Abstract
Alcohol drinking and smoking contain the risk of a carcinogenesis. Acetaldehyde is content in cigarette smoke and an ethanol metabolite. However the clear evidence for reactive oxygen species (ROS) generation by acetaldehyde in gastric cells in vitro is none. In this study, we elucidated acetaldehyde is an oxidative stress inducer on rat gastric epithelial cells by electron paramagnetic resonance measurement in living cells. We also confirmed whether acetaldehyde-induced cellular ROS was derived from mitochondria or not. The results of cellular ROS determination showed that an increment of cellular ROS was shown for 15 min in living cells from exposing 0.1% (v/v) acetaldehyde. Lipid peroxidation in cellular membrane also induced by 0.1% ethanol and the tendency is same in the results of cellular ROS determination. JC-1 stained showed the decrement of mitochondrial membrane potential. These results indicated that acetaldehyde is not merely a necrotizing factor for gastric epithelial cells, but also an oxidative stress inducer via injured mitochondria.
Keywords: acetaldehyde, reactive oxygen species, mitochondria, stomach cells, electron paramagnetic resonance
Introduction
Alcohol drinking and smoking contains the risk of a carcinogenesis. Cigarette smoke and an ethanol metabolite contain acetaldehyde, which is known as the abundant carcinogen.(1) Acetaldehyde is contained in cigarette smoke, and this is indirectly generated in drinking via an ethanol metabolism such as microsomal ethanol oxidizing system (MEOS).(2) Acetaldehyde is also known as an inducible factor for a bout of heartburn and nausea when we are drinking alcohol or smoking. Reactive oxygen species (ROS) relate with these symptoms directly or indirectly.(3,4) Therefore, ROS should become a key for understanding of harmful effects with an intake of acetaldehyde.
The oxidative stress, exposure of ROS, is a prospective disease risk for gastrointestinal system. For example, toxic effects of alcohol have been studying because excessive consumption of alcohol relates with alcohol hepatitis.(5,6) Of note, oxidative stress is important factor for the liver injury through MEOS with CYP2E1 (cytochrome P450 family).(6,7) In stomach, acidic environment by gastric juice induced oxidative stress with DNA and mitochondrial damage.(8) Acid is not only a necrotizing factor but also an oxidative stressor through an inhibition of mitochondrial electron transport, which generates superoxide anion in cells.(9) Bile acids and/or gastric acids induce oxidative stress and alter signaling pathways, such as mitogen-activated protein kinase (MAPK), nuclear factor-κB (NF-κB) and signal tranducer and activator of transcription 3 (STAT3).(10–13) While acid induced an oxidative stress in stomach, alcohol also induced the stress independently of acid.(14) We reported that exposure of ethanol generated ROS with mitochondrial damage in gastric cells directly.(15) In addition, Holownia et al.(16) reported that 2% of ethanol metabolizes to acetaldehyde in vitro. Several reports describe that acetaldehyde is a major toxic content, meanwhile, there is not enough evidences for toxic mechanisms of acetaldehyde.
ROS also relates with a carcinogenesis and malignancy such as cancer growth and invasion and metastasis.(17–19) We also suggested that mitochondrial ROS (mitROS) enhanced an invasion in gastric cancer cells.(20) Mitochondria in cancer cells are of mutations in mitochondrial electron transfer system of Complex I and III, which facilitates to generate excess ROS.(21,22) The identification of oxidative stressors should be important for an indication of cancer prevention, however, acetaldehyde is not enough understandings as an oxidative stressor in vitro.
Electron paramagnetic resonance (EPR) is unique beyond comparison to analyze ROS directly. Ikeda et al.(23) developed the compounds for evaluation of nuclear oxidative stress in living cells by EPR. In addition, Kamibayashi et al.(24) synthesized a spin trap agent 2-[5,5-dimethyl-2-oxo-2λ5-(1,3,2)dioxaphosphinan-2-yl]-2-methyl-3,4-dihydro-2H-pyrrole 1-oxide (CYPMPO) which can consummate the analysis of superoxide. In previous reports, we tried blending EPR measurement in living cells with CYPMPO. As a consequence of the combination, mitROS such as superoxide anion can be directly detected in living cells by EPR with CYPMPO.(20,25)
Herein, we have confirmed that acetaldehyde is an oxidative stressor through mitochondrial damage in a rat gastric mucosal cell line, RGM-1.(26) For this aim, we used EPR for a determination of ROS from living cells. EPR spectra clarified that acetaldehyde is an oxidative stressor. For clarifying the acetaldehyde-induced ROS is derived from mitochondria, we also performed the microscopic observation with fluorescent probes both indicators of mitochondrial electron potential and ROS.
Materials and Methods
Materials
Aminophenyl fluorescein (APF) (SEKISUI MEDICAL CO., LTD., Tokyo, Japan), CYPMPO (Radical Research Inc., Tokyo, Japan), β-nicotinamide adenine dinucleotide (NADH) (Life Technologies Co., Carlsbad, CA), D-glutamic acid (Life Technologies), malic acid (Wako Pure Chem. Ind., Ltd., Osaka, Japan), succinic acid (Life Technologies), diphenyl-1pyrenylphosphine (DPPP) (DOJINDO, Kumamoto, Japan), cell counting kit-8 (DOJINDO), MitoRed (DOJINDO) and ethanol (Wako Pure Chem. Ind.) were purchased. Alcohol-contained culture medium was prepared by mixing alcohol, and the culture medium was used after filter-sterilized (Millex 0.22 µm, Millipore Co., Billerica, MA).
Cell culture
RGM-1 was cultured in DMEM/F12 (Life Technologies). This culture medium contained 10% inactivated FBS and 1% penicillin/streptomycin. Cells were cultured in 5% CO2 cell culture incubator at 37°C.
Cell viability test by WST assay
Cell viability test was examined with cell counting kit-8 according to the manufacturer’s instructions. RGM-1 was dispersed in the 96-well dish at 10,000 cells/well and it was incubated for overnight. The medium was replaced to the acetaldehyde-contained culture medium which contained acetaldehyde of 0, 0.01, 0.02, 0.05, 0.1, 0.2, 0.5, 1, 2, 5% (v/v) and it was incubated for 30 min. After incubation, medium was replaced to the medium contained 10%-cell counting kit-8 of 100 µl and cells were incubated for 1 h. The absorbance of 450 nm was measured by Varioskan plate reader (Thermo Fisher Scientific K. K., Kanagawa, Japan).
Lipid peroxide determination by DPPP
The lipid peroxidation was measured by DPPP as follows; upon cells were dispersed at the concentration of 31,250 cells/cm2, cells were incubated for 24 h, and then acetaldehyde exposed to cells for 30 min. The culture medium was thereafter replaced to the culture medium contained 10 µM DPPP. After incubated for 15 min, cells were washed twice with cold phosphate buffer saline (PBS). The fluorescence intensities at Ex. 352 nm and Em. 380 nm of DPPP were measured by the plate reader.
Mitochondrial damage determination by JC-1
Mitochondrial damage was estimated by JC-1 stain, which indicated the mitochondrial membrane potential. Cells were prepared and stimulated same as a method of DPPP. The culture medium was thereafter replaced to the culture medium contained 10 µM JC-1. After incubated for 15 min, cells were washed twice with cold PBS. The fluorescence intensities at green fluorescence (Ex. 485 nm and Em. 535 nm) and red fluorescence (Ex. 560 nm and Em. 595 nm) of JC-1 were measured by the plate reader.
Intracellular ROS determination by APF
Free radicals (hydroxyl radical and peroxynitrite) were detected by APF. APF was diluted with PBS and it exposed to the cells at the concentration of 1 µM for 30 min following exposure of acetaldehyde for 30 min. After incubation, cells were washed using a cold PBS twice. The intensities of APF-fluorescent were measured by Varioskan at Ex.490 nm and Em. 515 nm.
Electron paramagnetic resonance (EPR) measurement
The methods of EPR measurement were consulted previous reports and so on.(27) Cells were cultured on the slide glass until confluent. The slide glass was immersed into different acetaldehyde-contained medium (0, 0.1 and 0.5% acetaldehyde) for 0, 15, 30 and 60 min in the 5% CO2 incubator at 37°C. After the incubation, the slide glass was put on the tissue glass (Radical Research Inc., Tokyo, Japan). 100 µl of the solution for EPR measurement, which was prepared that the respiratory substrates (5 mM succinic acid, 5 mM malic acid, 5 mM D-glutamic acid, 5 mM NADH) and 10 mM CYPMPO was dissolved in PBS, was poured in the tissue glass. And then the EPR spectra were recorded by using a JEOL-TE X-band spectrometer (JEOL, Tokyo, Japan). All EPR spectra were obtained under the following conditions: 10 mW incident microwave power, 0.1 mT modulation width, 8 min sweep time, 7.5 mT sweep width, 0.1 s time contrast, 333.5 mT center field, and 15 mT scan range. Spectral computer simulation was performed using a Win-Rad Radical Analyzer System (Radical Research).
Static analysis
Significant static value (p value) was calculated using ANOVA followed by Turkey HSD.
Results
Acetaldehyde induced the cell death
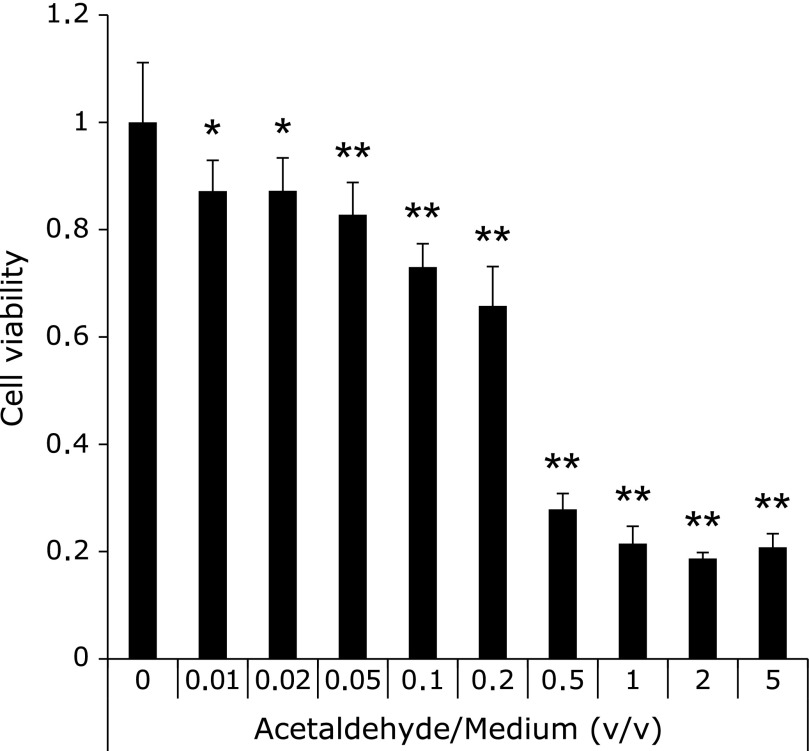
Cell death by acetaldehyde was determined by cell viability test in comparison with the normal rat gastric mucosa cells (RGM-1). Fig. 1 showed 0.01% acetaldehyde had cytotoxicity for 30 min exposure. RGM-1 died completely in the medium contained one hour exposure of more 0.5% (v/v) acetaldehyde, and we suggested that necrosis was involved on these cells. On the other hand, the cells survived environments under less than 0.2% acetaldehyde suggested that another kind of death was derived on these cells such as an apoptosis.
Fig. 1.
Cell viability after acetaldehyde exposure. Cell viability was evaluated by WST assay. The different acetaldehyde concentration medium was made by adding acetaldehyde to culture medium. The absorbance at 450 nm was measured by plate reader. Cell; RGM-1, n = 6, Error bar; SD. *p<0.05, **p<0.01.
Acetaldehyde induced lipid peroxidation
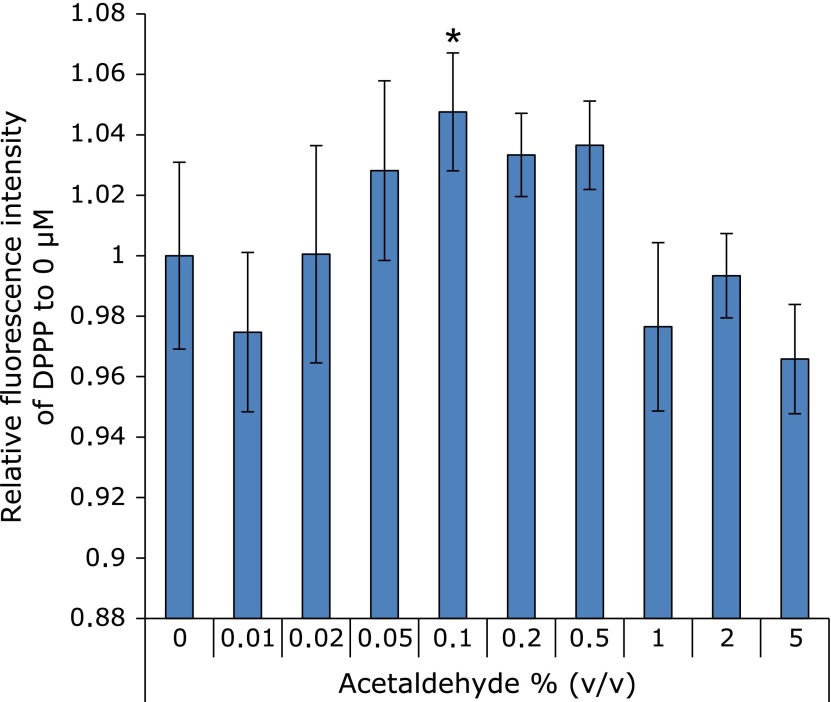
For determining the oxidative stress by acetaldehyde, Fig. 2 showed the amounts of lipid peroxidation in cellular membrane after half hour exposure with acetaldehyde. The graph shows the intensity of DPPP fluorescence. This result indicated that acetaldehyde between 0.05% and 0.5% induced lipid peroxidation as a result of oxidative stress.
Fig. 2.
The evaluation of oxidative stress based on lipid peroxide. Lipid peroxidation was significantly increased under the condition of 0–5% (v/v) acetaldehyde after incubation for 30 min. 0.5–5% acetaldehyde-exposed cells were completely death. n = 6, Error bar; SD. *p<0.05.
Acetaldehyde induced cellular ROS
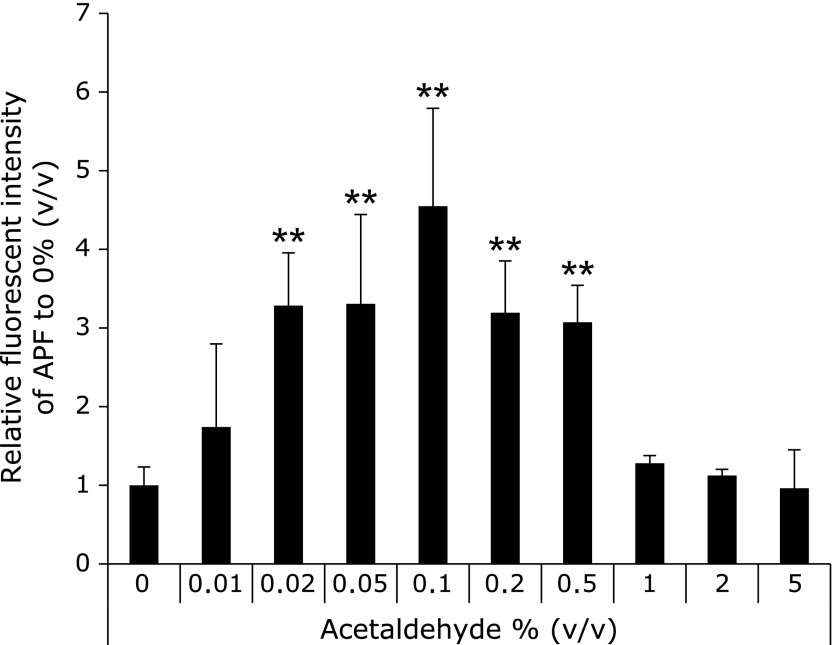
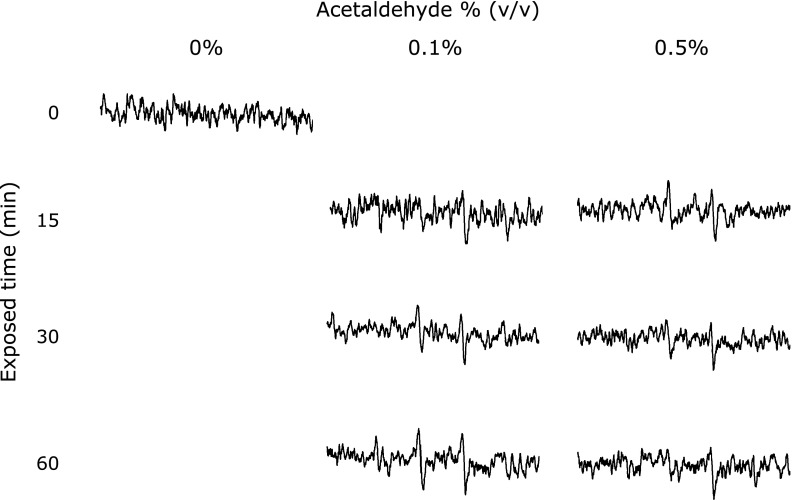
The ROS concentration from the cells was determined by both the AFP study and the EPR measurement using spin-trapping agents (CYPMPO). Fig. 3 showed the results of cellular ROS determination by APF. Cellular ROS was increased with acetaldehyde concentration. The amounts of cellular ROS in 0.02–0.5% ethanol exposing cells was significantly higher than that in the control cells. This result coincided with the study about lipid peroxidation (Fig. 2). Fig. 4 showed EPR signals intensities in RGM-1 exposed different concentrations of ethanol. These results showed the 15 min exposure of 0.1% acetaldehyde induced ROS from the RGM-1. While exposure of 0.1% acetaldehyde enhanced a cellular ROS production with time, 0.5% acetaldehyde attenuated the production after 15 min. Cells cannot be survive in 0.5% acetaldehyde, resulting in ROS production is attenuated after 30 min exposure.
Fig. 3.
The determination of intracellular ROS by APF. Intracellular ROS was significantly increased under the condition of 0.02–0.5% (v/v) acetaldehyde after incubation for 30 min. 0.5–5% acetaldehyde-exposed cells were completely death. n = 6, Error bar; SD. **p<0.01.
Fig. 4.
The EPR spectra from RGM-1 after acetaldehyde exposure. The intensity of EPR signals was strong after exposed-acetaldehyde. This phenomena was begun after incubated for 15 min. Spin-trapping agent; CYPMPO.
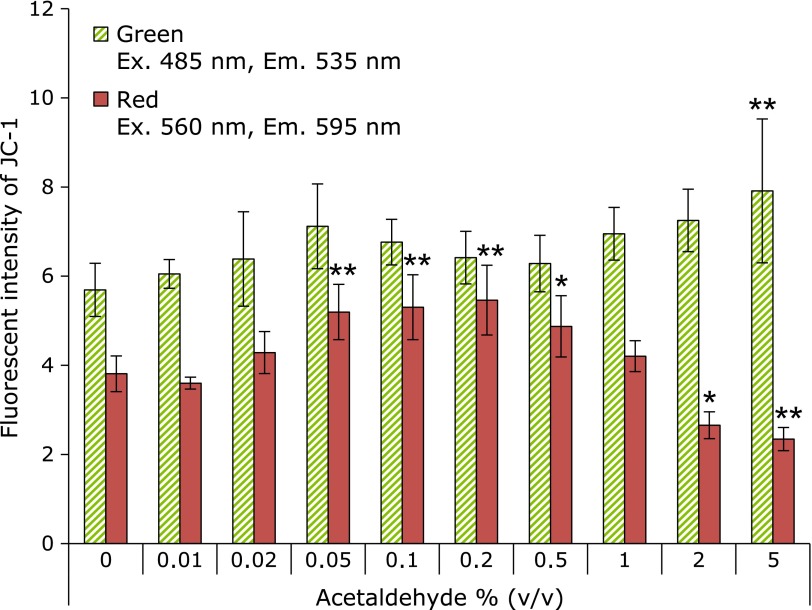
Acetaldehyde injured mitochondrion
We confirmed that acetaldehyde injured mitochondria. Fluorescence characteristics of JC-1 were changed in accordance with mitochondrial membrane potential dependence. Green and red fluorescence of JC-1 means injured mitochondria (decreasing membrane potential) and healthy mitochondria (normal membrane potential), respectively. Fig. 5 shows the results of JC-1 stained. Injured mitochondria were showed in 0.05% ethanol exposed cells. These results indicated that acetaldehyde injured mitochondrion.
Fig. 5.
Mitochondrial injury by exposing acetaldehyde. Mitochondrial injury was measured by JC-1 after exposed-ethanol for 1 h. Green fluorescence (Ex. 485 nm, Em. 535 nm) and red fluorescence (Ex. 560 nm, Em. 595 nm) show mitochondrial injury and healthy mitochondria, respectively. The fluorescence intensity was measured by plate reader. 0.5–5% acetaldehyde-exposed cells were completely death. n = 6, Error bar; SD. *p<0.05, **p<0.01.
Discussion
In this study, we demonstrated that acetaldehyde treatments involved on reactive ROS production, in particular superoxide anion, in gastric epithelial cells.
This study was performed under the condition from 0 to 5% acetaldehyde because ethanol used to be metabolized to 2% acetaldehyde.(16) The condition assumes an intake of from 0 to 20% ethanol which is popular alcohol’s ethanol concentration. If a main toxicity of ethanol is acetaldehyde, the cytotoxicity of acetaldehyde should be coincided with that of five to ten times greater concentration of ethanol. In fact, the cytotoxicity of acetaldehyde showed as expected (Fig. 1). In our previous study, more than 15% ethanol exposure caused immediate cell death within 1 h, while cells enhanced a ROS production from mitochondria under less than 10% ethanol condition, especially from 1 to 5%.(28) Fig. 2 and 3 showed moderate concentration of acetaldehyde, from 0.02 to 0.5%, was an oxidative stress. These results suggested that acetaldehyde is a main toxic metabolite from ethanol, and it should act as an oxidative stressor when ethanol exposed to cells. Gastrointestinal tracts including the stomach are called the first-pass metabolism of alcohol. Administration of a low dose ethanol have been reported to protect the gastric mucosa from gastric lesions.(29–31) In the metabolism, MEOS requires CYP2E1 for generating oxidized NADPH,(2,32) which used to localize in cytoplasm. CYP2E1 accelerates the expression of cyclooxygenase-2 (COX-2) in liver.(33) COX-2 produces prostaglandins, and it should protect gastric lesions in vivo. Ethanol is an inducer of CYP2E1, which metabolized ethanol to acetaldehyde.(6) We hypothesized that acetaldehyde causes negative or positive feedback for an expression of CYP2E1, however, exposure of acetaldehyde did not changed the expression (data not shown). In smoking, acetaldehyde is well known as a major toxic content.(34) Biogenic amines such as catecholamine and indoleamine were implicated by an intake of acetaldehyde, these are synthesized through a direct reaction with amines and acetaldehyde.(35) One of the differences between drinking and smoking are an indirectly and directly exposure, respectively. The results of CYP2E1 after an acetaldehyde exposure may indicate that a reduction of harmful effects by smoking is more difficult than by drinking.
Previous reports described that NSAIDs and bisphosphonate involved superoxide anion production by EPR measurement using separated mitochondria.(9,36) Additionally, ethanol induced a ROS production through mitochondrial damage in living cells. MitROS were likely to play a role to derive the cellular injury. MitROS such as oxygen-derived free radicals have been reported to be related with many diseases.(37) As one example, several reports indicates that mitROS production enhances tumor specific properties.(38) MitROS also indicates the relation with the expression of oncogene expression.(39) In present study, acetaldehyde is also an oxidative stressor through mitochondrial damage in living cells (Fig. 4 and 5). Gastric cancer is coincided with an infection of Helicobacter pylori, class I carcinogen, and daily foods of oxidative stressor such as salt promotes carcinogenesis under the infection.(40,41) Taking these suggestions into account, antioxidants probably become chemopreventive agents.
Recent studies report that microbially produced acetaldehyde from ethanol may become the risk of cancer, especially esophageal carcinogenesis.(42) Oral bacterial flora including Nesseria and Streptococcus and Candida metabolize ethanol to acetaldehyde through an activity of alcohol dehydrogenase (ADH).(43–45) Acetaldehyde in saliva is considered carcinogenic substance.(43) Drinking and smoking are also known to shift the balance of bacterial flora, resulting in an increment of acetaldehyde in the oral cavity.(43) Interestingly, isolates of Candida albicans from smoker produced acetaldehyde greater than that from non-smoker. Class IV ADH activity is an ADH isozyme for maintaining normal cellular proliferation and differentiation in various organs.(46) Although Helicobacter pylori infection and aging reduced Class IV ADH activities,(47) smoking might involve in the activities. The microbially produced acetaldehyde should also cause harmful effects in stomach. The clarification of the relations between daily foods and oral bacterial flora should provide a method of cancer prevention, including an involvement in oxidative stress.
In conclusion, acetaldehyde is not merely a necrotizing factor for gastric epithelial cells, but also an oxidative stress inducer. ROS after acetaldehyde treatment were involved from mitochondria. Now we are undergoing the study to prevent carcinogenesis and malignancy in gastric epithelial cells.
Acknowledgments
This study was partially supported by the Japan Society for the Promotion of Science (JSPS) and Grant-in-Aid for Scientific Research (KAKENHI) grant number 24106503 and 12J00241.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Salaspuro M. Acetaldehyde and gastric cancer. J Dig Dis. 2011;12:51–59. doi: 10.1111/j.1751-2980.2011.00480.x. [DOI] [PubMed] [Google Scholar]
- 2.Pronko P, Bardina L, Satanovskaya V, Kuzmich A, Zimatkin S. Effect of chronic alcohol consumption on the ethanol- and acetaldehyde-metabolizing systems in the rat gastrointestinal tract. Alcohol Alcohol. 2002;37:229–235. doi: 10.1093/alcalc/37.3.229. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Wo JM, Ellis S, Ray MB, Jones W, Martin RC. A novel external esophageal perfusion model for reflux esophageal injury. Dig Dis Sci. 2006;51:527–532. doi: 10.1007/s10620-006-3165-4. [DOI] [PubMed] [Google Scholar]
- 4.Oh TY, Lee JS, Ahn BO, et al. Oxidative damages are critical in pathogenesis of reflux esophagitis: implication of antioxidants in its treatment. Free Radic Biol Med. 2001;30:905–915. doi: 10.1016/s0891-5849(01)00472-5. [DOI] [PubMed] [Google Scholar]
- 5.Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 2012;56:704–713. doi: 10.1016/j.jhep.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63–S74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- 7.Zhu H, Jia Z, Misra H, Li YR. Oxidative stress and redox signaling mechanisms of alcoholic liver disease: updated experimental and clinical evidence. J Dig Dis. 2012;13:133–142. doi: 10.1111/j.1751-2980.2011.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res. 2005;589:47–65. doi: 10.1016/j.mrrev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Nagano Y, Matsui H, Tamura M, et al. NSAIDs and acidic environment induce gastric mucosal cellular mitochondrial dysfunction. Digestion. 2012;85:131–135. doi: 10.1159/000334685. [DOI] [PubMed] [Google Scholar]
- 10.Dvorak K, Chavarria M, Payne CM, et al. Activation of the interleukin-6/STAT3 antiapoptotic pathway in esophageal cells by bile acids and low pH: relevance to barrett’s esophagus. Clin Cancer Res. 2007;13:5305–5313. doi: 10.1158/1078-0432.CCR-07-0483. [DOI] [PubMed] [Google Scholar]
- 11.Souza R, Shewmake K, Terada L, Spechier SJ. Acid exposure activates the mitogen-activated protein kinase pathways in Barrett’s esophagus. Gastroenterology. 2002;122:299–307. doi: 10.1053/gast.2002.30993. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Latif MM, O’Riordan J, Windle HJ, et al. NF-kappaB activation in esophageal adenocarcinoma: relationship to Barrett’s metaplasia, survival, and response to neoadjuvant chemoradiotherapy. Ann Surg. 2004;239:491–500. doi: 10.1097/01.sla.0000118751.95179.c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song J, Futagami S, Nagoya H, et al. Apurinic/apyrimidinic endonuclease-1 (APE-1) is overexpressed via the activation of NF-κB-p65 in MCP-1-positive esophageal squamous cell carcinoma tissue. J Clin Biochem Nutr. 2013;52:112–119. doi: 10.3164/jcbn.12-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loguercio C, Tuccillo C, Federico A, Fogliano V, Del Vecchio Blanco C, Romano M. Alcoholic beverages and gastric epithelial cell viability: effect on oxidative stress-induced damage. J Physiol Pharmacol. 2009;60 (Suppl 7):87–92. [PubMed] [Google Scholar]
- 15.Matharu Z, Enomoto J, Revzin A. Miniature enzyme-based electrodes for detection of hydrogen peroxide release from alcohol-injured hepatocytes. Anal Chem. 2013;85:932–939. doi: 10.1021/ac3025619. [DOI] [PubMed] [Google Scholar]
- 16.Holownia A, Ledig M, Braszko JJ, Ménez JF. Acetaldehyde cytotoxicity in cultured rat astrocytes. Brain Res. 1999;833:202–208. doi: 10.1016/s0006-8993(99)01529-2. [DOI] [PubMed] [Google Scholar]
- 17.Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25:695–705. doi: 10.1007/s10555-006-9037-8. [DOI] [PubMed] [Google Scholar]
- 18.Nishikawa M. Reactive oxygen species in tumor metastasis. Cancer Lett. 2008;266:53–59. doi: 10.1016/j.canlet.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 19.Miura Y, Kozuki Y, Yagasaki K. Potentiation of invasive activity of hepatoma cells by reactive oxygen species is mediated by autocrine/paracrine loop of hepatocyte growth factor. Biochem Biophys Res Commun. 2003;305:160–165. doi: 10.1016/s0006-291x(03)00725-3. [DOI] [PubMed] [Google Scholar]
- 20.Tamura M, Matsui H, Tomita T, et al. Mitochondrial reactive oxygen species accelerate gastric cancer cell invasion. J Clin Biochem Nutr. 2013;54:12–17. doi: 10.3164/jcbn.13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majima HJ, Indo HP, Suenaga S, Matsui H, Yen HC, Ozawa T. Mitochondria as possible pharmaceutical targets for the effects of vitamin E and its homologues in oxidative stress-related diseases. Curr Pharm Des. 2011;17:2190–2195. doi: 10.2174/138161211796957490. [DOI] [PubMed] [Google Scholar]
- 22.Chomyn A, Attardi G. MtDNA mutations in aging and apoptosis. Biochem Biophys Res Commun. 2003;304:519–529. doi: 10.1016/s0006-291x(03)00625-9. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda M, Nakagawa H, Ban S, Tsumoto H, Suzuki T, MIyata N. Development of a DNA-binding TEMPO derivative for evaluation of nuclear oxidative stress and its application in living cells. Free Radic Biol Med. 2010;49:1792–1797. doi: 10.1016/j.freeradbiomed.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Kamibayashi M, Oowada S, Kameda H, et al. Synthesis and characterization of a practically better DEPMPO-type spin trap, 5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide (CYPMPO) Free Radic Res. 2006;40:1166–1172. doi: 10.1080/10715760600883254. [DOI] [PubMed] [Google Scholar]
- 25.Tamura M, Matsui H, Nagano YN, et al. Salt is an oxidative stressor for gastric epithelial cells. J Physiol Pharmacol. 2013;64:89–94. [PubMed] [Google Scholar]
- 26.Kobayashi I, Kawano S, Tsuji S, et al. RGM1, a cell line derived from normal gastric mucosa of rat. In Vitro Cell Dev Biol Anim. 1996;32:259–261. doi: 10.1007/BF02723056. [DOI] [PubMed] [Google Scholar]
- 27.Mukohda M, Ueno S, Kamibayashi M, Okada M, Yamawaki H, Hara Y. Influences of organic solvents on CYPMPO-electron spin resonance spectra in in vitro radical generating systems. J Vet Med Sci. 2010;72:1547–1550. doi: 10.1292/jvms.10-0232. [DOI] [PubMed] [Google Scholar]
- 28.Tamura M, Matsui H, Kaneko T, Hyodo I. Alcohol is an oxidative stressor for gastric epithelial cells: detection of superoxide in living cells. J Clin Biochem Nutr. 2013;53:75–80. doi: 10.3164/jcbn.13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka K, Nishimoto K, Tomisato W, et al. Adaptive cytoprotection induced by pretreatment with ethanol protects against gastric cell damage by NSAIDs. Dig Dis Sci. 2004;49:210–217. doi: 10.1023/b:ddas.0000017440.46863.56. [DOI] [PubMed] [Google Scholar]
- 30.Kopic S, Corradini S, Sidani S, et al. Ethanol inhibits gastric acid secretion in rats through increased AMP-kinase activity. Cell Physiol Biochem. 2010;25:195–202. doi: 10.1159/000276553. [DOI] [PubMed] [Google Scholar]
- 31.Robert A, Nezamis JE, Lancaster C, Davis JP, Field SO, Hanchar AJ. Mild irritants prevent gastric necrosis through ”adaptive cytoprotection” mediated by prostaglandins. Am J Physiol. 1983;245:G113–G121. doi: 10.1152/ajpgi.1983.245.1.G113. [DOI] [PubMed] [Google Scholar]
- 32.Julkunen RJ, Di Padova C, Lieber CS. First pass metabolism of ethanol—a gastrointestinal barrier against the systemic toxicity of ethanol. Life Sci. 1985;37:567–573. doi: 10.1016/0024-3205(85)90470-9. [DOI] [PubMed] [Google Scholar]
- 33.Uzma N, Kumar BS, Priyadarsini KI. Hepatoprotective, immunomodulatory, and anti-inflammatory activities of selenocystine in experimental liver injury of rats. Biol Trace Elem Res. 2011;142:723–734. doi: 10.1007/s12011-010-8807-x. [DOI] [PubMed] [Google Scholar]
- 34.Seeman JI, Dixon M, Haussmann HJ. Acetaldehyde in mainstream tobacco smoke: formation and occurrence in smoke and bioavailability in the smoker. Chem Res Toxicol. 2002;15:1331–1350. doi: 10.1021/tx020069f. [DOI] [PubMed] [Google Scholar]
- 35.Talhout R, Opperhuizen A, van Amsterdam JG. Role of acetaldehyde in tobacco smoke addiction. Eur Neuropsychopharmacol. 2007;17:627–636. doi: 10.1016/j.euroneuro.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Matsui H, Nagano Y, Shimokawa O, et al. Gastric acid induces mitochondrial superoxide production and lipid peroxidation in gastric epithelial cells. J Gastroenterol. 2011;46:1167–1176. doi: 10.1007/s00535-011-0434-6. [DOI] [PubMed] [Google Scholar]
- 37.Zeki S, Miura S, Suzuki H, et al. Xanthine oxidase-derived oxygen radicals play significant roles in the development of chronic pancreatitis in WBN/Kob rats. J Gastroenterol Hepatol. 2002;17:606–616. doi: 10.1046/j.1440-1746.2002.02733.x. [DOI] [PubMed] [Google Scholar]
- 38.Friday E, Oliver R, Welbourne T, Turturro F. Glutaminolysis and glycolysis regulation by troglitazone in breast cancer cells: relationship to mitochondrial membrane potential. J Cell Physiol. 2011;226:511–519. doi: 10.1002/jcp.22360. [DOI] [PubMed] [Google Scholar]
- 39.Ralph SJ, Rodríguez-Enríquez S, Neuzil J, Saavedra E, Moreno-Sánchez R. The causes of cancer revisited: ”mitochondrial malignancy” and ROS-induced oncogenic transformation—why mitochondria are targets for cancer therapy. Mol Aspects Med. 2010;31:145–170. doi: 10.1016/j.mam.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Ding S-Z, Minohara Y, Fan XJ, et al. Helicobacter pylori infection induces oxidative stress and programmed cell death in human gastric epithelial cells. Infect Immun. 2007;75:4030–4039. doi: 10.1128/IAI.00172-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kato S, Tsukamoto T, Mizoshita T, et al. High salt diets dose-dependently promote gastric chemical carcinogenesis in Helicobacter pylori-infected Mongolian gerbils associated with a shift in mucin production from glandular to surface mucous cells. Int J Cancer. 2006;119:1558–1566. doi: 10.1002/ijc.21810. [DOI] [PubMed] [Google Scholar]
- 42.Homann N, Tillonen J, Salaspuro M. Microbially produced acetaldehyde from ethanol may increase the risk of colon cancer via folate deficiency. Int J Cancer. 2000;86:169–173. doi: 10.1002/(sici)1097-0215(20000415)86:2<169::aid-ijc4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 43.Gainza-Cirauqui ML, Nieminen MT, Novak Frazer, Aguirre-Urizar JM, Moragues MD, Rautemaa R. Production of carcinogenic acetaldehyde by Candida albicans from patients with potentially malignant oral mucosal disorders. J Oral Pathol Med. 2013;42:243–249. doi: 10.1111/j.1600-0714.2012.01203.x. [DOI] [PubMed] [Google Scholar]
- 44.Muto M, Hitomi Y, Ohtsu A, et al. Acetaldehyde production by non-pathogenic Neisseria in human oral microflora: implications for carcinogenesis in upper aerodigestive tract. Int J Cancer. 2000;88:342–350. [PubMed] [Google Scholar]
- 45.Kurkivuori J, Salaspuro V, Kaihovaara P, et al. Acetaldehyde production from ethanol by oral streptococci. Oral Oncol. 2007;43:181–186. doi: 10.1016/j.oraloncology.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Matsumoto M, Yokoyama H, Suzuki H, Shiraishi-Yokoyama H, Hibi T. Retinoic acid formation from retinol in the human gastric mucosa: role of class IV alcohol dehydrogenase and its relevance to morphological changes. Am J Physiol Gastrointest Liver Physiol. 2005;289:G429–G433. doi: 10.1152/ajpgi.00502.2004. [DOI] [PubMed] [Google Scholar]
- 47.Matsumoto M, Yokoyama H, Shiraishi H, et al. Alcohol dehydrogenase activities in the human gastric mucosa: effects of Helicobacter pylori infection, sex, age, and the part of the stomach. Alcohol Clin Exp Res. 2001;25:29S–34S. doi: 10.1097/00000374-200106001-00008. [DOI] [PubMed] [Google Scholar]