SUMMARY
Group A Streptococcus (GAS) is a leading cause of infection-related mortality in humans. All GAS serotypes express the Lancefield group A carbohydrate (GAC), comprising a polyrhamnose backbone with an immunodominant N-acetylglucosamine (GlcNAc) side chain, which is the basis of rapid diagnostic tests. No biological function has been attributed to this conserved antigen. Here we identify and characterize the GAC biosynthesis genes,gacA-L. An isogenic mutant of the glycosyltransferase gacI, which is defective for GlcNAcside chain addition, is attenuated for virulence in two infection models, in association with increased sensitivity to neutrophil killing, platelet-derived antimicrobials in serum and the cathelicidin antimicrobial peptide LL-37. Antibodies to GAC lacking the GlcNAc side chain and containing only polyrhamnose promoted opsonophagocytic killing of multiple GAS serotypes and protected against systemic GAS challenge after passive immunization. Thus, the Lancefield antigen plays a functional role in GAS pathogenesis and its understanding has implications for vaccine development.
INTRODUCTION
Streptococcus pyogenes, commonly known as group A Streptococcus (GAS), is a preeminent human pathogen responsible for ~700 million cases of pharyngitis (‘strep throat’) annually worldwide and increasing numbers of severe invasive infections including necrotizing fasciitis (‘flesh-eating disease’) and streptococcal toxic shock syndrome (Carapetis et al., 2005). GAS is also responsible for the post-infectious immune-mediated disease rheumatic fever, which is a major cause of chronic heart disease and mortality in many parts of the developing world (Carapetis et al., 2005;Marijon et al., 2012). Serological classification of streptococci in groups is based upon expression of unique carbohydrate antigens in the bacterial cell wall (Lancefield, 1928), known only to play a structural role in cell wall biogenesis(Caliot et al., 2012;McCarty, 1952). All serotypes of GASexpress the Lancefield group A carbohydrate (GAC),comprising a polyrhamnose backbone with an immunodominantN-acetylglucosamine (GlcNAc) side chain(McCarty, 1952, 1956); this GlcNAc epitope of GAC is the basis of all rapid diagnostic testing for GAS infection. Remarkably, ~50% of the GAS cell wall by weight is made up of the GAC (McCarty, 1952); however, a specific biological function has yet to be attributed to this conserved and abundant eponymous antigen.
Despite a high global demand(Carapetis et al., 2005), there is currently no safe and efficacious commercial vaccine against GAS infection. Certain unique phenotypic features of the pathogen pose particular challenges to vaccination(Pandey et al., 2012), including the invariant GAS capsule composed of hyaluronic acid (Kendall et al., 1937), an immunologically inert carbohydrate ubiquitous in human connective tissues. In addition, immunodominant surface-anchored GAS M proteins are polymorphic (>200emm genotypes) (McMillan et al., 2013)and regions of their dimeric coiled-coil structure may provokean autoimmune response against cardiac tissue in rheumatic fever (Fae et al., 2005). Due to its prominence in the GAS cell wall and its conservation across all GAS strains, the GAC has been considered as a potential antigen for a universal GAS vaccine. Passive and active mouse immunization studies with protein conjugate vaccines using purified or synthetic versions of the WT GAC show significant efficacy against multiple GAS serotypes (Kabanova et al., 2010; Sabharwal et al., 2006). Moreover, anti-GAC antibodies are detected in human serum, high titers correlate with decreased colonization in children (Sabharwal et al., 2006), and peak around age 17 correlating to dropping GAS infection rates after this age (Zimmerman et al., 1971). However, theoretical concerns regarding autoreactivity of antibodies that recognize the native GAC GlcNAc side chain against human tissues have been raised by several groups, but remain a point of significant controversy. Glycoproteins from human heart valves, when used to immunize rabbits, engender antibodies that bind to GAC in a manner inhibited by GlcNAc (but not rhamnose and other sugars)(Goldstein et al., 1968) and persistence of anti-GlcNAc/GAC antibodies (from 1 to 20 years) are a marker of poor prognosis of heart valve problems in rheumatic heart disease (RHD), whereas antibodies against streptolysin O and the polyrhamnose core of GAC decline independently of valve complications (Ayoub et al., 1974; Dudding and Ayoub, 1968; Shulman et al., 1974). The specificity and persistence of elevated anti-GAC (GlcNAc) antibody titers for rheumatic mitral valve heart disease was also suggested in a study of 30 patients vs. equal sized control groups of control individuals, patients with congenital heart disease, and patients with non-rheumatic mitral valve prolapse (Appleton et al., 1985). Finally, anti-GlcNAc monoclonal antibodies, cross-reactive for heart or brain tissue have been derived from patients with rheumatic fever and its cardiac or neurological complications (Galvin et al., 2000; Kirvan et al., 2003).
Here, we identify the genes responsible for GAC biosynthesis, identify a role for the GlcNAc side chain in GAS resistance to host innate immunity, and discuss potential implications of this new genetic insight for GAS vaccine design.
RESULTS
Identification and Mutagenesis of GAC Biosynthesis GenesgacA-L
Analogous to a bioinformatic analysis of group B Streptococcus (GBS) (Sutcliffe et al. 2008), we searched the GAS chromosome for clusters enriched in genes encoding rhamnosepolysaccharide-related proteins, and identified a putative 12-gene GAC biosynthesis locus (Figure 1A) that is completely conserved among all sequenced GAS genomes published to date. Many of these genes, herein designated gacABCDEFGHIJKL, are predicted to encode proteins with functional annotations as glycosyltransferases (including rhamnosyltransferases) and polysaccharide transport proteins (Figure 1A). The same 12-gene gacA-L cluster was identified in a 16-kb non-homologous region in two Streptococcusdysgalactiae subsp. equisimilis (SDSE) strains from invasive human infections, which typically express group C or G carbohydrate antigens, but showed unexpected reactivity to the GAS latex agglutination test (McMillan et al., 2010) (Figure 2A-2C), implying a recent recombination event between GAS and a progenitor to the SDSE strains. Similar ‘hybrid strains’ have been described but a genetic basis has never been identified (Tanaka et al., 2008). Using a representative strain of the globally disseminated serotype M1T1 GAS clone(5448), we performed a plasmid integrational mutagenesis scan across the gene cluster. Nine of 12 mutants (targeting genes gacD-gacL) were viable. Three of nine mutants (disrupting gacI, gacJ, and gacK) lost reactivity in the diagnostic GAS latex agglutination test (Figure 1B) and no longer interacted with aGlcNAc-specific lectin, succinylated wheat germ agglutinin(sWGA) (Figure 1B), indicating loss of the hallmark side chain. Normal growth phenotypes were observed for the majority of mutants, except those targeting gacG (putative rhamnosyltransferase), which reached lower stationary phase optical density, and gacJ (annotated only as a membrane protein), which grew only upon addition of 0.5 M sucrose for osmotic stabilization (Figure S1A). Even with osmotic stabilization, we were unable to obtain mutants in gacA, predicted to generate activated rhamnose, nor the annotated rhamnosyltransferasesgacB and gacC, suggesting that all three genes are essential for production of the core polyrhamnose backbone.
Figure 1. Schematic Representation of the GAC Gene Cluster, Mutagenesis Scan, and Δ GacI mutant.
(A) Schematic representation of the GAS M1T1 strain 5448 group A carbohydrate (GAC) gene cluster M5005_Spy0602-0613, which was renamed gacA-L, and annotated gene functions based on analysis using the SEED tools (URL: pubseed.theseed.org). (B)Latex agglutination reaction with GAC-specific beads and GlcNAc-specific sWGAlectin staining of viable GAS inserional knockout mutants in genes gacD-L. Grey fill: medium control; black line:sWGA-FITC stain. Histogram numbers indicate geometric mean of fluorescence. (C) Latex agglutination reaction with GAC-specific beads and GlcNAc expression as assessed by sWGA stain as indicated on GAS WT, ΔGacImutant and GacI* complemented strain. HPLC tracing and linkage analysis with deduced schematic structure of the repeating unit of extracted GAC from (D) GAS WT and (E)ΔGacI mutant strain. See also Figure S1.
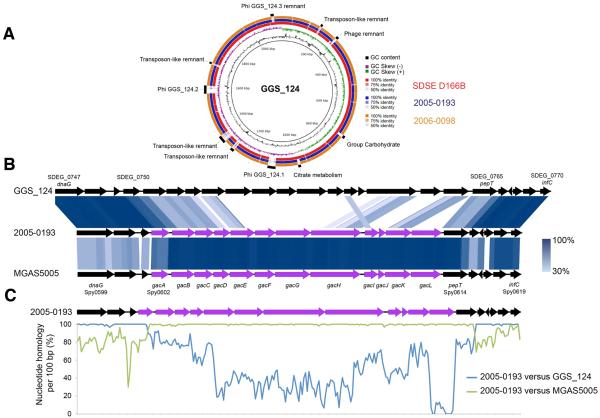
Figure 2. Streptococcus dysgalactiae subsp. equisimilis Testing Positive for GACPossess the gacA-gacK Gene Locus.
(A) Circular genome map of the group G carbohydrateexpressing Streptococcus dysgalactiae subsp. equisimilis (SDSE) GGS_124 genome (accession numberAP010935) with BLAST comparisons to the completely sequenced group G carbohydrate expressing SDSE strain SDSE_D166B genome (accession number CP002215) and the draft genomes of two SDSE strains expressing the GAC antigen, 2005-0193 and 2006-0098. The comparative map was created using BRIG. The inner most rings show GC content (black) and GC skew (purple/green) of GGS_124. The three outer rings show BLAST comparisons (using BLASTn and an E-value cutoff of 10.0) to SDSE_D166B (Red) and to the draft genome sequences of 2005-0193 (blue) and 2006-0098 (orange). The legend shows the percent identity of BLASTn hits to the GGS_124 reference. Both genomes share similar genome synteny with both GGS_124 and SDSE_D166B. Labels around the outer ring refer to genomic regions present in GGS_124 but not in the two SDSE strains expressing GAC, including the GAC carbohydrate gene cluster. (B) Genome architecture of the carbohydrate loci located between the dnaG toinfC gene sequences from 2005-0193 with GGS_124 and M1 strain MGAS5005. Regions of genetic similarity were determined using BLAST with graphical representation of syntenic gene content designated using Easyfig 2.1. Purple coding sequences refer to the GAC encoded gacA to gacL cluster. Blue shaded regions between the stacked genome sequences reflect conserved gene content with percenthomology indicated by the legend. (C) Similarity plot showing carbohydrate locus nucleotide sequence conservation between 2005-0193 versus GGS_124 (blue line) and 2005-0193 versus MGAS5005 (green line). Evidence of homologous recombination between a GAS donor and the SDSE progenitor is evident between the gacA and pepT genes. The plot was generated from a clustal alignment of sequence between the dnaG and infC using a sliding window of 100 bp with values plotted as an average over a 100 bp sliding window.
GlycosyltransferaseGacI is Essential for the Expression of the GAC GlcNAcSide Chain
To study the specific role of the GlcNAc side chain in GAS virulence phenotypes, we generated a precise in-frame allelic replacement mutant eliminating gacI, an annotated glycosyltransferase. The ΔGacI mutant lost reactivity in the diagnostic GAS latex agglutination test and interaction with GlcNAc-specific lectinsWGA (Figure 1C); both phenotypes were restored by reintroducing the gacI gene (marked with a silent mutation) into the ΔGacI mutant chromosome, yielding the reconstituted strain GacI* (Figure 1C). Extraction and purification of GAC from WT GAS, ΔGacI, and GacI* strains followed by glycan composition and linkage analysis unambiguously confirmed the absence of GlcNAc side chain in the ΔGacI mutant, leaving the polyrhamnose core intact (Figure1D and 1E, Figure S1B and S1C). Historically, ‘antigen-negative’ GAS strains are known as ‘A-variant’ strains and infrequently arise upon serial passage in mice and rabbits (McCarty and Lancefield, 1955; Wilson, 1945), but have never been isolated from humans, suggesting a crucial role for the side chain in human infection, the only natural host of GAS.
The TarOHomologue GacO Contributes to GAC Biosynthesis
In GBS, a neonatal pathogen, the enzyme encoded by gbcO catalyzes the transfer of GlcNAc-1-phosphate to bactoprenyl phosphate and is essential for synthesis of the GBS cell wall carbohydrate (GBC) (Caliot et al., 2012). A GBS gbcO knockout mutant lacks cell wall rhamnose, exhibits aberrant cell morphology and slow growth, and has impaired peptidoglycan polymerization leading to mutanolysin hypersensitivity (Caliot et al., 2012); all these phenotypes are reproduced in WT GBS by treatment with tunicamycin (Caliot et al., 2012) a specific inhibitor of gbcO-type transferases(Campbell et al., 2011). GAS has a homologue of gbcO(MGAS5005_spy0240, gacO) located at a distance in the chromosome from the 12-gene locus described here. As was seen with GBS(Caliot et al., 2012), treatment of WT GAS with tunicamycin inhibited growth, increased mutanolysin sensitivity, induced aberrant morphology, and eliminated cell wall rhamnose (Figure 3A - 3D), suggesting thatgacO is essential for synthesis of the GAC polyrhamnose core and bacterial viability. Similar defects in cell wall integrity were not observed with the ΔGacI mutant strain. To assess GAS cell wall integrity in more depth at the functional level, we exposed WT and ΔGacI mutant GAS to stresses that can disrupt a generally weakened cell wall. WT and ΔGacI GAS strains grew at similar rates under high salt (upto 300 mMNaCl) and varying pH (6.0 to 8.0) conditions (data not shown). The mutant showed no increase in autolysis (Figure S2A), and killing of the ΔGacI mutant by lysozyme or the cell wall-active antibiotics vancomycin and nafcillin, was even slightly slower than observed with the parent strain (FigureS2B - S2D). Also, in contrast to tunicamycin-treated GAS that completely lack GAC expression, the ΔGacI mutant is not more sensitive to mutanolysin(Figure S2E) nor defectivein peptidoglycan formationas measured by vancomycin-BODIPY labeling (Figure S2F).
Figure 3. TarO/GbcOHomologue M5005_Spy0240 Contributes to GAC Biosynthesis.
Tunicamycin is a specific inhibitor of UDP-GlcNAC:lipid phosphate transferases like TarO and GbcO and produces a similar phenotype to a gbcO knockout in group B Streptococcus (GBS). WT M1 GAS was treated with different concentrations of tunicamycin to inhibit the activity of homologous enzyme encoded by the gacO gene (M5005_Spy0240), which resulted in (A) growth inhibition (mean ± SEM of four independent experiments, one-way ANOVA), (B) increased sensitivity to mutanolysin (100 U/ml; mean ± SEM of two independent experiments, two-way ANOVA), *p< 0.05,**p< 0.01, ***p< 0.001. (C) changes in cell morphology, and (D) complete loss of rhamnose expression indicating a loss of GAC production. Abbreviations: Rha = Rhamnose; Man = Mannose; Glc = Glucose; GlcNAc =N-acetyl-D-glucosamine; PS = polysaccharide; Tun = tunicamycin at indicated concentration (g/ml). See also Figure S2.
Phenotypic Characterization of ΔGacI Expressing GlcNAc-Deficient GAC
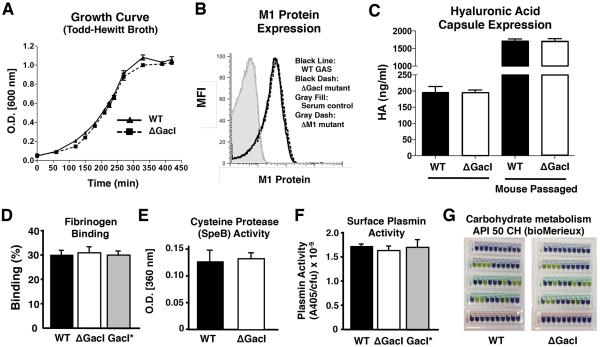
The isogenic ΔGacI mutant was compared to the WT GAS M1T1 parent strain to examine the phenotypic and functional consequences of loss of the GlcNAc side chain from the GAC. The mutant and parent strains grew equally well in bacteriologic media (Figure4A) and did not differ with respect to well-known virulence factors or traits including surface-anchored M1 protein expression, hyaluronic acid capsule production, fibrinogen binding, cysteine protease (SpeB) activity, surface plasmin acquisition due to streptokinase, or sugar metabolism (Figure4B - 4G). Analysis of total protein preparations by gel electrophoresis and silver stain showed a similar pattern for WT and ΔGacI mutant (Figure S3A). Interestingly, the ΔGacI mutant produced longer chains in stationary phase cultures (average 16.6 cocci) than the parent strain (average 6.7 cocci)(Figure S3B). Differences in average chain lengths were less pronounced in exponential phase cultures: WT = 11.2cocci versus ΔGacI = 15.6cocci. While the elongated chains indicate a fundamental problem in cell division or chaining, transmission electron microscopy of WT and ΔGacI revealed no apparent differences in the ultrastructural appearance of the bacterial cell walls (Figure S3C), findings consistent with a previous reporton‘A-variant’ strains isolated from mice(Swanson and Gotschlich, 1973).
Figure 4. GacI Mutant Bacteria Are Not Impaired in Expression of Several Known GAS Virulence Factors or Traits.
GAS WT and ΔGacI mutant bacteria were assessed for (A) Growth in Todd-Hewitt broth. (B) M1 protein expression: Grey fill: serum control, black line: WT GAS, black dash: GacI, grey dotted: GacI*reconstituted; (C) Capsule expression in lab grown and animal passaged bacteria (mean ± SEM of two independent experiments); (D) fibrinogen binding; (E)SpeB secretion; (F) plasmin accumulation of GASWT, GacI mutant and GacI*. Pooled normalized data from three independent experiments are shown(mean ± SEM; one-way ANOVA). (G) Carbohydrate metabolism of GAS WT and GacI mutant assessed using diagnostic API 50 CH test (BioMerieux; t = 48 h). Yellow color indicates the specific carbohydrate is fermented, whereas blue/purple color indicates the bacteria do not metabolize the sugar. See also Figure S3.
The GlcNAc Side Chain of GACPromotes GAS Survival in the Presence of Whole Blood, Serum, Neutrophils, and Antimicrobial Peptides
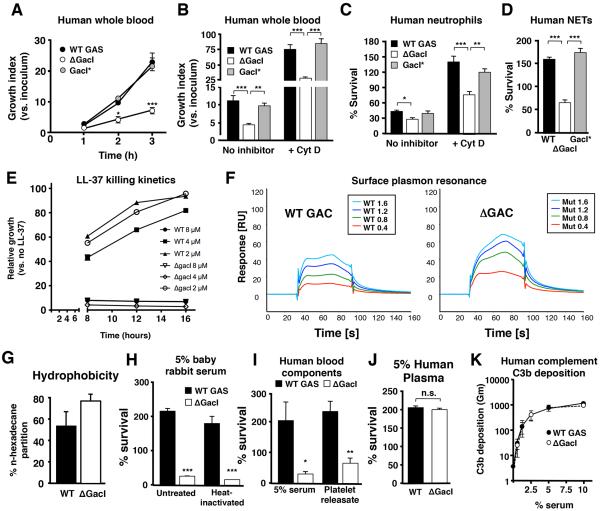
Since GAC is localized at the host-pathogen interface, we next assessed survival of the ΔGacImutant in assays modeling critical steps in invasive disease pathogenesis. Compared to the WT and GacI* complemented strains, the ΔGacImutant was attenuated in whole blood survival (Figure5A and 5B) and resistance to killing by isolated human neutrophils (Figure5C), despite similar phagocytic uptake (Figure S4A - S4C). Accelerated killing of the ΔGacImutant persisted in the presence of cytochalasin D (Figure 5B and 5C), an actin microfilament inhibitor that impairs phagocytosis. Thus, it appeared that the ΔGacImutant was sensitized to extracellular neutrophil killing, which predominantly occurs through DNA-based neutrophil extracellular traps (NETs)(Brinkmann et al., 2004), and potentially other antimicrobial components in human blood. Correspondingly, WT GAS and ΔGacI mutant strains were equally susceptible to hydrogen peroxide and superoxide (Figure S4D and S4E). Exposure of neutrophils to WT GAS and ΔGacImutant strains induced formation of NETs equally (Figure S4F); however, the mutant was more susceptible to killing within NETs (Figure 5D) and to the human cathelicidin antimicrobial peptide LL-37 (Figure 5E and Figure S4G). LL-37 is a component of neutrophil specific granules important for intracellular killing and is deployed within NETs. To examine the specific contribution of GAC and its GlcNAc side chain in modulating LL-37 affinity for GAS, we purified WT and mutant GAC and used surface plasmon resonance to measure its interaction with the host defense peptide. Both WT and ΔGacI mutant GACbound to immobilized LL-37; however, the binding of the ΔGacI mutant GAC to the host defense peptide was substantially stronger than WT GAC at all polysaccharide concentrations tested (Figure 5F). The calculated Kd values obtained by kinetic measurements using a 1:1 Langmuir fitting model were 130 μM for the WT GAC and 44 μM for the ΔGacI mutant GAC. This finding shows that the GlcNAcside chain of GAC itself markedly influences LL-37 affinity for its cell wall target. Increased cell surface hydrophobicity, as measured by n-hexadecane partition of the intact bacterium, suggested a potential basis for increased LL-37 binding and sensitivity(Clarke et al., 2007) of the ΔGacI mutant (Figure 5G).
Figure 5. Antigen-negative GAS Display Increased Sensitivity to Neutrophil and Platelet Immune Defenses.
(A, B)ΔGacI mutant bacteria are defective in whole blood proliferation both in the absence (A) and presence (B, +cytochalasin D, CytD 25 μg/ml) of phagocytosis. Data in (B) represent the 2 h time point. Pooled data from three independent experiments are shown (mean ± SEM; one-way ANOVA). (C) Total (no inhibitor) and extracellular (+Cyt D, 10 μg/ml) bacterial killing by isolated human neutrophils. Surviving CFU were quantified after 15 min and 30 min for total killing and extracellular killing, respectively. Combined data from three independent experiments using different donors are shown (Mean ± SEM; one-way ANOVA). (D)Survival of bacteria in neutrophil extracellular traps (NETs). Neutrophils were incubated with 25 nM PMA for 4 h and incubated with bacteria for 30 min. Pooled data from two independent experiments are shown (mean ± SEM, one-way ANOVA). (E) Kinetic analysis showing increased susceptibility to cathelidicin antimicrobial peptide LL-37 for the GacImutant compared to WT. (F) Surface plasmon resonance (SPR) analysis. LL-37 peptide was immobilized on a CM5 sensor chip by amine coupling. Various concentrations of purified WT (left) or GacI mutant GAC (right) were used as analytes to detect binding to LL-37. SPRsensorgrams were generated by subtraction of the reference flow cell and the signals obtained by injection of only the running buffer from the measured response units. (G) Increased hydrophobicity of the GacI mutantbacteria compared to GAS WT as assessed by the n-hexadecane partition assay. The Y-axis indicates the % of original inoculum recovered from the n-hexadecane layer. (H) Survival of GAS WT or ΔGacI mutant strain in 5% complement sufficient or heat-inactivated (HI) baby rabbit serum (BRS). Survival was quantified after 5 h incubation at 37°C. Representative data are shown. (I) Effects of human serum or thrombin-activated platelet supernatant on survival of GAS WT or ΔGacI mutant bacteria. Serum and platelets were collected from the same donor, processed as described in the Material and Methods, and added to a final concentration of 5% and 25%, respectively. Pooled data from 5 independent experiments are shown (mean ± SEM; ratio t-test). *p< 0.05, **p< 0.01, ***p< 0.001. (J) No difference in growth of GAS WT or ΔGacI mutant strain in 5% human plasma (mean ± SEM, t-test). (K) C3b deposition on GAS WT and ΔGacI bacteria after incubation with a range of serum concentrations. Pooled data from 4 independent experiments are shown (mean ± SEM). See also Figure S4.
Serum, but not plasma, possesses high levels of bactericidal activity against Gram-positive bacteria due to the presence of platelet-derived antimicrobials that are released during the clotting process(Hirsch, 1960). We initially examined baby rabbit serum (BRS), which lacks pre-existing anti-GAS antibodies. The GAS GacI mutant was sensitive to killing in BRS, a difference that persisted upon heat inactivation of complement activity (Figure 5H). Similarly, the GacI mutant had reduced growth in human serum (Figure 5I), but WT and mutant strains survived equally well in human plasma (Figure 5J) and accumulated similar levels of surface C3b (Figure 5K). Serum but not plasma sensitivity suggested a role for platelets, important in host defense against GAS (Hirsch, 1960; Yeaman, 2010). As inferred, the GacI mutant was found to be hypersensitive to killing by the releasate of thrombin-activated washed human platelets (Figure 5I).
The GAC GlcNAcSide Chainis Required for Full GAS Virulence In Vivo
Given the increased susceptibility of the GacI mutant to whole blood, neutrophil, cathelicidin, and serum (platelet-derived antimicrobial) killing, we examined whether loss of the GlcNAc side chain affected GAS pathogenicity in vivo. In a rabbit model of pulmonary infection, no mortality was seen following challenge with the GacI mutant at a dose where the WT strain produced 89% mortality (Figure 6A). Gross and microscopic examination of the lungs of WT GAS-infected rabbits revealed extensive hemorrhagic necrosis amid diffuse bacterial and leukocytic infiltrates, changes that were markedly reduced in the lungs of surviving ΔGacI-infected animals (Figure 6B and Figure S5A). Assessed early in infection (12 h), WT GAS infection was associated with higher fever (Figure 6C), greater bacterial load in the lung (Figure 6D), and increased lung TNF-α levels (Figure 6E) compared to GacI mutant-infected rabbits. Likewise, the GacImutant produced significantly lower mortality in a mouse systemic infection model (Figure 6F), in association with lower bacterial counts in blood (Figure 6G) and serum TNF-α levels (Figure S5B). Thus, the GlcNAc side chain of the GAC is a virulence determinant increasing bacterial survival and resistance to host immune clearance in vivo.
FIGURE 6. Loss of the GAC GlcNAcSide Chain Attenuates GAS Virulence.
(A) Survival curve of rabbits infected with GAS WT or ΔGacI mutant bacteria. Rabbits were infected with 4 × 109 CFU intrabronchially and survival was monitored for seven days (n = 9 rabbits of either sex per group in three independent experiments; log-rank test). (B) Gross lung appearance and microscopic H&E stain of rabbit lungs after infection with GAS WT or ΔGacI mutant bacteria. (C) Body temperature, (D) lung bacterial counts, and (E) lung TNF-α levels in lungs of infected rabbits 12 hours after intrapulmonary challenge; n = 4 rabbits per group; t-test; mean and 95% confidence interval. (F) Survival curve of mice upon systemic infection with GAS WT or ΔGacI mutant bacteria; survival was monitored for 6 days (n = 11 per group; log-rank test). (G) Blood CFU of mice 24 hours post systemic infection (n = 10 mice per group; t-test; mean and 95% confidence interval). *p< 0.05, ***p< 0.001. See also Figure S5.
The GAC Polyrhamnose Backboneis a Vaccine Antigen Candidate
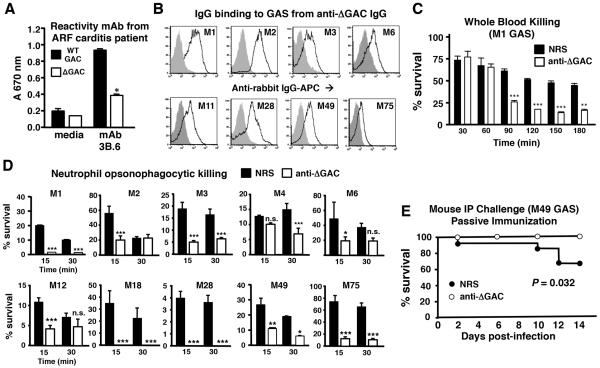
There is lack of consensus regarding the role of the GAC GlcNAc side chain in the immunopathogenesis of rheumatic fever. Using the GlcNAc-deficient GAC extracted from GacI mutant bacteria as a tool, we assessed recognition of this altered antigen by an autoreactive human monocolonal antibody (3B.6) derived from a patient with rheumatic carditis that binds to heart valve and myocardium, to GAS bacteria, and to GlcNAc-albumin, but not to albumin alone (Galvin et al., 2000). 3B.6 showed markedly reduced binding to the GacI mutant carbohydrate compared to the native GAC (Figure 7A), lending support to the hypothesized role of GACGlcNAc in the pathogenesis of rheumatic carditis. We considered whether the GlcNAc-deficient GAC (ΔGAC), as purified from the GacI mutant, could elicit an antibody response that facilitates opsonization and immune killing of diverse GAS serotypes. A conjugate vaccine antigen complex, consisting of a protein + GlcNAc-deficient GAC, was prepared using affinity interactions (Zhang et al., 2013) and then used to raise a polyclonal rabbit anti-ΔGAC antiserum. The final antiserum was highly reactive against the immunizing antigen, GacI mutant GAC (1:307,200, control non-immune serum 1:1,200), as well as the native GAC (1:307,200, control non-immune serum 1:1,200), indicating recognition was not shielded by presence of GlcNAc.
Figure 7. Antiserum Raised Against GlcNAc-Deficient GAC Promotes Opsonophagocytosis of Multiple GAS Serotypes.
(A) GlcNAc-specific human monoclonal antibody (mAb) 3B6 derived from a patient with rheumatic carditis(Galvin et al., 2000) shows significantly reduced cross-reactivity with GlcNAc-deficient GAC. *p< 0.05 (t-test; mean ± SEM). (B) Binding of polyclonal anti-ΔGAC IgG (black line) to WT GAS of 8 different disease-associated serotypes; grey fill = non-immune rabbit IgG control. Serotype is indicated in histogram. (C) Enhanced killing of WT M1 GAS in human whole blood upon addition of anti-ΔGAC rabbit antiserum versus normal rabbit serum (NRS). (D)Improved opsonophagocytic killing of multiple GAS serotypes by isolated human neutrophils upon addition of anti-ΔGAC antiserum versus NRS; mean ± SEM, t-test; *p< 0.05, **p< 0.01, ***p< 0.001, n.s not significant. (E)Mice are protected from infection with WT GAS M49 through passive immunization with ΔGAC antiserum vs. NRS (n = 12 per group; log-rank test). See also Figure S6.
Anti-ΔGACIgG efficiently bound to intact WT M1 GAS bacteria as well as GAS strains representing 7 additional common disease-associated serotypes (Figure 7B), and promoted bacterial killing by human whole blood (M1) and neutrophils(M1 + 8 additional GAS serotypes) (Figure 7C and 7D). Finally, as proof-of-concept, administration of the anti-ΔGAC antiserum provided passive protection in a murine systemic infection model with a heterologous(GAS M49) strain (Figure 7E). Efficacy of the anti-ΔGAC antiserum in promoting neutrophil opsonophagocytosis and passive immune protection compared favorably to an antiserum reactive against the WT GAC prepared through an identical procedure(Figure S6A - S6C). Neither rabbit post-immune antiserum demonstrated cross-reactivity to human cardiac antigens (Figure S6DandS6E) consistent with findings reported in post-immune mouse antisera against a WT GAC conjugate antigen(Sabharwal et al., 2006).
DISCUSSION
We have studied aspects of the biology of the hallmark GAC cell wall antigen of GAS through the identification and subsequent mutation of the genetic locus encoding its biosynthesis. An isogenic mutant GAS strain lacking the species-defining GlcNAc side chain had markedly reduced survival in human blood and systemic animal infection models, with increased sensitivity to neutrophil and serum killing identified as key elements underlying the virulence attenuation. Although phagocytic uptake, complement deposition, and killing of WT GAS and ΔGacI mutant were similar, the mutant was hyper-susceptible to human cathelicidin LL-37 and the antimicrobial action of factors released bythrombin-activated platelets. Loss of the GlcNAc side chain on GAC did not produce a general defect in GAS cell wall integrity, as the ΔGacI mutant did not differ from WT in susceptibility to autolysis, reactive oxygen species, lysozyme, nafcillin or vancomycin. GAS growth in bacteriologic media, tissue culture media, and plasma were unaffected by loss of the GlcNAc side chain, but the average chain length of the mutant strains was increased. Although several well characterized GAS virulence factors were unaltered (e.g. M1 protein, capsule, cysteine protease, surface plasmin acquisition), future studies may reveal additional mechanisms by which the GlcNAc side chain interacts with other bacterial surface components to influence the host-pathogen interaction. Elimination of a key structural feature of the most abundant GAS cell wall component no doubt changes the full context in which other GAS cell wall-associated are displayed, and potentially modulates their access to soluble factors, matrix components, or cellular receptors present in host tissues. Our studies were performed in an invasive GAS isolate representative of the globally disseminated serotype M1T1 clone, and the relative contribution of the GlcNAc side chain to virulence might vary dependent on the quorum of other innate immune resistance factors present in a given GAS serotype.
A variety of published clinical data, summarized in the introduction, has raised concern regarding the potential of the GAC GlcNAc side chain to provoke cross-reactive antibodies relevant to the immunopathogenesis of rheumatic fever. While anti-GlcNAc antibodies correlate with the presence and severity of rheumatic heart disease (Ayoub et al., 1974; Dudding and Ayoub, 1968; Shulman et al., 1974; Appleton et al. 1985), and anti-GlcNAc monoclonal antibodies cross-reactive for heart or brain tissue have been derived from patients with rheumatic fever (Galvin et al., 2000; Kirvan et al., 2003), these clinical associations have proven difficult to corroborate experimentally, since there is no faithful animal model of rheumatic fever, and cardiac cross-reactive antibodies are not readily elicited in experimental immunization of mice (Sabharwal et al., 2006) or rabbits (this study).
In 2004, the U.S. National Institute of Allergy and Infectious Diseases convened an expert workshop of scientists, clinicians, government agencies and the pharmaceutical to review the available data and to explore the microbiologic, immunologic, epidemiologic, and economic issues involved in development and implementation of a safe and effective GAS vaccine, a sentinel event toward the lifting of the 30-year Food and Drug Administration ban on GAS vaccine research set in place because of the suspected development of rheumatic fever in vaccine subjects in early trials. The report summarizing the deliberations of the workshop(Bisno et al., 2005)stated: “Molecular mimicry—sharing of antigenic determinants between the host and antigens of GAS—has been implicated in ARF and rheumatic heart disease and has represented a major obstacle for vaccine development. GAS antigens, including the M proteins and group A carbohydrate, have been shown to contain epitopes that mediate B and/or T cell cross-reactions with human tissue antigens. Because the precise role of molecular mimicry in the pathogenesis of ARF has not been established, every effort should be made to exclude tissue–cross-reactive epitopes during vaccine development”. Our proof-of-principle demonstration that antisera raised against the polyrhamnose core of GAC, as purified from the GacI mutant, may still provide significant broad-spectrum opsonophagocytic activity, is at minimum consistent with this encouragement.
In summary, we have demonstrated that the classical Lancefield antigen is not simply a structural component of the GAS cell wall but rather an important virulence determinant. In addition, the sidechain-deficient core backbone of the GAC, containing only the non-mammalian polyrhamnose structure and lacking the potentially autoimmune GlcNAc epitope, merits future exploration as a component of universal GAS vaccines.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Genetic Manipulations
Principal strains analyzed were GASM1T15448 (Kansal et al., 2000) and M49 NZ131 (Simon and Ferretti, 1991), and Streptococcus dysgalactiae subsp. equisimilis (SDSE) strains (2005-0193 and 2006-0098) reactive for GAC (McMillan et al., 2010). Other GAS serotype strains were obtained from the CDC Streptococcal Laboratory (B. Beall, Director). Insertional mutation of gacD-H, and gacJ-L was performed as described (Hollands et al., 2010) with osmotic protection in THB 0.5 M sucrose. Precise in-frame allelic replacement of the gacI gene was performed using established methodology(Pritzlaff et al., 2001). Genetic complementation of GacI with the gacI gene on multicopy plasmid vector pDCerm resulted in incomplete complementation suggesting perturbation of gene regulatory networks or improper stoichiometry of the enzymes involved GAC biosynthesis. Therefore, we performed genomic complementation with a ‘watermarked’ copy of gacI to allow discrimination from authentic WT cultures. Details of these techniques are provided in the Supplemental Material
Whole Blood Proliferation and Phagocytosis Assay
Bacteria were incubated with lepirudinanti coagulated blood from healthy donors in siliconized tubes at 37°C. For vaccine-related assays, rabbit anti-WT GAC serum, anti-ΔGAC serum or non-immune normal rabbit serum diluted 1:5 in PBS were added. Surviving CFU were quantified by dilution plating. Cytochalasin D (25μg/ml) was used to block phagocytosis. Whole blood phagocytosis was performed undershaking conditions with FITC-labeled bacteria and analyzed by flow cytometry.
Lectin Staining, M1 Protein Expression, and IgG Binding
Overnight cultures were centrifuged and resuspended in HEPES++buffer (20 mM HEPES, 140 mM NaCl, 5 mM CaCl2, 2.5 mM MgCl2, pH 7.4) + 0.1% bovine serumalbumin (BSA) (HEPES++0.1%BSA) to OD600=0.4. The bacterial suspension (100 μl) was pelleted and stained with FITC-labeled succinylated wheat germ agglutinin (sWGA) to assess GlcNAc expression. M1 protein expression was quantified on bacterial cultures at an OD600 = 0.6 using anti-M1 or sham mouse serum and PE-conjugated anti-mouse IgG. To test IgG binding, bacterial cultures were grown to OD600 of 0.4, washed, resuspended in buffer and pre-incubated with 10% heat-inactivated normal horse serum to block Fc binding proteins. After washing, samples were incubated with 0.1 mg/ml control rabbit IgG (Jackson Immunoresearch) or purified anti-ΔGAC rabbit IgG followed by allophycocyanin-conjugated goat anti-rabbit IgG. Staining was analyzed by flow cytometry.
Neutrophil Experiments
Human neutrophils were isolated from healthy donor blood using the Polymorphprep system (Axis-Shield). Neutrophils were seeded into 96-well flat-bottom tissue culture treated plates (Costar) in RPMI1640 (Invitrogen)+2%fetalbovineserum (FBS)heat-inactivated at 70°C. Bacteria were added at a multiplicity of infection (MOI) of 0.1, plates were centrifuged and incubated at 37°C with 5% CO2 for 30 min. Neutrophils were lysed and bacterial survival (vs. control wells lacking neutrophils) was determined by dilution plating. Extracellular neutrophil killing was assessed by pre-treating neutrophils with cytochalasin D (10 μg/ml). For opsonophagocytic assays, exponential phase bacteria were pre-incubated with anti-WTGAC rabbit serum, anti-ΔGACrabbit serum, or NRS diluted 1:5 in PBS and added to neutrophils at a multiplicity of infection of 0.1. NET quantification was determined by counting extracellular traps after staining with Sytox Orange. NET killing assays were performed as above with preincubation of neutrophils in 25nM phorbol12-myristate 13-acetate (PMA) for 3h to maximally induce NETs (Akong-Moore et al., 2012).
LL-37 Susceptibility
LL-37 minimum inhibitory concentrations (MICs) were determined by incubating log phase cultures in DMEM 10% THB with different concentrations of LL-37 for 24 h at 37°C. For kinetic analysis, GAS WT and ΔGacI mutant bacteria were grown and resuspended as for MIC assays, incubated with different concentrations of LL-37, and growth was recorded by measuring OD600 every 30 min for 20 h using the Bioscreen C MBR machine.
Serum, Plasma, and Platelet Survival Assays
Serum and/or plasma (lepirudin) were collected from healthy individuals and used immediately or stored at −80°C. Bacterial survival in 5% serum, plasma, or 25% platelet releasate was performed as described (Rooijakkers et al., 2010). Survival/growth was calculated as the ratio of bacteria CFU surviving after incubation compared to the initial inoculum.
GAC Purification and Carbohydrate Analysis
Cell pellets of bacterial strains were resuspended in cold 48% aqueous hydrogenfluoride (HF), sonicated, and stirred at 4°C for 48 h. Next, ice-cold H2O was added to each bacterial suspension and the material dialyzed against cold H2O. Dialyzed preparations were centrifuged to remove cellulardebris, and supernatant containing polysaccharide was lyophilized. Finally polysaccharide (HF-PS) was purified by size exclusion chromatography and positive fractions were pooled and lyophilized. Monosaccharide composition analysis was performed using gas chromatography/mass spectrometry as alditol acetate (AA) derivatives. Linkage analysis was performed on partially methylated alditol acetate (PMAA) derivatives.
In vivo Virulence
For the pulmonary infection model, rabbits (total n = 13 over four independent experiments, either sex; 8 male, 5 female) were infected with 4×109CFU of GAS WTor ΔGacI mutant bacteria intrabronchially, and survival monitored for 7 days (n=9 rabbits). Lungs from a fourth cohort were harvested after 12 h (n=4 rabbits; 2 male, 2 female) and homogenized for CFU counts and TNF-α levels(ELISA, R&D systems). For the mouse systemic infection model, log phase bacteria were resuspended in PBS 5% porcine gastric mucin. Female 10-week-old CD-1 mice were injected intraperitoneally (i.p.) with 2×106 CFU/200μl GASWT or ΔGacI mutant bacteria. Survival was monitored twice daily for 6 days. A second group of 10CD-1 female mice was similarly injected with GAS WT or ΔGacI and blood, serum and organs harvested at 24 h and homogenized for bacterial CFU counts.
Preparation of WT and ΔGAC Protein Conjugates and Rabbit Polyclonal Antisera
GAC purified from WT GAS (WT GAC) and the ΔGacI mutant (ΔGAC) were coupled to recombinant pneumococcal protein SP_0435 using streptavidin-biotin affinity interactions and the complexes purified by gelfiltration chromatography to >95% purity (Zhang et al., 2013). Polyclonal rabbit antibodies were raised against MAPS-conjugated WT GAC and GAC through Cocalico Biologicals (Reamstown, PA); see Supplemental Materials for details.
Mouse Passive Immunization and Challenge
Cohorts of female 10-week-old CD-1 mice (Charles River Labs) were immunized i.p. with 0.5ml anti-GAC rabbitserum(n=12) or normal rabbitserum (NRS;MP Biomedicals) (n=12) diluted 1:5 in PBS 3 h prior to infection with serotype M49 GAS strain NZ131. Log phase bacteria were resuspended in PBS plus5% porcinegastricmucin. Mice were challenged i.p. with WTM49 GAS (2.5×107CFU/200μl) and survival monitored twice daily for 10 days.
Ethics Statements
Human blood and neutrophils were collected after informed consent from healthy human volunteers as approved by the University of California San Diego (UCSD) Human Research Protection Program. Animal infection studies were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Protocols were approved by the Institutional Animal Care and Use Committees (IACUC)at the University of Minnesota (rabbits), University of Iowa (rabbits), and UCSD (mice). All efforts were made to minimize the suffering of animals employed in this study.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism version 5.0d (GraphPad Software Inc.),and display experimental data of at least two independent experiments performed in triplicate, displaying mean and standard error of mean (SEM) throughout the manuscript, except for animal-related experiments which display mean +/− 95% confidence intervals. The following statistical tests were applied: comparisons between three or more groups, one-way ANOVA; comparison between two groups, Student’s t-test; time course experiments, two-way ANOVA; animal survival curves, Log-rank test. Statistical significance was accepted at p< 0.05. *p< 0.05, **p< 0.01, ***p< 0.001.
Supplementary Material
HIGHLIGHTS.
The genetic locus for the Lancefield group A carbohydrate (GAC) is identified
The gacI gene is essential for expression of the signature GAC GlcNAc epitope
The GAC GlcNAc side chain is required for full GAS virulence in animal models
Antibodies against GlcNAc-deficient GAC protect against streptococcal challenge
ACKNOWLEDGEMENTS
This research was supported by NIH/NIAID grants to V.N., NHLBI-supported UCSD Program in Excellence in Glycosciences (V.N., B.C.), a Marie Curie International Incoming Fellowship and NIH T32 Training Grant to N.M.v.S, a CJ Martin Overseas Postdoctoral Training Fellowship514639 and Project Grant APP1033258 to J.N.C from the Australian National Health and Medical Research Council, the NIAID-supported Great Lakes Regional Center for Excellence in Biodefense and Emerging Diseases (AI57153) where P.M.S. and V.N. are members, and Collaborative Research Center (SFB) 765 to J.H. and B.L.. We thank David McMillan and Sri Sriprakash of the Queensland Institute for Medical Research for providing SDSE strains expressing GAC and Lingjun He, Department of Mathematics and Statistics, San Diego State University, for her statistical consultation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
N.M.v.S, J.N.C, K.K., A.H. A.K-F, L.L., E.T.M.B., F.Z., S.D, L.S., J.G., J.H., B.L., S.H.M.R., R.M., S.J.S., P.M.S, and V.N. participated in designing the experiments. N.M.v.S, J.N.C., K.K., S.D., L.L., L.S., and J.G. performed genetic manipulation of GAS and in vitro and in vivo assays. R.K.A. performed bioinformatics analysis of GAC, GCC loci. G.D., M.R.D., and M.J.W. collected, sequenced, and analyzed genome sequence data from hybrid GGAS strains. F.Z. and R.M. designed and constructed protein-polysaccharide complexes of mutant and WT GAC to raise rabbit antisera. M. C. provided monoclonal antibody from carditis patient and performed immunohistochemical analyses. P.M.S. and J.A.M. designed and performed rabbit infection experiments. E.T.M.B. and S.H.M.R designed and performed whole blood phagocytosis and complement assays. A.K-F and S.J.S. designed and participated in platelet experiments. J.H. and B.L. performed the surface plasmon resonance studies with LL-37 and purified native and mutant GAC. B.C. performed all the glycoanalysis. N.M.v.S. and V.N. wrote the manuscript and other authors provided comments.
REFERENCES
- Akong-Moore K, Chow OA, von Kockritz-Blickwede M, Nizet V. Influences of chloride and hypochlorite on neutrophil extracellular trap formation. PLoS One. 2012;7:e42984. doi: 10.1371/journal.pone.0042984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton RS, Victorica BE, Tamer D, Ayoub EM. Specificity of persistence of antibody to the streptococcal group A carbohydrate in rheumatic valvular heart disease. J Lab Clin Med. 1985;105:114–119. [PubMed] [Google Scholar]
- Ayoub EM, Taranta A, Bartley TD. Effect of valvular surgery on antibody to the group A streptococcal carbohydrate. Circulation. 1974;50:144–150. doi: 10.1161/01.cir.50.1.144. [DOI] [PubMed] [Google Scholar]
- Bisno AL, Rubin FA, Cleary PP, Dale JB. Prospects for a group A streptococcal vaccine: rationale, feasibility, and obstacles--report of a National Institute of Allergy and Infectious Diseases workshop. Clin Infect Dis. 2005;41:1150–1156. doi: 10.1086/444505. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Caliot E, Dramsi S, Chapot-Chartier MP, Courtin P, Kulakauskas S, Pechoux C, Trieu-Cuot P, Mistou MY. Role of the Group B antigen of Streptococcus agalactiae: a peptidoglycan-anchored polysaccharide involved in cell wall biogenesis. PLoS Pathog. 2012;8:e1002756. doi: 10.1371/journal.ppat.1002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J, Singh AK, Santa Maria JP, Jr., Kim Y, Brown S, Swoboda JG, Mylonakis E, Wilkinson BJ, Walker S. Synthetic lethal compound combinations reveal a fundamental connection between wall teichoic acid and peptidoglycan biosyntheses in Staphylococcus aureus. ACS Chem Biol. 2011;6:106–116. doi: 10.1021/cb100269f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- Clarke SR, Mohamed R, Bian L, Routh AF, Kokai-Kun JF, Mond JJ, Tarkowski A, Foster SJ. The Staphylococcus aureus surface protein IsdA mediates resistance to innate defenses of human skin. Cell Host Microbe. 2007;1:199–212. doi: 10.1016/j.chom.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Dudding BA, Ayoub EM. Persistence of streptococcal group A antibody in patients with rheumatic valvular disease. J Exp Med. 1968;128:1081–1098. doi: 10.1084/jem.128.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fae KC, Oshiro SE, Toubert A, Charron D, Kalil J, Guilherme L. How an autoimmune reaction triggered by molecular mimicry between streptococcal M protein and cardiac tissue proteins leads to heart lesions in rheumatic heart disease. J Autoimmun. 2005;24:101–109. doi: 10.1016/j.jaut.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Hemric ME, Ward K, Cunningham MW. Cytotoxic mAb from rheumatic carditis recognizes heart valves and laminin. J Clin Invest. 2000;106:217–224. doi: 10.1172/JCI7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I, Rebeyrotte P, Parlebas J, Halpern B. Isolation from heart valves of glycopeptides which share immunological properties with Streptococcus haemolyticus group A polysaccharides. Nature. 1968;219:866–868. doi: 10.1038/219866a0. [DOI] [PubMed] [Google Scholar]
- Hirsch JG. Comparative bactericidal activities of blood serum and plasma serum. J Exp Med. 1960;112:15–22. doi: 10.1084/jem.112.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollands A, Pence MA, Timmer AM, Osvath SR, Turnbull L, Whitchurch CB, Walker MJ, Nizet V. Genetic switch to hypervirulence reduces colonization phenotypes of the globally disseminated group A Streptococcus M1T1 clone. J Infect Dis. 2010;202:11–19. doi: 10.1086/653124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabanova A, Margarit I, Berti F, Romano MR, Grandi G, Bensi G, Chiarot E, Proietti D, Swennen E, Cappelletti E, et al. Evaluation of a group A Streptococcus synthetic oligosaccharide as vaccine candidate. Vaccine. 2010;29:104–114. doi: 10.1016/j.vaccine.2010.09.018. [DOI] [PubMed] [Google Scholar]
- Kansal RG, McGeer A, Low DE, Norrby-Teglund A, Kotb M. Inverse relation between disease severity and expression of the streptococcal cysteine protease, SpeB, among clonal M1T1 isolates recovered from invasive group A streptococcal infection cases. Infect Immun. 2000;68:6362–6369. doi: 10.1128/iai.68.11.6362-6369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall FE, Heidelberger M, Dawson MH. A serologically inactive polysaccharide elaborated by mucoid strains of group A hemolytic Streptococcus. J Biol Chem. 1937;118:61–69. [Google Scholar]
- Kirvan CA, Swedo SE, Heuser JS, Cunningham MW. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat Med. 2003;9:914–920. doi: 10.1038/nm892. [DOI] [PubMed] [Google Scholar]
- Lancefield RC. The antigenic complex of Streptococcus haemolyticus: I. Demonstration of a type-specific substance in extracts of Streptococcus haemolyticus. J Exp Med. 1928;47:91–103. doi: 10.1084/jem.47.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marijon E, Mirabel M, Celermajer DS, Jouven X. Rheumatic heart disease. Lancet. 2012;379:953–964. doi: 10.1016/S0140-6736(11)61171-9. [DOI] [PubMed] [Google Scholar]
- McCarty M. The lysis of group A hemolytic streptococci by extracellular enzymes of Streptomyces albus. II. Nature of the cellular substrate attacked by the lytic enzymes. J Exp Med. 1952;96:569–580. doi: 10.1084/jem.96.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty M. Variation in the group-specific carbohydrate of group A streptococci. II. Studies on the chemical basis for serological specificity of the carbohydrates. J Exp Med. 1956;104:629–643. doi: 10.1084/jem.104.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty M, Lancefield RC. Variation in the group-specific carbohydrate of group A streptococci. I. Immunochemical studies on the carbohydrates of variant strains. J Exp Med. 1955;102:11–28. doi: 10.1084/jem.102.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan DJ, Dreze PA, Vu T, Bessen DE, Guglielmini J, Steer AC, Carapetis JR, Van Melderen L, Sriprakash KS, Smeesters PR. Updated model of group A Streptococcus M proteins based on a comprehensive worldwide study. Clin Microbiol Infect. 2013;19:E222–229. doi: 10.1111/1469-0691.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan DJ, Vu T, Bramhachari PV, Kaul SY, Bouvet A, Shaila MS, Karmarkar MG, Sriprakash KS. Molecular markers for discriminating Streptococcus pyogenes and S. dysgalactiae subspecies equisimilis. Eur J Clin Microbiol Infect Dis. 2010;29:585–589. doi: 10.1007/s10096-010-0899-x. [DOI] [PubMed] [Google Scholar]
- Pandey M, Batzloff MR, Good MF. Vaccination against rheumatic heart disease: a review of current research strategies and challenges. Curr Infect Dis Rep. 2012;14:381–390. doi: 10.1007/s11908-012-0263-7. [DOI] [PubMed] [Google Scholar]
- Pritzlaff CA, Chang JC, Kuo SP, Tamura GS, Rubens CE, Nizet V. Genetic basis for the beta-haemolytic/cytolytic activity of group B Streptococcus. Mol Microbiol. 2001;39:236–247. doi: 10.1046/j.1365-2958.2001.02211.x. [DOI] [PubMed] [Google Scholar]
- Rooijakkers SH, Rasmussen SL, McGillivray SM, Bartnikas TB, Mason AB, Friedlander AM, Nizet V. Human transferrin confers serum resistance against Bacillus anthracis. J Biol Chem. 2010;285:27609–27613. doi: 10.1074/jbc.M110.154930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabharwal H, Michon F, Nelson D, Dong W, Fuchs K, Manjarrez RC, Sarkar A, Uitz C, Viteri-Jackson A, Suarez RS, et al. Group A Streptococcus (GAS) carbohydrate as an immunogen for protection against GAS infection. J Infect Dis. 2006;193:129–135. doi: 10.1086/498618. [DOI] [PubMed] [Google Scholar]
- Shulman ST, Ayoub EM, Victorica BE, Gessner IH, Tamer DF, Hernandez FA. Differences in antibody response to streptococcal antigens in children with rheumatic and non-rheumatic mitral valve disease. Circulation. 1974;50:1244–1251. doi: 10.1161/01.cir.50.6.1244. [DOI] [PubMed] [Google Scholar]
- Simon D, Ferretti JJ. Electrotransformation of Streptococcus pyogenes with plasmid and linear DNA. FEMS Microbiol Lett. 1991;66:219–224. doi: 10.1016/0378-1097(91)90336-9. [DOI] [PubMed] [Google Scholar]
- Sutcliffe IC, Black GW, Harrington DJ. Bioinformatic insights into the biosynthesis of the Group B carbohydrate in Streptococcus agalactiae. Microbiology. 2008;154:1354–1363. doi: 10.1099/mic.0.2007/014522-0. [DOI] [PubMed] [Google Scholar]
- Swanson J, Gotschlich EC. Electron microscopic studies on streptococci. II. Group A carbohydrate. J Exp Med. 1973;138:245–258. doi: 10.1084/jem.138.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka D, Isobe J, Watahiki M, Nagai Y, Katsukawa C, Kawahara R, Endoh M, Okuno R, Kumagai N, Matsumoto M, et al. Genetic features of clinical isolates of Streptococcus dysgalactiae subsp. equisimilis possessing Lancefield’s group A antigen. J Clin Microbiol. 2008;46:1526–1529. doi: 10.1128/JCM.02188-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AT. Loss of group carbohydrate during mouse passages of a group A hemolytic Streptococcus. J Exp Med. 1945;81:593–596. doi: 10.1084/jem.81.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman MR. Platelets in defense against bacterial pathogens. CellMolLifeSci. 2010;67:525–544. doi: 10.1007/s00018-009-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Lu YJ, Malley R. Multiple antigen-presenting system (MAPS) to induce comprehensive B- and T-cell immunity. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1307228110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman RA, Auernheimer AH, Taranta A. Precipitating antibody to group A streptococcal polysaccharide in humans. J Immunol. 1971;107:832–841. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.