Abstract
Approximately 100 years have passed since the Maillard reaction was first reported in the field of food chemistry as a condensation reaction between reducing sugars and amino acids. This reaction is thought to progress slowly primarily from glucose with proteins in vivo. An early-stage product, called the ”Amadori product”, is converted into advanced glycation end products. Those accumulate in the body in accordance with age, with such accumulation being enhanced by lifestyle-related diseases that result in the denaturation of proteins. Recent studies have demonstrated that intermediate carbonyls are generated by several pathways, and rapidly generate many glycation products. However, accurate quantification of glycation products in vivo is difficult due to instability and differences in physicochemical properties. In this connection, little is known about the relationship between the structure of glycation products and pathology. Furthermore, the interaction between proteins modified by glycation and receptors for advanced glycation end products is also known to induce the production of several inflammatory cytokines. Therefore, those inhibitors have been developed over the world to prevent lifestyle-related diseases. In this review, we describe the process of protein denaturation induced by glycation and discuss the possibility of using the process as a marker of age-related diseases.
Keywords: AGEs, glycation, post-translational modification, diabetic complications, aging
Introduction
Research on advanced glycation end products (AGEs), which are produced by a browning reaction that occurs between reducing sugars and the amino residues of proteins and free amino acids (Fig. 1), was first reported by Louis Camille Maillard in 1912. Therefore, this reaction is called the Maillard reaction, rather than glycation, in the field of food science, and the end products of this reaction are also called melanoidins. The Maillard reaction also proceeds in vivo by several reactions, including oxidation and condensation between reducing sugars and proteins more gradually than that observed in food processing, resulting in the induction of denaturation of proteins (Fig. 2). In other words, although carbohydrates are indispensable for ATP production, an excess of these molecules arises in irreversible functional disorders of proteins in patients with disordered metabolism. In fact, the level of hemoglobin A1c (HbA1c), an early-stage product of the Maillard reaction, is used worldwide as a clinical marker of glycemic control in patients with diabetes, as it reflects the blood glucose level over the previous 1–2 months. However, because the stability of and methods used to detect each AGE structure differ, the clinical application of AGE analyses has not fully progressed.
Fig. 1.
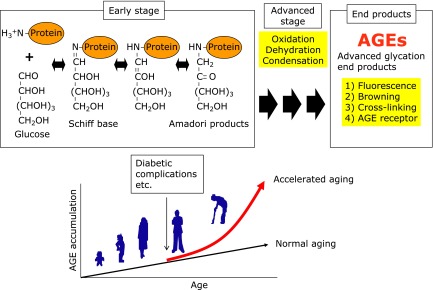
Maillard Reaction. Reducing sugars such as glucose and ribose react with amino residues of proteins and free amino acid, and reaction that occurs between reducing sugars and generate AGEs through formation of Schiff base and Amadori products. AGEs are characterized by a yellow-brown color, an autofluorescence, intra- and intermolecular cross-linkings. AGEs are recognized by several AGEs receptor such as receptor for AGE (RAGE), and AGEs-RAGE interaction is reported to activate cell signaling pathways. AGEs accumulate in the body in accordance with age, with such accumulation being enhanced by lifestyle-related diseases such as diabetic complications that result in the denaturation of proteins.
Fig. 2.
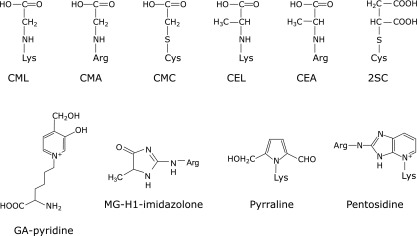
Possible pathway for AGEs formation and those biological impacts to protein modification. The Maillard reaction proceeds in vivo between reducing sugars and proteins, resulting in the induction of denaturation of proteins. Intermediate aldehydes such as glyoxal, methylglyoxal, glucosone and glycolaldehyde rapidly modify proteins in vivo.
Immunochemical Detection of AGEs
The quantification of AGEs in vivo was originally performed using the fluorescent characteristics of AGEs. Monnier et al.(1) reported that the accumulation of fluorescent AGEs increases in the dura mater of the brain in an age-dependent manner (Fig. 1). Subsequently, an anti-AGE antibody was developed as a more specific tool for detecting AGEs,(2) and the involvement of AGEs in various diseases has been reported. However, since the epitope structures of anti-AGEs antibodies were not identified until the 1990s,(3) little is known about the relationship between the structure and pathology of AGEs. We previously demonstrated that 6D12, which has been reported to be a monoclonal anti-AGE antibody in the 1990s,(3) recognizes both Nε-(carboxymethyl)lysine (CML) and Nε-(carboxyethyl)lysine (CEL) (Fig. 3).(4) CML is generated by the oxidative cleavage of Amadori products by hydroxyl radicals(5) and peroxynitrite,(6) thus suggesting that CML is an important biological marker of oxidative stress in vivo (Fig. 4). In contrast, CEL is generated from methylglyoxal via the Embden-Meyerhof pathway.(7)
Fig. 3.
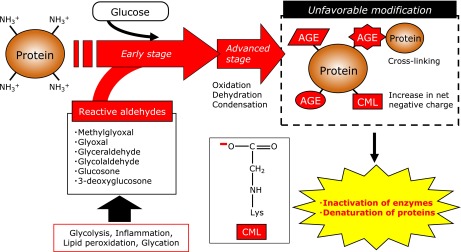
Reported AGEs structures. AGEs are generated not only from glucose but also from intermediate carbonyls via glycolysis, lipid peroxidation and inflammatory response. Typical AGEs structures were shown among AGE structures reported to date.
Fig. 4.
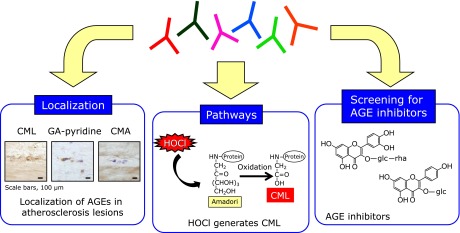
Usefulness of antibody library against AGEs structures. Monoclonal anti-AGEs antibodies that epitope structures were identified are useful for evaluating the biological significance of AGEs such as localization, pathway for AGE formation and screening of AGE inhibitors.
Since AGEs are modified amino acids with molecular weights of less than 500 Da, preparation of structure-specific anti-AGE antibody is difficult. Although 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) is the most conventional coupling reagent for small molecules and produces peptide bond between carrier protein and hapten, EDC-conjugated hapten-carrier adducts often fail to produce immune responses against small molecule haptens. Therefore, CML, a major antigenic AGE structure, was conjugated to human serum albumin (HSA) with three different cross-linkers, EDC, bis(sulfosuccinimidyl)suberate (BS3) and glutaraldehyde, and their efficacy in the production of antibodies was compared. Although all three CML-conjugated HSAs were strongly recognized by anti-CML antibody, only CML-conjugated HSA prepared by glutaraldehyde cross-linking produced an antibody against CML.(8) Similarly, antibodies against CEL, 2SC and CMC were also obtained by conjugation to carrier proteins using glutaraldehyde, indicating that glutaraldehyde is a promising cross-linker for production of antibody against small molecules.
We previously identified new AGE structure derived from glycolaldehyde (GA) in human atherosclerotic lesions. GA is formed from serine by action of myeloperoxidase and reacts with proteins to form several products. Prominent among them is CML. Because CML is formed from several pathways as described above, we attempted to identify unique structures characteristic of the reaction of GA with protein. To this end, monoclonal antibodies (GA5 and 1A12) and polyclonal antibody (non-CML-GA) specific for GA-modified proteins were prepared. These antibodies specifically reacted with GA- and hypochlorous acid-modified BSA, but not with BSA modified by other aldehydes, indicating that the epitope of these antibodies could be a specific marker for GA-modified protein. By HPLC purification, GA5-reactive compound was isolated and its chemical structure was characterized as 3-hydroxy-4-hydroxymethyl-1-(5-amino-5-carboxypentyl) pyridinium cation. This compound named as GA-pyridine (Fig. 3) was also recognized by both 1A12 and non-CML-GA, demonstrating that GA-pyridine is an important antigenic structure in GA-modified proteins.(9) Immunohistochemical studies with GA5 demonstrated the accumulation of GA-pyridine in the cytoplasm of foam cells and extracellularly in the central region of atheroma in human atherosclerotic lesions. These results suggest that GA-mediated protein modification may contribute to atherogenesis.(9) Taken together, anti-AGEs antibodies that epitope structures were identified are useful for evaluating the biological significance of AGEs (Fig. 4).
Difficulty of Measuring AGEs in Physiological Samples
Although the quantification of AGEs using instrumental analyses is superior to that of immunochemical analyses, anti-AGE antibodies are a convenient tool for estimating the content and examining the histological localization of AGEs. However, the immunochemical measurement of AGEs in physiological samples is associated with potential artifacts due to pretreatment techniques, such as heating and alkaline treatment. For instance, the pentosidine level in physiological samples is used as a sensitive marker for the early diagnosis of renal failure. In the quantitative measurements of pentosidine reported to date, a rapid enzyme-linked immunosorbent assay (ELISA) has been widely used to estimate the plasma/serum pentosidine levels in a number of clinical samples, because high performance liquid chromatography (HPLC) methods require multiple preparation steps before the analysis. However, the currently used clinical analysis of the plasma/serum pentosidine level by ELISA requires incubation of the plasma/serum at 100°C for 15 min to inactivate the protease,(10) which is required before the anti-pentosidine antibody can bind to the pentosidine. The pentosidine content, measured by HPLC, in the serum increased by heating in a temperature- and time-dependent manner.(11) Same tendency was also observed in CML formation. Thus, CML was generated from glycated HSA by heat treatment (above 80°C), and increased in a time-dependent manner.(12) These results demonstrated that AGEs could be generated artificially through the heating process.
Inhibitors for AGEs Formation
Recent studies have demonstrated that AGEs are generated not only from glucose but also from aldehydes such as glyoxal, methylglyoxal,(13) glucosone(6) and glycolaldehyde,(9) and those aldehydes rapidly modify proteins (Fig. 2). For instance, Mclellan et al.(14) demonstrated that plasma methylglyoxal concentrations in insulin-dependent diabetic patients were 7-times higher than those of healthy individuals. These reports indicate that the modification of proteins with aldehydes in vivo may contribute to the development of organ disorders such as diabetic nephropathy(15) and retinopathy(16) (Fig. 2). Therefore, AGE inhibitors have been developed over the world to prevent lifestyle-related diseases such as diabetic complications and atherosclerosis. Aminoguanidine is the first AGE inhibitor with an amino residue which traps the aldehyde group of reducing sugars.(17) Thiamine and its derivative, benfotiamine, are known to decrease methylglyoxal level in vivo and inhibit the development of incipient nephropathy(18) and retinopathy(19) in streptozotocin-induced diabetic rats. Pyridoxamine has been shown to significantly inhibit the progress of nephropathy(20) and retinopathy,(21) although the serum glucose concentration was not changed in a rat model of streptozotocin-induced diabetes.
It is known that preventive medicine is the most important approach to preventing the development of lifestyle-related diseases such as atherosclerosis and diabetic complications. We believe that the daily intake of AGEs inhibitors in natural products can play a beneficial role in preventing the pathogenesis of lifestyle-related diseases. Therefore, natural compounds were screened as potential inhibitors of AGEs formation (Fig. 4). We previously reported that Astragalus radix samples for the natural herb-derived compound inhibits formation of CML and pentosidine from the reaction of bovine serum albumin and ribose. Our findings confirmed that astragaloside significantly inhibits both of these AGEs,(22) although the inhibitory effect of the compound has not yet been confirmed in vivo. Furthermore, we also reported that high concentration (>1 mM) of catechol compounds such as epicatechin, gallic acid and 4-MC exhibits enhancing effects of CML formation by producing hydrogen peroxide, 0.01 mM of these compounds inhibits CML formation due to their high antioxidative activity.(23) This study provides the evidence that natural compounds containing catechol residues enhance CML formation and that high dose of flavonoid supplementation should be conducted with care to prevent any unfavorable aspects of antioxidants.
Discovery of New Post-translational Modification
Glycation research has recently attracted attention with respect to the study of lifestyle-related diseases, including metabolic syndrome, abnormalities in carbohydrate and/or lipid metabolism. In addition, although quantifying the amount of AGEs in vivo is difficult due to instability and differences in physicochemical properties, measuring the levels of various AGE structures in vivo is now possible due to advances in instrumental analysis techniques, such as gas or liquid chromatography tandem mass spectrometry (LC-MS/MS). As a result, the involvement of AGEs and their pathology have been evaluated at the molecular level.
We previously demonstrated that S-(2-Succinyl)cysteine (2SC) is formed by a reaction between the thiol group of proteins and fumarate, a Krebs cycle intermediate. The level of 2SC significantly increases during the maturation of 3T3-L1 fibroblasts to adipocytes. For example, the fumarate concentration increases >5-fold during adipogenesis in medium containing 30 mM glucose, producing a >10-fold increase in 2SC-proteins in adipocytes compared with undifferentiated fibroblasts grown in the same high-glucose medium(24) (Fig. 5). Furthermore, several proteins, including adiponectin and heat shock proteins, have been identified using matrix-assisted laser desorption ionization time-of-flight/time-of-flight mass spectrometry.(25) Although AGE structures, such as CML and pentosidine, are commonly measured in biological samples due to their stability and autofluorescent properties, 2SC is a prominent post-translational modification observed during the maturation of adipocytes (Fig. 5), demonstrating that the specific detection of each AGE component, including 2SC, is necessary in order to clarify the relationship between post-translational modifications and disease. 2SC is detected in human plasma and urine by gas chromatography mass spectrometry,(26) whereas the relationship between diseases and 2SC levels in plasma or urine has not been reported yet. We speculate that the increase in fumarate and 2SC is the result of mitochondrial stress in the adipocyte during adipogenesis and that 2SC may be a useful biomarker of mitochondrial stress in obesity, insulin resistance, and diabetes.
Fig. 5.
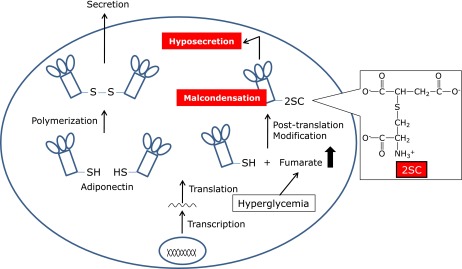
Succination of adiponectin proteins by fumarate affects the secretion and condensation in adipocytes. 2SC is formed by a reaction between the thiol group of proteins and fumarate, a Krebs cycle intermediate. 2SC-proteins such as adiponectin increased in adipocytes under hyperglycemic condition, resulting in the hyposecretion and malcondensation of adiponectin.
Question about AGE Receptors
The interaction between proteins modified by AGEs and AGE receptors, such as scavenger receptor(s) on macrophages and smooth muscle cells, is known to induce the production of several cytokines, including plasminogen activator and transforming growth factor-beta.(27) Although highly-modified AGE-bovine serum albumin (high-AGE-BSA) was significantly recognized by human monocyte-derived macrophages and Chinese hamster ovary cells which overexpress such scavenger receptors as CD36, SR-BI (scavenger receptor class B type-I), and LOX-1 (Lectin-like Ox-LDL receptor-1), the mildly-modified-AGE-BSA (mild-AGE-BSA) did not show any ligand activity to these cells. Furthermore, when 111In-labeled high- or mild-AGE-BSA were injected into the tail vein of mice, the high-AGE-BSA was rapidly cleared from the circulation whereas the clearance rate of the mild-AGE-BSA was very slow, similar to the native BSA.(28) Thornalley et al.(29) demonstrated that end-stage renal disease is associated with a significant increase in the molecular mass of HSA (+255 Da, relative to the control subjects) and ~3% of lysine residues were modified. However, our study using a MALDI-TOFMS analysis demonstrated that the molecular mass of the mild-AGE-BSA was 658 Da larger than the native BSA, indicating that our experimentally prepared mild-AGE-BSA is already more profoundly modified than physiological human serum albumin under (patho)physiological conditions. This study demonstrates the evidence that the ligand activity of the AGE-proteins to the scavenger receptors and its pharmacokinetic properties depend on their rate of modification by AGEs, and we should carefully prepare the AGE-proteins in vitro to clarify the physiological significance of the interaction between the AGE-receptors and AGE-proteins.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research (No. 24300260 and 24650482 to Ryoji Nagai) from the Ministry of Education, Science, Sports and Culture of Japan. This work was also supported in part by grants from the Japan Vascular Disease Research Foundation (2011–2013) and Central Research Institute of Fukuoka University (No.126004).
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Monnier VM, Kohn RR, Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci USA. 1984;81:583–587. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakayama H, Taneda S, Mitsuhashi T, et al. Characterization of antibodies to advanced glycosylation end products on protein. J Immunol Methods. 1991;140:119–125. doi: 10.1016/0022-1759(91)90133-z. [DOI] [PubMed] [Google Scholar]
- 3.Horiuchi S, Araki N, Morino Y. Immunochemical approach to characterize advanced glycation end products of the Maillard reaction. Evidence for the presence of a common structure. J Biol Chem. 1991;266:7329–7332. [PubMed] [Google Scholar]
- 4.Koito W, Araki T, Horiuchi S, Nagai R. Conventional antibody against Nepsilon-(carboxymethyl)lysine (CML) shows cross-reaction to Nepsilon-(carboxyethyl)lysine (CEL): immunochemical quantification of CML with a specific antibody. J Biochem. 2004;136:831–837. doi: 10.1093/jb/mvh193. [DOI] [PubMed] [Google Scholar]
- 5.Nagai R, Ikeda K, Higashi T, et al. Hydroxyl radical mediates Nε-(carboxymethyl)lysine formation from Amadori product. Biochem Biophys Res Commun. 1997;234:167–172. doi: 10.1006/bbrc.1997.6608. [DOI] [PubMed] [Google Scholar]
- 6.Nagai R, Unno Y, Hayashi MC, et al. Peroxynitrite induces formation of Nε-(carboxymethyl)lysine by the cleavage of Amadori product and generation of glucosone and glyoxal from glucose: novel pathways for protein modification by peroxynitrite. Diabetes. 2002;51:2833–2839. doi: 10.2337/diabetes.51.9.2833. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed MU, Brinkmann Frye E, Degenhardt TP, Thorpe SR, Baynes JW. Nε-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem J. 1997;324:565–570. doi: 10.1042/bj3240565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mera K, Nagai M, Brock JW, et al. Glutaraldehyde is an effective cross-linker for production of antibodies against advanced glycation end-products. J Immunol Methods. 2008;334:82–90. doi: 10.1016/j.jim.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Nagai R, Hayashi CM, Xia L, Takeya M, Horiuchi S. Identification in human atherosclerotic lesions of GA-pyridine, a novel structure derived from glycolaldehyde-modified proteins. J Biol Chem. 2002;277:48905–48912. doi: 10.1074/jbc.M205688200. [DOI] [PubMed] [Google Scholar]
- 10.Sanaka T, Funaki T, Tanaka T, et al. Plasma pentosidine levels measured by a newly developed method using ELISA in patients with chronic renal failure. Nephron. 2002;91:64–73. doi: 10.1159/000057606. [DOI] [PubMed] [Google Scholar]
- 11.Nakano M, Kubota M, Owada S, Nagai R. The pentosidine concentration in human blood specimens is affected by heating. Amino Acids. 2013;44:1451–1456. doi: 10.1007/s00726-011-1180-z. [DOI] [PubMed] [Google Scholar]
- 12.Miki Hayashi C, Nagai R, Miyazaki K, et al. Conversion of Amadori products of the Maillard reaction to Nε-(carboxymethyl)lysine by short-term heating: possible detection of artifacts by immunohistochemistry. Lab Invest. 2002;82:795–808. doi: 10.1097/01.lab.0000018826.59648.07. [DOI] [PubMed] [Google Scholar]
- 13.Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344:109–116. [PMC free article] [PubMed] [Google Scholar]
- 14.McLellan AC, Thornalley PJ, Benn J, Sonksen PH. Glyoxalase system in clinical diabetes mellitus and correlation with diabetic complications. Clin Sci (Lond) 1994;87:21–29. doi: 10.1042/cs0870021. [DOI] [PubMed] [Google Scholar]
- 15.Makino H, Shikata K, Hironaka K, et al. Ultrastructure of nonenzymatically glycated mesangial matrix in diabetic nephropathy. Kidney Int. 1995;48:517–526. doi: 10.1038/ki.1995.322. [DOI] [PubMed] [Google Scholar]
- 16.Murata T, Nagai R, Ishibashi T, Inomuta H, Ikeda K, Horiuchi S. The relationship between accumulation of advanced glycation end products and expression of vascular endothelial growth factor in human diabetic retinas. Diabetologia. 1997;40:764–769. doi: 10.1007/s001250050747. [DOI] [PubMed] [Google Scholar]
- 17.Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986;232:1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- 18.Babaei-Jadidi R, Karachalias N, Ahmed N, Battah S, Thornalley PJ. Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine. Diabetes. 2003;52:2110–2120. doi: 10.2337/diabetes.52.8.2110. [DOI] [PubMed] [Google Scholar]
- 19.Hammes HP, Du X, Edelstein D, et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9:294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- 20.Degenhardt TP, Alderson NL, Arrington DD, et al. Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int. 2002;61:939–950. doi: 10.1046/j.1523-1755.2002.00207.x. [DOI] [PubMed] [Google Scholar]
- 21.Stitt A, Gardiner TA, Alderson NL, et al. The AGE inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetes. Diabetes. 2002;51:2826–2832. doi: 10.2337/diabetes.51.9.2826. [DOI] [PubMed] [Google Scholar]
- 22.Motomura K, Fujiwara Y, Kiyota N, et al. Astragalosides isolated from the root of astragalus radix inhibit the formation of advanced glycation end products. J Agric Food Chem. 2009;57:7666–7672. doi: 10.1021/jf9007168. [DOI] [PubMed] [Google Scholar]
- 23.Fujiwara Y, Kiyota N, Tsurushima K, et al. Natural compounds containing a catechol group enhance the formation of Nε-(carboxymethyl)lysine of the Maillard reaction. Free Radic Biol Med. 2011;50:883–891. doi: 10.1016/j.freeradbiomed.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 24.Nagai R, Brock JW, Blatnik M, et al. Succination of protein thiols during adipocyte maturation: a biomarker of mitochondrial stress. J Biol Chem. 2007;282:34219–34228. doi: 10.1074/jbc.M703551200. [DOI] [PubMed] [Google Scholar]
- 25.Frizzell N, Rajesh M, Jepson MJ, et al. Succination of thiol groups in adipose tissue proteins in diabetes: succination inhibits polymerization and secretion of adiponectin. J Biol Chem. 2009;284:25772–25781. doi: 10.1074/jbc.M109.019257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alderson NL, Wang Y, Blatnik M, et al. S-(2-Succinyl)cysteine: a novel chemical modification of tissue proteins by a Krebs cycle intermediate. Arch Biochem Biophys. 2006;450:1–8. doi: 10.1016/j.abb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Higashi T, Sano H, Saishoji T, et al. The receptor for advanced glycation end products mediates the chemotaxis of rabbit smooth muscle cells. Diabetes. 1997;46:463–472. doi: 10.2337/diab.46.3.463. [DOI] [PubMed] [Google Scholar]
- 28.Nagai R, Mera K, Nakajou K, et al. The ligand activity of AGE-proteins to scavenger receptors is dependent on their rate of modification by AGEs. Biochim Biophys Acta. 2007;1772:1192–1198. doi: 10.1016/j.bbadis.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Thornalley PJ, Argirova M, Ahmed N, Mann VM, Argirov O, Dawnay A. Mass spectrometric monitoring of albumin in uremia. Kidney Int. 2000;58:2228–2234. doi: 10.1111/j.1523-1755.2000.00398.x. [DOI] [PubMed] [Google Scholar]


