Abstract
It is well recognized that adverse events in utero impair fetal development and lead to the development of obesity and metabolic syndrome in adulthood. To investigate the mechanisms linking impaired fetal growth to increased cholesterol, an important clinical risk factor characterizing the metabolic syndrome and cardiovascular disease, we examined the impact of maternal undernutrition on tumor necrosis factor-α (TNF-α)/c-jun N-terminal kinase (JNK) signaling pathway and the cholesterol 7α-hydroxylase (CYP7A1) expression in the livers of the offspring with a protein restriction model. The male offspring with intrauterine growth restriction (IUGR) caused by the isocaloric low-protein diet showed decreased liver weight at birth and augmented circulation and hepatic cholesterol levels at 40 weeks of age. Maternal undernutrition significantly upregulated cytokine TNF-α expression and JNK phospholytion levels in the livers from fetal age to adulthood. Elevated JNK phospholytion could be linked to downregulated hepatocyte nuclear factor-4α and CYP7A1 expression, subsequently led to higher hepatic cholesterol. This work demonstrated that intrauterine malnutrition-induced IUGR might result in intrinsic disorder in hepatic TNF-α/CYP7A1 signaling, and contribute to the development of hypercholesterolemia in later life.
Keywords: perinatal nutrition, TNF-α, JNK, CYP7A1, cholesterol
Introduction
Intrauterine growth restriction (IUGR) affects 3–10% of pregnancies, and is recognized as the leading cause of intrauterine fetal death and the second leading cause of neonatal death.(1,2) Numerous epidemiological studies have demonstrated a strong association between low birth weight and subsequent development of metabolic syndrome, consisting of type 2 diabetes mellitus, obesity, dyslipidaemia, hypertension and coronary artery disease (CHD).(3–9) These findings led to the ’fetal origins of adult disease hypothesis’, which is also described as the ’developmental origins of health and disease hypothesis’.(10) Studies of animal models are consistent with the concept that adverse events in utero predispose the offspring toward the later development of metabolic syndrome.(11) All these data suggested that although the underlying causes of the metabolic syndrome in humans are complicated and controversial, maternal undernutrition may be a causal factor.
Underlying molecular mechanisms of metabolic programming remains elusive. Poor nutrition in utero may alter life-long gene expression in major organs and the character of the genotype–environment interaction in later life.(10) There have been ample evidences suggesting that hepatic tissue may play a major role in linking IUGR to the subsequent development of adult diseases.(12,13) Various experimental animal models have expanded our knowledge of how poor intrauterine nutrition may influence liver development.(14,15) Among these, protein restriction in early life has been widely used as a rat model of metabolic programming to study the impact of prenatal malnutrition upon adult diseases. Maternal protein restriction exerts profound programming effects on major organs and tissues, such as liver, adipose and muscle. This effect is partly mediated through affecting the epigenetic modification of genes that regulate metabolism and transportation of glucose and lipid.(16–18) Our laboratory also developed an IUGR model in rats by maternal protein restriction, whereas the animals showed disturbed hepatic metabolism, in line with other studies.(19)
A large amount of evidence has recently suggested that birth weight is an independent risk factor for hypercholesterolemia, a proven risk factor for the CHD.(20,21) In the liver, cholesterol is converted to bile acids by a pathway consisting of abundant physiological reactions. Cholesterol 7α-hydroxylase (CYP7A1) is a liver-specific rate-limiting enzyme of this pathway. Previous studies have revealed programmed changes in the expression of CYP7A1 in IUGR rats caused by protein or iron restriction during pregnancy.(22,23) In hepatocytes, CYP7A1 expression could be regulated at the gene transcriptional level by bile acid and proinflammatory cytokines including tumor necrosis factor (TNF) α and interleukin 1.(24,25) TNF-α has been shown to play a pivotal role in the development of coronary artery disease, insulin resistance, type 2 diabetes, and dyslipidemia.(26) Experimental study has shown that TNF-α downregulates the expression of hepatic CYP7A1 by activating the c-jun N-terminal kinase (JNK) signaling pathway.(25) In brief, TNF-α binds to the receptor and phospholates the mitogen-activated protein (MAP) kinases JNK in the hepatocytes. Activated JNK inhibits hepatocyte nuclear factor (HNF)4α expression or phospholates HNF-4α and reduces its DNA binding and transactivation activity, ultimately decreases the transcription rate of CYP7A1. CYP7A1 catalyzes the conversion of cholesterol to two primary bile acids, cholic acid (CA) and chenodeoxycholic acid (CDCA), both of which are further excreted to bile by bile salt export pump (BSEP).(27) Studies of human have demonstrated that higher TNF-α level existed in the cord blood and trophoblasts of IUGR placenta.(28,29) Furthermore, studies of rodents have also shown that TNF-α were overproduced in the livers of IUGR offspring.(30) Therefore early life programming of the expression of key molecules in the TNF-α/JNK signaling pathway may play a role in determining postnatal cholesterol metabolism.
Thus, evidence exists to support the working hypothesis that reprogramming of long-term modifications of the expression of key factors of TNF-α/JNK signaling pathway involved in cholesterol and bile acid metabolism is associated with significant hepatic cholesterol accumulation in the aging IUGR offspring. The aim of the present study was therefore to elucidate the development of hypercholesterolemia and related underlying patterns of gene expression.
Materials and Methods
Animals
All experimental protocols were approved by the Animal Research Committee of China Medical University. Adult Wistar rats were housed under specific pathogen-free conditions in an environmentally-controlled clean room at the Experimental Animal Center (Shengjing Hospital, China Medical University). All animals were individually housed under standard conditions. Food and water were provided ad libitum throughout the study. The female rats were mated overnight. The presence of spermatozoids in the vaginal smear was verified the next morning to determine gestational day 0. The rats were then randomly divided into 2 groups: animals in the undernourished group received an isocaloric low-protein diet (8% protein) from day 0 of pregnancy until the birth of their pups as we described previously,(19) and control animals were maintained on standard diet (20% protein) during gestation. On the 20th day of gestation (E20), a subset of pups was delivered by caesarian section, the fetal blood was pooled (three or more) in a litter to quantify plasma total cholesterol (TC) and triglyceride (TG) levels, and the fetal livers were dissected out and frozen. IUGR referred to the pups whose birth weight was two standard deviations less than the mean value of the control group. Another subset of rats was allowed to deliver spontaneously. At birth, the litter size was reduced to eight-ten animals. The remaining pups were nourished by their own mothers until weaning at postnatal 3 weeks (PW3), and then fed under normal condition until 40 weeks (PW40). At PW3 and PW40, the pups were killed under ether anesthesia, and livers were dissected, snap frozen in liquid nitrogen, and then stored at –70°C until use. To avoid any sex and hormonal confounding interference, all the following analyses were performed with male pups only.
Histological examination of the liver
The left lobe of the liver specimens were fixed in 4% paraformaldehyde for over 24 h before processing by use of automatic tissue processor (ZT-12M, Wuhan, China) for paraffin embedding. Hemotoxylin and eosin staining were performed using standard techniques in 5-µm-thick sections. All images were analyzed with the assistance of image software NISElements BR 2.10 (Nikon, Tokyo, Japan).
Biochemical analysis
Plasma TG and TC contents were measured using enzymatic reagent kits from Jiancheng (Nanjing, China) according to the manufacturer’s instructions. Liver tissues (200 mg) from the central lobe were homogenized in 2 ml chloroform-methanol (2:1 vol/vol). The extract was collected, dried, and dissolved in ethanol as described by Folch et al.(31) Extracted lipids were then examined by colorimetry assay using enzymatic reagent kits for TG and TC (Jiancheng, Nanjing, China), and the result was expressed as µmol lipid per gram wet weight liver tissue.
Measurement of liver TNF-α
One hundred milligrams of liver tissues were homogenized in 1 ml PBS, and protein concentration was determined with a spectrophotometer. Assay for TNF-α was performed by Enzyme Linked Immuno-Sorbent Assay (ELISA) in the liver homogenate in all the groups using a commercially available kit (Invitrogen, Camirillo, CA), following the manufacturer’s instructions. TNF-α levels were expressed as picograms per milligram protein.
RNA isolation and real-time PCR
Total RNA from liver specimens was isolated at E20, PW3, and PW40 using the TRIzol reagent (Invitrogen), and quantified with the Nanovue instrument (GE Healthcare, Buckinghamshire, England). The RNA strand (2 µg) was reverse-transcribed into cDNA using the RNA PCR kit (TaKaRa, Dalian, China). The expression of the cDNAs of interest was measured by quantitative real-time PCR using the SYBR Premix Ex Taq kit (TaKaRa) and a Lightcycler detector (Roche, Basel, Switzerland), and was normalized with β-actin used for housekeeping. The results were expressed in arbitrary units relative to the mean value of the control group. The specific primer sets used are listed in Table 1.
Table 1.
Sequences of primers used in quantitative real-time PCR
| Target genes | Accession No. | Primer sequences (5'-3') | Amplicon size (bp) | Tm (°C) |
|---|---|---|---|---|
| TNF-α | NM_012675 | F: CTCAGATCATCTTCTCAAAA | 169 | 46.9 |
| R: AGGTACAGCCAATCTGCTAA | 52.7 | |||
| CYP7A1 | NM_012942.1 | F: TCACGGAAGGGATGTA | 202 | 45.5 |
| R: GCTTTATGTGCGGTCT | 45.3 | |||
| BSEP | NM_031760.1 | F: CAGGCGGAGGCAAGTCTTC | 150 | 58.8 |
| R: CCCATCACAACGTCATCTTGA | 60.4 | |||
| β-actin | NM_031144 | F: ACTATTGGCAACGAGCGGTT | 190 | 59.9 |
| R: TGTCAGCAATGCCTGGGTACA | 62.1 |
Immunoblot analysis
The liver tissues were homogenized in the RAPI buffer (50 mM Tris, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, sodium orthovanadate, sodium fluoride, EDTA, pH 7.4) with protease inhibitors, and centrifuged at 12,000 × g at 4°C for 20 min. The protein content was assessed using a BCA Protein Assay Kit (Beyotime, Shanghai, China). Antibodies to HNF-4α, JNK, and CYP7A1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies to phosphotyrosine JNK and β-actin were from Abcam (Cambridge, UK). Equal amounts of protein were separated by electrophoresis in 12% SDS polyacrylamide gel and then electroblotted from the gels on a PVDF membrane (Millipore, Billerica, MA) as described by Towbin et al.(32) After blocking, the membrane was probed with the primary antibody at 4°C overnight, followed by incubation with horseradish peroxidase-conjugated secondary antibody for 2 h at room temperature. The immune complexes were visualized using enhanced chemiluminescence reagents (GE Healthcare) and quantified with Quantity One software (Bio-Rad, Richmond, CA).
Statistical analysis
All data are presented as means ± SEM. Statistical significance was calculated by Student’s t test using SPSS ver. 17.0 (Chicago, IL). A statistical probability of p<0.05 was considered significant. The data are presented as a bar graph using the GraphPad Software (San Diego, CA).
Results
Weight and metabolic files
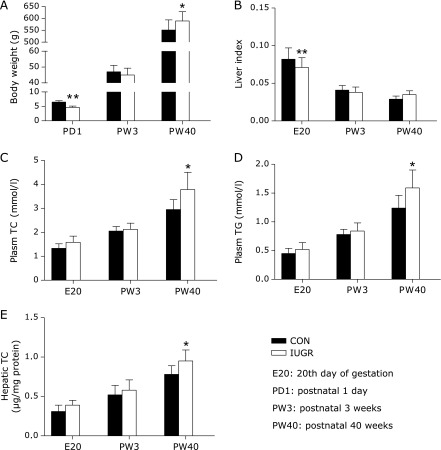
In a previous study we reported that protein restriction resulted in lower body weight in near-term fetuses (E20), with no observed change in litter size or sex ratio. In this study, postnatal body weight of IUGR animals was restored to normal during lactation (PW3), whereas it surpassed that of the control group at postnatal 40 weeks because of catch-up growth (Fig. 1A). In agreement with other reports, protein restriction resulted in a significant decrease in fetal liver index (liver weight as a percentage of body weight). However, the restored liver index was comparable with that of the same-aged control animals at PW3 and PW40 (Fig. 1B). Additionally, IUGR group showed a trend towards an augment in plasma TG, TC and hepatic TC concentration at E20, and a significant increase at PW40 (p<0.05) (Fig. 1C–E).
Fig. 1.
The influence of maternal protein restriction on offspring liver growth and metabolic profile. (A) Body weight of offspring rats at postnatal day 1 (PD1) (control, n = 93; and IUGR, n = 108), PW3 (control, n = 52; and IUGR, n = 48) and PW40 (control, n = 10 and IUGR, n = 12). (B) Liver weight is expressed as a percentage of body weight at E20 (control, n = 42; and IUGR, n = 45), PW3 (control, n = 8 and IUGR, n = 8) and PW40 (control, n = 8; and IUGR, n = 8). (C)–(E) Fasting plasma triglyceride (TG), total cholesterol (TC), and hepatic TC concentrations in all time points examined (n = 6 for both groups). Black bars, control group; white bars, IUGR group. Data are shown as means ± SE. Significant differences compared with controls of same age are indicated by *p<0.05 and **p<0.01.
Liver histology
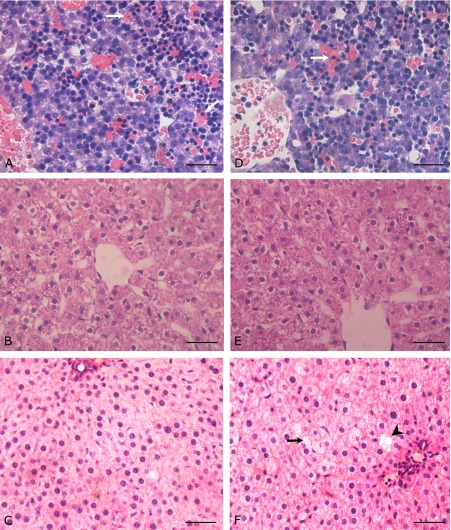
A subset of the liver sections obtained at all three ages was stained with hemotoxylin and eosin. In both control and IUGR fetal livers (Fig. 2A and D), there are many immature erythropoietic cells remaining in the perisinusoidal space compared with the livers of adult rats. Hepatic cords are two cells thick, with no lobular architecture observed in the fetal liver, consistent with previous reports.(33,34) In contrast, the liver consists mainly of hepatocytes, sinusoidal cells and bile ductile cells after birth, with the hepatocytes arranged as cords that are one cell thick. At day 20 of gestation, the livers of IUGR appeared histologically normal, and this remained the case at 3 weeks of age (Fig. 2B and E) without significant difference was detected between two groups. By PW40, however, IUGR animals exhibited mild hepatic steatosis with many microvesicular and a few macrovesicular deposits (Fig. 2C and F).
Fig. 2.
Evidence of hepatic steatosis in adult IUGR offsprings. HE staining was performed for the sections from rat livers at different developmental stages. (A)–(C) show representative sections from CON rats (40× original magnification) (A) E20, (B) PW3, (C) PW40. (D)–(F) show sections from IUGR rats (D) E20, (E) PW3, (F) PW40. White arrow indicates immature erythrocytes; black arrow indicates microvesicular fat droplets; black arrow head indicates macrovesicular fat droplet. Scale bars = 50 µm.
Effect of maternal malnutrition on the hepatic TNF-α
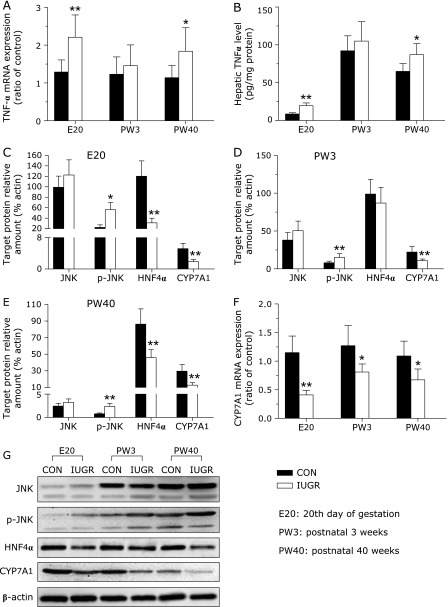
Maternal undernutrition significantly upregulated the TNF-α mRNA levels in the livers of IUGR fetal rats (p<0.05), which restored at PW3, but resumed to higher expression level at PW40 (Fig. 3A). To obtain further information of the protein content difference, we analyzed the cytokine TNF-α using ELISA. In general, mRNA and protein levels of TNF-α were comparable. Compared with the control group, hepatic TNF-α cytokine content was upregulated by 126.52% and 33.85% in the IUGR rats at E20 and PW40, respectively (p<0.05) (Fig. 3B).
Fig. 3.
The influence of IUGR on key molecules of TNF-α/CYP7A1 signaling pathway in rat livers at different developmental stages. (A) TNF-α mRNA levels in livers were analyzed by quantitative real-time PCR. Values are expressed relative to same-aged control. (B) TNF-α protein content was measured by ELISA. (C)–(E) Densitometric analysis of total JNK, phospho-JNK, HNF4α and CYP7A1 protein in rat livers at E20 (C), PW3 (D) and PW40 (E). (F) Hepatic mRNA expression of CYP7A1 at different developmental stages. (G) Representative Western blotting analyses of key molecules of TNF-α/CYP7A1 signaling pathway in rat livers. All protein expression levels were normalized relative to the expression level of β-actin. Data are presented as means ± SE for 6 observations per group. Black bars, control group; white bars, IUGR group. Significant differences compared with controls of same age are indicated by *p<0.05 and **p<0.01.
Effect of maternal malnutrition on the JNK/HNF/CYP7A1 signaling pathway
Cytokine TNF-α regulates CYP7A1 expression through the JNK signaling pathway. We further investigated the expression and activity of hepatic JNK in the IUGR offspring. Overproduction of TNF-α resulted in a remarkable increase in phosphorylation (Thr183/Tyr185) of JNK (p<0.05, Fig. 3). However no differences were observed in total JNK content at all time-points examined. To extend this study, we further measured HNF-4α and CYP7A1 expressions in the livers. In the IUGR group, there was a significant downregulation in hepatic HNF-4α expression at E20, with no significant change at PW3, and a significant decrease at PW40 compared with the control group (p<0.05, Fig. 3). This was accompanied by a statistically significant decrease in hepatic CYP7A1 expression at all time points of investigation (p<0.05, Fig. 3).
BSEP in the livers of offspring
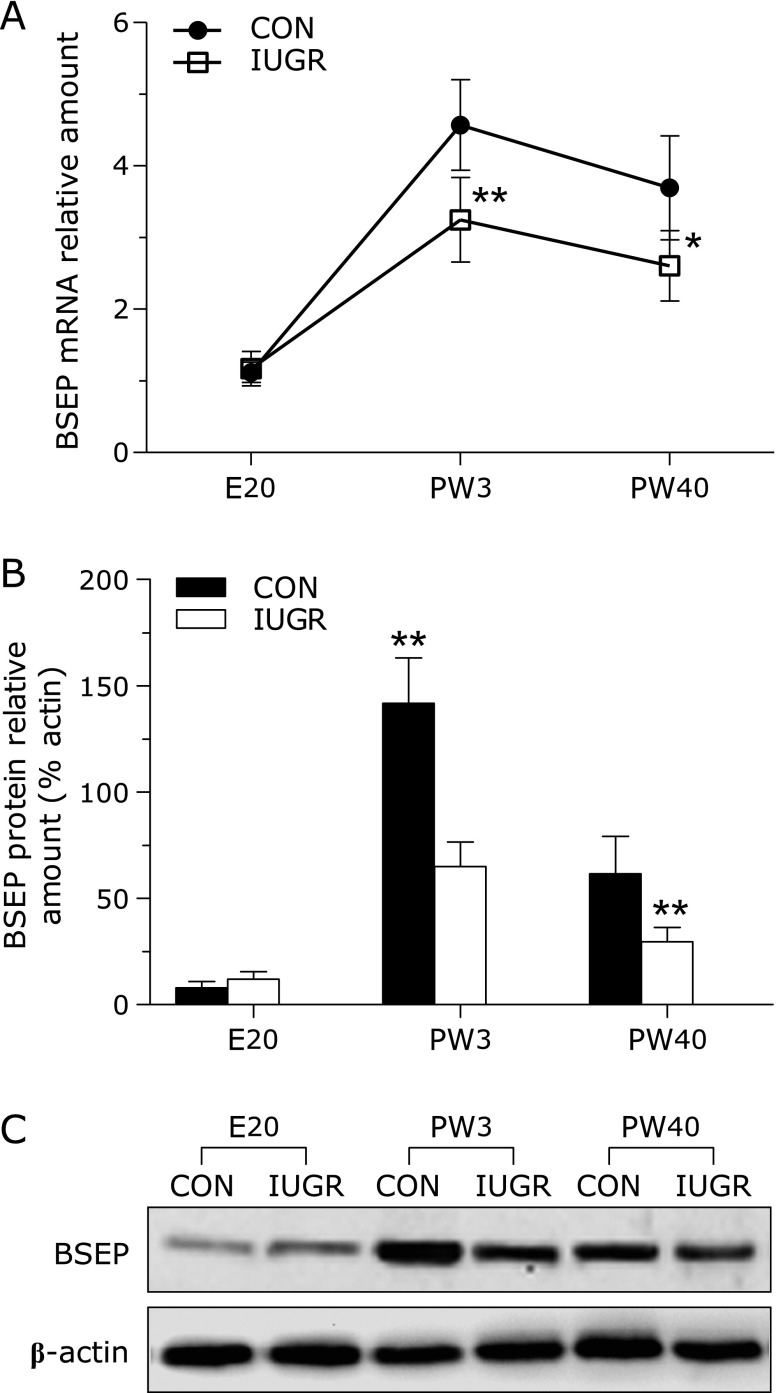
The expression patterns of BSEP mRNA in both groups were shown in Fig. 4A. In both groups, hepatic BSEP mRNA levels were remarkably low before birth, but increased at 3 weeks and 40 weeks of age. Compared to the control group, in the IUGR group there were remarkable decrease in BSEP mRNA levels at PW3 and PW40, with no significant change at E20. Consistent with the PCR data, by western hybridization, there were significant declines in BSEP protein expression at PW3 and PW40, when comparing IUGR with the control group (Fig. 4B and C).
Fig. 4.
The effect of maternal undernutrition on the expression of bile salt export pump (BSEP) gene in rat livers. (A) BSEP mRNA levels in both groups were determinated by quantitative real-time PCR, and results were expressed relative to the mean value of the E20 control. (B) and (C) Representative Western blotting and densitometric analysis of BSEP protein expression in the livers of both groups. BSEP protein expression level was normalized relative to the expression of β-actin. Data are presented as means ± SE for 6 observations per group. Black bars, control group; white bars, IUGR group. Significant differences compared with controls of same age are indicated by *p<0.05 and **p<0.01.
Discussion
The Barker’s ”fetal origins of adult disease hypothesis” implicates that fetal in-utero environment has a profound impact on one’s risk for major chronic diseases. Clinical studies have demonstrated that adverse intrauterine environment that results in IUGR can also alter or ”program” the physiological processes leading to the future adult diseases, such as coronary artery disease, hypertension, type 2 diabetes, and obesity. With regard to cholesterol, Forsdahl was the first to suggest a link between development and subsequent hypercholesterolemia in 1978.(35) To date, mounting evidence have confirmed there was apparent association between low birth weight and postnatal higher cholesterol, independent of social circumstance, current body weight, cigarette smoking, and alcohol intake.(20,36)
Various animal models, regardless of the type of intrauterine injury, have also demonstrated that IUGR could permanently alter offspring cholesterol homeostasis. However the mechanisms underlying these alterations remain largely unknown. Undoubtedly, elucidating the molecular mechanisms in this process will facilitate researchers to identify early life dietary and/or drug intervention strategies for the prevention of these metabolic diseases. In a well established IUGR model, we investigated the effect of protein-malnutrition during pregnancy on offspring cholesterol metabolism. Considering there was stronger association between IUGR and higher cholesterol in human males compared with females,(37) All the analyses were performed with male pups only. In keeping with previous reports, we found that birth weight of male pups from protein restriction dams were significantly lowered, however it exceeded that of control pups at postnatal 40 weeks because of catch-up growth. Furthermore, the liver index (liver weight as a percentage of body weight) was lowered at birth, but restored at PW3 and PW40 compared to the control animals. The IUGR group had hypercholesterolemia and significantly augmented accumulation of hepatic cholesterol in aging animals. Higher cholesterol concentration in hepatic was linked with aberrant gene expression and/or kinase activity in the TNF-α/CYP7A1 signaling pathway, which regulates the bile acid biosynthesis.
Cholesterol is an important biological molecule for membrane structure as well as serving as a precursor for the biosynthesis of the steroid hormones, bile acids, and vitamin D. However, an excessive accumulation of this molecule has profound negative impacts on cardiovascular disease. The biosynthesis of the bile acids is the predominant pathway of cholesterol elimination in liver, which is called the classic pathway. CYP7A1, the first and rate-limiting enzyme in the bile acid synthetic pathway is expressed normally only in hepatocytes and is highly regulated. Human deficiency of CYP7A1 manifests with a spectrum of phenotypes ranging from hypercholesterolemic and premature gallstones to premature coronary disease. CYP7A1 gene knockout mice had elevated total cholesterol as well as LDL and severely reduced bile acid synthesis.(38–41) Studies of humans and experimental animals have indicated that cytokine TNF-α limits cholesterol elimination from the body by inhibiting the expression and activity of CYP7A1. The mechanism involves the activation of the JNK-dependent signaling pathway that subsequently inhibits the expression and activity of HNF4, ultimately decreasing the transcription rate of CYP7A1.(24)
Previous works have shown that impaired fetal growth caused by protein or iron restriction in pregnancy leads to higher hepatic cholesterol in fetus or aging animals. These are linked to decreased expression of CYP7A1.(22,23) Using a pregnancy protein restriction model, we showed that hepatic CYP7A1 protein amount was persistently reduced from fetal to aged rats, consistent with previous reports. Furthermore we observed higher TNF-α concentration and enhanced phospholytion level of JNK, but lower HNF4α expression in the livers of IUGR offspring. These data suggested that intrauterine protein restriction might upregulate cytokine TNF-α expression; subsequently activate JNK signaling cascade, and eventually diminish the HNF4-mediated transcription activation of CYP7A1.
TNF-α is a multifunctional cytokine that in the liver acts as a cytotoxic agent in many types of hepatic injury. Kupffer cells, the resident macrophages which constitute 15% of the liver cell population, are major sources of TNF-α and other inflammatory cytokines.(42) Pretranscription regulation of TNF-α gene expression within liver follows activation of extracellular signal-regulated kinase (ERK) signaling pathway by growth factors and cytokines, as well as reactive oxygen species (ROS).(43–46) Studies have demonstrated that placental insufficiency induces oxidative stress in the offspring liver that creates a self-perpetuating process in which overproduction of ROS,(47) and increases hepatic ERK1/2 phosphorylation.(15,48) Thus it is possible that IUGR-induced oxidative stress activates ERK signaling and increases the expression of TNF-α in liver. Further studies are needed to confirm this speculation. After released from Kupffer cells, TNF-α activates TNF receptors on hepatocytes to induce JNK and inhibitor kappa beta kinase (IKK) activation, as well as more ROS production. Besides limiting cholesterol elimination, activation of JNK by TNF-α impairs insulin action by inhibiting the activity of insulin receptor substrate (IRS)-1.(49) Studies of several animal models showed deactivation of IRS-1 in IUGR offspring livers,(48,50) thence the potential relationship between TNF-α expression and the insulin signaling in the IUGR liver deserves further investigation.
BSEP, a member of the ATP-binding cassette (ABC) family of transporters (identified as ABCB11) is responsible for exporting bile acids out of hepatocytes as part of the hepatic detoxification process, while bile acids stimulate hepatic BSEP gene expression in turn.(51) Exposure to TNF-α caused downregulations of both BSEP mRNA and protein levels in rat hepatocytes. Administration of LPS in vivo triggered release of the proinflammatory cytokines including TNF-α and caused the decrease of BSEP protein in rat liver.(52) We observed similar expression pattern for BSEP and TNF-α gene in the livers of IUGR animals. Thus we speculated that the mechanism responsible for aberrant BSEP expression in IUGR animals maybe caused directly by increased TNF-α outproduction, which invites further investigation.
In short, this work demonstrated that intrauterine malnutrition-induced IUGR led to enhanced cholesterol in later life, through reprogramming of lifelong expression of key factors involved in the regulation of cholesterol and bile acid metabolism. The fundamental mechanisms underlying these changes and their influence on the metabolic syndrome remain unclear. Given the plasticity of the liver in fetal and neonatal stages, understanding the effects of intrauterine malnutrition on cholesterol dysregulation may be potentially helpful for developing early life dietary and drug treatment strategies to reduce the incidence of the metabolic syndrome in adulthood.
Acknowledgments
This study was supported by NSFC grant (81100432, 81370716). And the authors would like to thank Dr Aiyuan Wang (China Medical University, China) for critically reviewing the manuscript.
Abbreviations
- BSEP
bile salt export pump
- CYP7A1
cholesterol 7α-hydroxylase
- ERK
extracellular signal-regulated kinase
- HNF4α
hepatocyte nuclear factor 4α
- IKK
inhibitor kappa beta kinase
- IL-1β
interleukin-1β
- IRS
insulin receptor substrate
- IUGR
intrauterine growth restriction
- JNK
c-Jun N-terminal kinase
- TNF-α
tumor necrosis factor α
- ROS
reactive oxygen species
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Gagnon R. Placental insufficiency and its consequences. Eur J Obstet Gynecol Reprod Biol. 2003;110 (Suppl 1):S99–S107. doi: 10.1016/s0301-2115(03)00179-9. [DOI] [PubMed] [Google Scholar]
- 2.Pryor J. The identification and long term effects of fetal growth restriction. Br J Obstet Gynaecol. 1997;104:1116–1122. doi: 10.1111/j.1471-0528.1997.tb10933.x. [DOI] [PubMed] [Google Scholar]
- 3.Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70:811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- 4.Leon DA, Johansson M, Rasmussen F. Gestational age and growth rate of fetal mass are inversely associated with systolic blood pressure in young adults: an epidemiologic study of 165,136 Swedish men aged 18 years. Am J Epidemiol. 2000;152:597–604. doi: 10.1093/aje/152.7.597. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman PD, Hanson MA, Pinal C. The developmental origins of adult disease. Matern Child Nutr. 2005;1:130–141. doi: 10.1111/j.1740-8709.2005.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simmons R. Perinatal programming of obesity. Semin Perinatol. 2008;32:371–374. doi: 10.1053/j.semperi.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson JG. Epidemiology, genes and the environment: lessons learned from the Helsinki Birth Cohort Study. J Intern Med. 2007;261:418–425. doi: 10.1111/j.1365-2796.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson JG. Early growth and coronary heart disease and type 2 diabetes: findings from the Helsinki Birth Cohort Study (HBCS) Am J Clin Nutr. 2011;94:1799S–1802S. doi: 10.3945/ajcn.110.000638. [DOI] [PubMed] [Google Scholar]
- 10.Langley-Evans SC. Developmental programming of health and disease. Proc Nutr Soc. 2006;65:97–105. doi: 10.1079/pns2005478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langley-Evans SC, Bellinger L, McMullen S. Animal models of programming: early life influences on appetite and feeding behaviour. Matern Child Nutr. 2005;1:142–148. doi: 10.1111/j.1740-8709.2005.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warner MJ, Ozanne SE. Mechanisms involved in the developmental programming of adulthood disease. Biochem J. 2010;427:333–347. doi: 10.1042/BJ20091861. [DOI] [PubMed] [Google Scholar]
- 13.Faienza MF, Brunetti G, Ventura A, et al. Nonalcoholic fatty liver disease in prepubertal children born small for gestational age: influence of rapid weight catch-up growth. Horm Res Paediatr. 2013;79:103–109. doi: 10.1159/000347217. [DOI] [PubMed] [Google Scholar]
- 14.VandeHaar MJ, Moats-Staats BM, Davenport ML, et al. Reduced serum concentrations of insulin-like growth factor-I (IGF-I) in protein-restricted growing rats are accompanied by reduced IGF-I mRNA levels in liver and skeletal muscle. J Endocrinol. 1991;130:305–312. doi: 10.1677/joe.0.1300305. [DOI] [PubMed] [Google Scholar]
- 15.Thorn SR, Regnault TR, Brown LD, et al. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology. 2009;150:3021–3030. doi: 10.1210/en.2008-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bagley HN, Wang Y, Campbell MS, Yu X, Lane RH, Joss-Moore LA. Maternal docosahexaenoic acid increases adiponectin and normalizes IUGR-induced changes in rat adipose deposition J Obes 2013. DOI: 10.1155/2013/312153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erhuma A, Salter AM, Sculley DV, Langley-Evans SC, Bennett AJ. Prenatal exposure to a low-protein diet programs disordered regulation of lipid metabolism in the aging rat. Am J Physiol Endocrinol Metab. 2007;292:E1702–E1714. doi: 10.1152/ajpendo.00605.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langley-Evans SC, Daniel ZC, Wells CA, Ryan KJ, Plant R, Welham SJ. Protein restriction in the pregnant mouse modifies fetal growth and pulmonary development: role of fetal exposure to {beta}-hydroxybutyrate. Exp Physiol. 2011;96:203–215. doi: 10.1113/expphysiol.2010.054460. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Qi Y, Gao H, et al. Maternal protein restriction induces alterations in insulin signaling and ATP sensitive potassium channel protein in hypothalami of intrauterine growth restriction fetal rats. J Clin Biochem Nutr. 2013;52:43–48. doi: 10.3164/jcbn.12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ. 1990;301:259–262. doi: 10.1136/bmj.301.6746.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 22.Sohi G, Marchand K, Revesz A, Arany E, Hardy DB. Maternal protein restriction elevates cholesterol in adult rat offspring due to repressive changes in histone modifications at the cholesterol 7alpha-hydroxylase promoter. Mol Endocrinol. 2011;25:785–798. doi: 10.1210/me.2010-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Lewis RM, Wang C, Hales N, Byrne CD. Maternal dietary iron restriction modulates hepatic lipid metabolism in the fetuses. Am J Physiol Regul Integr Comp Physiol. 2005;288:R104–R111. doi: 10.1152/ajpregu.00343.2004. [DOI] [PubMed] [Google Scholar]
- 24.De Fabiani E, Mitro N, Anzulovich AC, Pinelli A, Galli G, Crestani M. The negative effects of bile acids and tumor necrosis factor-alpha on the transcription of cholesterol 7alpha-hydroxylase gene (CYP7A1) converge to hepatic nuclear factor-4: a novel mechanism of feedback regulation of bile acid synthesis mediated by nuclear receptors. J Biol Chem. 2001;276:30708–30716. doi: 10.1074/jbc.M103270200. [DOI] [PubMed] [Google Scholar]
- 25.Li T, Jahan A, Chiang JY. Bile acids and cytokines inhibit the human cholesterol 7 alpha-hydroxylase gene via the JNK/c-jun pathway in human liver cells. Hepatology. 2006;43:1202–1210. doi: 10.1002/hep.21183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11:212–217. doi: 10.1016/s1043-2760(00)00272-1. [DOI] [PubMed] [Google Scholar]
- 27.Stieger B. Recent insights into the function and regulation of the bile salt export pump (ABCB11) Current Opinion in Lipidology. 2009;20:176–181. doi: 10.1097/MOL.0b013e32832b677c. [DOI] [PubMed] [Google Scholar]
- 28.Kilani RT, Mackova M, Davidge ST, Winkler-Lowen B, Demianczuk N, Guilbert LJ. Endogenous tumor necrosis factor alpha mediates enhanced apoptosis of cultured villous trophoblasts from intrauterine growth-restricted placentae. Reproduction. 2007;133:257–264. doi: 10.1530/REP-06-0080. [DOI] [PubMed] [Google Scholar]
- 29.Raghupathy R, Al-Azemi M, Azizieh F.Intrauterine growth restriction: cytokine profiles of trophoblast antigen-stimulated maternal lymphocytes Clinical and Developmental Immunology 2012. DOI:10.1155/2012/734865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Equils O, Singh S, Karaburun S, Lu D, Thamotharan M, Devaskar SU. Intra-uterine growth restriction downregulates the hepatic toll like receptor-4 expression and function. Clin Dev Immunol. 2005;12:59–66. doi: 10.1080/17402520400008905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 32.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Nat Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.David H. The hepatocyte. Development, differentiation, and ageing. Exp Pathol Suppl. 1985;11:1–148. [PubMed] [Google Scholar]
- 34.Vassy J, Kraemer M, Chalumeau MT, Foucrier J. Development of the fetal rat liver: ultrastructural and stereological study of hepatocytes. Cell Differ. 1988;24:9–24. doi: 10.1016/0045-6039(88)90082-6. [DOI] [PubMed] [Google Scholar]
- 35.Forsdahl A. Living conditions in childhood and subsequent development of risk factors for arteriosclerotic heart disease. The cardiovascular survey in Finnmark 1974–75. Journal of Epidemiology and Community health. 1978;32:34–37. doi: 10.1136/jech.32.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osmond C, Barker DJ. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ Health Perspect. 2000;108 (Suppl 3):545–553. doi: 10.1289/ehp.00108s3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 38.Post SM, Groenendijk M, Solaas K, Rensen PC, Princen HM. Cholesterol 7alpha-hydroxylase deficiency in mice on an APOE*3-Leiden background impairs very-low-density lipoprotein production. Arterioscler Thromb Vasc Biol. 2004;24:768–774. doi: 10.1161/01.ATV.0000121572.21122.59. [DOI] [PubMed] [Google Scholar]
- 39.Pullinger CR, Eng C, Salen G, et al. Human cholesterol 7-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J Clin Invest. 2002;110:109–117. doi: 10.1172/JCI15387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russell DW. Nuclear orphan receptors control cholesterol catabolism. Cell. 1999;97:539–542. doi: 10.1016/s0092-8674(00)80763-1. [DOI] [PubMed] [Google Scholar]
- 41.Erickson SK, Lear SR, Deane S, et al. Hypercholesterolemia and changes in lipid and bile acid metabolism in male and female cyp7A1-deficient mice. J Lipid Res. 2003;44:1001–1009. doi: 10.1194/jlr.M200489-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Aldred A, Nagy LE. Ethanol dissociates hormone-stimulated cAMP production from inhibition of TNF-alpha production in rat Kupffer cells. Am J Physiol. 1999;276:G98–G106. doi: 10.1152/ajpgi.1999.276.1.G98. [DOI] [PubMed] [Google Scholar]
- 43.Saile B, DiRocco P, Dudas J, et al. IGF-I induces DNA synthesis and apoptosis in rat liver hepatic stellate cells (HSC) but DNA synthesis and proliferation in rat liver myofibroblasts (rMF) Lab Invest. 2004;84:1037–1049. doi: 10.1038/labinvest.3700116. [DOI] [PubMed] [Google Scholar]
- 44.McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- 45.Son Y, Cheong Y-K, Kim N-H, Chung H-T, Kang DG, Pae H-O.Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J Signal Transduct 2011. DOI:10.1155/2011/792639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kishore R, Hill JR, McMullen MR, Frenkel J, Nagy LE. ERK1/2 and Egr-1 contribute to increased TNF-alpha production in rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2002;282:G6–G15. doi: 10.1152/ajpgi.00328.2001. [DOI] [PubMed] [Google Scholar]
- 47.Peterside IE, Selak MA, Simmons RA. Impaired oxidative phosphorylation in hepatic mitochondria in growth-retarded rats. Am J Physiol Endocrinol Metab. 2003;285:E1258–E1266. doi: 10.1152/ajpendo.00437.2002. [DOI] [PubMed] [Google Scholar]
- 48.Fu Q, McKnight RA, Yu X, Callaway CW, Lane RH. Growth retardation alters the epigenetic characteristics of hepatic dual specificity phosphatase 5. FASEB J. 2006;20:2127–2129. doi: 10.1096/fj.06-6179fje. [DOI] [PubMed] [Google Scholar]
- 49.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rueda-Clausen CF, Dolinsky VW, Morton JS, Proctor SD, Dyck JR, Davidge ST. Hypoxia-induced intrauterine growth restriction increases the susceptibility of rats to high-fat diet-induced metabolic syndrome. Diabetes. 2011;60:507–516. doi: 10.2337/db10-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng X, Buckley D, Klaassen CD. Regulation of hepatic bile acid transporters Ntcp and Bsep expression. Biochemical pharmacology. 2007;74:1665–1676. doi: 10.1016/j.bcp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diao L, Li N, Brayman TG, Hotz KJ, Lai Y. Regulation of MRP2/ABCC2 and BSEP/ABCB11 expression in sandwich cultured human and rat hepatocytes exposed to inflammatory cytokines TNF-α, IL-6, and IL-1β. J Biol Chem. 2010;285:31185–31192. doi: 10.1074/jbc.M110.107805. [DOI] [PMC free article] [PubMed] [Google Scholar]