Abstract
Reverse cholesterol transport (RCT) is a mechanism critical to the anti-atherogenic property of HDL. Although citrulline contributes to the amelioration of atherosclerosis via endothelial nitric oxide production, it remains unclear whether it affects RCT. This study was undertaken to clarify the effects of citrulline on expressions of specific transporters such as ATP binding cassette transporters (ABC)A1 and ABCG1, and the cholesterol efflux from macrophages to apolipoprotein (apo) A-I or HDL in vitro and ex vivo. Citrulline increased ABCA1 and ABCG1 mRNA and protein levels in THP-1 macrophages, translating into enhanced apoA-I- and HDL-mediated cholesterol efflux. In the human crossover study, 8 healthy male volunteers (age 30–49 years) consumed either 3.2 g/day citrulline or placebo for 1 week. Citrulline consumption brought about significant increases in plasma levels of citrulline and arginine. Supporting the in vitro data, monocyte-derived macrophages (MDM) differentiated under autologous post-citrulline sera demonstrated enhancement of both apoA-I- and HDL-mediated cholesterol efflux through increased ABCA1 and ABCG1 expressions, compared to MDM differentiated under pre-citrulline sera. However, the placebo did not modulate these parameters. Therefore, in addition to improving endothelium function, citrulline might have an anti-atherogenic property by increasing RCT of HDL.
Keywords: citrulline, cholesterol, macrophage, HDL, reverse cholesterol transport
Introduction
HDL has been shown to be inversely associated with the risk of atherosclerotic cardiovascular disease (CVD) and is thus considered to be an anti-atherogenic lipoprotein.(1,2) However, failure in recent clinical trials with HDL-C raising agents such as nicotinic acid and the cholesteryl ester transfer protein inhibitor torcetrapib to provide cardiovascular benefits despite raising HDL-C levels, indicate that the focus should be on quality (functionality), and not necessarily quantity (HDL-C levels) regarding therapeutic targets.(3) Anti-atherogenic functions of HDL include anti-inflammatory,(4) anti-oxidative,(5) anti-coagulative,(6) and endothelial-improving functions,(7) as well as enhancement of macrophage reverse cholesterol transport (RCT), a process by which cholesterol is transported from macrophages to the liver for ultimate fecal excretion. Although these functions may comprehensively be involved in the prevention of atherosclerosis progression,(8,9) RCT itself has been postulated to play a major role in HDL-mediated atheroprotection.(10) Indeed, quantitative measures of macrophage RCT have been shown to be more strongly associated with atherosclerosis than plasma HDL-C concentrations in mice and humans.(11,12) The first critical step of macrophage-derived foam cell RCT involves efflux of cellular cholesterol to circulating HDL particles.
Research in recent years has documented a specific role for the macrophage transporters ATP-binding cassette subfamilies (ABC) A1 and ABCG1 in cholesterol efflux. ABCA1 has been shown to play an important role in apolipoprotein (apo) A-I-mediated cholesterol efflux from peripheral cells and macrophages while ABCG1 promotes cholesterol efflux from macrophages to HDL particles.(13,14) These findings have stimulated efforts to target the macrophage at the cellular level as a means of enhancing overall RCT.
Endothelial dysfunction is a key feature of early atherosclerotic lesions in both humans and animal models and elevated plasma concentrations of risk factors for CVD.(15–18) Citrulline is unusually abundant in watermelon (Citrullus vulgaris) and arginine is synthesized from it in endothelial cells.(19) The conversion of citrulline to nitric oxide (NO) via arginine forms part of a recycling pathway.(20–22) NO is synthesized by NO synthase (NOS), which produces citrulline, a precursor of arginine. Chronic consumption of citrulline-rich food has been known to be effective in increasing plasma concentrations of arginine in healthy humans,(23) and Hayashi et al.(24) reported that citrulline decreased the progression of atherosclerosis in rabbits fed a high cholesterol diet, via endothelial NOS (eNOS). Although citrulline contributes to anti-atherosclerosis via endothelial nitric oxide, it remains unclear whether it affects the anti-atherogenic properties of HDL-mediated RCT functions, or how it might do this. Therefore, the present study was undertaken to investigate the effects of citrulline on ABCA1 and ABCG1 expressions and HDL-mediated cholesterol efflux from macrophages, both in vitro and ex vivo situations.
Materials and Methods
Materials
Actinomysin D (ActD) and human apoA-I were purchased from Sigma (St. Louis, MO). NG-nitro-l-arginine methyl ester (L-NAME) and phorbol 12-myristate 13-acetate (PMA) were purchased from Wako Pure Chemical (Tokyo, Japan). HDL was isolated by sequential ultracentrifugation and acetylated LDL (acLDL) was prepared according to the previously reported methods.(25,26) L-Citrulline for the in vitro and in vivo study was purchased from Kanto Chemical CO., INC. (Tokyo, Japan). Placebo and citrulline for the ex vivo study were kindly donated by Kyowa Hakko Bio Co., Ltd. (Tokyo, Japan).
Cell culture
THP-1 cells (Riken Cell Bank, Tsukuba, Japan) were maintained in RPMI 1640 (Sigma) containing 10% fetal bovine serum (FBS). The differentiation of THP-1 monocytes into macrophages was induced in the presence of 320 nM of PMA for 72 h. RAW264.7 cells (Riken Cell Bank, Tsukuba, Japan) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma) containing 10% FBS.
Quantitative real-time PCR
At the indicated hours after treatment with the compounds, total RNA was extracted from the cells, and first-strand cDNA was synthesized from the total RNA (250 ng) by placing in a Reverse Transcription Reagent (Applied Biosystems, Foster City, CA). Quantitative real-time PCR was performed using an ABI 7900 PCR machine, TaqMan PCR master mix and FAM-labeled TaqMan probes (Assays-on-Demand, Applied Biosystems) for human ABCA1, ABCG1, scavenger receptor class B type I (SR-BI) and 18S ribosomal RNA. The expression data were normalized for 18S levels.
Western blot analyses
The cells were harvested and protein extracts prepared as previously described.(25–27) They were then subjected to Western blot analyses (10% SDS-PAGE; 30 µg protein per lane) using rabbit anti-ABCG1 (Novus Biologicals, Littleton, CO) and anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies. Rat anti-ABCA1 was kindly donated by Dr. S, Yokoyama. The proteins were visualized and quantified using a chemiluminescence method (ECL Pus Western Blotting Detection System; GE Healthcare UK Ltd., Buckinghamshire, UK) and the NIH image analysis software program.
Determination of cholesterol efflux
Cholesterol efflux experiments were performed as previously described.(25–27) The cells were labeled with [3H] cholesterol (1.0 µCi/ml) in media containing 0.1% bovine serum albumin (BSA) for 24 h. The cells were washed with phosphate buffered saline (PBS) and incubated in RPMI 1640 containing 0.1% BSA in the presence and absence of apoA-I (10 µg/ml) or HDL (50 µg/ml) for 24 h. The percentage cholesterol efflux was calculated by dividing the media-derived radioactivity by the sum of the radioactivity in the media and the cells.
Human study
Eight healthy male volunteers (age 30–49 years) were recruited for a crossover study. After an overnight (12 h) fast, the study subjects consumed placebo or 1.6 g citrulline twice daily for 1 week.(28) Blood samples were taken at 0 and 1 week after consumption of placebo or citrulline. All subjects provided written informed consent. The study was approved by the Ethical Committee of the National Defense Medical College.
Human monocyte isolation and macrophage differentiation
Human monocytes were isolated as previously described.(25–27,29) The remaining blood was drawn into heparinized blood collection tubes. Human peripheral blood monocytes were isolated using the method of Fogelman et al.(30) with Ficoll/Hypaque gradient centrifugation. The mononuclear cells were re-suspended in RPMI 1640 (Sigma) supplemented with 20% autologous serum, plated on to serum-treated 10-cm dishes and incubated for 2 h. Non-adherent cells were removed by washing three times with PBS, and adherent cells were then detached by incubation in PBS containing 10% autologous serum and 0.02% EDTA at 4°C for 30 min. The adherent cells were then washed extensively and re-suspended in RPMI 1640 supplemented with 10% autologous serum. They were then plated on 12-well plates and incubated for 10 days so that they would differentiate into monocyte derived macrophages (MDM). The culture media was changed every 3–4 days during this 10-days period.
Analysis of citrulline and arginine in plasma
Blood samples were rapidly centrifuged and deproteinized with a 5% (w/v) sulfosalicylic acid solution. The supernatant fractions were stored at –80°C for analysis of amino acids. Amino acids were separated and quantified by ion exchange chromatography using an amino acid autoanalyser (Amino Tac, JLC-300; Jeol Ltd, Tokyo, Japan) and the detection sensitivity was 125–500 nM. Amino acid analysis was performed using an LC-R-6 column (6.0 × 90 mm I,D., Mitsubishi Chemical Co., Tokyo, Japan). The mobile phase was performed using lithium citrate buffer solution.(28) The autoanalyzer system consisted of the reaction coil (0.25 × 10 m I.D.) and a 570 and 440 nm at 132°C.
Serum TC, TG, LDL-C, HDL-C, glucose and NO3
Serum total cholesterol (TC), triglycerides (TG), LDL-cholesterol (LDL-C) and HDL-C levels were determined by standard enzymatic methods (Measure L, Kyowa Mediceed, Tokyo, Japan). The serum concentration of glucose was determined using an Olympus AU 600 analyser. Blood nitrate (NO3) was quantified by the HPLC-UV method.(31,32)
Statistical analysis
Statistical analyses were performed using the Stat View ver. 5.0 software package (SAS Institute Inc., NC). For the cell culture study, two-way factorial ANOVA (Fig. 1A and B) or one-way ANOVA, followed by post-hoc analysis using Bonferroni/Dunn test, with a value of p<0.05 considered to be significant. All results were expressed as the mean ± SEM.
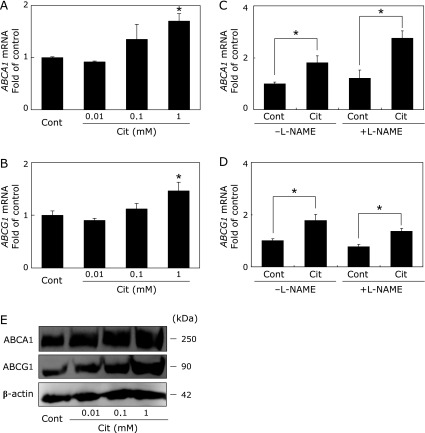
Fig. 1.
Citrulline increases expression of ABCA1 and ABCG1 in macrophages. A, B, THP-1 macrophages were treated with control (Cont) or 0.01, 0.1, 1 mM of citrulline (Cit) for 6 h. RNA extraction and quantitative real-time PCR were performed as described in Materials and Methods. The results from 3 separately performed experiments are expressed relative to the vehicles and presented as the mean ± SEM. Within each treatmentgroup, means without a common letter, p<0.05. C, D, THP-1 macrophages were treated with Cont or 1 mM of Cit with or without 5 mM of L-NAME for 6 h. The results from 3 separately performed experiments are expressed relative to Cont and presented as the mean ± SEM. *Different from Cont, p<0.05. RNA extraction and quantitative real-time PCR were performed as described in Materials and Methods. E, THP-1 macrophages were lysed and subjected to Western blot analysis 24 h after treatment with Cont or the indicated doses of Cit. The results are from 3 separately performed experiments that yielded similar results.
Results
Upregulation of ABCA1 and ABCG1 expressions by citrulline
The effect of citrulline on ABCA1 and ABCG1 mRNA levels in THP-1 macrophages was first investigated. As shown in Fig. 1A and B, 1 mM of citrulline increased ABCA1 and ABCG1 mRNA levels by 74.5 ± 21.5% (p<0.05) and 58.3 ± 22.0% (p<0.05), respectively. In contrast, citrulline did not affect SR-BI expression in THP-1 macrophages (data not shown). To further investigate an association between ABCA1 and ABCG1 expressions and NOS, we performed experiments using the NOS inhibitor L-NAME. To our surprise, citrulline-induced ABCA1 and ABCG1 mRNA expression with or without 5 mM of L-NAME in THP-1 macrophages (Fig. 1C and D), indicating that citrulline-induced ABCA1 and ABCG1 expressions were NO-independent. Mirroring the increased mRNA levels, citrulline dose-dependently increased the protein levels of ABCA1 and ABCG1 (Fig. 1E).
Effect of ABCA1 and ABCG1 mRNA stability by citrulline
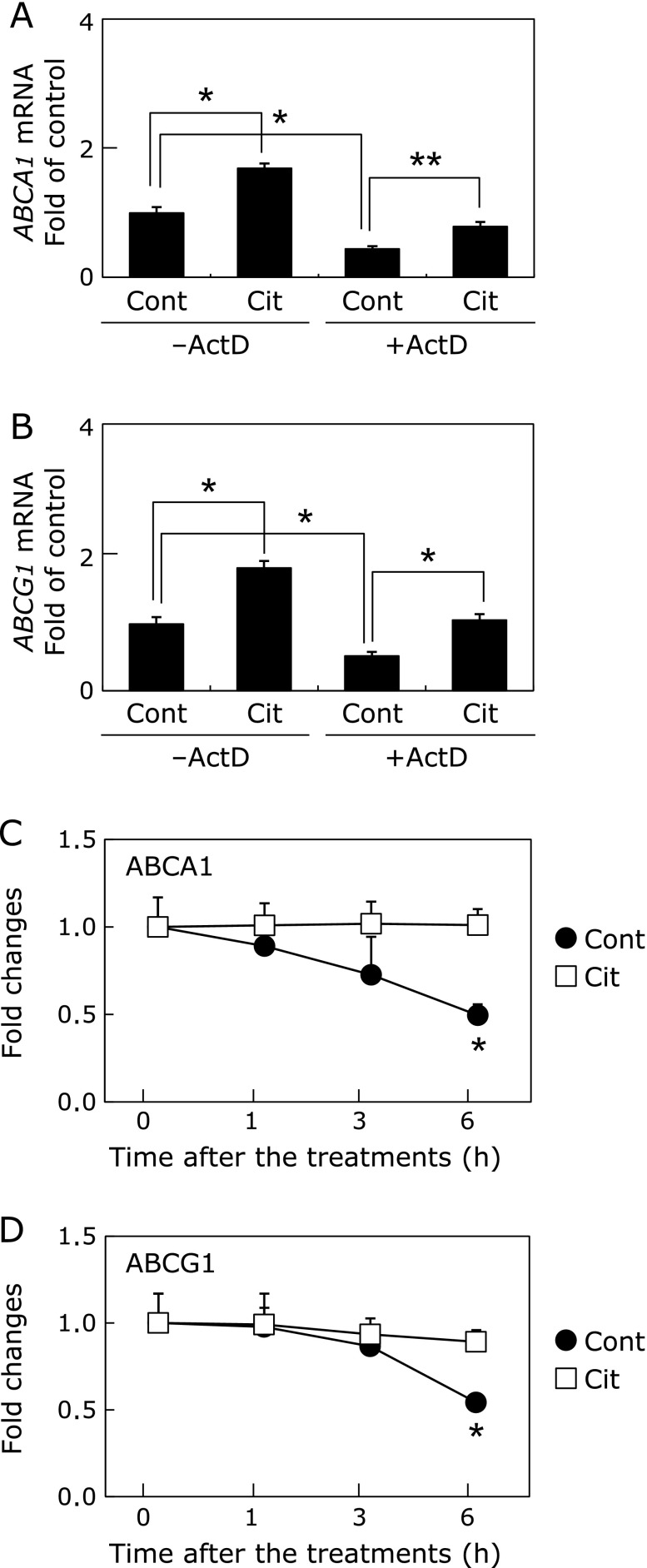
To further explore the mechanisms by which the citrulline increased ABCA1 and ABCG1 mRNA levels in the macrophages, we investigated whether they transcriptionally induced these genes using Act D. As shown in Fig. 2A and B, treatment with 10 µg/ml of ActD did not abolish the inducible effects of citrulline on ABCA1 and ABCG1 expression in THP-1 macrophages. Further, ABCA1 and ABCG1 mRNA decay induced by ActD were inhibited in the presence of the citrulline (Fig. 2C and D), thus indicating they enhanced mRNA stability of ABCA1 by 44.5 ± 7.0% (p<0.05) and ABCG1 by 39.1 ± 5.5% (p<0.05). These data indicated that citrulline enhanced mRNA stability of ABCA1 and ABCG1.
Fig. 2.
ABCA1 and ABCG1 mRNA stability enhanced by citrulline in macrophages. A, B, THP-1 macrophages were pretreated with 10 µg/ml of ActD 0.5 h before the treatment with control (Cont) or 1 mM of citrulline (Cit) with or without 10 µg/ml of ActD for 6 h. The results from 3 separately performed experiments are expressed relative to the vehicles and presented as the mean ± SEM. *Different from Cont or ActD, p<0.05, p<0.05. RNA extraction and quantitative real-time PCR were performed as described in Materials and Methods. C, D, THP-1 macrophages were pretreated with 10 µg/ml of ActD 0.5 h before the treatment with Cont or 1 mM of Cit with 10 µg/ml of ActD for 1, 3 and 6 h. The results from 3 separately performed experiments are expressed relative to the vehicles and presented as the mean ± SEM. *Different from Cont, p<0.05. RNA extraction and quantitative real-time PCR were performed as described in Materials and Methods.
Citrulline enhanced ApoA-I and HDL-mediated cholesterol efflux in THP-1 macrophages
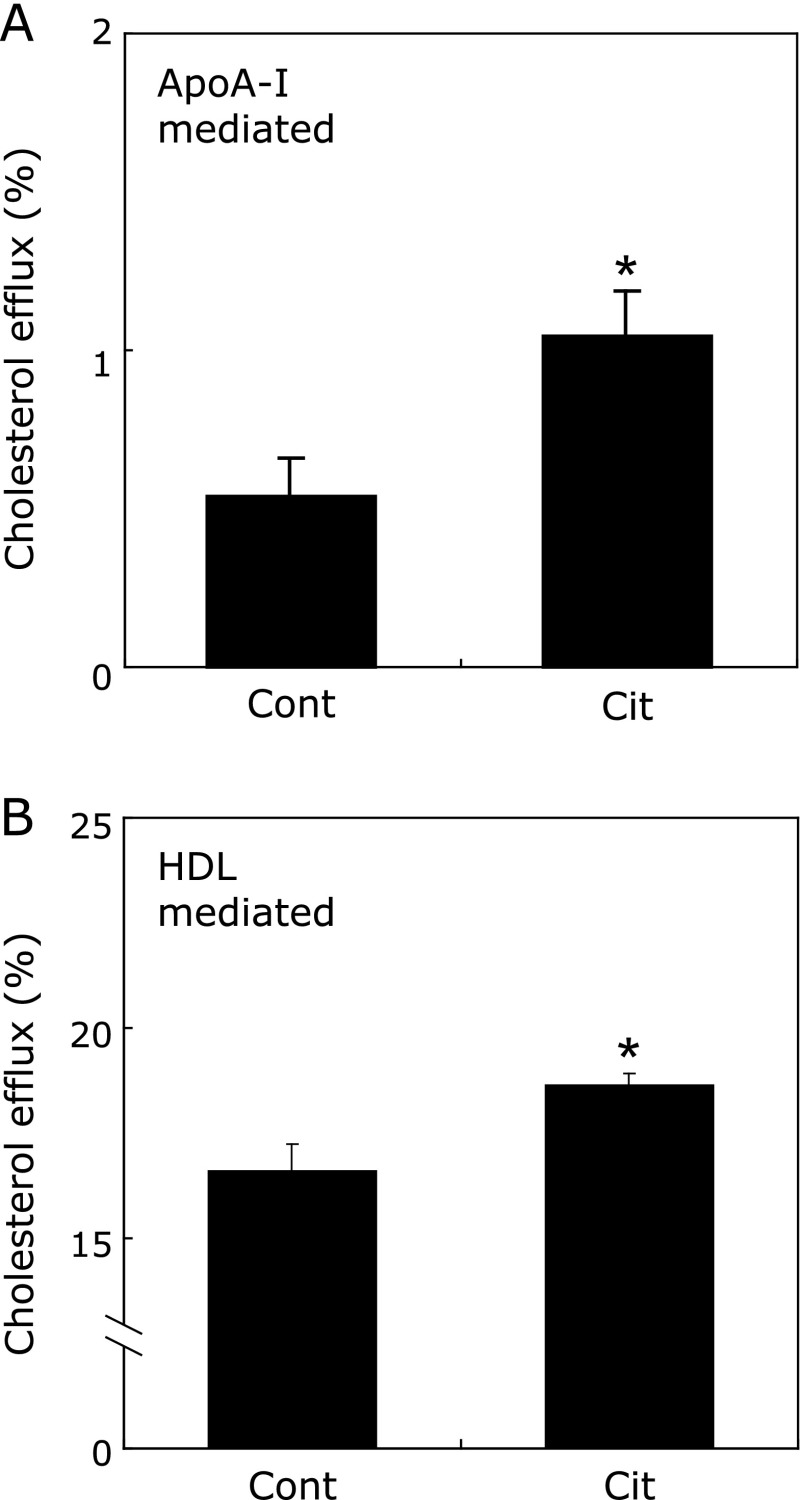
Next, we investigated the effect of citrulline on cholesterol efflux from THP-1 macrophages mediated by apoA-I or HDL. Citrulline enhanced both apoA-I- and HDL-mediated cholesterol efflux from THP-1 macrophages by 96.1 ± 26.1% (p<0.05) and 14.4 ± 2.5% (p<0.05), respectively, compared to control (Fig. 3A and B).
Fig. 3.
Citrulline enhances ApoAI- and HDL-mediated cholesterol efflux in macrophages. The indicated doses of citrulline (Cit) or control (Cont) were added to the cultures after [3H] cholesterol labeling, which were then incubated in the presence of 10 µg/ml of human apoA-I (A) or 50 µg/ml of HDL (B) for 24 h. Determination of cholesterol efflux from the cells was performed as described in Materials and Methods. The results for 6 samples are presented as the mean ± SEM. *p<0.05 vs Cont.
Citrulline increases serum citrulline and arginine, but has no effects on serum TC, TG, LDL-C, HDL-C, glucose or NO3
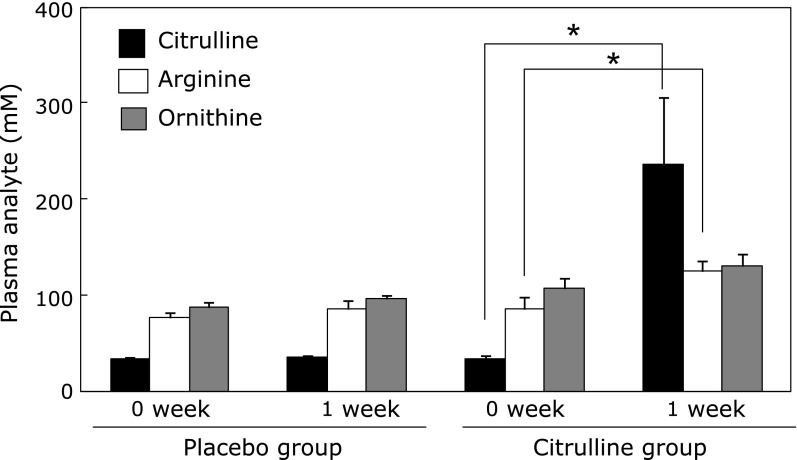
Our next objective was to clarify the matter of whether the above in vitro observations translate into human physiology. As shown in Fig. 4, 1 week of citrulline consumption markedly increased its concentration, by 595% (p<0.05). Further, arginine and ornithine, which are metabolically linked with citrulline through the Krebs-Henseleit cycle,(33) were also increased, by 44.7% (p<0.05) and 12.4%, respectively. As expected, placebo consumption did not produce any changes. We also found that 1 week of citrulline consumption did not change other parameters, including plasma lipids, glucose, and NO3 (a metabolite of NO) (data not shown).
Fig. 4.
Citrulline consumption results in increased levels of citrulline/arginine in human plasma. A, plasma was isolated from blood obtained from 8 subjects before and 1 week after consumption of placebo or citrulline. The plasma concentrations of citrulline, arginine and ornithine were determined by amino acid analyzer as described in Materials and Methods. The results are presented as the mean ± SEM. *Different from 0 week, p<0.05.
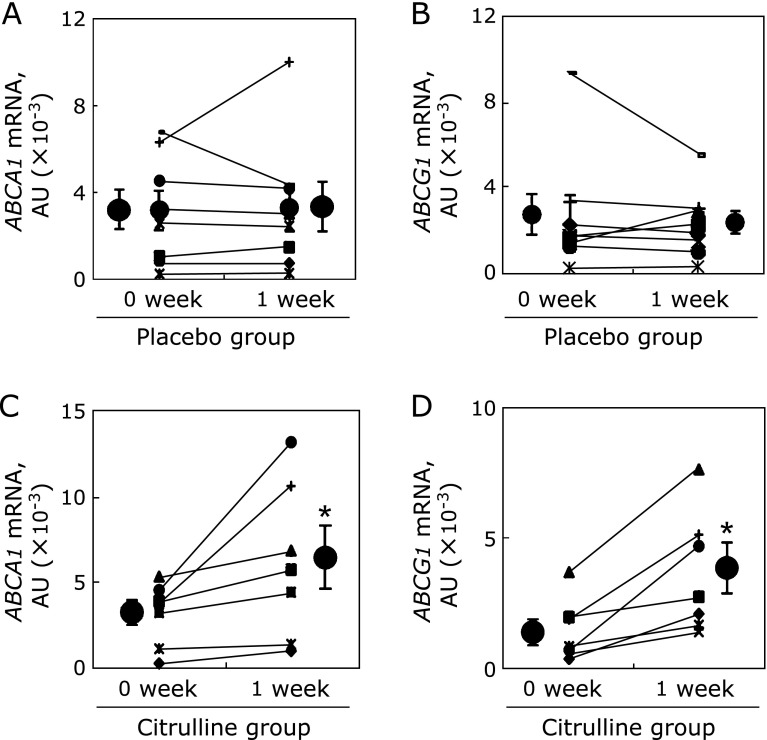
Increased ABCA1 and ABCG1 expression in MDM in presence of post-citrulline sera
Individual changes in ABCA1 and ABCG1 mRNA levels in the MDM differentiated using autologous sera obtained before and after citrulline or placebo consumption are shown in Fig. 5. Although there was some individual variation, ABCA1 and ABCG1 mRNA levels were increased in the MDM treated with post-citrulline sera as compared to pre-citrulline cells and sera combinations by 95.9% (p<0.05) and 162% (p<0.05), respectively, in most study subjects. As expected, placebo did not affect the expression of the MDM genes. As shown in Fig. 6, parallel increases in ABCA1 and ABCG1 protein levels were observed. ABCA1 and ABCG1 protein levels in MDM cultured under post-citrulline sera were significantly increased, by 85.7 ± 24.1% (p<0.05) and 34.7 ± 10.5% (p<0.05), respectively (Fig. 6C and D). Again, there was no difference between pre- and post-placebo cells and sera (Fig. 6A and B).
Fig. 5.
Citrulline consumption results in increased mRNA levels of ABCA1 and ABCG1 in human monocyte derived macrophages (MDM). The monocytes were cultured and differentiated into macrophages in the presence of autologous sera obtained from 8 subjects before and 1 week after placebo (A, B) or citrulline (C, D) consumption. RNA extraction and quantitative real-time PCR were performed as described in Materials and Methods. The results are expressed as the mean ± SEM. *Different from 0 week-citrulline, p<0.05.
Fig. 6.
Citrulline results in increased protein levels of ABCA1 and ABCG1 in human monocyte derived macrophages (MDM). The monocytes were cultured and differentiated into macrophages in the presence of autologous sera obtained from 8 subjects before and 1 week after placebo or citrulline consumption as describe in Materials and Methods. MDM were lysed and subjected to a Western blot analysis. Protein levels were quantified as described in Materials and Methods. Independent experiments from 8 different subjects are expressed as the mean ± SEM. *Different from 0 week-citrulline, p<0.05.
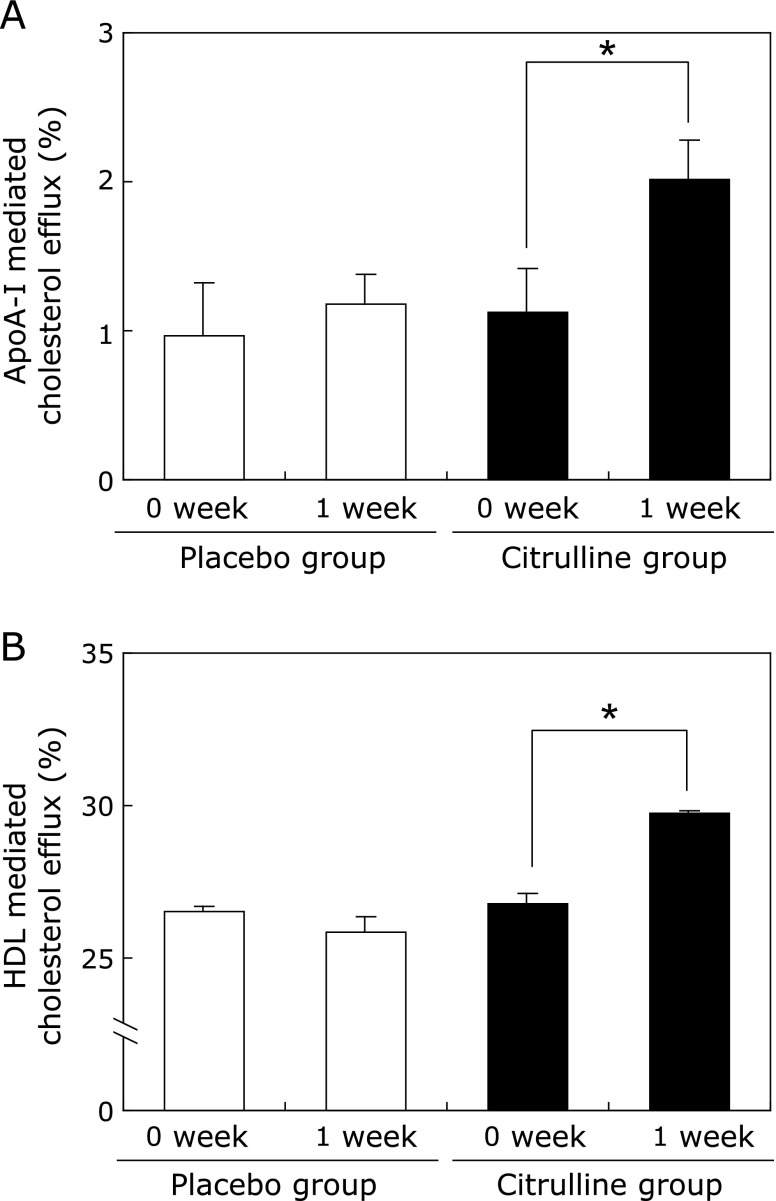
Enhanced ApoA-I and HDL-mediated cholesterol efflux in MDM treated with post-citrulline sera
Finally, we performed an HDL-mediated cholesterol efflux assay using human MDM cultured in media containing sera obtained before and after citrulline consumption. Consistent with the increased ABCA1/G1 expressions observed above, ApoA-I and HDL-mediated cholesterol effluxes were also significantly increased, by 78.0% (p<0.05) and 11.0% (p<0.05), respectively, in MDM cultured under post-citrulline sera, as compared to pre-citrulline cells and sera (Fig. 7A and B). Again, no changes were observed for placebo.
Fig. 7.
Citrulline consumption enhances ApoAI- and HDL-mediated cholesterol efflux in human monocyte derived macrophages (MDM). The monocytes were cultured and differentiated into macrophages under pooled sera obtained before and 1 week after placebo or citrulline consumption as described in Materials and Methods. After [3H] cholesterol labeling, MDM were incubated in the presence of 10 µg/ml of ApoA-I (A) and 50 µg/ml of HDL (B) for 24 h. Determination of cholesterol efflux from the cells was performed as described in Materials and Methods. The results for HDL-mediated efflux in quadruplicate and ApoA-I-mediated efflux in sextuplicate are presented as the mean ± SEM. *Different from 0 week-citrulline, p<0.05.
Discussion
There is a strong inverse correlation between plasma concentrations of HDL-C and risk of atherosclerotic cardiovascular disease.(34) Although the mechanism by which HDL may exert a direct protective effect against the development of atherosclerosis is not yet well understood, it has been postulated that HDL facilitates the efflux of cholesterol from peripheral tissues and transports it back to the liver in a process called RCT.(35) Here we showed, for the first time, that citrulline consumption lead to increased ApoA-I- and HDL-mediated cholesterol efflux from macrophages by inducing ABCA1 and ABCG1 expressions, both in an in vitro and ex vivo study.
With regard to mechanisms by which citrulline-mediated induction of ABCA1 and ABCG1 gene expression is transcriptionally regulated, preliminary results showed that citrulline did not increase these promoter activities of well-defined human ABCA1 (−940 to +110 bp relative to transcription start site) and ABCG1 (−1104 to +38 bp relative to transcription start site in exon 1) or (−1180 to +144 bp in exon 5) promoter-luciferase constructs (data not shown).(27,36,37) We then investigated effects of citrulline on mRNA stability of ABCA1 and ABCG1 by using ActD to find that citrulline enhanced ABCA1 and ABCG1 mRNA stability. Indeed, we showed that citrulline enhanced ABCA1 and ABCG1 mRNA stability (Fig. 2C and D), thus resulting in increased steady-state expression of these genes in the macrophage.
Consistent with the in vitro findings, the ex vivo human study showed that MDM differentiated under autologous post-citrulline sera enhanced apoA-I- and HDL-mediated cholesterol efflux by increasing ABCA1 and ABCG1 expression without changing plasma HDL-C levels. In theory, since ex vivo experiments can provide a situation that is closer to the in vivo situation in humans than the in vitro setting, comparable results from in vitro and in vivo studies would provide a reasonable basis for hypothesizing that citrulline promotes RCT by enhancing cholesterol efflux via ABCA1/G1 pathways in humans in vivo. This has been highlighted as an important concept in the recent study by Rader et al.(11) which found that quantitative measures of macrophage RCT are more strongly associated with atherosclerotic disease morbidity than plasma HDL-C concentrations. Enthusiasm concerning cholesterol efflux and its modulation began about 10 years ago when ABCA1 was discovered as the mutation causing human HDL deficiency in Tangier disease. However, although research efforts have been intensely focused on enhancing ABCA1 function, drugs that achieve this are still not available. In the meantime, natural products that enhance cholesterol efflux might be realistic alternatives.
Several studies have reported an association between citrulline and anti-atherosclerosis. The activation of adhesion molecules elicited by oxidized LDL and the uptake of oxidized LDL by macrophages are key steps in the progression of atherothrombotic cardiovascular diseases.(38) L-citrulline itself is an efficient radical scavenger and is a strong antioxidant.(39) In addition, NO has potent antioxidant properties that exert anti-inflammatory effects and can inhibit LDL oxidation.(40) Recently, Morita et al.(41) reported that 800 mg/day of L-citrulline intake for 8 weeks reduced serum oxidized LDL and lectin-like oxidized LDL receptor 1 (LOX-1) ligand containing ApoB, an indicator of the biological activity of oxidized lipoprotein binding to LOX-1 in humans with vasospastic angina. NO is a widespread signaling molecule in the cardiovascular system, which functions in multiple ways to protect against the initiation and progression of atherosclerosis.(42–44) Interestingly, in our study, citrulline selectively induced ABCA1 and ABCG1 independently of L-NAME. Also, citrulline consumption did not induce any changes in serum NO3 levels (data not shown), indicating that citrulline increases HDL-mediated cholesterol efflux from macrophages via an NOS-independent pathway. Thus, overall, our results do not completely exclude the possibility that enhanced cholesterol efflux due to citrulline is NO dependent. We will therefore need to perform an in vivo RCT assay using NOS knockout mice as a further study.
There are several limitations in the present study. First, as mentioned above, the subjects in the human study were healthy volunteers. Therefore, it is difficult to generalize the findings to dyslipidemic patients who are at increased risk for atherosclerosis. Second, 1 week of oral citrulline represents an acute effect, which may differ from the effect of taking it habitually. Third, the present study did not test for the presence of other factors potentially affecting cholesterol efflux nor factors along the pathway by which citrulline consumption could affect NO. Likewise, arginine might increase cholesterol efflux by citrulline administration because citrulline administration increased serum arginine concentration, although arginine concentration is very lower than citrullin concentration. Finally, we did not directly examine whether citrulline actually removed oxidized cholesterol, oxidized fatty acids or oxidized phospholipids from peripheral tissues. Such interesting a posteriori considerations deserve future studies.
In conclusion, the comparable results from the in vitro and ex vivo studies indicate that potential anti-atherogenic properties of citrulline could be explained, at least in part, as being due to up-regulated cholesterol efflux from macrophages mediated through ABCA1 and ABCG1 pathways. Combined with its previously-proven endothelial NO-related properties, this favorable effect of citrulline on HDL suggests great synergistic potential with regard to cardioprotective properties.
Acknowledgments
We thank Fumiko Watanabe of KYOWA HAKKO Bio Co., Ltd., for her helpful assistance. Supported, in part, by a research grant from KYOWA HAKKO Bio Co., Ltd. Placebo and Citrulline for the ex vivo study were kindly donated by KYOWA HAKKO Bio Co., Ltd.
Abbreviations
- ABCA1
ATP-binding cassette subfamily A member
- ABCG1
ATP-binding cassette subfamily G member 1
- acLDL
acetylated LDL
- ActD
actinomycin D
- Apo
apolipoprotein
- BSA
bovine serum albumin
- cAMP
cyclic AMP
- CVD
cardiovascular disease
- eNOS
endothelial nitric oxide synthase
- L-NAME
NG-nitro-l-arginine methyl ester
- LOX-1
lectin-like oxidized LDL receptor 1
- LXR
liver X receptor
- MDM
monocyte derived macrophages
- NO
nitric oxide
- NO3
nitrate
- NOS
nitric oxide synthase
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- PMA
phorbol 12-myristate 13-acetate
- PPAR
peroxisome proliferator-activated receptor
- RCT
reverse cholesterol transport
- SR-BI
scavenger receptor class B type I
- TC
total cholesterol
- TG
triglycerides
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 2.Wilson PW, Abbott RD, Castelli WP. High density lipoprotein cholesterol and mortality. The Framingham Heart Study. Arteriosclerosis. 1988;8:737–741. doi: 10.1161/01.atv.8.6.737. [DOI] [PubMed] [Google Scholar]
- 3.AIM-HIGH Investigators, Boden WE, Probstfield JL, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki M, Pritchard DK, Becker L, et al. High-density lipoprotein suppresses the type I interferon response, a family of potent antiviral immunoregulators, in macrophages challenged with lipopolysaccharide. Circulation. 2010;122:1919–1927. doi: 10.1161/CIRCULATIONAHA.110.961193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parthasarathy S, Barnett J, Fong LG. High-density lipoprotein inhibits the oxidative modification of low-density lipoprotein. Biochim Biophys Acta. 1990;1044:275–283. doi: 10.1016/0005-2760(90)90314-n. [DOI] [PubMed] [Google Scholar]
- 6.Murphy AJ, Woollard KJ. High-density lipoprotein: a potent inhibitor of inflammation. Clin Exp Pharmacol Physiol. 2010;37:710–718. doi: 10.1111/j.1440-1681.2009.05338.x. [DOI] [PubMed] [Google Scholar]
- 7.Sorrentino SA, Besler C, Rohrer L, et al. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation. 2010;121:110–122. doi: 10.1161/CIRCULATIONAHA.108.836346. [DOI] [PubMed] [Google Scholar]
- 8.Li XP, Zhao SP, Zhang XY, Liu L, Gao M, Zhou QC. Protective effect of high density lipoprotein on endothelium-dependent vasodilatation. Int J Cardiol. 2000;73:231–236. doi: 10.1016/s0167-5273(00)00221-7. [DOI] [PubMed] [Google Scholar]
- 9.Kuvin JT, Patel AR, Sidhu M, et al. Relation between high-density lipoprotein cholesterol and peripheral vasomotor function. Am J Cardiol. 2003;92:275–279. doi: 10.1016/s0002-9149(03)00623-4. [DOI] [PubMed] [Google Scholar]
- 10.Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 2006;113:2548–2555. doi: 10.1161/CIRCULATIONAHA.104.475715. [DOI] [PubMed] [Google Scholar]
- 11.Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50 (Suppl):S189–S194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khera AV, Cuchel M, de la. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang N, Silver DL, Thiele C, Tall AR. ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J Biol Chem. 2001;276:23742–23747. doi: 10.1074/jbc.M102348200. [DOI] [PubMed] [Google Scholar]
- 14.Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci U S A. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeiher AM, Drexler H, Wollschläger H, Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991;83:391–401. doi: 10.1161/01.cir.83.2.391. [DOI] [PubMed] [Google Scholar]
- 16.Förstermann U, Mügge A, Alheid U, Haverich A, Frölich JC. Selective attenuation of endothelium-mediated vasodilation in atherosclerotic human coronary arteries. Circ Res. 1988;62:185–190. doi: 10.1161/01.res.62.2.185. [DOI] [PubMed] [Google Scholar]
- 17.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 18.Marliss EB, Chevalier S, Gougeon R, et al. Elevations of plasma methylarginines in obesity and ageing are related to insulin sensitivity and rates of protein turnover. Diabetologia. 2006;49:351–359. doi: 10.1007/s00125-005-0066-6. [DOI] [PubMed] [Google Scholar]
- 19.Rimando AM, Perkins-Veazie PM. Determination of citrulline in watermelon rind. J Chromatogr A. 2005;1078:196–200. doi: 10.1016/j.chroma.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Creager MA, Gallagher SJ, Girerd XJ, Coleman SM, Dzau VJ, Cooke JP. L-arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans. J Clin Invest. 1992;90:1248–1253. doi: 10.1172/JCI115987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu G, Bazer FW, Cudd TA, et al. Pharmacokinetics and safety of arginine supplementation in animals. J Nutr. 2007;137:1673S–1680S. doi: 10.1093/jn/137.6.1673S. [DOI] [PubMed] [Google Scholar]
- 22.Tangphao O, Chalon S, Moreno H,, Jr, Hoffman BB, Blaschke TF. Pharmacokinetics of L-arginine during chronic administration to patients with hypercholesterolaemia. Clin Sci (Lond) 1999;96:199–207. [PubMed] [Google Scholar]
- 23.Collins JK, Wu G, Perkins-Veazie P, et al. Watermelon consumption increases plasma arginine concentrations in adults. Nutrition. 2007;23:261–266. doi: 10.1016/j.nut.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi T, Juliet PA, Matsui-Hirai H, et al. l-Citrulline and l-arginine supplementation retards the progression of high-cholesterol-diet-induced atherosclerosis in rabbits. Proc Natl Acad Sci U S A. 2005;102:13681–13686. doi: 10.1073/pnas.0506595102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayaori M, Sawada S, Yonemura A, et al. Glucocorticoid receptor regulates ATP-binding cassette transporter-A1 expression and apolipoprotein-mediated cholesterol efflux from macrophages. Arterioscler Thromb Vasc Biol. 2006;26:163–168. doi: 10.1161/01.ATV.0000193513.29074.52. [DOI] [PubMed] [Google Scholar]
- 26.Uto-Kondo H, Ayaori M, Ogura M, et al. Coffee consumption enhances high-density lipoprotein-mediated cholesterol efflux in macrophages. Circ Res. 2010;106:779–787. doi: 10.1161/CIRCRESAHA.109.206615. [DOI] [PubMed] [Google Scholar]
- 27.Nakaya K, Ayaori M, Hisada T, et al. Telmisartan enhances cholesterol efflux from THP-1 macrophages by activating PPARgamma. J Atheroscler Thromb. 2007;14:133–141. doi: 10.5551/jat.14.133. [DOI] [PubMed] [Google Scholar]
- 28.Schwedhelm E, Maas R, Freese R, et al. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: impact on nitric oxide metabolism. Br J Clin Pharmacol. 2008;65:51–59. doi: 10.1111/j.1365-2125.2007.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka N, Momiyama Y, Ohmori R, et al. Effect of atorvastatin on plasma osteopontin levels in patients with hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2006;26:e129–e130. doi: 10.1161/01.ATV.0000229701.42828.73. [DOI] [PubMed] [Google Scholar]
- 30.Fogelman AM, Haberland ME, Seager J, Hokom M, Edwards PA. Factors regulating the activities of the low density lipoprotein receptor and the scavenger receptor on human monocyte-macrophages. J Lipid Res. 1981;22:1131–1141. [PubMed] [Google Scholar]
- 31.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi H, Nakanishi T, Nishimura M, Tanaka H, Yoshimura M. Measurements of serum levels of nitrate ions in men and women: implications of endothelium-derived relaxing factor in blood pressure regulation and atherosclerosis. J Cardiovasc Pharmacol. 1992;20 (Suppl 12):S214–S216. doi: 10.1097/00005344-199204002-00061. [DOI] [PubMed] [Google Scholar]
- 33.Elsen A. Proceedings: Krebs-Henseleit urea cycle in cultured human diploid fibroblasts. Arch Int Physiol Biochim. 1975;83:204–205. [PubMed] [Google Scholar]
- 34.Gordon DJ, Rifkind BM. High-density lipoprotein—the clinical implications of recent studies. N Engl J Med. 1989;321:1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 35.Glomset JA. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968;9:155–167. [PubMed] [Google Scholar]
- 36.Uehara Y, Miura S, von Eckardstein A, et al. Unsaturated fatty acids suppress the expression of the ATP-binding cassette transporter G1 (ABCG1) and ABCA1 genes via an LXR/RXR responsive element. Atherosclerosis. 2007;191:11–21. doi: 10.1016/j.atherosclerosis.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 37.Sabol SL, Brewer HB,, Jr, Santamarina-Fojo S. The human ABCG1 gene: identification of LXR response elements that modulate expression in macrophages and liver. J Lipid Res. 2005;46:2151–2167. doi: 10.1194/jlr.M500080-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Sawamura T, Kume N, Aoyama T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 39.Akashi K, Miyake C, Yokota A. Citrulline, a novel compatible solute in drought-tolerant wild watermelon leaves, is an efficient hydroxyl radical scavenger. FEBS Lett. 2001;508:438–442. doi: 10.1016/s0014-5793(01)03123-4. [DOI] [PubMed] [Google Scholar]
- 40.Napoli C, Ackah E, De Nigris. Chronic treatment with nitric oxide-releasing aspirin reduces plasma low-density lipoprotein oxidation and oxidative stress, arterial oxidation-specific epitopes, and atherogenesis in hypercholesterolemic mice. Proc Natl Acad Sci U S A. 2002;99:12467–12470. doi: 10.1073/pnas.192244499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morita M, Sakurada M, Watanabe F, et al. Effects of Oral L-Citrulline supplementation on lipoprotein oxidation and endothelial dysfunction in humans with vasospastic angina. Immun Endocr & Metab Agents in Med Chem. 2013;13:214–220. doi: 10.2174/18715222113139990008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ignarro LJ, Napoli C. Novel features of nitric oxide, endothelial nitric oxide synthase, and atherosclerosis. Curr Atheroscler Rep. 2004;6:281–287. doi: 10.1007/s11883-004-0059-9. [DOI] [PubMed] [Google Scholar]
- 43.Cooke JP. Flow, NO, and atherogenesis. Proc Natl Acad Sci U S A. 2003;100:768–770. doi: 10.1073/pnas.0430082100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ignarro LJ, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]