Abstract
Oxidative stress is associated with both healthy aging and age-related disease states. In connection with oxidative stress, immunity is also a major component as a result of the chronic, low-grade inflammation associated with the development of tissue aging. Here we show that long-term treatment with the antioxidant tempol extends life-span in mice. Tempol-treated mice exhibited a reduction in mortality at 20 months. Tempol drinking did not have any effect on body weight, amount of visceral adipose tissue, or plasma biochemical parameters in aged mice. Body temperature of aged control mice (which drank only water) was significantly lower than young mice, but this reduction of body temperature was partially restored in aged mice which drank tempol. Plasma thiobarbituric acid-reactive substances and C-reactive protein were significantly increased in the control aged mice compared with young mice, but levels of both were normalized by tempol drinking. One of the endogenous antioxidants, ascorbic acid, was significantly increased in the plasma of mice which consumed tempol. The proportion of CD4 lymphocytes in the blood of aged tempol-treated mice was partially increased in comparison to aged control mice. These results suggest that the reduction of mortality by tempol is due to amelioration of chronic inflammation and improved function of the immune system through antioxidant effects.
Keywords: oxidative stress, antioxidant, aging, inflammation, immunity
Introduction
Oxidative stress is associated with both healthy aging and age-related disease states.(1–3) For example, mitochondria are one of the major sources of reactive oxygen species (ROS) relevant to the aging process. Cells using oxygen generate ROS during the process of ATP production; increased ROS levels lead to a vicious cycle where ROS produced by the mitochondrial electron transport chain damage the mitochondria, leading to an exponential increase in ROS production and mitochondrial damage.(4,5) In addition to mitochondria, NADPH oxidase or xanthine oxidase are major sources of ROS associated with aging. Several reports suggested that oxidative stress associated with aging is related to increased expression and activity of NADPH oxidase(6,7) or xanthine oxidase(8,9) in a range of tissues.
In connection with increased oxidative stress, inflammation stands out as a determinant process in the development of tissue aging.(2) The redox-regulated and oxidant stress-activated transcription factor nuclear factor-κB (NF-κB) has been reported to be active in the tissues of aged experimental animals,(10,11) and this active NF-κB was shown to correlate with expression of the NF-κB regulated genes IL-6, IL-12, macrophage migration inhibitory factor, cyclooxygenase-2, and tumor necrosis factor-a in aged mice.(12) Spin trapping agents also decrease inflammatory factors such as steady state cyclooxygenase-2 (both at the mRNA level, and its catalytic activity), and inducible nitric oxide synthase (at the mRNA level) in macrophage cell culture.(13) Immunosenescence is understood as an imbalance between inflammatory and anti-inflammatory mechanisms. The immune system is a major component of chronic, low-grade inflammation associated with normal aging and age-related disease.(14,15) Chen et al.(16) reported that a decrease in the proportion of the CD4 T cell subset in peripheral blood was a general phenotype for the aged immune system.
4-Hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (hydroxy-TEMPO, or tempol), which is a well-known antioxidant, has a protective effect on age-related phenomena. Chronic treatment with tempol has been shown to restore the sensitivity of pharmacological preconditioning in aged rats, and reduce the myocardial infarct size.(17) Short-term treatment with tempol also normalizes the age-associated increase in arterial superoxide production, and ameliorates large elastic artery stiffness and carotid artery endothelial dysfunction in aged mice.(18) Tempol prevents the age-related impairment in cardiovascular disease or vascular disease animal models,(17–19) however other age-related effects, for example on the immune system, remain unknown. Therefore, in this study we tested whether tempol treatment would ameliorate the decrease in proportion of the CD4 T cell subset with aging, in order to identify an association between oxidative stress and immunosenescence.
Materials and Methods
Chemicals
Ascorbic acid, 2,6-di-t-butyl-4-methyphenol, 1,1,3,3,-tetraethoxypropane, and thiobarbituric acid (TBA) were obtained from Wako Pure Chemical Industries (Osaka, Japan). Glucose, cholesterol, triglyceride, and non-esterified fatty acid assay kits were also obtained from Wako Pure Chemical Industries. C-reactive protein (CRP) assay kit was obtained from Kamiya Biomedical Company (Seattle, WA). All other chemicals were of the highest grade available.
Animals
C57BL/6 male mice (7 weeks of age) were purchased from Kyudo Co., Ltd. (Saga, Japan). The mice were housed in a temperature- and humidity-controlled room, and fed commercial normal diet (MF, Oriental Yeast Co., Tokyo, Japan) ad libitum. After an acclimation period of 1 week, the mice were divided into two groups: control animals, which continued on regular drinking water, or treated animals, which had tempol supplemented at a dose (6 mM) previously used in vivo.(20) Individual body weights were determined every 1 to 2 weeks. Mice were grouped by age, either young (2 months of age, water only), or aged (20–23 months of age, water or tempol) for the experiments. All mice were housed in an animal care facility on a 12 h:12 h light-dark cycle.
All procedures and animal care were approved by the Committee on Ethics of Animal Experiments, Graduate School of Pharmaceutical Sciences, Kyushu University, and were conducted according to the Guidelines for Animal Experiments of the Graduate School of Pharmaceutical Sciences, Kyushu University.
Survival
A survival analysis was performed in control (n = 38) and tempol-treated (n = 37) mice. During the study period of 20 months, cages were inspected daily for deceased animals.
Biochemical determination
Blood was carefully collected from the inferior vena cava using a heparinized syringe. Plasma was separated by centrifugation at 800 × g for 15 min at 4°C and stored at –80°C until analyses. Plasma glucose, cholesterol, triglyceride, urea nitrogen, and CRP were determined using assay kits. Measurements of CD4 and CD8 population were performed at SRL Inc. (Tokyo, Japan).
Measurement of plasma ascorbic acid concentration
Plasma ascorbic acid concentration was determined using a fluorophore–nitroxide probe as follows.(21) Five µl of plasma was added to an assay solution containing 85 µl of distilled water and 10 µl of probe stock solution (500 mM in dimethylsulfoxide). The solution was then measured at 310 nm (excitation) and 430 nm (emission) wave lengths. The concentrations were calculated from a standard curve.
Level of lipid peroxidation
The concentration of plasma thiobarbituric acid-reactive substances (TBARS) was measured. The plasma was pre-treated with 10% phosphotungstic acid and 1/12 N sulfuric acid. The samples were mixed to give a final concentration of 7.5% acetic acid, 2 mM EDTA, and 0.4% SDS; it was then reacted with 0.3% thiobarbituric acid in a bath of boiling water for 45 min. After cooling, the chromogen was extracted in n-butanol/pyridine (15:1, v/v). The TBARS concentration was calculated from the absorption values of the butanol-extracted supernatant at 532 nm. The concentration of TBARS was calculated using 1,1,3,3,-tetraethoxypropane as a standard.
Statistical analysis
Data are expressed as mean ± SEM. Survival analysis was performed using the Kaplan–Meier method, and between-group differences in survival were tested by the log-rank test. A between-group comparison of means was analyzed using the Tukey–Kramer test. A probability value of 0.05 was set as the minimum level of statistical significance.
Results
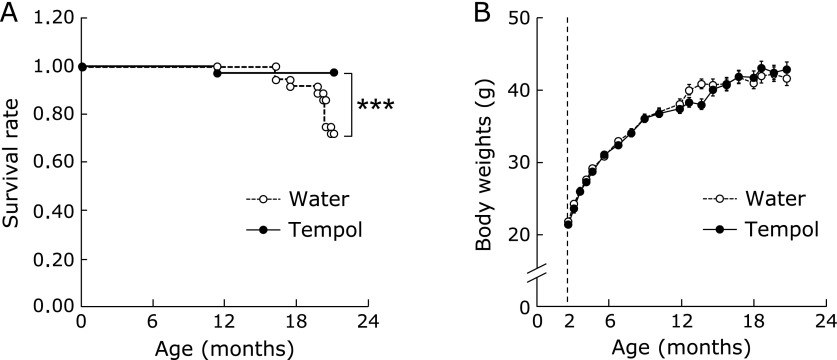
To characterize its effect on mortality in mice, tempol was added to the drinking water. As shown in Fig. 1A, tempol-treated mice exhibited a reduction in mortality at 20 mouths, which was the experimental endpoint to determine biochemical changes. The body weight of tempol-treated mice was the same as control mice (Fig. 1B). Food and water consumption did not differ between the two groups (data not shown). These results suggested that there is no caloric restriction-like effect, as has been observed in a previous study.(20)
Fig. 1.
Survival rate of control and tempol-treated mice (A) and body weight as a function of time (B). Tempol concentration was 6 mM in drinking water. Open and closed circles represent mice treated with either water (n = 38) or tempol (n = 37), respectively. *p<0.05 vs aged control group.
Animal characteristics are shown in Table 1. At 20 months, the body weight of the aged mice had increased significantly in comparison with young mice. Plasma total cholesterol and triglyceride of aged mice were significantly lower than in young mice. Plasma glucose and blood urea nitrogen levels of aged mice were not different from those in young mice. Tempol drinking did not have any effect on body weight, amount of visceral adipose tissue, or plasma biochemical parameters in aged mice. Body temperature of aged control mice was significantly lower than that of young mice, but this reduction in body temperature was partially restored in the tempol-treated aged mice.
Table 1.
Animal characteristics
| Young | Old | ||
|---|---|---|---|
| Water | Tempol | ||
| Body weight (g) | 21.8 ± 0.5 | 35.1 ± 1.6** | 37.4 ± 2.2** |
| Body fat (%) | 1.4 ± 0.3 | 6.0 ± 0.9** | 6.1 ± 1.3** |
| Body temperature (°C) | 38.1 ± 0.2 | 35.9 ± 0.1** | 36.7 ± 0.3**,# |
| Glucose (mg/dl) | 136.4 ± 2.8 | 122.5 ± 3.8 | 120.1 ± 6.0 |
| Total cholesterol (mg/dl) | 61.7 ± 6.3 | 40.5 ± 3.3* | 39.5 ± 5.1* |
| Triglyceride (mg/dl) | 62.0 ± 7.4 | 48.9 ± 4.8 | 46.8 ± 3.2 |
| Blood urea nitrogen (mg/dl) | 20.8 ± 0.9 | 23.2 ± 1.1 | 21.4 ± 1.1 |
Each value represents the mean ± SEM. The data were obtained from 6–8 animals.*p<0.05 and **p<0.005 vs young group. #p<0.05 vs aged control group.
To determine whether tempol drinking affects systemic oxidative stress in aged mice, lipid peroxidation was estimated by the TBARS method. Plasma TBARS were significantly increased in the aged control mice compared to the young mice, but this increase was normalized by tempol consumption (Fig. 2A). Ascorbic acid, one of the endogenous antioxidants, was significantly increased in the plasma of tempol-treated mice (Fig. 2B). Other plasma antioxidant enzymes such as superoxide dismutase did not change between the groups (data not shown). CRP has been reported to be associated with oxidative stress.(22) The plasma level of CRP was increased in aged control mice, but that of tempol-treated mice was the same as the control mice (Fig. 2C).
Fig. 2.
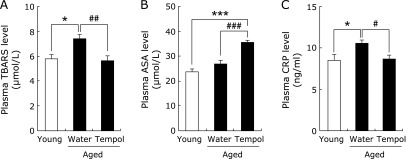
Effect of tempol on plasma TBARS (A), ascorbic acid (B), and CRP (C) levels. Open and closed column represents young (n = 7) and aged (n = 8) mice, respectively. *p<0.05 and **p<0.005 vs young group. #p<0.05, ##p<0.01, and ###p<0.005 vs aged control group.
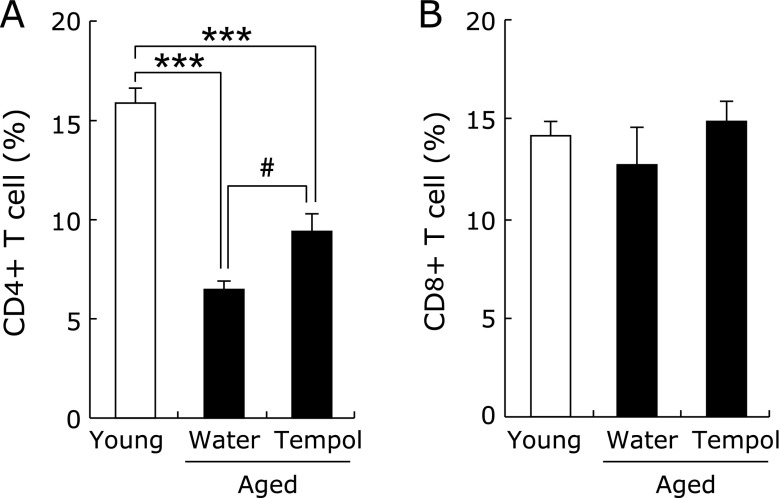
The proportion of CD4 lymphocytes decreased significantly with age in the peripheral blood of mice, and tempol drinking partially ameliorated this reduction (Fig. 3A). The proportion of CD8 lymphocytes did not change between groups (Fig. 3B).
Fig. 3.
Effect of tempol on peripheral blood leukocyte composition: CD4 (A) and CD8 (B). Open and closed columns represent young (n = 6) and aged (n = 5–6) mice, respectively. **p<0.005 vs young group. #p<0.05 vs aged control group.
Discussion
Advanced age is associated with oxidative stress, chronic inflammation, and dysregulation of the immune system. Here we report that tempol has a systemic antioxidant effect in aged mice, and is beneficial in reducing mortality up to 20 months of age. One of the mechanisms by which this may occur is the change of plasma CRP level and peripheral blood CD4 cells in aged mice, both of which were ameliorated by tempol consumption.
Tempol is a redox-cycling nitroxyl radical that promotes the scavenging of many ROS.(23–26) Tempol can shuttle between the nitroxyl radical, reduced hydroxylamine, and oxidized oxoammonium cation forms through one- and two-electron-transfer reactions. The oxoammonium cation is generated if the hydroxyl radical or superoxide reacts with the nitroxyl group of tempol at almost the diffusion-controlled rate, and is reduced to the hydroxylamine by NADH.(23,25) In addition to the direct scavenging effect for free radicals, tempol acts as an antioxidant through increasing SOD activity.(17) In this study, ascorbic acid was significantly increased in the plasma of aged tempol-treated mice (Fig. 2). Ascorbic acid is one of most important antioxidants in vivo. Ascorbic acid can scavenge the superoxide anion, hydroxyl and other radicals, and couples with glutathione to detoxify hydrogen peroxide.(27–29) Tempol may therefore act as both a direct and indirect antioxidant in aged mice, normalizing plasma TBARS and CRP levels.
Aging is associated with a chronic, low-grade inflammatory state, which appears to be a major characteristic of the immune-aging process. In fact a reduced peripheral blood CD4 lymphocyte proportion is one component of the ageing phenotype.(15,16,30) The effect on the proportion of CD4 lymphocytes in aged mice is muted by caloric restriction(30) or resveratrol treatment.(15) In this study, the proportion of CD4 lymphocytes in the peripheral blood was significantly decreased with age, and this was partially restored by tempol (Fig. 3A). These results suggest that antioxidants delay age-dependent changes, resulting in life-span extension.
An effect of drinking a high concentration of tempol (58 mM) on life-span extension in C3H mice has been reported.(20) In C3H mice, tempol obviated weight gain, elevated levels of mitochondrial UCP-2, and decreased age-related spontaneous tumor incidence. In this study, we show that a relatively low concentration of tempol (6 mM) had a life-extending effect without a reduction of body weight. The anti-aging effect of low concentration tempol may be due to the amelioration of chronic inflammation and beneficial effects on the immune system through its antioxidant effects.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science, by Adaptable and Seamless Technology Transfer Program through Target-driven R&D, Japan Science and Technology Agency and by the Platform for Drug Discovery, Informatics, and Structural Life Science from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Abbreviations
- CRP
C-reactive protein
- ROS
reactive oxygen species
- TBARS
thiobarbituric acid reactive substances
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Dato S, Crocco P, D'Aquila P, et al. Exploring the role of genetic variability and lifestyle in oxidative stress response for healthy aging and longevity. Int J Mol Sci. 2013;14:16443–16472. doi: 10.3390/ijms140816443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Assar, Angulo J, Rodríguez-Mañas L. Oxidative stress and vascular inflammation in aging. Free Radic Biol Med. 2013;65:380–401. doi: 10.1016/j.freeradbiomed.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi Y, Yoshida M, Yamato M, et al. Reverse of age-dependent memory impairment and mitochondrial DNA damage in microglia by an overexpression of human mitochondrial transcription factor a in mice. J Neurosci. 2008;28:8624–8634. doi: 10.1523/JNEUROSCI.1957-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flynn JM, Melov S. SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic Biol Med. 2013;62:4–12. doi: 10.1016/j.freeradbiomed.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang CH, Wu SB, Wu YT, Wei YH. Oxidative stress response elicited by mitochondrial dysfunction: implication in the pathophysiology of aging. Exp Biol Med (Maywood) 2013;238:450–460. doi: 10.1177/1535370213493069. [DOI] [PubMed] [Google Scholar]
- 6.McCrann DJ, Yang D, Chen H, Carroll S, Ravid K. Upregulation of Nox4 in the aging vasculature and its association with smooth muscle cell polyploidy. Cell Cycle. 2009;8:902–908. doi: 10.4161/cc.8.6.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oudot A, Martin C, Busseuil D, Vergely C, Demaison L, Rochette L. NADPH oxidases are in part responsible for increased cardiovascular superoxide production during aging. Free Radic Biol Med. 2006;40:2214–2222. doi: 10.1016/j.freeradbiomed.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Aranda R, Doménech E, Rus AD, et al. Age-related increase in xanthine oxidase activity in human plasma and rat tissues. Free Radic Res. 2007;41:1195–1200. doi: 10.1080/10715760701481461. [DOI] [PubMed] [Google Scholar]
- 9.Vida C, Corpas I, De la, González EM. Age-related changes in xanthine oxidase activity and lipid peroxidation, as well as in the correlation between both parameters, in plasma and several organs from female mice. J Physiol Biochem. 2011;67:551–558. doi: 10.1007/s13105-011-0100-8. [DOI] [PubMed] [Google Scholar]
- 10.Helenius M, Hänninen M, Lehtinen SK, Salminen A. Changes associated with aging and replicative senescence in the regulation of transcription factor nuclear factor-kappa B. Biochem J. 1996;318:603–608. doi: 10.1042/bj3180603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helenius M, Hänninen M, Lehtinen SK, Salminen A. Aging-induced up-regulation of nuclear binding activities of oxidative stress responsive NF-kB transcription factor in mouse cardiac muscle. J Mol Cell Cardiol. 1996;28:487–498. doi: 10.1006/jmcc.1996.0045. [DOI] [PubMed] [Google Scholar]
- 12.Spencer NF, Poynter ME, Im SY, Daynes RA. Constitutive activation of NF-kappa B in an animal model of aging. Int Immunol. 1997;9:1581–1588. doi: 10.1093/intimm/9.10.1581. [DOI] [PubMed] [Google Scholar]
- 13.Kotake Y, Sang H, Miyajima T, Wallis GL. Inhibition of NF-kappaB, iNOS mRNA, COX2 mRNA, and COX catalytic activity by phenyl-N-tert-butylnitrone (PBN) Biochim Biophys Acta. 1998;1448:77–84. doi: 10.1016/s0167-4889(98)00126-8. [DOI] [PubMed] [Google Scholar]
- 14.Sansoni P, Vescovini R, Fagnoni F, et al. The immune system in extreme longevity. Exp Gerontol. 2008;43:61–65. doi: 10.1016/j.exger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Wong YT, Gruber J, Jenner AM, Tay FE, Ruan R. Chronic resveratrol intake reverses pro-inflammatory cytokine profile and oxidative DNA damage in ageing hybrid mice. Age (Dordr) 2011;33:229–246. doi: 10.1007/s11357-010-9174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Flurkey K, Harrison DE. A reduced peripheral blood CD4(+) lymphocyte proportion is a consistent ageing phenotype. Mech Ageing Dev. 2002;123:145–153. doi: 10.1016/s0047-6374(01)00347-5. [DOI] [PubMed] [Google Scholar]
- 17.Zhu J, Rebecchi MJ, Wang Q, Glass PS, Brink PR, Liu L. Chronic Tempol treatment restores pharmacological preconditioning in the senescent rat heart. Am J Physiol Heart Circ Physiol. 2013;304:H649–H659. doi: 10.1152/ajpheart.00794.2012. [DOI] [PubMed] [Google Scholar]
- 18.Fleenor BS, Seals DR, Zigler ML, Sindler AL. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell. 2012;11:269–276. doi: 10.1111/j.1474-9726.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller SJ, Coppinger BJ, Zhou X, Unthank JL. Antioxidants reverse age-related collateral growth impairment. J Vasc Res. 2010;47:108–114. doi: 10.1159/000235965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell JB, Xavier S, DeLuca AM, et al. A low molecular weight antioxidant decreases weight and lowers tumor incidence. Free Radic Biol Med. 2003;34:93–102. doi: 10.1016/s0891-5849(02)01193-0. [DOI] [PubMed] [Google Scholar]
- 21.Matsuoka Y, Yamato M, Yamasaki T, Mito F, Yamada K. Rapid and convenient detection of ascorbic acid using a fluorescent nitroxide switch. Free Radic Biol Med. 2012;53:2112–2118. doi: 10.1016/j.freeradbiomed.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 22.Park S, Kim M, Paik JK, Jang YJ, Lee SH, Lee JH. Oxidative stress is associated with C-reactive protein in nondiabetic postmenopausal women, independent of obesity and insulin resistance. Clin Endocrinol (Oxf) 2013;79:65–70. doi: 10.1111/j.1365-2265.2012.04512.x. [DOI] [PubMed] [Google Scholar]
- 23.Krishna MC, Grahame DA, Samuni A, Mitchell JB, Russo A. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. Proc Natl Acad Sci U S A. 1992;89:5537–5541. doi: 10.1073/pnas.89.12.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishna MC, Samuni A, Taira J, Goldstein S, Mitchell JB, Russo A. Stimulation by nitroxides of catalase-like activity of hemeproteins. Kinetics and mechanism. J Biol Chem. 1996;271:26018–26025. doi: 10.1074/jbc.271.42.26018. [DOI] [PubMed] [Google Scholar]
- 25.Kudo W, Yamato M, Yamada K, et al. Formation of TEMPOL-hydroxylamine during reaction between TEMPOL and hydroxyl radical: HPLC/ECD study. Free Radic Res. 2008;42:505–512. doi: 10.1080/10715760802112809. [DOI] [PubMed] [Google Scholar]
- 26.Willson RL. Reaction of triacetoneamine-N-oxyl with hydroxyl radicals. Int J Radiat Biol Relat Stud Phys Chem Med. 1972;21:401–403. doi: 10.1080/09553007214550471. [DOI] [PubMed] [Google Scholar]
- 27.Bánhegyi, Braun L, Csala M, Puskás F, Mandl J. Ascorbate metabolism and its regulation in animals. Free Radic Biol Med. 1997;23:793–803. doi: 10.1016/s0891-5849(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 28.Basu S, Som S, Deb S, Mukherjee D, Chatterjee IB. Dehydroascorbic acid reduction in human erythrocytes. Biochem Biophys Res Commun. 1979;90:1335–1340. doi: 10.1016/0006-291x(79)91182-3. [DOI] [PubMed] [Google Scholar]
- 29.Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86:6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller RA. Age-related changes in T cell surface markers: a longitudinal analysis in genetically heterogeneous mice. Mech Ageing Dev. 1997;96:181–196. doi: 10.1016/s0047-6374(97)01893-9. [DOI] [PubMed] [Google Scholar]