Abstract
Photodynamic therapy using hematoporphyrin and its derivatives is clinically useful for cancer treatments. It has been reported that cancer cells incorporate hematoporphyrin and its derivatives via heme carrier protein 1, which is a proton-coupled folate transporter. However, the mechanism of this protein expression has not been elucidated. In general, the concentration of reactive oxygen species in cancer cells is higher than that in normal cells. We previously reported that reactive oxygen species from mitochondria involved in the expression of peptide transporter 1 and accelerate the uptake of 5-aminolevulinic acid, which is a precursor of protoporphyrin IX. We suggested mitochondrial reactive oxygen species also regulated the expression of heme carrier protein 1. In this study, we used a rat gastric mucosal cell line RGM1 and its cancer-like mutated cell line RGK1. We clarified the expression of heme carrier protein 1 increased in cancer cells and it decreased in manganese superoxide dismutase expressed cancer cells. In addition, the uptake level of hematoporphyrin and photodynamic therapeutic effect were also decreased in manganese superoxide dismutase expressed cancer cells in comparison with cancer cells. Thus, we concluded that mitochondrial reactive oxygen species regulated heme carrier protein 1 expression and photodynamic therapeutic effect.
Keywords: mitROS, heme carrier protein 1 (HCP-1), gastric epithelial cell, hematoporphyrin derivatives (HpD), photodynamic therapy
Introduction
Photodynamic therapy (PDT) is one of cancer patients’ treatments which irradiates laser light to activate photosensitizers and induces cytotoxicity.(1) It is an effective method for cancer treatment without severe complications. Hematopophyrin and its derivatives (HpD) used to be commonly utilized for the therapy(2) because they show preferential tumor selective localization and light-activated tumor destruction via photosensitization.(3) Although several researchers have elucidated the detail mechanism of tumor cellular death induced by photo-irradiation, one of tumor specific HpD accumulation is not confirmed.
Recently, a proton-coupled folate transporter, SLC46A1, was identified as a heme transporter named as heme carrier protein 1 (HCP-1).(4) We have previously reported that HCP-1 was a major transporter of HpD in neoplastic cells.(5) Since the amounts of HpD in tumor tissue is most likely to decide the efficiency of HpD-PDT, it is important to clear the expression mechanism of this transporter. HCP-1 has been reported to be expressed in the duodenum, kidney and intestinal cells of mammals. Additionally, HCP-1 expression is significantly influenced by hypoxic condition where mitochondria generate reactive oxygen species (ROS).(6,7) Thus, ROS derived from mitochondria (mitROS) may affect the expression of HCP-1.
We have investigated the relation between mitROS and cancer cellular invasion using rat gastric mucosal cells,(8) RGM1,(9) its cancer-like mutant cells induced by oncogenic chemicals, RGK1(10) and manganese superoxide dismutase (MnSOD)-expressed RGK cells, RGK-MnSOD, which we have established previously.(8) MnSOD is a nuclear-encoded primary antioxidant enzyme which has an ability to scavenge superoxide radicals in mitochondria selectively.(11,12) Since the overexpression of MnSOD significantly restrained the invasive phenomenon, we have concluded that mitROS played an important role to involve this cancer specific characteristic in our previous study.
In this study, we have elucidated that the relation between mitROS and HCP-1 expression using RGM1, RGK1 and RGK-MnSOD. We used three clones of MnSOD expressed cells: MnSOD2, MnSOD3 and MnSOD9. These cell clones express both different amounts of MnSOD and ROS concentrations in cells respectively. We also studied the difference of HpD-PDT effects depending on the HCP-1 expression in these cells.
Materials and Methods
Materials
Hematoporhyrin dihydrochloride (Wako Pure Chem. Ind., Ltd., Osaka, Japan), Cell Counting Kit-8 (DOJINDO LABORATORIES, Kumamoto, Japan), Trizma® base (Sigma-Aldrich Japan K.K., Tokyo, Japan), NaCl (Wako Pure Chem. Ind., Ltd., Osaka, Japan), Triton X-100 (Sigma-Aldrich Japan K.K.) sodium dodecyl sulfate (SDS) (Wako Pure Chem. Ind., Ltd.), deoxycholic acid (Wako Pure Chem. Ind., Ltd.), hydrochloric acid (Wako Pure Chem. Ind., Ltd.), NuPAGE® Novex® 12% Bis-Tris gels (Life Technologies Japan Ltd., Tokyo, Japan), PVDF Blocking Reagent for Can Get Signal® (TOYOBO CO., LTD., Osaka, Japan), Tris Buffered Saline with Tween® 20 (TBST-10X, Cell Signaling Technology Japan, K.K., Tokyo, Japan), HCP-1 antibody (Santa Cruz Biotechnology, Inc., Dallas, TX), Can Get Signal® Immunoreaction Enhancer Solution 1 (TOYOBO CO., LTD.) horseradish peroxidase (HRP) linked anti-rabbit IgG antibody (Cell Signaling Technology Japan, K.K.), Lumina forte western HRP substrate (Millipore Co., Billerica, MA), anti-β-actin (Cell Signaling Technology Japan, K.K.), and Can Get Signal® Immunoreaction Enhancer Solution 2 (TOYOBO CO., LTD.) were purchased and used without further purification or modification.
Cell culture
Rat normal gastric mucosal cell line (RGM1) and its chemically oncogenic cancer-like mutant cell line (RGK1) were cultured in DMEM/F12 with L-glutamine (Life Technologies Japan Ltd.) and DMEM/F12 without L-glutamine (Sigma-Aldrich Japan K.K.), respectively. These culture media contained 10% of inactivated fatal bovine serum (FBS) (Biowest LLC, Kansas City, MO) and 1% of penicillin/streptomycin (Life Technologies Japan Ltd.). All cells were cultured in 5% CO2 cell culture incubator at 37°C. MnSOD expressed RGK cells were cultured by means of the same media as RGK cells. We used three types of MnSOD-expressed RGK cells; RGK-MnSOD2, RGK-MnSOD3, RGK-MnSOD9. The numbers mean different clones and the expression level of MnSOD is greater in the order of 9, 3 and 2.(8)
Cell viability test
Cell viability test was examined using Cell Counting Kit-8 according to manufacturer’s protocol. Cells were cultured on 96-well plate at 5 × 103 cells/well and incubated for overnight. The medium was replaced to flesh one containing 0, 5, 10, 20 and 50 µM of HpD dissolved in ethanol, which final concentration was 0.5% (v/v), and incubated at 37°C for 24 h. After incubation, medium was replaced to the medium containing 10% of Cell Counting Kit-8 and cells were further incubated. The absorbance of 450 nm was measured by DTX880 multi-mode microplate reader (Beckman Coulter, Inc., Brea, CA).
Cellular uptake study of HpD
Cellular uptake study of HpD was performed as follow; Cells were incubated for overnight on 12-well plate at 2 × 105 cells/well. The medium was exchanged to flesh one which contained 20 µM of HpD and incubated for 0.5, 1, 3 and 6 h. After incubation, cells were dissolved with 100 µl of self-prepared RIPA buffer.(13) The cell homogenates were transferred to 96-well plate and then the fluorescence intensity of HpD was measured by a Varioskan micro plate reader (Thermo Fisher Scientific K.K., Kanagawa, Japan). The measurement wavelength of excitation and emission were 415 nm and 625 nm, respectively.
Western blotting analysis of HCP-1
Western blotting analysis was performed according to previous reports.(13) Briefly, 15 µl of cell lysed solution (10 µg) from each cells were prepared with NuPAGE LDS Sample buffer containing Sample Reducing Agent (Life Technologies Japan Ltd.) and boiled at 70°C for 10 min. For SDS-polyaclylamidegel electrophoresis (SDS-PAGE), the cell lysed solutions were added into well of NuPAGE® Novex® 12% Bis-Tris gels. Since the gel was electrophoresed at 200 V for 30 min, protein were transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore Co.) by electrophoresis at 4.0 A·cm−2 for 10 min. The sandwich immune assay was performed by SNAP i.d. (Millipore Co.), which is a suction type immune reaction system and antibody reaction steps could be performed for about 30 min. After 15 ml of PVDF Blocking Reagent for Can Get Signal® was exposed to membrane and aspirated, an anti-rabbit HCP-1 antibody (1:500), which was primary antibody in this experiment, was added in Can Get Signal® Immunoreaction Enhancer Solution 1 and the solution was exposed to the membrane for 10 min. After the primary antibody solution was aspirated, the membrane was washed three times by 15 ml of 1 X TBST. HRP-linked anti-rabbit IgG antibody was cultured in Can Get Signal® Immunoreaction Enhancer Solution 2. The solution was exposed to the membrane for 10 min. The membrane was immersed in Lumina forte western HRP substrate (Millipore Co.), luminescence was then observed by LAS4000 (GE Health Care Japan, Tokyo, Japan). β-actin was detected with anti-β-actin as a control for protein loading.
Cell viability test after PDT
Cell viability test after PDT was performed according to manufacturer’s protocol and previous report.(13) Briefly, cells were incubated for overnight on 96-well plate at 2 × 104 cells/well. The medium was exchanged to flesh one which contained 20 µM of HpD and incubated for 0.5, 1, 3 and 6 h. After incubation, cells were washed by PBS twice and the flesh medium without phenol red (Life Technologies Japan Ltd.) was added. Subsequently, cells were irradiated to excimer dye laser light (630 nm, 1 J/cm2) for PDT by use of PDT EDL-1 (Hamamatsu Photonics K.K., Hamamatsu, Japan). After photo-irradiation, cells were incubated for 24 h. The medium was replaced to flesh one containing 10% of Cell Counting Kit-8 and then further incubated for 2 h. The absorbance of 450 nm was measured by the Varioskan micro plate reader (Thermo Fisher Scientific K.K.).
Static analysis
Static significant value (p value) was calculated using SPSS software (IBM Corp., NY) followed by Scheffe’s method.
Results
Cytotoxicity of HpD
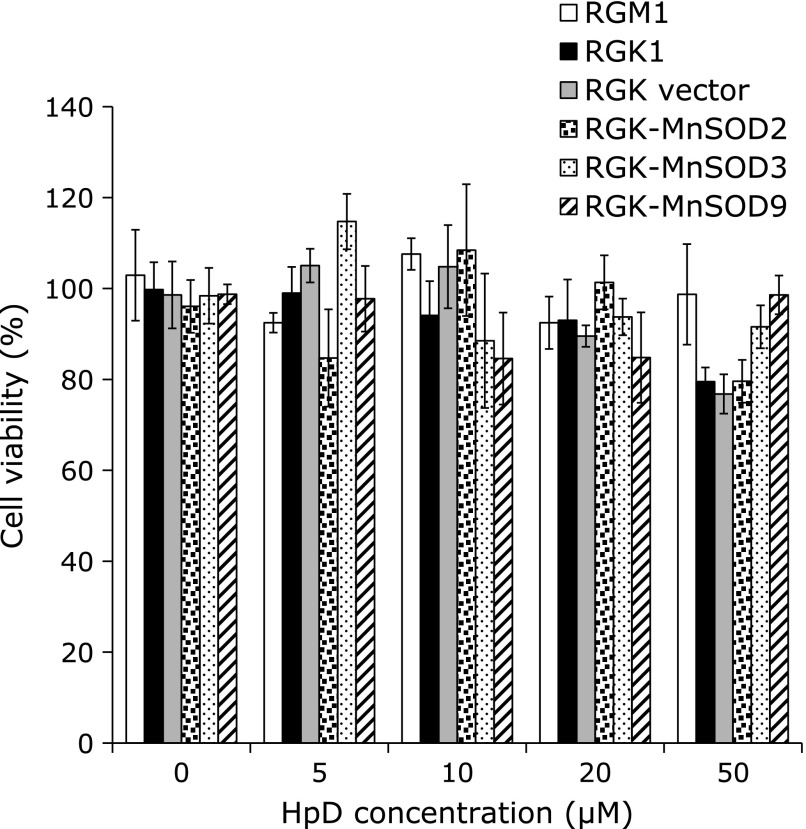
To evaluate the cytotoxicity after exposing HpD, WST assay with each cell was performed. In all cell lines, HpD did not show acute toxicity below the concentration of 20 µM (Fig. 1). From this result, following study was performed with 20 µM HpD when it was exposed to cells.
Fig. 1.
Cell viability after 24 h HpD exposure. Cell viability was evaluated by WST assay. The different HpD concentration medium was made by adding HpD dissolved in ethanol to culture medium. The absorbance at 450 nm was measured by plate reader. n = 4, Error bar; SD.
Cancer cellular specific uptake of HpD
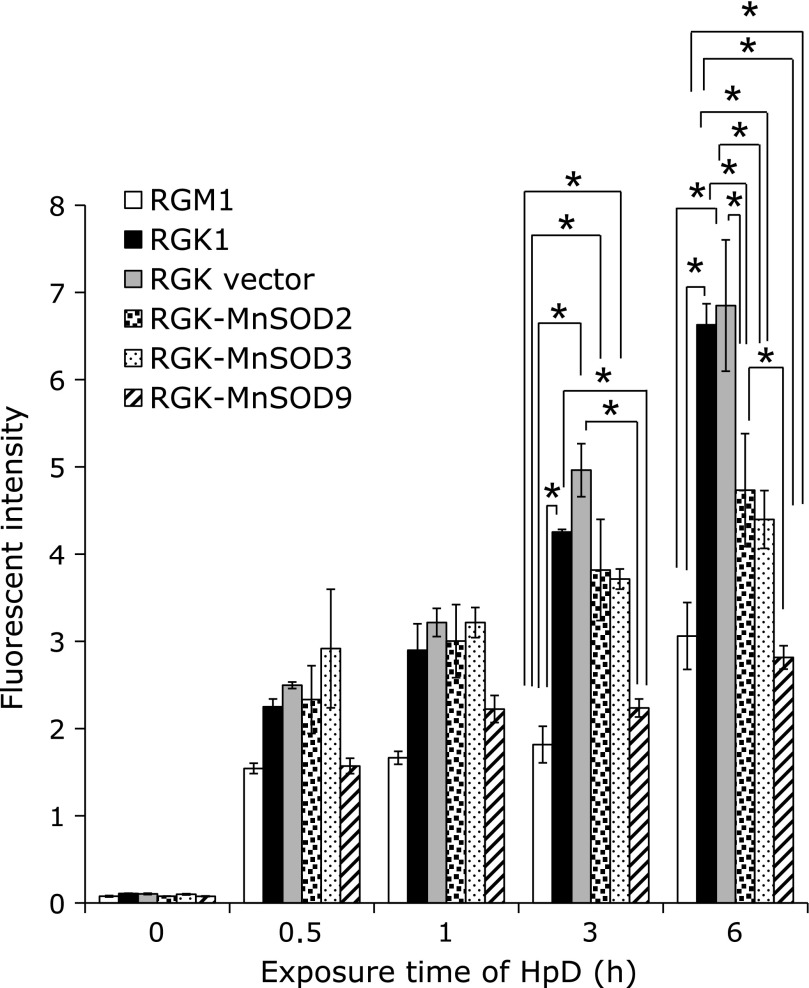
In order to investigate cellular uptake manner of HpD in association with mitROS, cellular uptake of HpD was examined by measuring fluorescent intensities of HpD after exposing it to cells. Fig. 2 showed the relation between an exposure time of HpD and the 625 ± 6 nm intensity of fluorescence. A HpD fluorescence intensity in cancer cells was greater than that of normal cells. Furthermore, these fluorescent intensities in RGK-MnSOD cells were less than cancer cells: of note, the intensity in RGK-MnSOD9 was about half in cancer cells’.
Fig. 2.
Cellular uptake study of HpD was performed. Cells were exposed to culture medium containing 20 µM of HpD for 0, 0.5, 1, 3, 6 h respectively and the fluorescence of HpD incorporated in cells was measured by microplate reader. This figure clearly showed cancer cell specific HpD uptake and it was suppressed by MnSOD expression. n = 4, Error bar; SD. *p<0.01.
HCP-1 protein expression
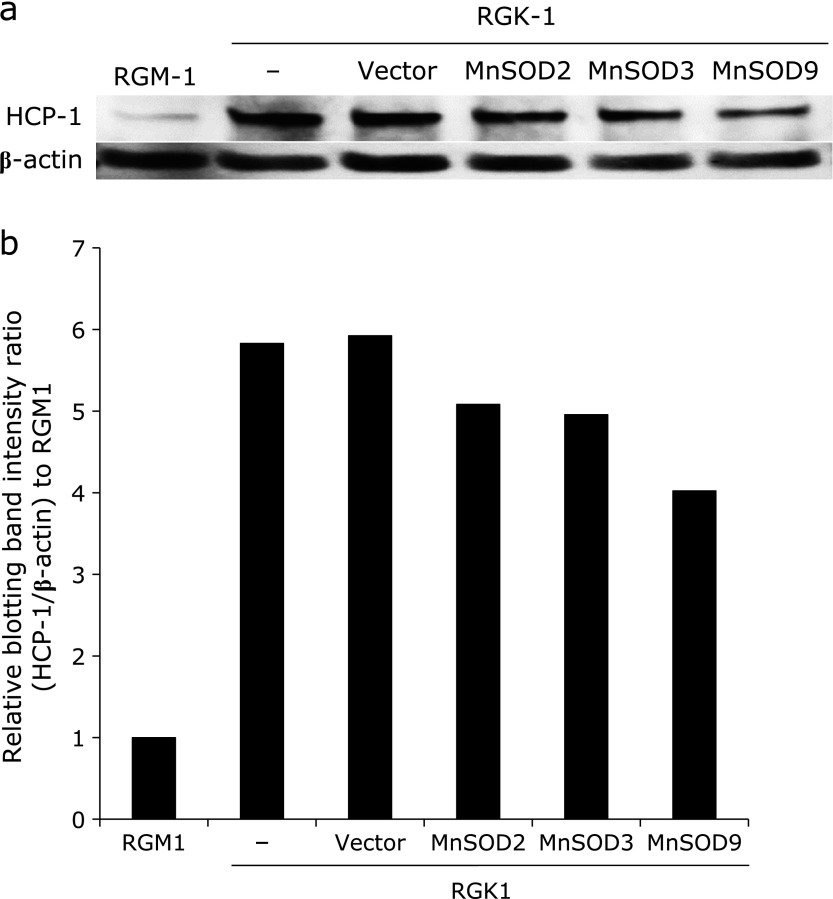
To elucidate the relation between the uptake of HpD and ROS, HCP-1 protein expression level was analyzed by western blotting. Fig. 3a showed the protein expression of HCP-1 and β-actin. Fig. 3b showed the expression ratio of HCP-1 and β-actin. HCP-1 expression level in RGK1 was greater than that in RGM1. Besides, the amounts of protein expression were decreased in RGK-MnSOD clones suggesting that mitROS was likely to upregulate the expression of HCP-1 using these cells.
Fig. 3.
Western blotting analysis of HCP-1 expression in each cell. HCP-1 protein bands of each cell (a), and HCP-1 expression levels were represented by use of graph (b). HCP-1 was expressed greater in cancer cells and suppressed in normal and MnSOD expressing cells. In addition, HCP-1 expression decreased with increasing the expression of MnSOD.
Cancer cell specific death by PDT
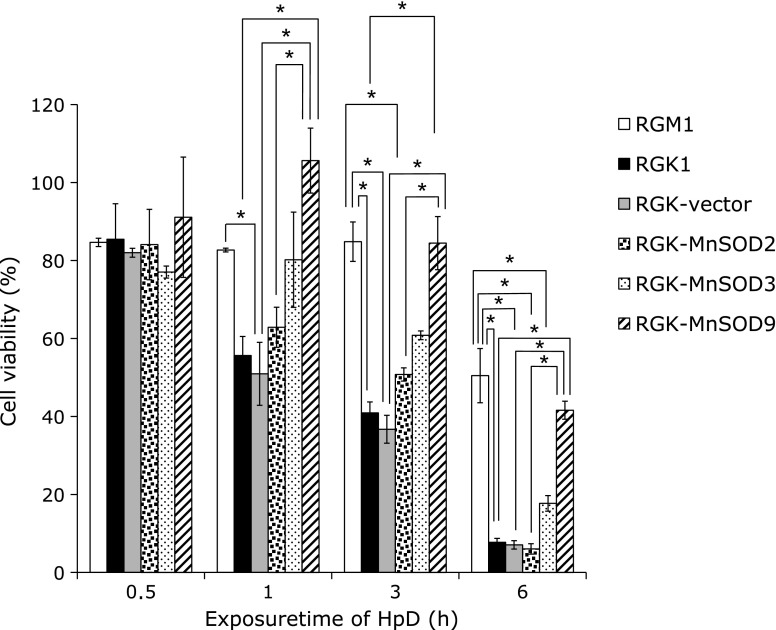
Due to the evaluation of PDT effect for gastric cell lines, the laser light was irradiated to cells after the treatment with HpD, and the cell viability was estimated by WST assay. Fig. 4 showed the viability decreased depending on the treatment time of HpD. Especially, cellular death was remarkably observed in cancer cells and it was suppressed by expression of MnSOD at 6 h.
Fig. 4.
PDT effect was examined after exposing HpD. Cell viability was evaluated after HpD exposure and photo-irradiation. Cancer cells specific death was observed and the death was suppressed by expressing MnSOD. n = 4, Error bar; SD. *p<0.01.
Discussion
In this study, we demonstrated for the first time that mitROS play an important role for cellular uptake of HpD in gastric epithelial cells. We examined cellular uptake of HpD using normal gastric epithelial cells RGM1, its mutated cancer-like cells with chemical carcinogen RGK1 and MnSOD overexpressing RGK1 (RGK-MnSOD). We used three MnSOD expressed RGK clones, which have different MnSOD expression levels(8) in order to study the relation between mitROS and HpD uptake. The amounts of incorporated HpD in RGK1 were more than twice compared to RGM1 after 6 h exposing 20 µM HpD, and the amounts were significantly suppressed in MnSOD expressed RGK cells (Fig. 2). We previously reported that MnSOD expression level in RGK-MnSOD clones were greater in the order of clone9, clone3 and clone2(8) and the uptake level was related to the MnSOD expression. These results indicated that mitROS play an important role to accelerate HpD uptake in cancer cells. As described above, we have reported that HCP-1 is most likely to transport HpD in cancer cells. Taken together, HpD accumulation was suggested to be regulated by mitROS via the regulation of HCP-1 expression. In fact, HCP-1 expressions were related to the MnSOD expression. We found that HCP-1 expression level in MnSOD clones were also in the order of clone9, clone3 and clone2 after western blotting analysis. HCP-1 is expressed in human cancer cells such as Caco-2 cells and Hela cells.(14) Furthermore, we have previously reported that the HCP-1 expressed in brain tumor: these expression levels in glioma are dependent on tumor malignant grade.(16) ROS were highly generated in tumor cells according to their mitochondrial dysfunction, especially complex I and III of electron transport chain,(16) and were related to the malignancy.(8) In this study, Fig. 3 shows that the expression of HCP-1 was highest in cancer cells and that was decreased according to the MnSOD expression level in RGK-MnSOD clones. Thus we concluded that HCP-1 expression level was regulated by mitROS.
Since ROS concentration is higher in cancer cells and DNA injuries used to occur more frequently in cancer cells, repair system for these phenomena should be desired. Thymidine is an essential nucleotide for DNA repair after oxidative stresses. This nucleotide is synthesized from folate. Thus lager amount of folate is required in cancer cells. Heme is also an important protein as an active site of redox enzymes such as cytochrome c. Exogenous heme is strongly desired because ferrocheletase, a key enzyme in heme biosynthesis, used to be inactivated in cancer cells. These are possibly the reasons why HCP-1, a carrier of both folate and heme, involved in cancer cells.
The hHCP1 (hPCFT) gene is localized to chromosome 17Q11.2 and consist of five exons. Nuclear respiratory factor 1 (NRF-1) used to regulate anti-oxidatve stress proteins via anti-oxidative stress responsible elements (ARE). NrF-1 has been also reported to bind and trans-activate HCP1 gene, leading to an increase in HCP1 mRNA levels via NrF-1 binding sites in the HCP1 minimal promoter (positions –108 to –97, –93 to –82 and –10 to +1).(17) Moreover, the activation of nuclear respiratory factor 2 (Nrf2) also leads anti-oxidative stress genes in response to ROS.(18) Since folate and heme are required for cellular anti-oxidation, these redox signaling pathway may increase HCP-1 expression in large amounts of ROS generating cancer cells. In addition, mitROS has been reported to induce pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β).(19) These cytokines activate nuclear factor-κB (NF-κB) and promote additional ROS production.(20,21)
We also studied influence of mitROS on HpD-PDT effect with MnSOD overexpressed cells. PDT using HpD had effective result in many cancer cells such as lung cancer,(22) osteosarcoma cells(23) and gastric cancer.(24) In this study, cancer cell specific great PDT effect was also observed (Fig. 4). In our previous study, we reported that accumulation of porphyrins by the metabolism of 5-aminolevulinic acid was related to mitROS, and according to the effect, PDT was enhanced in gastric epithelial cells.(13) Besides, expression of MnSOD made these effects weaken in both ALA-PDT and HpD-PDT. Therefore, we concluded that mitROS was one of the important factors to decide the PDT efficiency.
In conclusion, we have demonstrated that mitROS regulated the expression of a porphyrin transporter, HCP-1, and they decide the efficacy of HpD-PDT. We are now investigating the detail of signaling pathway which controls the expression of HCP-1.
Acknowledgments
This work was partially supported by the Japan Society for the Promotion of Science (JSPS) and Grant-in-Aid for Scientific Research (KAKENHI) #24106503, #70272200.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Nonaka T, Nanashima A, Nonaka M, et al. Advantage of laserphyrin compared with photofrin in photodynamic therapy for bile duct carcinoma. J Hepatobiliary Pancreat Sci. 2011;18:592–600. doi: 10.1007/s00534-011-0377-6. [DOI] [PubMed] [Google Scholar]
- 2.Chekulayeva LV, Shevchuk IN, Chekulayev VA. Influence of temperature on the efficiency of photodestruction of Ehrlich ascites carcinoma cells sensitized by hematoporphyrin derivative. Exp Oncol. 2004;26:125–139. [PubMed] [Google Scholar]
- 3.Gomer CJ, Jester JV, Razum NJ, Szirth BC, Murphree AL. Photodynamic therapy of intraocular tumors: examination of hematoporphyrin derivative distribution and long-term damage in rabbit ocular tissue. Cancer Res. 1985;45:3718–3725. [PubMed] [Google Scholar]
- 4.Shayeghi M, Latunde-Dada GO, Oakhill JS, et al. Identification of an intestinal heme transporter. Cell. 2002;122:789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 5.Hiyama K, Matsui H, Tamura M, et al. Cancer cells uptake porphyrins via heme carrier protein 1. J Porph Phtal. 2013;17:36–43. [Google Scholar]
- 6.Krishnamurthy P, Xie T, Schuetz JD. The role of transporters in cellular heme and porphyrin homeostasis. Pharmacol Ther. 2007;114:345–358. doi: 10.1016/j.pharmthera.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Chandel NS. Mitochondrial regulation of oxygen sensing. Adv Exp Med Biol. 2010;661:339–354. doi: 10.1007/978-1-60761-500-2_22. [DOI] [PubMed] [Google Scholar]
- 8.Tamura M, Matsui H, Tomita T, et al. Mitochondrial reactive oxygen species accelerate gastric cancer cell invasion. J Clin Biochem Nutr. 2013;54:12–17. doi: 10.3164/jcbn.13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi I, Kawano S, Tsuji S, et al. RGM1, a cell line derived from normal gastric mucosa of rat. In Vitro Cell Dev Biol Anim. 1996;32:259–261. doi: 10.1007/BF02723056. [DOI] [PubMed] [Google Scholar]
- 10.Shimokawa O, Matsui H, Nagano Y, et al. Neoplastic transformation and induction of H+,K+-adenosine triphosphatase by N-methyl-N'-nitro-N-nitrosoguanidine in the gastric epithelial RGM-1 cell line. In Vitro Cell Dev Biol Anim. 2008;44:26–30. doi: 10.1007/s11626-007-9067-8. [DOI] [PubMed] [Google Scholar]
- 11.Majima HJ, Oberley TD, Furukawa K, et al. Prevention of mitochondrial injury by manganese superoxide dismutase reveals a primary mechanism for alkaline-induced cell death. J Biol Chem. 1998;273:8217–8224. doi: 10.1074/jbc.273.14.8217. [DOI] [PubMed] [Google Scholar]
- 12.Motoori S, Majima J, Ebara M, et al. Overexpression of mitochondrial manganese superoxide dismutase protects against radiation-induced cell death in the human hepatocellular carcinoma cell line HLE. Cancer Res. 2001;61:5382–5388. [PubMed] [Google Scholar]
- 13.Ito H, Tamura M, Matsui H, Majima HJ, Indo HP, Hyodo I. Reactive oxygen species involved cancer cellular specific 5-aminolevulinic acid uptake in gastric epithelial cells. J Clin Biochem Nutr. 2014;54:81–85. doi: 10.3164/jcbn.13-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latunde-Dada GO, Takeuchi K, Simpson RJ, McKie AT. Haem carrier protein 1 (HCP-1): expression and functional studies in cultured cells. FEBS Lett. 2006;580:6865–6870. doi: 10.1016/j.febslet.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 15.Takada T, Tamura M, Yamamoto T, Matsui H, Matsumura A. Selective accumulation of hematoporphyrin derivative in glioma through proton-coupled folate transporter SLC46A1. J Clin Biochem Nutr. 2014;54:26–30. doi: 10.3164/jcbn.13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verschoor ML, Wilson LA, Singh G. Mechanisms associated with mitochondrial-generated reactive oxygen species in cancer. Can J Physiol Pharmacol. 2010;88:204–219. doi: 10.1139/Y09-135. [DOI] [PubMed] [Google Scholar]
- 17.Gonen N, Assaraf YG. The obligatory intestinal folate transporter PCFT (SLC46A1) is regulated by nuclear respiratory factor 1. J Biol Chem. 2010;285:33602–33613. doi: 10.1074/jbc.M110.135640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itoh K, Wakabayashi N, Katoh Y, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208:417–420. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gloire G, Legrand-Poels S, Piette J. NF-κB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Handa O, Naito Y, Takagi T, et al. Tumor necrosis factor-alpha-induced cytokine-induced neutrophil chemoattractant-1 (CINC-1) production by rat gastric epithelial cells: role of reactive oxygen species and nuclear factor-kappaB. J Pharmacol Exp Ther. 2004;309:670–676. doi: 10.1124/jpet.103.062216. [DOI] [PubMed] [Google Scholar]
- 22.Yang SG, Chang JE, Shin B, Park S, Nad K, Shim CK. 99mTc-hematoporphyrin linked albumin nanoparticles for lung cancer targeted photodynamic therapy and imaging. J Mater Chem. 2010;20:9042–9046. [Google Scholar]
- 23.Zeng H, Sun M, Zhou C, et al. Hematoporphyrin monomethyl ether-mediated photodynamic therapy selectively kills sarcomas by inducing apoptosis. PLoS ONE. 2013;8:e77727. doi: 10.1371/journal.pone.0077727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayata Y, Kato H, Okitsu H, Kawaguchi M, Konaka C. Photodynamic therapy with hematoporphyrin derivative in cancer of the upper gastrointestinal tract. Semin Surg Oncol. 1985;1:1–11. doi: 10.1002/ssu.2980010103. [DOI] [PubMed] [Google Scholar]