with TBS to remove unbound antibodies at 14,000×g for 15 minutes, deposited in a formvar-coated copper grid followed by negative staining with 2% phosphotungstic acid and examined directly with transmission electron microscopy.
Antibody concentration and correlation
An independent set of experiments was performed to establish the effect of antibody concentration on the binding yield of antibody to the particles (see supplemental files for details).
Ex-vivo nanoparticle binding studies for elastase treated aorta
Aortas from Sprague-Dawley rats were explanted, rinsed and treated with high purity porcine pancreatic elastase (Elastin products company, Owensville, Missouri) for 10, 20, 30, 60 mins. The aortas were clamped on either ends, and EL-NPs/IgG-NPs loaded with DIR dye were injected intra-luminally for 1 hour at room temperature (10 mg polymer/aorta). After washing, NPs attached to the aorta were quantified with DIR dye HPLC.
A separate set of experiments was conducted to evaluate the effect of antibody concentration on nanoparticle surface and binding efficiency to elastase treated aorta.
Cytotoxicity and cellular uptake of EL-NPs
Rat aortic vascular smooth muscle cells (passage 6) were treated with 2.5, 10 and 25 μg elastin antibody concentration and 100, 500 μg/ml PLA concentrations to evaluate cytotoxicity and cellular uptake of EL-NPs. Cells were seeded at 10,000 cells/cm2. At 70% confluency, cells were incubated with nanoparticles for 4 hours following which cell viability was determined using a LIVE/DEAD Cell Viability assay (Molecular Probes, Grand Island, NY). Cells fluorescing green are considered alive while cells fluorescing red are considered dead. Additionally, proliferation of cells was estimated using the MTT (3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) assay.
To visualize the internalization of nanoparticles, nanoparticles of different sizes and surface charge and loaded with DIR dye were prepared and tested for cellular uptake. Cells were visualized by the lipophilic membrane stain DiI (Invitrogen, Carlsbad, CA), and the nuclear dye 4 ,6-diamidino-2-phenyindole (DAPI; Vector Laboratories, Burlingame, CA).
In vivo nanoparticle targeting: Elastin degradation created by local periadventitial CaCl2 application
Vascular injury was created in rats (n=6) by periadventitial application of 0.5M CaCl2 for 15 minutes to the infra-renal abdominal aorta using a strip of pre-soaked sterile cotton gauze. The abdominal cavity was closed with subcutaneous suture. The elastin degradation was allowed to develop for 10 days. After 10 days, the rats were anesthetized, PLA anti-elastin nanoparticles (EL-NPs) or PLA anti-IgG control nanoparticles (IgG-NPs) were either injected in 0.3% rat serum albumin (Sigma Aldrich, St.Louis, MO) through the tail vein of the rats (10 mg of polymer/kg body weight) or delivered locally by intraluminal treatment of the isolated aorta. The injured infra-renal aorta was clamped on either ends, catheterized, and particles (10 mg polymer/kg body weight) were locally perfused intra-luminally for 5 minutes (100 μl). The rats were euthanized 24 hours post injection. The harvested aortae were imaged using Caliper IVIS Lumina XR (Hopkinton, MA) with Ex/Em of 745/795.
Brain, heart, blood, muscle skin, liver, kidneys, spleen, and lungs, were examined for fluorescence for determining bio-distribution. Only the organs that presented with fluorescence were lyophilized and the total fluorescence was normalized to the total dry weight of each organ. Targeting was calculated as follows:
In vivo nanoparticle targeting: Atherosclerotic plaque created in ApoE −/− mice by feeding high fat diet
Male apoE knockout (KO) mice (B6.129P2-Apoetm1Unc/J, Jackson Labs (No. 002052)), 9.5-11 months of age on western diet, were used in these experiments. Healthy mice were used as control groups. For the in vivo imaging study, mice (n=4) were anesthetized with 2% isoflurane and injected retro-orbitally with PLA anti-elastin nanoparticles (EL-NPs) in 0.3% mouse serum albumin (Sigma Aldrich, St.Louis, MO) in a total volume of 250 μl. At 24 hours after tracer injection, the mice were euthanized by CO2 asphyxiation and perfused with heparinized normal saline at ~100 mm Hg via a left ventricular puncture. The aortic trees were harvested, imaged ex vivo on the IVIS Spectrum, and frozen for histology. Additional organs (brain, heart, blood, muscle skin, liver, kidneys, spleen, and lungs) were harvested at the same time to assess bio-distribution of the injected nanoparticles.
In vivo nanoparticle targeting: Warfarin + Vitamin K model for systemic calcification
6 week old male Sprague Dawley rats (n=6) were given subcutaneous injections Vitamin K1 (10 mg/ml, 15 mg.kg-1.day-1 subcutaneous injection, every other day) and Warfarin (20 mg.kg-1.day-1) in drinking water. Small needles (25 G or smaller) were used and the subcutaneous injection sites were rotated between the 4 quadrants of the back to reduce stress. This routine was maintained for 3 weeks. The control group rats were age-matched and maintained normally with no treatment. At the end of 3 weeks, EL-NPs were injected through the tail vein of the rats. Following 24 hours of circulation, whole animals were euthanized and imaged using Calliper IVIS imaging system. The individual organs were also imaged to calculate the biodistribution and targeting of the nanoparticles. Explanted aorta were rinsed with phosphate buffered saline (PBS), embedded in Tissue CT compound, and frozen at −80°C.
The animal study was carried out in accordance with the recommendations in the Guide for the Humane Care and Use of Laboratory Animals of the National Institutes of Health and all efforts were made to minimize suffering. All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committees.
Statistical data analysis
Results are expressed as means ± standard error of the mean (SEM). Statistical analyses of the data were performed using single-factor analysis of variance (ANOVA). Subsequently, differences between means were determined using the least significant difference (LSD) with an alpha value of 0.05.
Results
Characterization of antibody-nanoparticle conjugates
We prepared poly L-lactic acid (PLA) nanoparticles (NPs) with surface bound PEG maleimide groups. The maleimide groups were reacted with either thiolated elastin antibodies (abbreviated as EL-NPs) or thiolated control IgG antibodies (abbreviated as IgG-NPs) to coat surfaces with antibodies (Figure 1A). Incorporation of DIR dye into the nanoparticles facilitated visualization and tracking of NPs. The DIR dye was permanently associated with the particles and did not leach out over seven days of buffer incubation (data not shown). Particle size was determined using dynamic light scattering (DLS) for bare and antibody coated nanoparticles. Over multiple experiments, all nanoparticle groups consistently sized approximately 200 ± 16 nm and had negatively charged surfaces (Table 1). The antibody conjugated nanoparticles were generally spherical with uniform size when examined under AFM (data not shown).
Table I.
Characterization of nanoparticles
| Type of nanoparticle | Size (nm) | Poly dispersity index | ζ- potential (mV) |
|---|---|---|---|
| Blank NP | 222 | .136 | −27.91 |
| NP + PEGmaleimide + DIR | 226 | .152 | −66 |
| NP + PEGmaleimide + DIR 4μg elastin antibody |
238 | .26 | −41.93 |
Antibody binding yield depends on initial antibody concentration
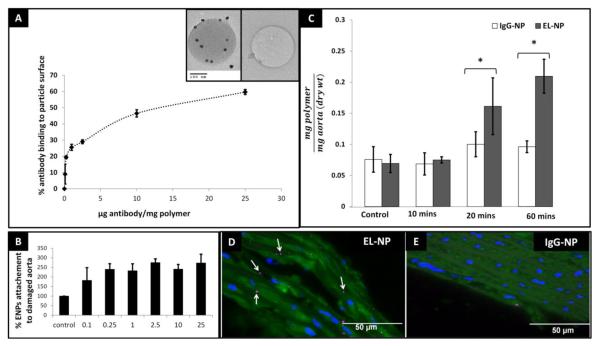
A direct positive correlation was established between the binding yield of the antibody and initial antibody concentration (Figure 2A). Increasing antibody binding leads to decrease in free PEG hydroxyl groups on the surface. Thus, to balance the amount of antibody and PEG groups so that optimum circulation time (provided by PEG) and site specific binding (provided by antibody) could be achieved, we chose the 4 μg/mg concentration for further examination which demonstrated ~30% binding yield. NP-antibody conjugation was studied through immunogold staining with TEM. Successful antibody conjugation was visualized as dark 10 nm gold particles under TEM. Control particles without antibody on the surface showed no gold staining (inset Figure 2A).
Figure 2.
(A) Antibody binding yield to nanoparticles increases with increasing starting antibody concentration. Insets show TEM for immunogold labeling of antibody coated nanoparticles gold-stained IgG (seen as small dark spots) indicate the presence of antibody on the particle surface and negative control without antibody on surface. (B) Increasing antibody surface density enhances target attachment efficiency up to 0.25 μg /mg polymer and stabilizes with further increase in surface antibody density. (C) Ex-vivo attachment of EL-NPs specifically to degraded elastin in rat aorta. Rat aorta treated with elastase for various times showing NP attachment. IgG-NPs/EL-NPs were administered intraluminally and incubated for 60 minutes. Significantly higher EL-NP attachment was seen as compared to IgG-NPs. (D) Histological assessment of EL-NPs attachment to elastase treated aorta (seen as pink dots) compared to lack of IgG-NPs adherence in elastase treated aorta (E).
Ex-vivo determination of EL-NP binding specificity to degraded elastic matrix
Isolated rat aortae were treated with elastase in vitro for 60 min to mimic elastin degradation. An increase in EL-NPs attachment efficiency to the elastase treated aorta was found with increase in surface antibody concentration when NPs were intraluminally delivered (Figure 2B). At 2.5 μg/ mg polymer ~2.8 fold increase in attachment was recorded when compared to IgG-NP control group. Further increase in surface antibody concentration did not increase attachment efficiency. To test NPs targeting in vitro, isolated rat aortae were treated with elastase for 10, 20, and 60 minutes respectively, creating various degrees of elastic lamina damage. Elastic fiber degradation was confirmed by Verhoeff’s Van Gieson (VVG) stain (data not shown). On intraluminal injection of EL-NPs and IgG-NPs, an increasing adherence of EL-NPs with greater elastic damage was observed (Figure 2B) as assessed by quantitative fluorescence in the tissue. After 60 minutes of elastase mediated elastin degradation, significantly higher EL-NP attachment in comparison with IgG-NP groups was observed. Elastase untreated aortae (control groups) showed negligible adherence of NPs demonstrating the necessity of elastic fiber degradation for EL-NP targeting. EL-NP attachment to the aorta was further confirmed through histological assessment, where NPs were visualized as purple dots along the fragmented media that were evidently absent in the control IgG-NP groups (Figure 2 C and D).
EL-NP internalization by vascular smooth muscle cells: In-vitro cell culture studies
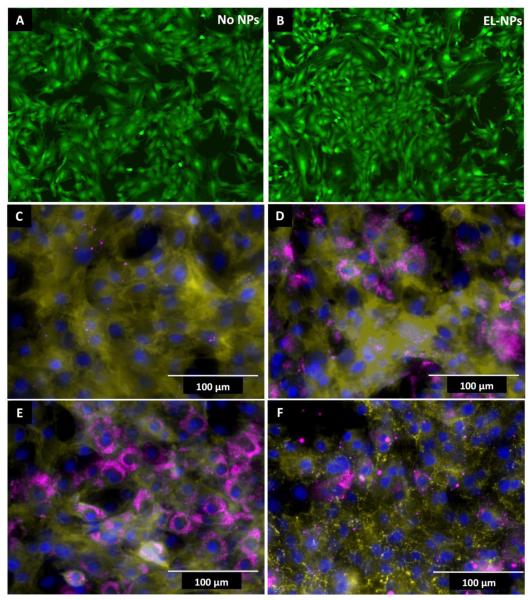
The effect of elastin antibody coated NPs on the viability of rat aortic smooth muscle cells in vitro was assessed using quantitative (MTT) and qualitative (LIVE-DEAD) assays after culturing cells in the presence of nanoparticle for four hours. Both assays showed no acute cytotoxicity associated with the nanoparticles (Live-Dead assay shown in Figure 3A-B). Next we examined the effect of particle size and charge on the intracellular uptake of DIR loaded-ELNPs. A majority of the nanoparticles (>200nm and negative chage) were confined to the extracellular space at the end of 24 hours (Figure 3C). To test if surface charge or size affects cellular uptake, we reduced the size to below 100 nm while maintaining negative charge. These NPs were taken up by cells (Figure 3D). To determine the effect of surface charge on cellular uptake of NPs, EL-NPs were incubated with poly-L-lysine to create positively charged particle surface (ζ-potential 28.76 mV). When cells were presented with these positively charged particles, an enhanced cellular uptake was found; this uptake was more pronounced in smaller positively charged EL-NPs (Figure 3E) as compared to larger positively charged EL-NPs (Figure 3F). Overall, both size (>200 nm) and negative charge of the EL-NPs were essential to minimize cellular internalization.
Figure 3.
Cell viability and evaluation of cellular uptake of EL-NPs. (A and B) Live-dead assay on cells treated with and without EL-NPs showing no change in cell viability; green = viable cells, red = dead cells. (C) Cellular exclusion of EL-NP of large (> 200nm) nanoparticles with negative surface charge and (D) uptake of small (<100nm) nanoparticles with negative surface charge, (E) small particles treated with poly-L-lysine to create positive surface charge and (F) large particles treated with poly-L-lysine positive surface charge. Negatively charged EL-NPs are excluded by cells when size >200nm compared to <100nm particles. Increasing positive surface charge enhances cellular uptake even of larger particles. Yellow = DiI stained cell membrane, Blue = DAPI stained nucleus, Purple = DIR dye encapsulated nanoparticles.
In vivo models for targeting NPs to diseased vasculature
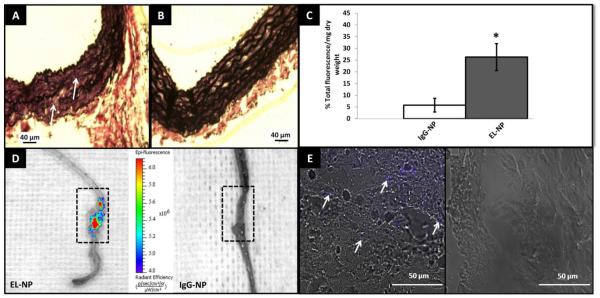
To evaluate the targeting efficiency of EL-NPs, we used three different animal models representing three prevalent vascular diseases; namely, aortic aneurysms (calcium chloride injury rat model), atherosclerosis (fat fed apoE−/− mice), and vascular medial calcification (systemic delivery of warfarin and vitamin K) in rats. First, we created a Calcium Chloride (CaCl2) mediated perivascular injury in the infra-renal abdominal aorta of rats that has previously been shown to stimulate degradation of the elastic lamina 18, 19 Single perivascular application of 0.5 mol/L CaCl2 to the infra-renal abdominal aorta for 15 minutes induced elastic fiber degradation after 10 days (Figure 4A) showing a distinct discontinuity and thinning of elastic fibers in the medial layer of the artery while un-injured control aorta showed undisturbed, wavy elastin fibers (Figure 4B). Ten days after calcium chloride injury, EL-NPs/IgG-NPs were administered intravenously to evaluate targeting efficiency. Based on in-vitro cytotoxicity experiments, we determined ~100 μg/ml nanoparticle concentration is well within the tolerable range. Assuming 20 ml total blood volume in rats, we administered 10 mg of NPs/kg body weight suspended in PBS (250 μl) as one time bolus intravenous injection and allowed NPs to circulate and target for 24 hours. Nanoparticles with surface bound elastin antibody (EL-NP) showed ~5 fold greater targeting to the injured abdominal aorta as compared to nanoparticles with surface IgG antibody (IgG-NP) as measured by % total fluorescence/g tissue weight (Figure 4C). When whole aortae were imaged with IVIS instrument, a strong fluorescence in the area of elastin degradation site in EL-NP group (Figure 4D, boxed area) was observed. In comparison, control nanoparticles with surface IgG antibody showed no fluorescence in the impaired aorta (Figure 4D). Histological assessment further revealed the penetration of EL-NPs into the medial arterial layer (evidenced by the purple coloration in Figure 4E), clearly absent in IgG-NP control group. This data strongly indicated the precise spatial accumulation of EL-NPs to sites of elastic damage, even under high-shear hemodynamic conditions. Importantly, no NPs attachment was observed in the healthy vasculature where elastic lamina was intact.
Figure 4.
Targeting of EL-NPs in aneurysm model. (A) VVG stain of rat aorta 10 days after calcium chloride treatment, (arrow marks indicate fragmented elastin fibers) (B) healthy rat aorta. (C) Targeting of elastin antibody coated nanoparticles (EL-NPs) to the abdominal aorta was five-fold higher than IgG antibody coated nanoparticles (IgG-NPs) 24 hours after intravenous injection (n=6) (D) IVIS imaging of whole aorta 24 hours after intravenous injection of IgG-NPs/EL-NPs. Boxed area indicates the site of elastic damage where highest fluorescence was detected while no fluorescence was seen in healthy parts of aorta. (E) Fluorescent microscopy of abdominal aorta. Purple coloration indicates the presence of nanoparticles in ELNP group while no staining in IgG-NP group.
Next, we assessed organ distribution of NPs by measuring fluorescence. At the end of 24 hours, no residual fluorescence was found in the blood, heart, brain, muscle and skin. A bio-distribution study revealed high intensity of signal in the spleen (Table 2). Splenic uptake was ~ 1.7 times lower in the EL-NP group than IgG-NP groups, possibly because of the higher accumulation in the impaired aorta. It must be noted that rabbit-anti-rat antibodies were utilized for this experiment which might be a major contributing factor to the splenic activation. Most of the injected nanoparticles were cleared from the organs eight days after NP injections with the liver showing 40 times lower fluorescence and a ~ 50% reduction in the spleen (data not shown).
Table II.
Organ distribution of fluorescent nanoparticles
| Organ | % total fluorescence/g dry weight (IgG-NP) |
% total fluorescence/g dry weight (EL-NP) |
|---|---|---|
| liver | 7.86 ± 1.40 | 15.08 ± 2.15 |
| aorta | 5.78 ± 5.05 | 26.30 ± 5.74 |
| lungs | 6.36 ± 1.15 | 9.66 ± 4.69 |
| kidneys | 1.86 ± 1.95 | 0.78 ± .17 |
| spleen | 78.14 ± 7.08 | 48.19± 8.14 |
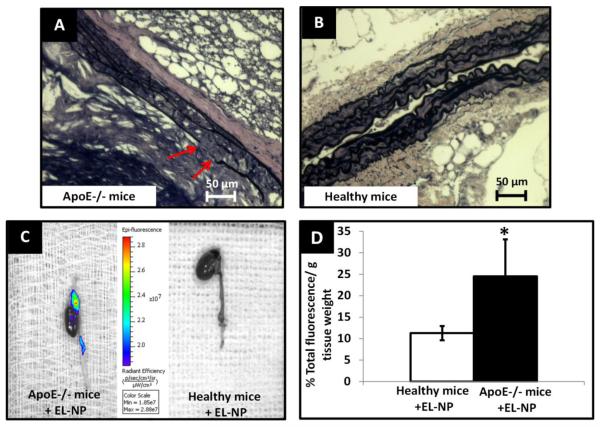
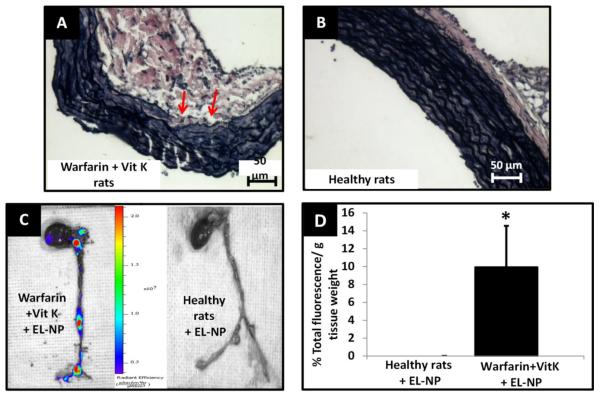
Our next goal was to test these nanoparticles in physiologically relevant animal models wherein elastic lamina damage is a result of biochemical events rather than local chemical injury, as observed in calcium chloride injury model. We chose two different, well-established models to test such situations. First, ApoE−/− mice--maintained under a fat fed diet for 10 months, deemed a sufficient time to create severe atherosclerotic lesions specifically in aortic arch and iliac bifurcation, were used for the experiments. Histological VVG stain for elastin revealed random thinning and disruption of elastin fibers in the medial layer (Figure 5A) which was evidently absent in aorta of healthy mice (Figure 5B). When EL-NPs were delivered systemically, we observed a strong fluorescent signal in the aortic arch indicating the presence of EL-NPs at 24 hours after the injection (Figure 5C) which, again was completely absent in healthy mice aorta (Figure 5C). EL-NPs showed 2.2-fold greater attachment to elastin degradation sites in atherosclerotic mice compared to the healthy mice aorta with quantitative fluorescence analyses (Figure 5D). Next, we used the established model of systemic administration of Warfarin and Vitamin K to create systemic vascular elastic lamina degradation in rats.20 VVG staining confirmed the elastic lamina damage in medial layer after three weeks of daily injections of Warfarin + Vitamin K, particularly in aortic arch and iliac bifurcation (Figure 6A) which was clearly absent in untreated rats (Figure 6B). After three weeks, EL-NPs were delivered intravenously (10 mg/kg body weight) and allowed to circulate for 24 hours. At explants, an accumulation of EL-NPs was observed as seen by bright fluorescence within the aortic arch and at bifurcation of abdominal aorta (the sites known to be subject to elastic lamina damage) whereas healthy rats showed no particle retention (Figure 6C). Quantitatively, EL-NPs achieved ~ 10 times greater targeting in diseased rats compared to healthy rats (Figure 6D).
Figure 5.
Targeting of EL-NPs in atherosclerosis model. (A) VVG histological stain for ApoE−/−mouse aorta after 10 months of fat fed diet (arrow marks indicate fragmented elastin fibers, black) (B) healthy mouse aorta. (C) IVIS imaging of whole heart and aorta at 24 hours after intravenous injection of elastin antibody coated nanoparticles (EL-NPs). Strong fluorescent signal indicates the localization of EL-NPs at aortic arch while no targeting in healthy animals. (D) Targeting of EL-NPs in healthy vs. diseased aorta 24 hours after intravenous injection showing significantly higher fluorescence in the aortic arch (n=5).
Figure 6.
Targeting of EL-NPs in vascular calcification model (A) VVG histological stain for rat aorta after 3 weeks of warfarin + vitamin K (WK) treatment (arrow marks indicate fragmented elastin fibers, black) (B) healthy rat aorta. (C) IVIS imaging of whole heart and aorta at 24 hours after intravenous injection of EL-NPs. Strong fluorescent signal indicates the localization of ELNPs at specific sites of elastic lamina fragmentation. (D) Targeting of EL-NPs in healthy Vs. WK animals at 24 hours after intravenous injection showing significantly higher fluorescence in the aortic arch (n=6).
Discussion
There are a number of challenges associated with developing nanoparticles that specifically target the site of a diseased artery for drug/gene therapy or imaging applications. First, the heterogeneous nature of vascular diseases poses a significant challenge for spatial and temporal delivery of therapeutic/imaging agents to the diseased site. Second, the hemodynamic environment in major arteries exposes the delivery vehicle to both convective and diffusive forces, which retard target-specific binding and local retention. Third, arterial cellular and molecular targets are intermittently expressed during different stages of the pathology; although useful for molecular imaging, such expression may prove suboptimal for site-specific delivery of therapeutic payload.
Most previous research has been focused primarily on delivering drugs and genes to arteries by targeting vascular cells. Particularly, endothelial cell adhesion molecules (CAMs) have been extensively studied as potential targets for homing drug-delivery vehicles.4, 5 Anti-vascular cell adhesion molecule-1 (VCAM-1) liposome targeting has shown some promising results in an ldlr−/− mice model in mitigating atherosclerosis.21 Although these constitutively expressed endothelial markers show several-fold increase in surface density in local sites of inflammation, they pose a major obstacle in serving as consistent and heterogeneous molecular targets due to their shedding from the plasma membrane as a negative feedback mechanism to prevent leukocyte adhesion.22 Furthermore, cellular targeting leads to uptake and intracellular delivery of drugs. Our intent was to develop a delivery system for agents meant to function in the extracellular environment. For example, MMP inhibitors known to prevent degradation of matrix in diseases such as aortic aneurysm must be present in the ECM for effective function.23 Similarly, compounds that specifically bind to elastic fibers such as polyphenols and prevent elastic fiber degradation 19 must be delivered to the ECM with minimal-to-no cellular uptake. In the case of coronary thrombosis, deliveries of fibrinolytic agents require an extracellular drug release for rapid clot dissolution. In such cases, negligible cellular uptake of drug-loaded nanoparticles maximizes the therapeutic benefit. Although previous studies have utilized exposed basement-membrane collagen type IV as a viable vascular target,24 insufficient quantity of the fibrous protein may provide suboptimal matrix targeting.
Cellular/molecular targets are transient and inconsistent through the disease progression; however, elastin degradation is a consistent feature of vascular diseases. It persists throughout diseases such as aortic aneurysms, atherosclerosis, and medial vascular calcification making it an ideal target. Elastic fiber consists of two main components— a core of amorphous cross-linked elastin protein making up 90% of the mature elastic fiber (Figure 1B), with the remaining 10% consisting of 10-12 nm fibrils located around the periphery of the amorphous elastin. These fibrils include fibrillins, fibulins, and some other glycoproteins like microfibril-associated glycoproteins (MAGPs).7, 8 It has been shown that MMPs in vascular diseases specifically degrade the peripheral glycoprotein cover on elastic fibers prior to elastin degradation.25 This proteolytic degradation exposes the hydrophobic core of elastin, allowing us to target only the elastic-fiber degradation site and not the healthy aorta (Figure 1B). Therefore, we developed NPs that specifically target degraded elastin and prevent nonspecific attachment to native elastic fibers, which would localize NPs to the diseased site only and prevent attachment to elastic lamina throughout the vasculature.
We first tested NP targeting in ex-vivo conditions using whole rat aortas. Inducing elastase-mediated elastic lamina damage elicited a nanoparticle adherence that was elastin-specific (high in the EL-NP group and minimal in IgG-NP group) and damage-dependent (minimal EL-NP attachment in healthy unimpaired aorta) (Figure 2B). This data clearly suggested that EL-NPs were specifically attaching to degraded elastic lamina and not to the healthy aorta. The data also confirmed that intraluminal delivery was possible as NPs could pass through intact endothelium.
Next, we decided to test if such NPs with surface elastin antibody could be targeted to diseased aorta in vivo. One of the biggest barriers to nanoparticle-mediated targeted therapy in vivo is the rapid clearance of particles by the mononuclear phagocytic system (MPS),26 especially by the liver and spleen.27, 28 It has been established that PEGylation of the carrier surface significantly increases circulation time, minimizes immune responses, and increases flexibility and hydrophilicity28, 29, thus we chose to PEGylate the NP surface. In our preliminary studies, we used non-PEGylated PLA nanoparticles and confirmed a rapid hepatic clearance (<1 hour) as opposed to PEGylated EL-NPs (>24 hours) (data not shown). We specifically chose ~200 nm particle size and negatively charged surfaces to avoid cellular uptake. Nanoparticle size is an important determinant in cellular uptake and tissue accumulation. Studies have found that 100 nm sized nanoparticles show greater uptake compared to 500 nm nanoparticles in vascular smooth muscle cells.30 Similarly, when delivered endoluminally, particle size plays an important role in penetrating the endothelium. In an ex-vivo canine carotid artery model, smaller size nanoparticles have been shown to (~ 100 nm) achieve 3-fold greater arterial uptake compared to larger (~ 275 nm) nanoparticles.31, 32 Our results indicated that nanoparticles of ~200 nm size were able to penetrate both the endothelium and the basement membrane. Another vital parameter that determines endocytosis, or the lack thereof, is the surface charge of nanoparticles. Due to the inherent negative charge on the mammalian cell membrane, positively charged nanoparticles show superior cellular uptake when compared to negatively charged particles.33 We confirmed this in our studies. On the whole, we found that limiting size to ~ 200 nm while coupling with sufficient surface negative charge, allowed sufficient retention of nanoparticles in the extracellular matrix with minimum cellular uptake by SMCs. Although our results suggest that size and charge are important parameters in deciding the cellular uptake of nanoparticles, other critical factors like surface-protein density, nanoparticle concentration, antibody affinity and shear rate may all contribute to the phagocytic effect of nanoparticles.
Our results largely indicate that specifically designed NPs with elastin-targeting antibodies on the surface can be used to deliver agents to the site of elastic-lamina damage. One of the most exciting observations was that NPs accumulated only where elastic-lamina injury had occurred, while the remaining healthy vasculature was spared (Figure 4D). In addition, EL-NPs delivered in healthy rats failed to display targeting (Figure 5C and 6C), proving the specificity of EL-NPs to elastic-tissue degradation as seen in various vascular disease pathologies such as aortic aneurysms, atherosclerosis, and vascular calcification. However, several unanswered questions still need to be addressed with further research. The possible assimilation of NPs by inflammatory cells like macrophages presented locally at the site of vascular disease is unclear. The maximum duration of NP retention at the impaired site is also unknown; however, such systems could be used to deliver imaging agents6 or drugs that act quickly such as elastin stabilizing compounds.34 Moreover, this study was performed with single dose; one can envision using multiple doses to maintain supply. We used antibody-mediated elastin targeting. The presence of antibodies on a surface makes nanoparticles highly prone to Fc-receptor-mediated phagocytosis, which causes rapid clearance by liver and spleen.27, 35 Antibodies have been investigated extensively in the last two decades, and antibody-mediated tissue targeting for clinical practice has been approved by the FDA.36-38 With advancements in hybridoma technology, antibodies can now be engineered and humanized; fully human antibodies with minimal immunogenicity have been shown for active site targeting.39 Also, this study was performed with an encapsulated fluorescent labeled dye for tracking nanoparticle distribution and targeting. Further studies with drug- or imaging-agent-loaded particles are underway to study therapeutic effectiveness of targeting in vivo.
We show that nanoparticles can be designed to specifically target degraded elastic lamina, a key feature in a number of vascular diseases, for site-specific delivery of therapeutics and imaging agents. After several optimization experiments, we developed a minimally cytotoxic nanoparticle system that displayed ex-vivo and in vivo specificity to impaired elastic lamina in the vasculature. Our results indicate that elastin-antibody-coated PLA nanoparticles are excluded by cells, while displaying high affinity to degraded elastin when delivered ex vivo in rat aorta or or injected intravenously in rodents. Nanoparticles with irrelevant control antibody (IgG-NPs) did not show such specificity. The EL-NP attachment was specific to injury site, and healthy elastic lamina was spared. Such nanoparticles could be loaded with drugs/genes and/or imaging agents to deliver therapeutic agents to diseased vasculature, which was difficult in the past.
Supplementary Material
Acknowledgement
The authors would like to thank Dr. Guzelya Korneva for all HPLC procedures, Dida Weeks for elastic fiber schematic, and Dr. Frank Alexis for gifting the low molecular PLA for our experiments.
This work was partially supported by the grants from National Institutes of Health (R01HL070969-08, R21HL084267, P20GM103444) and Hunter Endowment at Clemson University.
Non-standard abbreviations and acronyms
- PLA
Polylactic acid
- DIR
1, 1-dioctadecyl-3, 3, 3, 3-tetramethylindotricarbocyanine iodide
- DMSO
Dimethyl sulfoxide
- IgG
Immunoglobulin G
- AFM
Atomic force microscopy
- TEM
Transmission electron microscopy
- EL-NP
Elastin antibody-coated nanoparticles
- IgG-NP
IgG antibody-coated nanoparticles
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gupta AS. Nanomedicine approaches in vascular disease: A review. Nanomedicine: Nanotechnology, Biology, and Medicine. 2011;7:763–779. doi: 10.1016/j.nano.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Schulman S, Beyth RJ, Kearon C, Levine MN. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American college of chest physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133:257S–298S. doi: 10.1378/chest.08-0674. [DOI] [PubMed] [Google Scholar]
- 3.Baxter BT, Pearce WH, Waltke EA, Littooy FN, Hallett JW, Jr., Kent KC, Upchurch GR, Jr., Chaikof EL, Mills JL, Fleckten B, Longo GM, Lee JK, Thompson RW. Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: Report of a prospective (phase ii) multicenter study. J Vasc Surg. 2002;36:1–12. doi: 10.1067/mva.2002.125018. [DOI] [PubMed] [Google Scholar]
- 4.Muro S, Dziubla T, Qiu W, Leferovich J, Cui X, Berk E, Muzykantov VR. Endothelial targeting of high-affinity multivalent polymer nanocarriers directed to intercellular adhesion molecule 1. Journal of Pharmacology and Experimental Therapeutics. 2006;317:1161–1169. doi: 10.1124/jpet.105.098970. [DOI] [PubMed] [Google Scholar]
- 5.Rossin R, Muro S, Welch MJ, Muzykantov VR, Schuster DP. In vivo imaging of 64cu-labeled polymer nanoparticles targeted to the lung endothelium. Journal of Nuclear Medicine. 2008;49:103–111. doi: 10.2967/jnumed.107.045302. [DOI] [PubMed] [Google Scholar]
- 6.Majmudar MD, Yoo J, Keliher EJ, Truelove JJ, Iwamoto Y, Sena B, Dutta P, Borodovsky A, Fitzgerald K, Di Carli MF, Libby P, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M. Polymeric nanoparticle pet/mr imaging allows macrophage detection in atherosclerotic plaques. Circulation Research. 2013;112:755–761. doi: 10.1161/CIRCRESAHA.111.300576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenbloom JAW, Mecham R. Extracellular matrix 4: The elastic fiber. FASEB J. 1993 Oct;7(13):1208–1218. [PubMed] [Google Scholar]
- 8.Sakai LY, Keene DR, Engvall E. Fibrillin, a new 350-kd glycoprotein, is a component of extracellular microfibrils. The Journal of Cell Biology. 1986;103:2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pai As GCM. Matrix remodeling in vascular calcification associated with chronic kidney disease. Journal of the American Society of Nephrology : JASN. 2010;21:1637–1640. doi: 10.1681/ASN.2010040349. [DOI] [PubMed] [Google Scholar]
- 10.Dao Hh ERBCMP. Evolution and modulation of age-related medial elastocalcinosis: Impact on large artery stiffness and isolated systolic hypertension. Cardiovascular research. 2005;66:307–317. doi: 10.1016/j.cardiores.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Edmonds ME. Medial arterial calcification and diabetes mellitus. Z Kardiol. 2000;89:S101–S104. doi: 10.1007/s003920070107. [DOI] [PubMed] [Google Scholar]
- 12.Salusky IB, Goodman WG. Cardiovascular calcification in end stage renal disease. Nephrology Dialysis Transplantation. 2002;17:336–339. doi: 10.1093/ndt/17.2.336. [DOI] [PubMed] [Google Scholar]
- 13.Aikawa E, Aikawa M, Libby P, Figueiredo J-L, Rusanescu G, Iwamoto Y, Fukuda D, Kohler RH, Shi G-P, Jaffer FA, Weissleder R. Arterial and aortic valve calcification abolished by elastolytic cathepsin s deficiency in chronic renal disease. Circulation. 2009;119:1785–1794. doi: 10.1161/CIRCULATIONAHA.108.827972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun J, Sukhova GK, Yang M, Wolters PJ, MacFarlane LA, Libby P, Sun C, Zhang Y, Liu J, Ennis TL, Knispel R, Xiong W, Thompson RW, Baxter BT, Shi G-P. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. The Journal of clinical investigation. 2007;117:3359–3368. doi: 10.1172/JCI31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sims FH. The initiation of intimal thickening in human arteries. Pathology - Journal of the RCPA. 2000;32:171–175. 110.1080/pat.1032.1083.1171.1175. [PubMed] [Google Scholar]
- 17.Raines EW, Koyama H, Carragher NO. The extracellular matrix dynamically regulates smooth muscle cell responsiveness to pdgfa. Annals of the New York Academy of Sciences. 2000;902:39–52. doi: 10.1111/j.1749-6632.2000.tb06299.x. [DOI] [PubMed] [Google Scholar]
- 18.Basalyga DM, Simionescu DT, Xiong W, Baxter BT, Starcher BC, Vyavahare NR. Elastin degradation and calcification in an abdominal aorta injury model: Role of matrix metalloproteinases. Circulation. 2004;110:3480–3487. doi: 10.1161/01.CIR.0000148367.08413.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isenburg JC, Simionescu DT, Starcher BC, Vyavahare NR. Elastin stabilization for treatment of abdominal aortic aneurysms. Circulation. 2007;115:1729–1737. doi: 10.1161/CIRCULATIONAHA.106.672873. [DOI] [PubMed] [Google Scholar]
- 20.Price PA, Faus SA, Williamson MK. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arteriosclerosis, Thrombosis, and Vascular Biology. 1998;18:1400–1407. doi: 10.1161/01.atv.18.9.1400. [DOI] [PubMed] [Google Scholar]
- 21.Homem de Bittencourt PI, Jr, Lagranha DJ, Maslinkiewicz A, Senna SM, Tavares AMV, Baldissera LP, Janner DR, Peralta JS, Bock PM, Gutierrez LLP, Scola G, Heck TG, Krause MS, Cruz LA, Abdalla DSP, Lagranha CJ, Lima T, Curi R. Lipocardium: Endothelium-directed cyclopentenone prostaglandin-based liposome formulation that completely reverses atherosclerotic lesions. Atherosclerosis. 2007;193:245–258. doi: 10.1016/j.atherosclerosis.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 22.Melis M, Pace E, Siena L, Spatafora M, Tipa A, Profita M, Bonanno A, Vignola AM, Bonsignore G, Mody CH, Gjomarkaj M. Biologically active intercellular adhesion molecule-1 is shed as dimers by a regulated mechanism in the inflamed pleural space. American Journal of Respiratory and Critical Care Medicine. 2003;167:1131–1138. doi: 10.1164/rccm.200207-654OC. [DOI] [PubMed] [Google Scholar]
- 23.Bartoli MA, Parodi FE, Chu J, Pagano MB, Mao D, Baxter BT, Buckley C, Ennis TL, Thompson RW. Localized administration of doxycycline suppresses aortic dilatation in an experimental mouse model of abdominal aortic aneurysm. Annals of Vascular Surgery. 2006;20:228–236. doi: 10.1007/s10016-006-9017-z. [DOI] [PubMed] [Google Scholar]
- 24.Chan JM, Zhang L, Tong R, Ghosh D, Gao W, Liao G, Yuet KP, Gray D, Rhee J-W, Cheng J, Golomb G, Libby P, Langer R, Farokhzad OC. Spatiotemporal controlled delivery of nanoparticles to injured vasculature. Proceedings of the National Academy of Sciences. 2010 doi: 10.1073/pnas.0914585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sluijter JPG, de Kleijn DPV, Pasterkamp G. Vascular remodeling and protease inhibition–bench to bedside. Cardiovascular research. 2006;69:595–603. doi: 10.1016/j.cardiores.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 26.Jones SW, Roberts RA, Robbins GR, Perry JL, Kai MP, Chen K, Bo T, Napier ME, Ting JPY, DeSimone JM, Bear JE. Nanoparticle clearance is governed by th1/th2 immunity and strain background. The Journal of clinical investigation. 2013;123:3061–3073. doi: 10.1172/JCI66895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Derksen JTP, Morselt HWM, Scherphof GL. Uptake and processing of immunoglobulin-coated liposomes by subpopulations of rat liver macrophages. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1988;971:127–136. doi: 10.1016/0167-4889(88)90184-x. [DOI] [PubMed] [Google Scholar]
- 28.Vonarbourg A, Passirani C, Saulnier P, Benoit J-P. Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials. 2006;27:4356–4373. doi: 10.1016/j.biomaterials.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 29.Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angewandte Chemie International Edition. 2010;49:6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 30.Sivaraman B, Ramamurthi A. Multifunctional nanoparticles for doxycycline delivery towards localized elastic matrix stabilization and regenerative repair. Acta Biomaterialia. 2013;9:6511–6525. doi: 10.1016/j.actbio.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song C, Labhasetwar V, Cui X, Underwood T, Levy RJ. Arterial uptake of biodegradable nanoparticles for intravascular local drug delivery: Results with an acute dog model. Journal of Controlled Release. 1998;54:201–211. doi: 10.1016/s0168-3659(98)00016-9. [DOI] [PubMed] [Google Scholar]
- 32.Westedt U, Barbu-Tudoran L, Schaper A, Kalinowski M, Alfke H, Kissel T. Deposition of nanoparticles in the arterial vessel by porous balloon catheters: Localization by confocal laser scanning microscopy and transmission electron microscopy. AAPS PharmSci. 2002;4:206–211. doi: 10.1208/ps040441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho EC, Au L, Zhang Q, Xia Y. The effects of size, shape, and surface functional group of gold nanostructures on their adsorption and internalization by cells. Small. 2010;6:517–522. doi: 10.1002/smll.200901622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isenburg JC, Karamchandani NV, Simionescu DT, Vyavahare NR. Structural requirements for stabilization of vascular elastin by polyphenolic tannins. Biomaterials. 2006;27:3645–3651. doi: 10.1016/j.biomaterials.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Aragnol D, Leserman LD. Immune clearance of liposomes inhibited by an anti-fc receptor antibody in vivo. Proceedings of the National Academy of Sciences. 1986;83:2699–2703. doi: 10.1073/pnas.83.8.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabizon AA. Pegylated liposomal doxorubicin: Metamorphosis of an old drug into a new form of chemotherapy. Cancer Investigation. 2001;19:424–436. doi: 10.1081/cnv-100103136. [DOI] [PubMed] [Google Scholar]
- 37.Schrama D, Reisfeld RA, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov. 2006;5:147–159. doi: 10.1038/nrd1957. [DOI] [PubMed] [Google Scholar]
- 38.Kaminski MS, Zasadny KR, Francis IR, Milik AW, Ross CW, Moon SD, Crawford SM, Burgess JM, Petry NA, Butchko GM, Glenn SD, Wahl RL. Radioimmunotherapy of b-cell lymphoma with [131i]anti-b1 (anti-cd20) antibody. New England Journal of Medicine. 1993;329:459–465. doi: 10.1056/NEJM199308123290703. [DOI] [PubMed] [Google Scholar]
- 39.Hudson PJ, Souriau C. Engineered antibodies. Nat Med. 2003;9:129–134. doi: 10.1038/nm0103-129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.