Abstract
Calcium signals regulate many critical processes during vertebrate brain development including neurogenesis, neurotransmitter specification, and axonal outgrowth. However, the identity of the ion channels mediating Ca2+ signaling in the developing nervous system is not well defined. Here, we report that embryonic and adult mouse neural stem/progenitor cells (NSCs/NPCs) exhibit store-operated Ca2+ entry (SOCE) mediated by Ca2+ release-activated Ca2+ (CRAC) channels. SOCE in NPCs was blocked by the CRAC channel inhibitors La3+, BTP2, and 2-APB and Western blots revealed the presence of the canonical CRAC channel proteins STIM1 and Orai1. Knock down of STIM1 or Orai1 significantly diminished SOCE in NPCs, and SOCE was lost in NPCs from transgenic mice lacking Orai1 or STIM1 and in knock-in mice expressing the loss-of-function Orai1 mutant, R93W. Therefore, STIM1 and Orai1 make essential contributions to SOCE in NPCs. SOCE in NPCs was activated by epidermal growth factor and acetylcholine, the latter occurring through muscarinic receptors. Activation of SOCE stimulated gene transcription through calcineurin/NFAT (nuclear factor of activated T cells) signaling through a mechanism consistent with local Ca2+ signaling by Ca2+ microdomains near CRAC channels. Importantly, suppression or deletion of STIM1 and Orai1 expression significantly attenuated proliferation of embryonic and adult NPCs cultured as neurospheres and, in vivo, in the subventricular zone of adult mice. These findings show that CRAC channels serve as a major route of Ca2+ entry in NPCs and regulate key effector functions including gene expression and proliferation, indicating that CRAC channels are important regulators of mammalian neurogenesis.
Keywords: calcium, CRAC channels, Orai1, progenitor cell, proliferation, STIM1
Introduction
Calcium is a ubiquitous second messenger that regulates numerous developmental events in the brain, including neural induction (Leclerc et al., 2000), neurotransmitter specification (Spitzer et al., 2004), growth cone motility (Zheng and Poo, 2007), and neuronal maturation (Nakanishi and Okazawa, 2006). Both spontaneous and agonist-induced Ca2+ signals occur in neural stem/progenitor cells (NSCs/NPCs) and the temporal and spatial patterns of these Ca2+ signals are widely presumed to control the types of downstream genetic programs that are activated (Gu and Spitzer, 1995; Owens and Kriegstein, 1998; Maric et al., 2003; Weissman et al., 2004; Belmadani et al., 2005; Fiorio Pla et al., 2005; Mishra et al., 2006; Lin et al., 2007; Platel et al., 2008). However, the functional architecture of the NSC/NPC Ca2+ signaling network and, in particular, the identity and organization of ion channels mediating the Ca2+ signals is not well understood. Some studies have implicated voltage-gated Ca2+ channels as an important route of Ca2+ entry in late stage neural precursors (Maric et al., 2000; D'Ascenzo et al., 2006). However, because NPCs are of neuroectodermal lineage and are largely nonexcitable, it is likely that these cells also use alternate Ca2+ influx pathways of unknown identity.
In many nonexcitable and some excitable cells, store-operated Ca2+ entry (SOCE) is a major mechanism for Ca2+ mobilization (Hogan et al., 2010). Store-operated channels (SOCs) open in response to depletion of Ca2+ in the endoplasmic reticulum (ER) after stimulation of cell surface receptors coupled to G-proteins and tyrosine kinases (Lewis, 2011). The best-described SOCs are the Ca2+ release-activated Ca2+ (CRAC) channels, consisting of the pore-forming Orai proteins that are activated by the ER Ca2+ sensors STIM1 and STIM2 (Prakriya, 2009). CRAC channel activation occurs through a mechanism by which STIM1 translocates from the bulk ER to junctional ER sites and binds directly to Orai1, resulting in accumulation of these two proteins in opposite membranes (Lewis, 2011).
Historically, physiological studies of CRAC channels focused on hematopoetic cells such as T cells and mast cells, a consequence of the discovery of CRAC currents in these cells (Lewis, 2011). However, recent studies also implicate SOCE in modulating several neuronal functions, including neurotransmitter release (Emptage et al., 2001), synaptic plasticity (Baba et al., 2003), Ca2+ oscillations (Singaravelu et al., 2008), and firing of flight motorneurons in Drosophila (Venkiteswaran and Hasan, 2009). Moreover, aberrant SOCE has been implicated in hypoxia-mediated neuronal death (Berna-Erro et al., 2009), epilepsy (Steinbeck et al., 2011), and the response to axonal injury (Gemes et al., 2011). Nevertheless, the molecular mechanisms of SOCE during early neural development and its physiological functions remain poorly understood. In this study, we show that store-operated CRAC channels encoded by Orai1 and STIM1 are a major mechanism for Ca2+ entry in mouse subventricular zone NPCs. We also found that CRAC channels regulate calcineurin/nuclear factor of activated T cells (NFAT)-mediated gene expression and regulate the proliferation of NPCs. These findings establish CRAC channels as a novel mechanism for regulation of Ca2+ signaling, gene expression, and proliferation in NPCs.
Materials and Methods
Cells and transgenic mice.
C57BL/6 mice were cared for in accordance with the Guide for the Care and Use of Laboratory Animals. Animals were pair-housed in a sterile ventilated facility. All research protocols were approved by the Northwestern University Institutional Animal Care and Use Committee. Primary NPCs were isolated from mice of various ages, as indicated in the figures. The first day of the presence of a plug was counted as embryonic day 0 (E0) of gestation. Neurospheres were cultured from the ganglionic eminence (embryonic and neonatal mice) or the anterior subventricular zone (adult mice). Individual cells were dissociated from the tissues of interest and grown at 50,000 cells/ml in DMEM:F12 (1:1) growth medium supplemented with, B-27, N-2, 2 mm l-glutamine, 2 μg/ml heparin, and either 10 ng/ml recombinant human bFGF (<E15 NPCs), 20 ng/ml recombinant human EGF (>E15 NPCs), or 10 ng/ml each of bFGF and EGF (E15 and adult NPCs). After 4–5 d in culture, primary neurospheres were dissociated with 0.05% trypsin for 2 min at 37°C, followed by trypsin inhibitor (Sigma-Aldrich) and seeded again. Secondary neurospheres generated from these cells were used for our studies. Orai1R93W/+ and STIM1fl/+:CMV-Cre mice have been described previously (Oh-Hora et al., 2008; Bergmeier et al., 2009). STIM1fl/+:CMV-Cre mice were bred with STIM1fl/+ mice to obtain STIM1fl/− mice. CMV-Cre was bred out.
Tissue-specific deletion of Orai1 in the brain was achieved by crossing Orai1fl/fl mice (Amgen) with nestin-Cre mice (The Jackson Laboratory). To generate the Orai1fl/fl mice, genomic DNA containing Orai1 was retrieved from a BAC clone. The overall scheme is detailed in Figure 3. The first loxP site was inserted into intron 1 at ∼1 kb upstream of exon 2 and the second loxP site, along with the Frt-PGKneo-Frt cassette, was inserted ∼0.3 kb 3′ of exon 3 downstream of the polyA signal sequence and 3′ UTR. The targeting vector was linearized by NotI digestion, purified, and electroporated into C57BL/6N ES cells. Cells that integrated the targeting vector were selected with 200 mg/ml G418 and selected clones were screened by PCR. Four potential clones were expanded and screened by Southern blot for the 5′-, 3′-, and Neo-containing portions of the vector. Two of the four clones were confirmed by Southern blot to contain the targeted vector. Flp electroporation was performed for both clones to delete the Neo cassette. Six Neo-deleted clones were identified after selection and confirmed upon expansion by PCR analysis using the following primers: Neo DelF 5′-CAGCGTGCATAATATACCTAACTCTACCCG-3′ Neo DelR 5′-GTATTGATGAGGAGAGCAAGCGTGAATC-3′). The presence of the 3′ Lox site in these clones was also confirmed by PCR (LoxPF 5′-GAAATGGCTCGGGGACAAAACACTA-3′ LoxPR 5′-GCCATTTCTGGTCTTCTGGAGACTCTG-3′). Blastocyst injection was performed with two of the Neo-deleted clones to generate male chimeras that were subsequently bred to wild-type (WT) C57BL/6N females to produce heterozygous animals suitable for expansion of the line and breeding to homozygosity. Genotyping was performed by PCR using the NeoDel primers. WT animals generate a single band of ∼0.22 kb and loxP animals produce a band of ∼0.36 kb using these primers.
Figure 3.
Orai1 is critical for SOCE in adult NPCs. A, Schematic of the targeting strategy for conditional deletion of Orai1. The Orai1-targeting construct inserted an FRT2-PGK neocassette flanked by LoxP sites around exons 2 and 3 into the WT locus. The genomic organization of that insertion is shown as the recombinant allele. PGKNeo was removed through Flp electroporation of ES cells verified for the insertion by PCR and Southern blot and is shown as the final floxed allele after recombination. Deletion of exons 2 and 3 removes the entire coding region of Orai1. B, PCR of genomic DNA isolated from various tissues in Orai1+/+:Nes-Cre, Orai1fl/+:Nes-Cre, and Orai1fl/fl:Nes-Cre mice showing deletion of Orai1 in brain tissue (top). Bottom, Deletion of Orai1 specifically in brain tissue in the Orai1fl/fl:Nes-Cre mouse. OB, Olfactory bulb; CTX, cortex; HC, hippocampus; Panc, pancreas (20-week-old mice representative of 2 PCR experiments from 2 sets of mice). C, SOCE is significantly diminished in adult (24-week-old) Orai1fl/fl:Nes-Cre NPCs. Ca2+ influx rates (D) and [Ca2+]i (E) are strongly attenuated after re-addition of external Ca2+ (12- to 22-week-old mice; ***p < 0.001, unpaired t test, n = 122–237 cells, 3–8 experiments).
Interestingly, in addition to conditional deletion of Orai1 in the brain, the Orai1fl/fl × nestin-Cre cross also produced germline transmission in a few instances, yielding Orai1fl/− genotypes. Orai1fl/− mice were then intercrossed to obtain Orai1−/− germline-knock-out (KO) embryos. Deletion was verified using the following primers: 5′-GGG ACA AAA CAC TAA CCT GTC AT-3′, 5′-GGA GTA GAA TTC AGT GGG AGA GT-3′, and 5′-TAT GGT AAG GCT GGG AGA CAC-3′. The expected size of the PCR products are as follows: WT ∼131 bp, floxed ∼257 bp, and KO ∼207 bp.
Solutions and chemicals.
The standard Ringer's solution used for Ca2+-imaging studies contained the following (in mm): 155 NaCl, 4.5 KCl, 10 d-glucose, 5 HEPES, 1 MgCl2 and 2 CaCl2. The Ca2+-free Ringer's solution contained 3 mm MgCl2, 1 mm EGTA (Sigma-Aldrich), and no added CaCl2. pH was adjusted to 7.4 with 1.0 n NaOH. Stock solutions of thapsigargin, nifedipine, 2-aminoethyldiphenylborate, ionomycin, phorbol 12,13-dibutyrate (PDBu), cyclosporine A, tetrodotoxin (all Sigma-Aldrich) and YM-58483 (BTP-2; Calbiochem) were dissolved in DMSO and used at the indicated concentrations. SKF96365, muscarine chloride, methyllycaconitine citrate, and dihydro-β-erythroidine (all Sigma-Aldrich) were dissolved in water. EGTA-AM and BAPTA-AM were obtained from Invitrogen.
Plasmids and nucleofection.
NPCs were nucleofected using the Amaxa nucleofection kit (Lonza) according to the manufacturer's instructions. Dissociated neurospheres were grown for 12–16 h before nucleofection. The E106A Orai1-YFP plasmid has been described previously (Navarro-Borelly et al., 2008). GFP-NFAT1 was a kind gift from Anjana Rao (Harvard University). The pGL3-NFAT-Luc and pRL-Tk-Luc vectors were obtained from Richard Lewis (Stanford University). siSTIM1 and siOrai1 were purchased from Ambion. Experiments were performed 24–48 h after nucleofection.
Calcium imaging.
Dissociated neurospheres were plated onto poly-d-lysine-coated glass coverslips in growth medium containing low bFGF (0.5 ng/ml) or low EGF (1.0 ng/ml). Cells were incubated with 2 μm fura-2-AM (Invitrogen) in growth medium for 15 min at 37°C. Excess fura-2 was washed off and cells were incubated for an additional 15 min at 37°C before imaging. Single-cell Ca2+ measurements were performed within 4–10 h of plating to prevent confounding effects due to differentiation of NPCs. Ca2+ imaging was performed as described previously (McNally et al., 2012). All experiments were performed at room temperature. TTX (0.25 μm) was added to Ringer's solutions to prevent any depolarization-induced Ca2+ influx. [Ca2+]i was estimated using the standard equation (Grynkiewicz et al., 1985) as follows:
where R is the F340/F380 fluorescence ratio and values of Rmin and Rmax were estimated from an in situ calibration of fura-2. Rmin and Rmax were determined from the fluorescence ratios with solutions containing 1 mm EGTA or 20 mm Ca2+ Ringer's solutions together with 2 μm ionomycin, respectively. Q was determined from the Fmin/Fmax ratio at 380 nm and Kd is the apparent dissociation constant of fura-2 binding to Ca2+ (135 nm). The values of these parameters were as follows: Rmin = 0.32 ± 0.04, Rmax = 3.09 ± 0.13, and Q = 5 ± 0.32.
Western blots.
Neurospheres were washed with cold PBS and lysed in buffer containing 150 mm NaCl, 50 mm Tris, 1% Triton X-100, 0.1% SDS, and 1× protease inhibitor mixture (Sigma-Aldrich) by incubating on ice for 15 min. Lysates were centrifuged at 4°C for 30 min and supernatants were stored at −80°C. Isolated protein samples were heated to 65°C in Laemmli sample buffer (Bio-Rad) containing 5% β-mercaptoethanol and run on 10% SDS-polyacrylamide gel. Protein was transferred onto nitrocellulose membrane and Orai1 and STIM1 were detected using purified polyclonal primary antibodies (Gwack et al., 2008; Oh-Hora et al., 2008) and peroxidase-labeled secondary antibodies. Nestin antibody was purchased from BD PharMingen. Western blot images were analyzed using the image analysis software ImageJ.
NFAT-luciferase assays.
NFAT activity in NPCs was assayed using luciferase activity of an NFAT-dependent luciferase construct, pGL3-NFAT (Dolmetsch et al., 1998). The NFAT-driven firefly luciferase activity was normalized to the constitutively active renilla luciferase (pRL-Tk-Luc) activity to correct for variations in cell density. NPCs were nucleofected with the pGL3-NFAT-luc and pRL-Tk-Luc vectors at a ratio of 3:1 24–48 h before experiments. Cells were treated with CRAC channel inhibitors for ∼3 h, followed by stimulation with 0.5 μm thapsigargin (TG) and 100 nm PDBu for 6–13 h; 0.5 μm TTX and 5 μm nifedipine were present throughout to prevent Ca2+ influx through voltage-gated Ca2+ channels. Cells were lysed and luciferase activity was measured using the Dual Luciferase Reporter Assay kit (Promega) and a single tube luminometer (Berthold Instruments).
Immunostaining.
Dissociated NPCs were plated on coverslips and fixed 16 h after plating with 4% PFA in PBS for 15 min at 4°C. Cells were washed with PBS, permeabilized using 0.1% Triton X-100 for 30 min, followed by blocking in 1% bovine serum albumin in PBS for 1 h. Cells were incubated with primary antibodies at 1:500 for nestin (BD PharMingen), 1:500 for Orai1 (mAb 266.1; Amgen), 1:1000 for GFAP (Sigma-Aldrich), and 1:500 for doublecortin (Abcam) for 1 h, followed by secondary antibody tagged to Alexa Fluor 488 or Alexa Fluor 594 (Invitrogen) at 1:500 or 1:1000 for 1 h. Nuclei were labeled with DAPI.
Proliferation assays.
In vitro NPC proliferation was assessed using the Click-iT 5-ethynyl-2′-deoxyuridine (EdU) proliferation kit (Invitrogen). Briefly, secondary neurospheres were treated with EdU for ∼3 h, followed by dissociation, fixation, and staining for EdU using an Alexa Fluor 488 conjugate according to manufacturer's instructions (Morte et al., 2013). DNA was labeled using 7-amino-actinomycin D (7-AAD). Flow analysis was performed on an LSR Fortessa Analyzer (BD). Alexa Fluor 488 and 7-AAD were excited at 488 and 552 nm, respectively, and emission signals were collected using 530 and 670 nm band-pass filters, respectively. At least 20,000 cells were collected for each sample. Analysis was restricted to single cells; doublet discrimination was achieved using area and width signals in the 7-AAD channels or using area and height signals in the forward scatter. MTT proliferation assays were performed using the MTT assay kit (Promega). Cells were plated at a density of 5000 cells per well of a 96-well plate; 15 μl of MTT reagent was added to each well at various time points after plating. The solubilization buffer was added 3 h later and absorbance was measured at 595 nm.
In vivo bromodeoxyuridine (BrdU) incorporation assays in tissue slices were performed on 4- to 6-week-old mice. Mice were injected intraperitoneally with a single dose of 50 mg/kg BrdU dissolved in saline and killed 24 h later after anesthetization with isofluorane. Anesthetized mice were perfused intracardially with saline and then 4% PFA in PBS and decapitated. Brains were postfixed for 24 h in 4% PFA, cryoprotected for 24 h in 30% sucrose at 4°C, embedded in optimal cutting temperature medium, and cryosectioned at 20 μm thickness. Immunolabeling for BrdU was performed on every third section containing the anterior SVZ. Sections were incubated in sheep anti-BrdU (Fitzgerald Laboratories) at a 1:500 dilution overnight at 4°C, followed by biotinylated donkey anti-sheep (Jackson Laboratories) at 1:250 for 2 h at room temperature (RT) and Alexa Fluor 546-conjugated streptavidin (Invitrogen) at 1:100 for 45 min at RT. Nuclei were stained using DAPI. Immunolabeled sections were imaged using an Olympus FV10i confocal microscope at a magnification of 60× and image thickness of 1 μm. BrdU+ cells were counted in each section and averaged per section.
Apoptosis assays.
The fraction of apoptotic cells in cultured NPCs was estimated using a kit (Invitrogen) that measures the differential permeability of the DNA dyes 7-AAD and PO-PRO-1 (Benzoxazolium, 3-methyl-2-[[1-[3-(trimethylammonio)propyl]-4(1H)-pyridinylidene]methyl]-, diiodide) to live, dead, and apoptotic cells. Dissociated neurospheres were washed with 1× cold PBS, incubated with 2.5 μm PO-PRO-1 and 1 μg/ml 7-AAD for 30 min on ice, and analyzed by flow cytometry. The detection of apoptotic cells in brain sections was estimated by terminal deoxynucleotidyl transferase (TUNEL) staining, which fluorescently labels DNA fragmentation. Briefly, sections were permeabilized using 0.1% sodium citrate/ 0.1% Triton for 2 min at 4°C, and then washed with 1× PBS. Positive control sections were treated with DNaseI (at 500 U/ml) for 10 min at room temperature. All sections were treated with a mixture of TUNEL enzyme and fluorescein-labeled nucleotides except for the negative control, which was treated with a mixture lacking enzyme. The reaction was performed at 1 h at 37°C. Sections were washed in 1× PBS and mounted with Vectashield mounting medium containing DAPI. Images were taken on the FV10i Olympus confocal microscope.
Data analysis.
All data are expressed as mean ± SEM. Statistical analysis was performed using either a two-tailed t test (paired or unpaired as indicated) for comparison between the control and test groups or ANOVA (one- or two-factor as indicated in the figure legends) at 5% confidence interval, followed by post hoc two-tailed paired t tests to compare between conditions. The data were assumed to be parametric. Results with p ≤ 0.05 were considered significant.
Results
Many types of Ca2+ signals have been described in the immature nervous system (Gu and Spitzer, 1995; Owens and Kriegstein, 1998; Weissman et al., 2004); however, the identity of the Ca2+ channels that generate these signals is not well understood. Here, we investigated the role of store-operated CRAC channels for Ca2+ signaling and effector function in NPCs. For these studies, we cultured NPCs and analyzed brain slices from C57BL/6 mice from various developmental ages. The contribution of CRAC channels for SOCE and effector function was examined through pharmacological and genetic approaches using transgenic mice lacking CRAC channel proteins or expressing a nonfunctional CRAC channel, R93W Orai1 (Bergmeier et al., 2009).
Mouse NPCs exhibit SOCE
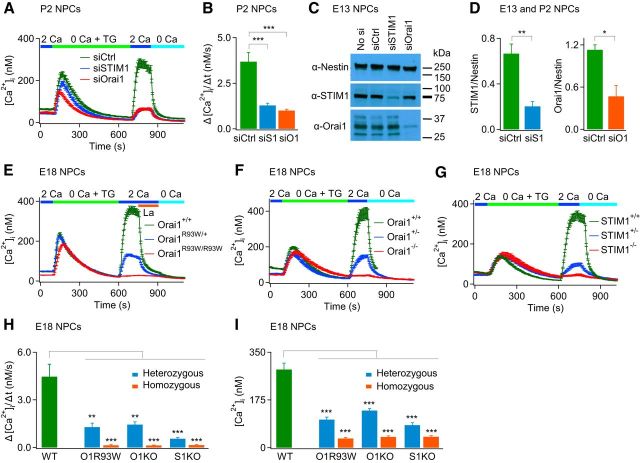
SOCs are activated by reduction in the ER Ca2+ concentration (Lewis, 2011). To determine whether NPCs exhibit SOCE, we depleted ER stores using 1 μm TG in a Ca2+-free Ringer's solution and studied Ca2+ influx after re-addition of extracellular Ca2+ (Fig. 1). This experiment revealed prominent SOCE in NPCs after re-addition of external Ca2+ (Fig. 1B). We found that SOCE was present in NPCs derived from embryonic (E13–E18), neonatal (P0-P2, P5), and adult mice (Figs. 1, 2, 3 and data not shown). Moreover, the amplitude of SOCE was not different between embryonic and adult NPCs (cf. Figs. 2H,I, 3D,E), indicating that this Ca2+ entry mechanism is conserved throughout development and is not unique to NPCs from mice of a particular age.
Figure 1.
Mouse NPCs exhibit SOCE with pharmacological properties consistent with CRAC channels. A, Depletion of ER Ca2+ stores evokes La3+-sensitive SOCE. ER Ca2+ stores were depleted with 1 μm TG in a Ca2+-free Ringer's solution and extracellular Ca2+ was re-added to evoke SOCE. SOCE is blocked by 2 μm LaCl3 (E18; ***p < 0.001 by 2-tailed unpaired t test; n = 80 cells; representative of experiments from 4 cultures of NPCs cultured from E18 embryos). B, The CRAC channel modulator 2-APB (10 μm) produces dual effects on SOCE in NPCs. Administration of 2-APB caused transient potentiation, followed by inhibition, as described previously for CRAC channels (Prakriya and Lewis, 2001; E13, **p < 0.01, 2-tailed unpaired t test; n = 14 cells, representative of 3 cultures of E13 NPCs). C, The CRAC channel inhibitor BTP-2 attenuated SOCE in NPCs. NPCs were pretreated with BTP-2 (1 μm) for 5 h before Ca2+ imaging (P2; ***p < 0.001 2-sample unpaired t test, n = 23–29 cells, representative of 2 cultures of P2 NPCs).
Figure 2.
STIM1 and Orai1 are essential for SOCE in embryonic and neonatal NPCs. A, B, SOCE is significantly diminished in NPCs treated with siSTIM1 and siOrai1 (P2; *p < 0.0001 determined by one-way ANOVA followed by 2-sample unpaired t test, n = 35–82 cells, representative of 3 cultures). C, Western blots showing expression of STIM1 and Orai1 in E13 NPCs and after knock down of these proteins by treatment with siRNA. Nestin was used as the loading control. D, Summary graphs showing relative expression of STIM1 and Orai1 in NPCs after suppression of these molecules (E13 and P2; **p < 0.01, *p < 0.05, unpaired t test, n = 3 blots from 2 cultures). E–G, SOCE is abolished in NPCs derived from homozygous Orai1R93W/R93W, Orai1−/−, and STIM1−/− mice and significantly diminished in heterozygous Orai1R93W/+, Orai1+/−, and STIM1−/− NPCs. H, Summary of the rate of Ca2+ entry after re-addition of extracellular Ca2+ in the indicated transgenic mice. Ca2+ influx rates were estimated by measuring the initial slope of Ca2+ entry over 30 s (E18; p < 0.0001, one-way ANOVA followed by post hoc 2-sample unpaired t tests between WT and mutant pairs, ***p < 0.001, **p < 0.01, average data from 77–852 cells from 4–13 experiments, from 2–11 mice). I, Summary of the average [Ca2+]i measured 120 s after addition of extracellular Ca2+ (E18; p-values determined as in H; [Ca2+]i was averaged over a 30 s duration).
The best-described SOCs are CRAC channels, which are distinguished by high Ca2+ selectivity and a unique pharmacological profile (Lewis, 2011; McNally and Prakriya, 2012; Jairaman and Prakriya, 2013). We found that SOCEs in NPCs shared many pharmacological hallmarks of CRAC channels, including blockade by a low concentration (2 μm) of La3+ (Fig. 1A) and modulation by the compound 2-aminoethyldiphenylborate (2-APB). In the latter case, a low dose of 2-APB (5 μm) potentiated influx (data not shown), whereas higher doses of 10 μm or above caused transient potentiation followed by inhibition (Fig. 1B), as described previously for CRAC channels (Prakriya and Lewis, 2001). Furthermore, the potent CRAC channel inhibitor BTP-2 (Zitt et al., 2004) strongly inhibited SOCE in NPCs (Fig. 1C). Therefore, the pharmacological profile of SOCE in mouse NPCs is consistent with those of CRAC channels (Jairaman and Prakriya, 2013).
Suppressing STIM1 and Orai1 expression/function diminishes SOCE in NPCs
Prototypic CRAC channels are a two-component system composed of STIM1, the ER Ca2+ sensor, and Orai1, the pore-forming protein (Lewis, 2011). mRNA and protein expression studies indicate that STIM1 and Orai1 are widely expressed in many organ systems, including the nervous system (Gross et al., 2007; Gwack et al., 2007; Klejman et al., 2009; Skibinska-Kijek et al., 2009), but whether these proteins are expressed and functional in the neurogenic regions of the brain is unclear. We therefore examined the contribution of STIM1 and Orai1 for SOCE in NPCs using several approaches. In one approach, we suppressed expression of STIM1 and/or Orai1 using siRNA knock down. Knock down of STIM1 and Orai1 significantly reduced SOCE in NPCs, reducing the rate of Ca2+ influx after re-addition of extracellular Ca2+ from 3.68 ± 0.51 to ∼1 nm/s in siSTIM1- and siOrai1-treated cells (Fig. 2A,B). In a second approach, analysis of protein expression by Western blot revealed the presence of STIM1 and Orai1 that was sensitive to siRNA mediated knock down (Fig. 2C). For Orai1, protein expression was observed as a series of bands of ∼25 kDa, as expected from the glycosylation of Orai1 and the presence of an alternate translational initiation site in the N terminus (McNally et al., 2009; Fukushima et al., 2012). Finally, overexpression of a dominant-negative Orai1 mutant, E106AOrai1, strongly inhibited SOCE (from 4.73 ± 1.11 nm/s in control cells to 0.09 ± 0.03 nm/s in E106AOrai1-expressing NPCs; p < 0.0001; data not shown). Together, these studies indicate that NPCs express store-operated Ca2+ entry that is encoded by the canonical CRAC channel proteins STIM1 and Orai1.
The residual SOCE in siRNA-treated cells could be due either to incomplete suppression of STIM1 and Orai1 (Fig. 2A–D) or to contribution from other STIM and Orai paralogs (STIM2/Orai2/Orai3). To determine whether the residual SOCE is due to incomplete suppression of STIM1 and Orai1 knock down, we examined SOCE in NPCs derived from transgenic mice lacking functional STIM1 or Orai1. In one set of studies, we examined NPCs isolated from Orai1R93W/R93W knock-in mice. These mice contain the loss-of-function R93W mutation in Orai1, equivalent to the R91W mutation described in human SCID patients (Feske et al., 2006; Bergmeier et al., 2009). Compared with Orai1+/+ NPCs, SOCE was significantly suppressed in Orai1R93W/+ NPCs and abolished in Orai1R93W/R93W NPCs (Fig. 2E,H,I). Likewise, germline deletion of one or both alleles of Orai1 resulted in suppression or complete loss of SOCE in embryonic NPCs (Fig. 2F,H,I). Qualitatively similar results were observed in STIM1 KO mice (Fig. 2G–I). The extent of SOCE loss exhibited a clear gene dosage effect in which the peak amplitude and the initial rate of [Ca2+]i entry after extracellular Ca2+ re-addition were attenuated to a much greater extent in the homozygous mice (Orai1R93W/R93W, Orai1−/−, and STIM1−/−) compared with heterozygous mice (Orai1R93W/+, Orai1+/−, and STIM1+/−). Collectively, these results indicate that STIM1 and Orai1 are essential for SOCE in embryonic NPCs.
Emerging evidence indicates that Ca2+ signals regulate neurogenesis and gene transcription, not only in embryonic NPCs, but also in adult NPCs (Deisseroth et al., 2004; Pathania et al., 2010). We therefore sought to examine the molecular properties of SOCE in adult NPCs. However, these experiments were complicated by the high postnatal mortality of transgenic mice lacking STIM1 or Orai1 (Gwack et al., 2008; Oh-Hora et al., 2008) or expressing the loss-of-function Orai1R93W mutant protein (Bergmeier et al., 2009), with most mice dying in the first few hours after birth. Therefore, we generated a conditional brain-specific KO of Orai1 by crossing Orai1fl/fl mice to transgenic mice expressing Cre-recombinase under the nestin promoter. As described previously, nestin-Cre deleter mice are widely used to generate brain-specific deletion of target genes (Bates et al., 1999; Groszer et al., 2001). PCR analysis of genomic DNA isolated from various tissues showed deletion of Orai1 specifically in brain tissue derived from Orai1fl/fl:Nes-Cre and Orai1fl/+:Nes-Cre mice, but not in the control mice (Fig. 3B). Conditional deletion of Orai1 in the brain did not cause the perinatal lethality that is observed in whole animal germline KOs of Orai1, enabling us to investigate Ca2+ influx mechanisms in adult brain NPCs.
These studies revealed that SOCE in adult NPCs was markedly diminished in cells from Orai1fl/− and Orai1fl/−:Nes-Cre mice compared with WT littermates (Fig. 3C–E). Further, as seen in embryonic mice, a haplotype insufficiency phenotype is clearly evident, with the Orai1fl/−:Nes-Cre mice exhibiting markedly diminished Ca2+ entry after re-addition of extracellular Ca2+ compared with heterozygous Orai1fl/− mice (Fig 3C–E). In contrast to embryonic mice, however, NPCs from adult mice showed a very small residual Ca2+ influx after extracellular Ca2+ re-addition. We did not further investigate the molecular identity of this small residual Ca2+ entry, but did find that it was sensitive to blockade by low concentrations of La3+ (2 μm; Fig. 3C), suggesting that it also arises from CRAC channels, albeit of a different molecular composition (possibly Orai2 or Orai3). Together, these studies indicate that SOCE in both embryonic and adult NPCs is encoded predominantly by the canonical CRAC channel proteins STIM1 and Orai1.
Maturation of NPCs regulates SOCE
As progenitor cells develop and differentiate into mature neurons and glia, the ion channel repertoire of these cells is modified through increased expression of voltage- and ligand-activated conductances to enhance membrane excitability (Yasuda and Adams, 2010). To explore how CRAC channel expression and function is altered during this process, we next examined SOCE and Orai1 expression in subpopulations of NPCs. A key attribute of NSCs/NPCs is the expression of the filamentous proteins nestin and GFAP (Lendahl et al., 1990; Doetsch et al., 1999). Immunostaining revealed that 78 ± 4% of NSCs/NPCs grown as neurospheres also expressed the intermediate filament nestin (Fig. 4A). Because the majority of cells grown as neurospheres (>90%) also displayed SOCE, this result indicates that there is high overlap between the progenitor marker (nestin) and functional presence of SOCE. Moreover, a fraction (∼28 ± 4%) of the cultured NPCs also showed expression of GFAP (Fig. 4B). GFAP is commonly associated with differentiated astrocytes, but is also a marker for proliferating NSCs/NPCs, especially in neurospheres cultured from postnatal and adult forebrains (Imura et al., 2003; Bonaguidi et al., 2005). Importantly, immunolabeling for Orai1 and GFAP revealed that all (100 ± 0%) GFAP+ cells were also positive for Orai1 (Fig. 4B and data not shown). Therefore, these results reaffirm that the machinery for SOCE is strongly expressed in nestin- and GFAP-expressing NSC/NPCs.
Figure 4.
NPC maturation regulates SOCE. A, Neurospheres cultured from E13 NPCs express the progenitor marker nestin. Cells were fixed and immunolabeled for nestin ∼16 h after plating. Nuclei were labeled using DAPI. B, Costaining of NPCs with GFAP and Orai1. Cells were imaged at the coverslip interface (images show the cell footprint). NPCs were cultured from P21 mice. C, Orai1 labeling is absent in the Orai1fl/−:Nes-Cre mice, confirming the deletion of Orai1 in these mice (P21) D, Costaining of NPCs for the neuroblast marker DCX and for Orai1. E, Staining of NPCs from Orai1fl/−:Nes-Cre mice for DCX and Orai1. No Orai1 labeling is seen in the Orai1 fl/−:Nes-Cre mice, as expected from the genetic ablation of Orai1. F, SOCE is diminished in NPCs expressing DCX-GFP compared with GFP− cells. NPCs were isolated from DCX-GFP mice and [Ca2+]i was measured using fura-2 as in Figure 1. G, Summary of SOCE amplitude after re-addition of extracellular Ca2+ (E15 mice; unpaired t test, ***p < 0.001, n = 13–143 cells, representative of 3 experiments from 2 cultures). H, Images of NPCs expressing DCX-GFP loaded with fura-2. The fura-2 signal is at the 380 nm excitation wavelength. DIC, Dfferential interference contrast.
NPCs derived from neurospheres differentiate into neuroblasts or glia and, at the neuroblast stage, they express the microtubule-associated protein doublecortin (DCX). To determine how SOCE changes during maturation to the neuronal phenotype, we examined SOCE in NPCs derived from DCX-EGFP reporter mice. In these mice, EGFP is expressed under the control of the DCX promoter, providing a method to conveniently track the cells normally expressing DCX (Bhattacharyya et al., 2008). These experiments indicated that SOCE is strongly downregulated in DCX-EGFP+ cells compared with DCX-EGFP− cells (Fig. 4F,G). To better understand the mechanistic basis of this downregulation, we costained cultured NPCs for DCX and Orai1 by immunocytochemistry (Fig. 4D,E). These experiments revealed that, in contrast to the 100% labeling of Orai1 seen in GFAP+ cells, only 33 ± 5% of the DCX+ cells costained for Orai1 (Fig. 4D and data not shown). Therefore, the decrease in SOCE seen in the DCX-EGFP NPCs is likely related to the reduced expression of Orai1. Together, these results suggest that the SOCE is regulated by the maturation state of NPCs and is specifically downregulated in cells expressing the neuroblast marker DCX due to diminished expression of the CRAC channel protein Orai1.
EGF and muscarine activate CRAC channels in NPCs
CRAC channels are typically activated after stimulation of cell surface receptors coupled to the phospholipase C signaling and subsequent depletion of intracellular Ca2+ stores through the generation of inositol-1,4,5-trisphosphate (IP3; Hogan et al., 2010; Lewis, 2011). To determine whether CRAC channels contribute to the effector responses of growth factors and neurotransmitters linked to PLC signaling in NPCs, we tested several agonists of cell surface receptors for their ability to stimulate CRAC channel activation in NPCs. We applied these ligands in a Ca2+-free Ringer's solution and examined SOCE after re-addition of external Ca2+. This screen revealed that EGF and muscarine are strong activators of SOCE in NPCs (Table 1). EGF is a potent mitogen that regulates proliferation of a wide variety of cell types (Prenzel et al., 2001) and is essential for the proliferation of NPCs (Reynolds et al., 1992). EGF acts via tyrosine kinase receptors, leading to stimulation of phospholipase C and IP3 signaling, which in turn would be expected to deplete ER Ca2+ stores (Ullrich and Schlessinger, 1990). Likewise, cholinergic activation of muscarinic ACh receptors (mAChRs) mobilizes Ca2+ signaling in many cells (Felder, 1995). mAChR subunits are expressed in the neurogenic regions of rodent brains (Ma et al., 2000; Kaneko et al., 2006) and cholinergic signaling has been implicated in many aspects of neurogenesis (Resende and Adhikari, 2009).
Table 1.
Agonists stimulating SOCE in NPCs
| Ligand | Cells responding (%) | Cells tested (no.) |
|---|---|---|
| EGF | 65.3 | 754 |
| Acetylcholine | 49.5 | 101 |
| Muscarine | 51.7 | 267 |
| L-glutamate | 60.2 | 266 |
| SDF-1α | 23.1 | 368 |
| UTP | 6.5 | 77 |
| Caffeine | 5.9 | 34 |
| Wnt-5α | 4.7 | 107 |
| Baclofen | 2.5 | 40 |
| GABA | 1.7 | 290 |
| BDNF | 1.0 | 196 |
| BMP | 0.0 | 31 |
| FGF | 0.0 | 218 |
Wild-type NPCs derived from embryonic (E12, E13, E15, E18), neonatal (P0–P3), or adult (∼8 weeks old) mice were treated with the indicated ligands in a Ca2+-free Ringer's solution. Extracellular Ca2+ was then restored (2 mm) and the amplitude of the [Ca2+]i was examined. Cells were considered responders if the [Ca2+]i elevation was >2× SEM above the resting [Ca2+]i.
Administration of EGF (100 ng/ml) in a Ca2+-free solution produced a transient Ca2+ elevation suggestive of Ca2+ release from intracellular stores (Fig. 5A). Subsequent re-addition of extracellular Ca2+ elicited a significant Ca2+ elevation that was blocked by La3 (2 μm, Fig. 5A). In a variation of this test, application of EGF in the presence of extracellular Ca2+ produced a biphasic Ca2+ signal, the sustained component of which was eliminated in Orai1fl/fl:nes-CRE NPCs (Fig. 5B,C). These results indicate that EGF mobilizes Ca2+ elevations in NPCs through release of intracellular Ca2+ stores followed by activation of SOCE via Orai1 channels. Similarly, a saturating concentration of acetylcholine (300 μm) triggered Ca2+ signals consistent with SOCE (Fig. 5D). The ACh-induced Ca2+ signal was blocked by low concentration of La3+ (2 μm) and the mAChR antagonist atropine, suggesting involvement of metabotropic ACh receptors (Fig. 5D). Direct tests with muscarine (50 μm), a selective agonist of metabotropic ACh receptors, indicated that mAChR activation causes robust SOCE (Fig. 5E). Consistent with an essential role for Orai1 in this pathway, the sustained Ca2+ elevations caused by muscarine were diminished in the heterozygous Orai1R93W/+ NPCs and eliminated in the Orai1R93W/R93W NPCs (Fig. 5F).
Figure 5.
Stimulation of EGF and muscarinic ACh receptors activates SOCE in NPCs. A, Application of EGF (100 ng/ml) in a Ca2+-free Ringer's solution followed by re-addition of extracellular Ca2+ elicits a [Ca2+]i elevation that is blocked by La3+ (2 μm). The two traces show averages of cells that responded with a rise in [Ca2+]i and those that failed to respond after application of EGF. P1 mice, P-values determined by 2-sample unpaired t test, n = 17/29 cells responded to EGF (Resp), while the remainder of the cells did not show increases in [Ca2+]i (No Resp). ***p < 0.001, unpaired t test, 17/29 cells responded to EGF (Resp). B, Administration of EGF (100 ng/ml) in the presence of extracellular Ca2+ produces a biphasic [Ca2+]i elevation. C, The sustained component of the EGF-stimulated Ca2+ signal is eliminated in Orai1-deficient (Orai1fl/fl:Nes-Cre) NPCs (adult 8 weeks old, *p < 0.05, unpaired t test, n = 13–14 cells for each condition, representative of 2 experiments). D, Application of ACh (300 μm) in a Ca2+-free Ringer's solution stimulates a transient [Ca2+]i elevation. Re-addition of extracellular Ca2+ produces Ca2+ entry that is inhibited by atropine (20 nm) and La3+ (2 μm). Methyllycaconitine citrate (MLA) and dihydro-β-erythroidine (DHBE) were used in all solutions to block nAChR subunits (8-week-old NPCs, ***p < 0.001 determined by paired t tests, n = 26/33 cells responded to ACh). E, Administration of muscarine (50 μm) in Ca2+-free solution triggers ER Ca2+ store release. Subsequent re-addition of extracellular Ca2+ causes Ca2+ influx that is inhibited by La3+ (2 μm; adult 8 weeks old, ***p < 0.001, 2-tailed paired t test, n = 10/15 cells responded to muscarine). F, Application of muscarine (50 μm) in the standard extracellular Ringer's solution produces a biphasic [Ca2+]i elevation. The sustained phase of the [Ca2+]i elevation is suppressed in Orai1R93W/+ NPCs and abolished in Orai1R93W/R93W NPCs. (P0, one-way ANOVA, p < 0.0001, followed by unpaired t test between WT and each test condition, ***p < 0.001 n = 10–21 cells, representative of 5 experiments from 2 cultures). G, Single-cell Ca2+ responses after application of 200 μm muscarine. Ca2+ oscillations are seen in ∼50% of the Orai1R93W/+ NPCs that responded to muscarine (P0, n = 16–18 cells for each condition).
Interestingly, unlike cells derived from WT mice, which exhibited only sustained Ca2+ signals in response to muscarine stimulation, a significant fraction of the responders in the heterozygous Orai1R93W/+ NPCs exhibited Ca2+ oscillations that were not present in the homozygous Orai1R93W/R93W NPCs (Fig. 5G). Previous studies have shown that the strength and specificity of cellular effector responses, including gene transcription, differs between oscillatory and sustained Ca2+ signals (Dolmetsch et al., 1998). Therefore, these results raise the possibility that the Ca2+ oscillations caused by submaximal Ca2+ entry through CRAC channels stimulate cellular functions apart from sustained signals elicited by maximal CRAC channel activation. Together, these results indicate that CRAC channels are activated by both mitogens and neurotransmitters in NPCs.
CRAC channels activate NFAT-dependent gene expression in NPCs
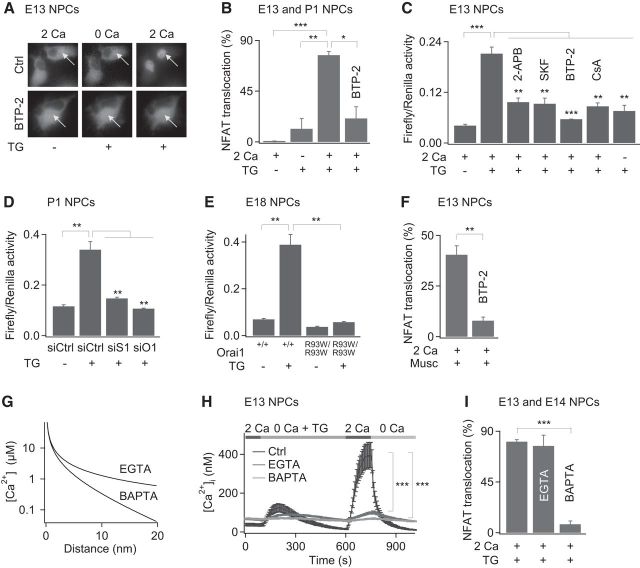
A key mechanism through which Ca2+ signals regulate downstream effector functions, including cell growth and differentiation, is through the NFAT family of transcription factors (Crabtree and Olson, 2002; Hogan et al., 2003). NFAT proteins are normally phosphorylated and reside primarily in the cytoplasm at rest. However, Ca2+ elevations trigger the dephosphorylation of NFAT through the phosphatase calcineurin, causing NFAT to translocate into the nucleus, where it can initiate gene transcription in diverse cell types (Crabtree and Olson, 2002; Hogan et al., 2003). In NPCs, the physiological role of calcineurin/NFAT signaling is presently unknown. However, the central role of NFAT signaling for effector function in many nonexcitable cells led us to consider whether NPCs exhibit NFAT activity and if CRAC channels regulate its activity. We approached this question in two ways. First, we examined the nuclear translocation of exogenously transfected GFP-NFAT1. Activation of SOCE by depletion of ER Ca2+ stores with 1 μm TG resulted in nuclear translocation of GFP-NFAT1 in a majority of NPCs (Fig. 6A,B). Nuclear translocation of GFP-NFAT1 was significantly diminished in the absence of external Ca2+ or by the CRAC channel inhibitor BTP-2 (Fig. 6A,B), indicating that Ca2+ influx through CRAC channels is necessary for NFAT translocation. In a second method, we studied the activation of the endogenous NFAT in NPCs using an NFAT-luciferase reporter assay. Activation of SOCE caused robust stimulation of NFAT-mediated gene expression, which was abolished by the CRAC channel inhibitors 2-APB, SKF96365, and BTP-2 and was absent in nominally Ca2+-free medium (Fig. 6C). Moreover, siRNA knock down of Orai1 and STIM1 significantly reduced NFAT activity in NPCs (Fig. 6D), and NFAT activation was completely lost in NPCs derived from Orai1R93W/R93W knock-in mice (Fig. 6E). Finally, as expected from the dependence of NFAT activation on calcineurin, the calcineurin antagonist cyclosporine A abrogated stimulation of NFAT-dependent gene transcription (Fig. 6C). Therefore, these results indicate that Ca2+ influx through CRAC channels activates NFAT-dependent gene expression in NPCs. In addition to direct depletion of ER Ca2+ stores by thapsigargin, [Ca2+]i elevations resulting from stimulation of mAChRs were also effective in stimulating nuclear translocation of GFP-NFAT1 (Fig. 6F). The muscarine-induced nuclear translocation of GFP-NFAT1 was largely prevented by the CRAC channel inhibitor BTP-2 (Fig. 6F), indicating that CRAC channels serve as a key effector mechanism to activate the calcineurin-NFAT pathway after stimulation of metabotropic receptors.
Figure 6.
CRAC channels activate NFAT-dependent gene expression in NPCs. A, NPCs were nucleofected with GFP-NFAT1 and imaged 24–48 h later. Images were acquired in 2 mm extracellular Ca2+, 15 min after application of 1 μm TG, in a Ca2+-free solution, and 15 min after re-addition of 2 mm Ca2+. Where indicated, BTP-2 (1 μm) was applied for 3–5 h before the experiment. B, Summary of the percentage of cells showing nuclear translocation of GFP-NFAT1. (E13 and P2; one-way ANOVA, p = 0.0003, followed by unpaired t test between indicated test conditions, *p < 0.05, **p < 0.01, ***p < 0.001, n = 93–133 cells from 3–4 experiments). C, NFAT luciferase activity stimulated by depletion of ER Ca2+ stores is significantly suppressed by pretreatment with the CRAC channel inhibitors 2-APB (50 μm), SKF96365 (20 μm), and BTP-2 (0.5 μm) in the presence of low external Ca2+ and by cyclosporine A (1 μm; E13; one-way ANOVA, p < 0.0001; unpaired t test between indicated test conditions, **p < 0.01, ***p < 0.001, representative of 3 cultures). D, E, NFAT luciferase activity is diminished by siRNA treatment against STIM1 and Orai1 (P1; one-way ANOVA, p < 0.0001; unpaired t test, **p < 0.01, representative of 3 cultures; D) and in NPCs derived from Orai1R93W/R93W mice (E18; one-way ANOVA, p < 0.0001; unpaired t test, **p < 0.01; E). F, Muscarine stimulates nuclear translocation of GFP-NFAT1, which is abolished by BTP-2 (1 μm; E13, p < 0.01, unpaired t test, n = 219–461 cells from 4 experiments). G, Theoretical [Ca2+]i profiles as a function of distance from an open CRAC channel. The [Ca2+] profiles were calculated as described previously (Neher, 1986; Stern, 1992). The single-channel CRAC current was assumed to be 5 fA. H, [Ca2+]i elevations produced by SOCE in EGTA- or BAPTA-treated cells. NPCs were loaded with 20 μm EGTA-AM or 20 μm BAPTA-AM for 25–35 min at 37°C. (E13, ***p < 0.001, unpaired t test, n = 58–73 cells; from 2 experiments). I, Nuclear translocation is not significantly affected by intracellular EGTA, but is suppressed by BAPTA (E13 and E14; one-way ANOVA, p = 0.0002; unpaired t test, p = 0.73 for EGTA, and ***p < 0.001 for BAPTA-treated cells, n = 200–233 cells from 3 experiments).
Previous studies have indicated that, in cells expressing multiple subtypes of Ca2+ channels, specificity of gene expression is controlled at least in part by local Ca2+ signals that are sensed by Ca2+-binding proteins located in close proximity to the preferred Ca2+ channel (Deisseroth et al., 1998; Dolmetsch et al., 2001; Di Capite et al., 2009). In this paradigm, Ca2+ acts locally near the plasma membrane to activate a second signal, which in turn leads to stimulation of gene expression in the nucleus. The ability of CRAC channels to signal to the nucleus through calcineurin-NFAT in NPCs therefore led us next to consider whether calcineurin-NFAT signaling may be coupled through a local Ca2+ signal emanating from the CRAC channel. To test this, we studied the differential effects of the slow and fast Ca2+ chelators EGTA and BAPTA, respectively. EGTA and BAPTA have comparable affinities for Ca2+ (∼250 nm; Neher, 1986; Stern, 1992). However, the 150-fold slower Ca2+ binding on-rate of EGTA is expected to produce a region of relatively unbuffered [Ca2+]i around individual Ca2+ channels (Neher, 1986; Stern, 1992). In contrast, the faster Ca2+ binding on-rate of BAPTA would cause [Ca2+]i to drop very steeply to resting levels a few tens of nanometers away from a Ca2+ channel (Fig. 6G). Therefore, whereas BAPTA is expected to suppress both local and global [Ca2+]i elevations, EGTA should selectively suppress the global Ca2+ signal (Neher, 1986; Stern, 1992). The differential effects of EGTA and BAPTA have been widely used to map the local coupling of Ca2+ channels to Ca2+-activated K+ channels (Roberts et al., 1990; Prakriya et al., 1996; Prakriya and Lingle, 2000), the neurotranmitter release machinery (Adler et al., 1991; Rozov et al., 2001), and gene transcriptional machinery (Deisseroth et al., 1996; Kar et al., 2011).
Figure 6, H and I, shows the effects of EGTA and BAPTA on the bulk Ca2+ signal as measured using fura-2 Ca2+ imaging and on translocation of GFP-NFAT1. Both EGTA and BAPTA strongly suppressed the global [Ca2+]i signal resulting from SOCE (Fig. 6H). However, whereas BAPTA nearly completely eliminated the nuclear translocation of GFP-NFAT1, EGTA had no effect (Fig. 6I). The failure of EGTA to suppress GFP-NFAT nuclear translocation while strongly attenuating the global rise in [Ca2+]i indicates that the global rise in [Ca2+]i after CRAC channel activation is poorly correlated with NFAT activation. Therefore, these results are consistent with the hypothesis that NFAT is activated by a local Ca2+ signal closely coupled to CRAC channels. Because NFAT is activated by calmodulin (CaM)-calcineurin-dependent dephosphorylation, these results suggest that calcineurin-NFAT signaling is turned on by a local Ca2+ signal, likely through local activation of CaM-calcineurin by CRAC channels. We will consider potential mechanisms that mediate this coupling in the Discussion.
Suppressing CRAC channels diminishes NPC proliferation
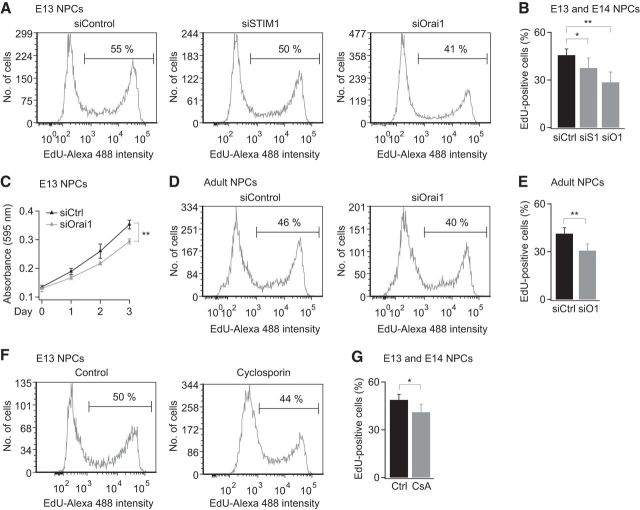
Previous studies have shown that Ca2+ elevations induced by mitogens and neurotransmitters have a powerful influence on the proliferation of NPCs (Ma et al., 2000; Maric et al., 2003; Fiorio Pla et al., 2005). The finding that EGF and muscarine activate CRAC channels in NPCs (Fig. 5) therefore led us to consider whether CRAC channels contribute to NPC proliferation. To examine this possibility, we first tested the effects of STIM1 and Orai1 knock down on actively proliferating NPCs labeled with EdU, a thymidine analog that is incorporated into the DNA of dividing cells in the S-phase of the cell cycle. These experiments revealed that siRNA-mediated knock down of STIM1 or Orai1 results in significant reduction in the fraction of EdU+ cells in both embryonic (Fig. 7A,B) and adult NPCs (Fig. 7D,E). Moreover, MTT assays to monitor total cell densities showed a significant decrease in the proliferation of siOrai1-treated cells (Fig. 7C). Interestingly, proliferation of NPCs was also diminished by cyclosporine A (Fig. 7F,G), an antagonist of calcineurin that inhibits NFAT activation and is a drug widely used for immune suppression (Crabtree and Olson, 2002; Hogan et al., 2003). Therefore, these results indicate that STIM1 and Orai1 regulate NPC proliferation through a mechanism that likely involves calcineurin-NFAT signaling.
Figure 7.
Suppression of STIM1 or Orai1 expression diminishes NPC proliferation. A, NPCs were nucleofected with the indicated siRNA constructs and incorporation of EdU was analyzed by flow cytometry. B, Summary of EdU incorporation for the indicated conditions (E13 and E14 mice; p-values determined by ANOVA followed by post hoc paired t tests, *p = 0.05, **p < 0.01, n = 6 experiments). C, Knockdown of Orai1 expression diminishes NPC cell density. Cell density was assessed by an MTT colorimetric assay. The x-axis shows duration in days after nucleofection and seeding of NPCs at day 0 (E13, p = 0.01 at day 5, 2-sample unpaired t test, representative of 3 experiments). D, NPCs derived from adult mice show diminished EdU incorporation after Orai1 knock down. E, Summary of the effects of siRNA knock down of Orai1 (adult mice, 7–10 wk, p = 0.008, 2-sample paired t test, n = 4 experiments from 3 cultures). F, G, The calcineurin inhibitor cyclosporin A (5 μm) diminishes NPC proliferation. NPCs were treated with CsA once every 24 h for 48 h before EdU incorporation (E13 and E14, *p < 0.05, paired t test, n = 3 experiments).
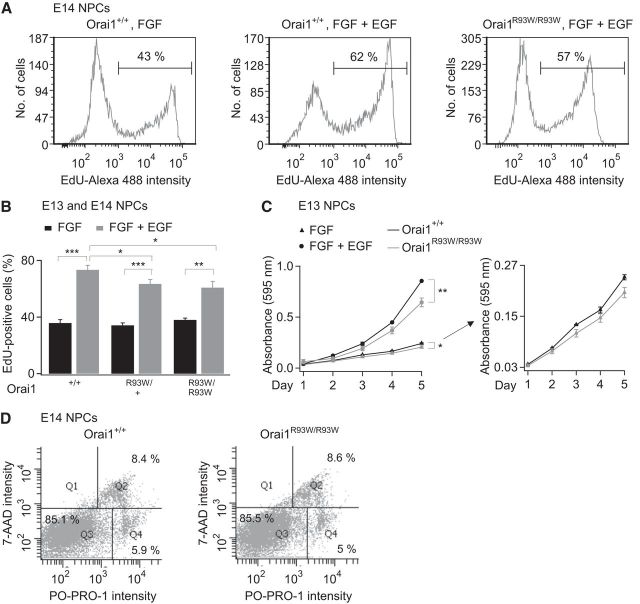
To examine the contributions of CRAC channels for NPC proliferation in the Orai1R93W/R93W knock-in mice, we performed two types of analyses. In one method, we examined the proliferation of NPCs from embryonic mice grown in FGF alone, which reflects the basal proliferation of these cells. EdU labeling of NPCs derived from embryonic Orai1R93W/R93W mice grown in FGF alone did not reveal a difference in basal proliferation (Fig. 8B). Nonetheless, examination of cell growth curves using MTT assays revealed a small but significant decrease in average cell densities in Orai1R93W/R93W NPCs grown in FGF compared with WT cells (Fig. 8C). In a second method, we studied the enhanced proliferation of NPCs stimulated by the mitogen EGF. Stimulation of NPCs (E14 embryos) with EGF resulted in strong enhancement of NPC proliferation as assessed by EdU incorporation and MTT cell growth assays (Fig. 8A–C). The enhancement of EdU incorporation stimulated by EGF was diminished both in the heterozygous Orai1R93W/+ and in homozygous Orai1R93W/R93W mice (Fig. 8B). Likewise, MTT cell growth assays revealed a significant loss of proliferation in Orai1R93W/R93W mice (Fig. 8C). Therefore, loss of Orai1 function attenuates proliferation of NPCs, demonstrating a role for SOCE in regulating both basal and EGF-stimulated NPC proliferation. The decrease in the fraction of actively dividing NPCs in CRAC-channel-deficient cells is not an indirect consequence of increased apoptosis, because estimates of the fraction of apoptotic cells were comparable in WT and CRAC-channel-deficient NPCs (Fig. 8D). Together, these results indicate that Ca2+ signaling through CRAC channels regulates NPC proliferation.
Figure 8.
Proliferation is diminished in NPCs from transgenic mice lacking functional Orai1 channels. A, EdU analysis of embryonic NPCs from Orai1+/+ and Orai1R93W/R93W mice grown in FGF (10 ng/ml) or FGF and EGF (10 ng/ml each). NPCs were grown for 24–48 h before EdU labeling (3–8 h). B, Summary of the fraction of EdU-labeled cells under different growth conditions. EGF-stimulated proliferation of NPCs is attenuated in Orai1R93W/R93W NPCs (E13–E14, 2-way ANOVA followed by post hoc t test, n = 8–19 experiments/mice for each genotype, 5 cultures; *p < 0.05, **p < 0.01, ***p < 0.001). C, MTT cell growth assays show decreased NPC cell densities in Orai1R93W/R93W mice. Right, Same experiment on a magnified y-axis scale to better visualize the difference between Orai1R93W/R93W and WT cells grown in FGF alone (E13, *p < 0.05, **p < 0.01 unpaired t test, n = 3 experiments, representative of 3 cultures). D, Apoptosis is not significantly different in NPCs lacking functional CRAC channels. Representative flow cytometry analysis depicting the fraction of live (Q3), apoptotic (Q4), and dead cells (Q2) in WT and Orai1R93W/R93W NPCs. The percentage of apoptotic cells is indicated in the bottom right box. E14 embryos were used for this experiment.
One possible explanation for the differing effects of Orai1 deficiency in NPCs cultured in the presence of FGF alone between the two EdU tests and MTT assays in the Orai1R93W-transgenic mice (Fig. 8B,C) may lie in the differing methodologies of the assays: because EdU tests only provide a snapshot of EdU incorporation in the S-phase, a population of NPCs that asymmetrically divide into quiescent cells will also incorporate EdU to the same extent as progenitors that retain proliferative capacity and continue to divide. In contrast, MTT assays reveal cell densities over a period of days and thus better reflect overall differences in rates of proliferation taking into account possible variations in progenitor populations. The high concentrations of growth factors (10–20 ng/ml) in which these cells are grown may also partially overcome deficits in basal proliferation, as assessed by EdU incorporation, due to chronic CRAC channel deficiency. Nonetheless, the decrease in cell growth revealed by the MTT assays indicates that Orai1 loss-of-function or deficiency modulates NPC proliferation.
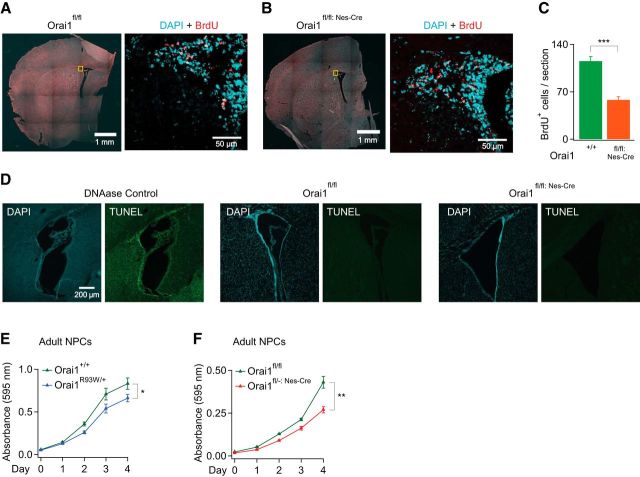
Although growing NPCs in culture as neurospheres is a popular and useful method for studying stem and progenitor cell behaviors in vitro, the properties of NPCs grown in cell culture are not likely to be identical to those expressed in vivo in their native milieu (Reynolds and Rietze, 2005; Pastrana et al., 2011). We therefore also investigated whether deleting CRAC channel expression affects the proliferation of NSCs in the subventricular zone in vivo in adult mice. For these studies, actively dividing cells were labeled by injecting mice intraperitoneally with a single injection of the thymidine analog BrdU. Mice were killed 24 h after injection and BrdU incorporation into the rapidly dividing cells was examined in fixed brain sections by counting the total number of BrdU+ cells in matched Orai1fl/fl:Nes-Cre and Orai1fl/fl coronal sections. This analysis of adult mice revealed a marked decrease in the number of proliferating cells in the subventricular zone in Orai1fl/fl:Nes-Cre mice (Fig. 9E). BrdU+ cells were distributed, as expected, along the lateral ventricle wall in both cases, indicating that the Orai1 KO brains have a generalized decrease in the number of proliferating cells in the SVZ and that this is not due to selective loss of proliferation in a particular subregion within the SVZ. TUNEL staining did not reveal significant levels of apoptosis in either Orai1fl/fl nor Orai1fl/fl:Nes-Cre brains (Fig. 9D), indicating that, as in cultured NPCs (Fig. 8D), the diminished BrdU labeling is not due to enhanced apoptosis.
Figure 9.
Adult mice lacking Orai1 exhibit diminished NSC proliferation in vivo and in vitro. A, B, Whole-mount images of coronal brain sections containing the SVZ from Orai1fl/fl (A) or Orai1fl/fl:Nes-Cre mice (B; low-magnification images are on shown on the left). Example confocal images at a high magnification (60×) of BrdU immunostaining (red) and DAPI (cyan) in the dorsolateral SVZ are shown on the right. Images were collected in the dorsolateral SVZ. C, Summary of the average number of BrdU-labeled cells per section in WT and KO mice (adult mice, 6 weeks old, ***p < 0.001, unpaired t test; 12–13 sections analyzed from 3 mice for each condition). D, TUNEL staining does not show significant levels of ongoing apoptosis or necrosis in Orai1fl/fl or Orai1fl/fl:Nes-Cre mice. E, F, MTT colorimetric cell growth assays in NPCs obtained from adult Orai1R93W/+ NPCs (9 weeks old) or Orai1fl/fl:Nes-Cre mice (11 weeks old; *p < 0.05, **p < 0.01, unpaired t test, n = 3 experiments). Cells were seeded at day 0.
The attenuation of NPC proliferation in vivo could arise because lack of CRAC channel function diminishes proliferation of adult progenitors directly. However, it could also be a consequence of a developmental deficit in which adult mice lacking Orai1 contain reduced numbers of progenitors at the outset due to depletion of progenitors during embryonic development. Unfortunately, our attempts to examine this issue directly using an inducible KO system of tamoxifen injections in adult STIM1 Cre-ERT2 mice were complicated by increases in [Ca2+]i caused by tamoxifen itself (data not shown) (Zhang et al., 2000; Chang et al., 2002). To overcome this problem and to gain insight into the role of CRAC channels for regulating the intrinsic proliferative potential of adult NPCs, we examined NPCs cultured as neurospheres from adult Orai1fl/fl:Nes-Cre mice. This analysis revealed strong suppression of proliferation in NPCs derived from adult Orai1fl/fl:Nes-Cre mice. Likewise, NPCs grown from heterozygous Orai1R93W/+ mice (which survive into adulthood, unlike the homozygous Orai1R93W/R93W mice) also showed significant deficits in proliferation. Therefore, these results demonstrate that ablation or loss-of-function of Orai1 channels results in an intrinsic defect in NPC proliferation in adult NPCs, suggesting that the reduced BrdU labeling seen in the adult Orai1fl/fl:Nes-Cre mice is unlikely to be solely due to a developmental deficit. Together with the in vitro results described for the embryonic cells (Fig. 8), these results indicate that CRAC channels play a significant role in regulating both embryonic and adult NPC proliferation.
Discussion
Ca2+ signaling is crucial for many aspects of neurogenesis, including proliferation, migration, and differentiation (Komuro and Rakic, 1996; Spitzer et al., 2004; Weissman et al., 2004; Hanson et al., 2008). Ca2+ signals are shaped by interactions among ion channels, pumps, and buffers and regulation of these molecules offers abundant opportunities for signal modulation and manipulation of downstream responses (Clapham, 2007). Growing evidence indicates that aberrant Ca2+ signals are detrimental for the proper “choreography” of events in neural development (Spitzer et al., 2005), which has sparked considerable interest over the years to understand the molecules and mechanisms of Ca2+ signaling in neurons and glia. However, the functional architecture of the Ca2+-signaling networks in NPCs—the molecular entities and their organization and how this machinery regulates the physiology of these cells—is not well understood. Previous studies indicate that voltage-gated Ca2+ channels emerge gradually at later stages in the evolution of NPCs (Maric et al., 2000; Cai et al., 2004; Fiorio Pla et al., 2005; Moe et al., 2005; D'Ascenzo et al., 2006; Yasuda et al., 2008), leading to the possibility that early stage NPCs must use alternate Ca2+ influx mechanisms to generate Ca2+ signals. In this study, we find that subventricular zone NPCs exhibit SOCE mediated by the CRAC channel proteins STIM1 and Orai1. The ensuing Ca2+ signal regulates Ca2+-dependent gene expression through the calcineurin/NFAT pathway and proliferation of NPCs. In addition to revealing a novel mechanism for the regulation of embryonic and adult neurogenesis, these results identify CRAC channels as a new target that could be relevant for current efforts to develop new therapies using NSCs/NPCs.
Mouse NPCs exhibit SOCE mediated by CRAC channels
We demonstrate that SVZ NPCs exhibit SOCE that is encoded by the canonical CRAC channel proteins STIM1 and Orai1 (Figs. 1, 2, 3). These results confirm and extend previous reports showing that neuroepithelial, neuroblastoma, and glioblastoma cells express SOCE and its machinery (Sakaki et al., 1997; Brown et al., 2005; Liu et al., 2011; Li et al., 2012; Motiani et al., 2013). However, our data do not rule out a role for the other CRAC channel molecules STIM2, Orai2, and Orai3. In fact, brain-specific deletion of Orai1 in adult mice does not completely abolish SOCE (Fig. 3C), and the residual Ca2+ entry is inhibited by low concentrations of La3+ (2 μm), consistent with the possibility that other Orai paralogs (Orai2/3) may also be expressed and contribute to Ca2+ signaling in adult NPCs. It is worth noting that the conspicuous role for Orai1 found in our studies differs from most previous published studies and mapping efforts of the Allen Institute (http://mouse.brain-map.org/), which implicate Orai2 and Orai3 as the key Orai paralogs expressed in brain (Gross et al., 2007; Gwack et al., 2007; Wissenbach et al., 2007; Berna-Erro et al., 2009). The published evidence, however, is based largely on Western blot or mRNA analysis of whole-brain lysates and could mask heterogeneity between different cell types. These differing results highlight the need for more in-depth analysis of the expression of Orai and STIM proteins in the nervous system using specific reagents such as antibodies and KO mice.
The majority of the NPCs we tested in culture for SOCE also expressed the progenitor marker nestin and a high fraction of the adult NPCs also expressed the classical astrocyte marker GFAP (Fig. 4). This result is consistent with long-standing observations that GFAP+ neurogenic progenitors are present in the SVZ and the subgranular zone of the dentate gyrus in the adult brains (Altman and Das, 1965; Altman, 1969; Doetsch et al., 1999). These GFAP+ cells remain in the cell cycle and are the predominant source of constitutive adult neurogenesis (Garcia et al., 2004). Therefore, the presence of SOCE appears to be well correlated with classical markers for progenitors with neurogenic potential. In contrast, SOCE was significantly attenuated in progenitors expressing the DCX-GFP transgene, and immunostaining revealed low frequency of coexpression of DCX and Orai1 (Fig. 4). Therefore, these results suggest that maturation of progenitors towards a neuronal potential is associated with downregulation of SOCE. This finding, however, does not necessarily imply that mature neurons do not express SOCE. On the contrary, there is considerable evidence for the presence of both SOCE and the expression of STIM and Orai proteins in many mature neurons (Berna-Erro et al., 2009; Gemes et al., 2011), suggesting that at least subpopulations of mature neurons regain the machinery for SOCE following developmental maturation.
CRAC channels activate NFAT-dependent gene expression in NPCs
NFAT is widely expressed in the nervous system (Graef et al., 2003; Kao et al., 2009), yet its functional roles in the brain are only now beginning to be understood. NFAT-directed gene transcription regulates the expression of IP3Rs, GluRs, and pronociceptive genes in neurons (Groth and Mermelstein, 2003; Groth et al., 2007; Groth et al., 2008). Moreover, calcineurin-NFAT signaling is critical for neurotrophin-mediated axonal outgrowth (Nguyen and Di Giovanni, 2008) and Schwann-cell-mediated myelination of peripheral nerve fibers (Kao et al., 2009). However, the sources of Ca2+ entry that activate NFAT are largely unknown in the brain. Here, we demonstrate that CRAC channels stimulate nuclear translocation of NFAT and NFAT-dependent gene transcription in NPCs. Therefore, CRAC channel activation of calcineurin-NFAT signaling may be a key mechanism that underlies the effector response by Ca2+ signals in the nervous system. Interestingly, we find that NFAT activation is preferentially stimulated by local Ca2+ signals after CRAC channel activation (Fig. 6H,I). This finding is reminiscent of reports showing a tight spatial association between store-operated channels and NFAT activation in HEK293 cells (Kar et al., 2011) and between voltage-activated l-type Ca2+ channels and NFAT in hippocampal neurons (Oliveria et al., 2007). In hippocampal neurons, calcineurin is directly tethered to l-type Ca2+ channels via the anchoring protein AKAP79/150 (Oliveria et al., 2007). Therefore, it is possible that a similar molecular mechanism involving tethers between the calcineurin/NFAT machinery and Orai1 channels may underlie local activation of NFAT by CRAC channels in NPCs.
CRAC channels regulate NPC proliferation
Many growth factors and neurotransmitters that regulate neurogenesis are mechanistically linked to Ca2+ signaling. For example, EGF, a well known regulator of NPC proliferation and differentiation, mobilizes Ca2+ signaling via activation of a receptor tyrosine kinase coupled to the PLCγ-IP3 pathway (Ullrich and Schlessinger, 1990; Maric et al., 2003). Likewise, cholinergic signaling, which triggers Ca2+ mobilization, has long been reported to regulate neurogenesis and proliferation (Ma et al., 2000; Li et al., 2001; Calza et al., 2003; Resende and Adhikari, 2009). However, the cellular machinery that mediates Ca2+ entry in response to these and other factors has remained unclear. Our findings indicate that CRAC channels are a major mechanism for the sustained and oscillatory Ca2+ signals activated by EGF and ACh (Fig. 5) and raise the possibility that CRAC channels serve as a key mechanism in the regulation of neurogenesis by mitogens, growth factors, and neurotransmitters.
Consistent with this possibility, we find that deletion of CRAC channel expression or loss of CRAC channel function significantly diminishes both constitutive and EGF-stimulated NPC proliferation (Figs. 7, 8, 9). The suppression of NPC proliferation is observed, not only in vitro in NPCs cultured as neurospheres (Figs. 7, 8), but also in vivo in tissue slices from Orai1-deficient mice (Fig. 9). Moreover, attenuation of NPC proliferations is not an indirect consequence of enhanced apoptosis. These novel findings indicate that CRAC channels regulate the self-renewal of NSCs/NPCs.
How do CRAC channels regulate NPC proliferation? It is known that Ca2+ signals accelerate the progression of cells through the cell cycle, specifically by speeding exit from quiescence in the early G1 and G1/S transition phases (Berridge, 1995; Munaron et al., 2004). This effect occurs through modulation of the expression and function of several cell cycle proteins, including cyclins (Sée et al., 2004; Karpurapu et al., 2008; Karpurapu et al., 2010; Singh et al., 2010). Previous studies have shown that the expression of cyclins is transcriptionally regulated by NFAT (Karpurapu et al., 2008; Karpurapu et al., 2010; Singh et al., 2010). Therefore, it is tempting to speculate that CRAC channels regulate NPC proliferation through control of calcineurin/NFAT signaling. Consistent with this possibility, cyclosporine A diminishes NPC proliferation (Fig. 7G). Therefore, these findings are compatible with the scenario that CRAC channels regulate neurogenesis, at least in part through transcriptional control of the expression of cell cycle proteins.
In summary, we describe a novel signaling pathway that regulates NPC proliferation and neurogenesis. Because the ventricular zones in the mammalian brain are niches for neural precursors that continue to proliferate into adulthood (Merkle and Alvarez-Buylla, 2006), the regulation of NPC proliferation by CRAC channels may have implications not only for brain development, but also for harnessing the full therapeutic potential of NPCs after brain injury and the treatment of neurodegenerative diseases.
Footnotes
This work was supported by the National Institutes of Health (NIH Grants NS057499 and AI097302) and the Brain Research Foundation. A.S. was supported by an American Heart Association predoctoral fellowship and A.K.S. by an NIH predoctoral training grant. We thank Lauren T. Sybert, Tammy McGuire, and Paul Mireles for technical help; members of the Prakriya, Kessler, and Miller laboratories for helpful discussions; and Anna Toth and Amit Jairaman for comments on the manuscript.
H.J.M. was an employee of Amgen, Inc. during the design and execution of this work. The remaining authors declare no competing financial interests.
References
- Adler EM, Augustine GJ, Duffy SN, Charlton MP. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991;11:1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Baba A, Yasui T, Fujisawa S, Yamada RX, Yamada MK, Nishiyama N, Matsuki N, Ikegaya Y. Activity-evoked capacitative Ca2+ entry: implications in synaptic plasticity. J Neurosci. 2003;23:7737–7741. doi: 10.1523/JNEUROSCI.23-21-07737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates B, Rios M, Trumpp A, Chen C, Fan G, Bishop JM, Jaenisch R. Neurotrophin-3 is required for proper cerebellar development. Nat Neurosci. 1999;2:115–117. doi: 10.1038/5669. [DOI] [PubMed] [Google Scholar]
- Belmadani A, Tran PB, Ren D, Assimacopoulos S, Grove EA, Miller RJ. The chemokine stromal cell-derived factor-1 regulates the migration of sensory neuron progenitors. J Neurosci. 2005;25:3995–4003. doi: 10.1523/JNEUROSCI.4631-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmeier W, Oh-Hora M, McCarl CA, Roden RC, Bray PF, Feske S. R93W mutation in Orai1 causes impaired calcium influx in platelets. Blood. 2009;113:675–678. doi: 10.1182/blood-2008-08-174516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berna-Erro A, Braun A, Kraft R, Kleinschnitz C, Schuhmann MK, Stegner D, Wultsch T, Eilers J, Meuth SG, Stoll G, Nieswandt B. STIM2 regulates capacitive Ca2+ entry in neurons and plays a key role in hypoxic neuronal cell death. Sci Signal. 2009;2:ra67. doi: 10.1126/scisignal.2000522. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Calcium signalling and cell proliferation. Bioessays. 1995;17:491–500. doi: 10.1002/bies.950170605. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya BJ, Banisadr G, Jung H, Ren D, Cronshaw DG, Zou Y, Miller RJ. The chemokine stromal cell-derived factor-1 regulates GABAergic inputs to neural progenitors in the postnatal dentate gyrus. J Neurosci. 2008;28:6720–6730. doi: 10.1523/JNEUROSCI.1677-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, McGuire T, Hu M, Kan L, Samanta J, Kessler JA. LIF and BMP signaling generate separate and discrete types of GFAP-expressing cells. Development. 2005;132:5503–5514. doi: 10.1242/dev.02166. [DOI] [PubMed] [Google Scholar]
- Brown AM, Riddoch FC, Robson A, Redfern CP, Cheek TR. Mechanistic and functional changes in Ca2+ entry after retinoic acid-induced differentiation of neuroblastoma cells. Biochem J. 2005;388:941–948. doi: 10.1042/BJ20042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Cheng A, Luo Y, Lu C, Mattson MP, Rao MS, Furukawa K. Membrane properties of rat embryonic multipotent neural stem cells. J Neurochem. 2004;88:212–226. doi: 10.1046/j.1471-4159.2003.02184.x. [DOI] [PubMed] [Google Scholar]
- Calza L, Giuliani A, Fernandez M, Pirondi S, D'Intino G, Aloe L, Giardino L. Neural stem cells and cholinergic neurons: regulation by immunolesion and treatment with mitogens, retinoic acid, and nerve growth factor. Proc Natl Acad Sci U S A. 2003;100:7325–7330. doi: 10.1073/pnas.1132092100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HT, Huang JK, Wang JL, Cheng JS, Lee KC, Lo YK, Liu CP, Chou KJ, Chen WC, Su W, Law YP, Jan CR. Tamoxifen-induced increases in cytoplasmic free Ca2+ levels in human breast cancer cells. Breast Cancer Res Treat. 2002;71:125–131. doi: 10.1023/A:1013807731642. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109:S67–79. doi: 10.1016/S0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- D'Ascenzo M, Piacentini R, Casalbore P, Budoni M, Pallini R, Azzena GB, Grassi C. Role of L-type Ca2+ channels in neural stem/progenitor cell differentiation. Eur J Neurosci. 2006;23:935–944. doi: 10.1111/j.1460-9568.2006.04628.x. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/S0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Heist EK, Tsien RW. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 1998;392:198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/S0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- Di Capite J, Ng SW, Parekh AB. Decoding of cytoplasmic Ca2+ oscillations through the spatial signature drives gene expression. Curr Biol. 2009;19:853–858. doi: 10.1016/j.cub.2009.03.063. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/S0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- Emptage NJ, Reid CA, Fine A. Calcium stores in hippocampal synaptic boutons mediate sort-term plasticity, store-operated Ca(2+) entry, and spontaneous transmitter release. Neuron. 2001;29:197–208. doi: 10.1016/S0896-6273(01)00190-8. [DOI] [PubMed] [Google Scholar]
- Felder CC. Muscarinic acetylcholine receptors: signal transduction through multiple effectors. FASEB J. 1995;9:619–625. [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Fiorio Pla A, Maric D, Brazer SC, Giacobini P, Liu X, Chang YH, Ambudkar IS, Barker JL. Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1-induced Ca2+ entry and embryonic rat neural stem cell proliferation. J Neurosci. 2005;25:2687–2701. doi: 10.1523/JNEUROSCI.0951-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima M, Tomita T, Janoshazi A, Putney JW. Alternative translation initiation gives rise to two isoforms of Orai1 with distinct plasma membrane mobilities. J Cell Sci. 2012;125:4354–4361. doi: 10.1242/jcs.104919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Gemes G, Bangaru ML, Wu HE, Tang Q, Weihrauch D, Koopmeiners AS, Cruikshank JM, Kwok WM, Hogan QH. Store-operated Ca2+ entry in sensory neurons: functional role and the effect of painful nerve injury. J Neurosci. 2011;31:3536–3549. doi: 10.1523/JNEUROSCI.5053-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef IA, Wang F, Charron F, Chen L, Neilson J, Tessier-Lavigne M, Crabtree GR. Neurotrophins and netrins require calcineurin/NFAT signaling to stimulate outgrowth of embryonic axons. Cell. 2003;113:657–670. doi: 10.1016/S0092-8674(03)00390-8. [DOI] [PubMed] [Google Scholar]
- Gross SA, Wissenbach U, Philipp SE, Freichel M, Cavalié A, Flockerzi V. Murine ORAI2 splice variants form functional Ca2+ release-activated Ca2+ (CRAC) channels. J Biol Chem. 2007;282:19375–19384. doi: 10.1074/jbc.M701962200. [DOI] [PubMed] [Google Scholar]
- Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- Groth RD, Mermelstein PG. Brain-derived neurotrophic factor activation of NFAT (nuclear factor of activated T-cells)-dependent transcription: a role for the transcription factor NFATc4 in neurotrophin-mediated gene expression. J Neurosci. 2003;23:8125–8134. doi: 10.1523/JNEUROSCI.23-22-08125.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth RD, Coicou LG, Mermelstein PG, Seybold VS. Neurotrophin activation of NFAT-dependent transcription contributes to the regulation of pro-nociceptive genes. J Neurochem. 2007;102:1162–1174. doi: 10.1111/j.1471-4159.2007.04632.x. [DOI] [PubMed] [Google Scholar]
- Groth RD, Weick JP, Bradley KC, Luoma JI, Aravamudan B, Klug JR, Thomas MJ, Mermelstein PG. D1 dopamine receptor activation of NFAT-mediated striatal gene expression. Eur J Neurosci. 2008;27:31–42. doi: 10.1111/j.1460-9568.2007.05980.x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375:784–787. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-hora M, Neems DS, Hogan PG, Rao A. Biochemical and functional characterization of Orai proteins. J Biol Chem. 2007;282:16232–16243. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- Gwack Y, Srikanth S, Oh-Hora M, Hogan PG, Lamperti ED, Yamashita M, Gelinas C, Neems DS, Sasaki Y, Feske S, Prakriya M, Rajewsky K, Rao A. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol Cell Biol. 2008;28:5209–5222. doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MG, Milner LD, Landmesser LT. Spontaneous rhythmic activity in early chick spinal cord influences distinct motor axon pathfinding decisions. Brain Res Rev. 2008;57:77–85. doi: 10.1016/j.brainresrev.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura T, Kornblum HI, Sofroniew MV. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci. 2003;23:2824–2832. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jairaman A, Prakriya M. Molecular pharmacology of store-operated CRAC channels. Channels (Austin) 2013;7:402–414. doi: 10.4161/chan.25292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko N, Okano H, Sawamoto K. Role of the cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb. Genes Cells. 2006;11:1145–1159. doi: 10.1111/j.1365-2443.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- Kao SC, Wu H, Xie J, Chang CP, Ranish JA, Graef IA, Crabtree GR. Calcineurin/NFAT signaling is required for neuregulin-regulated Schwann cell differentiation. Science. 2009;323:651–654. doi: 10.1126/science.1166562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar P, Nelson C, Parekh AB. Selective activation of the transcription factor NFAT1 by calcium microdomains near Ca2+ release-activated Ca2+ (CRAC) channels. J Biol Chem. 2011;286:14795–14803. doi: 10.1074/jbc.M111.220582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpurapu M, Wang D, Singh NK, Li Q, Rao GN. NFATc1 targets cyclin A in the regulation of vascular smooth muscle cell multiplication during restenosis. J Biol Chem. 2008;283:26577–26590. doi: 10.1074/jbc.M800423200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpurapu M, Wang D, Van Quyen D, Kim TK, Kundumani-Sridharan V, Pulusani S, Rao GN. Cyclin D1 is a bona fide target gene of NFATc1 and is sufficient in the mediation of injury-induced vascular wall remodeling. J Biol Chem. 2010;285:3510–3523. doi: 10.1074/jbc.M109.063727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klejman ME, Gruszczynska-Biegala J, Skibinska-Kijek A, Wisniewska MB, Misztal K, Blazejczyk M, Bojarski L, Kuznicki J. Expression of STIM1 in brain and puncta-like co-localization of STIM1 and ORAI1 upon depletion of Ca2+ store in neurons. Neurochem Int. 2009;54:49–55. doi: 10.1016/j.neuint.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Intracellular Ca2+ fluctuations modulate the rate of neuronal migration. Neuron. 1996;17:275–285. doi: 10.1016/S0896-6273(00)80159-2. [DOI] [PubMed] [Google Scholar]
- Leclerc C, Webb SE, Daguzan C, Moreau M, Miller AL. Imaging patterns of calcium transients during neural induction in Xenopus laevis embryos. J Cell Sci. 2000;113:3519–3529. doi: 10.1242/jcs.113.19.3519. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-X. [DOI] [PubMed] [Google Scholar]
- Lewis RS. Store-operated calcium channels: new perspectives on mechanism and function. Cold Spring Harb Perspect Biol. 2011;3:a003970. doi: 10.1101/cshperspect.a003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BS, Ma W, Zhang L, Barker JL, Stenger DA, Pant HC. Activation of phosphatidylinositol-3 kinase (PI-3K) and extracellular regulated kinases (Erk1/2) is involved in muscarinic receptor-mediated DNA synthesis in neural progenitor cells. J Neurosci. 2001;21:1569–1579. doi: 10.1523/JNEUROSCI.21-05-01569.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Chen C, Zhou Z, Xu S, Yu Z. A TRPC1-mediated increase in store-operated Ca2+ entry is required for the proliferation of adult hippocampal neural progenitor cells. Cell Calcium. 2012;51:486–496. doi: 10.1016/j.ceca.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Lin JH, Takano T, Arcuino G, Wang X, Hu F, Darzynkiewicz Z, Nunes M, Goldman SA, Nedergaard M. Purinergic signaling regulates neural progenitor cell expansion and neurogenesis. Dev Biol. 2007;302:356–366. doi: 10.1016/j.ydbio.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Hughes JD, Rollins S, Chen B, Perkins E. Calcium entry via ORAI1 regulates glioblastoma cell proliferation and apoptosis. Exp Mol Pathol. 2011;91:753–760. doi: 10.1016/j.yexmp.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Ma W, Maric D, Li BS, Hu Q, Andreadis JD, Grant GM, Liu QY, Shaffer KM, Chang YH, Zhang L, Pancrazio JJ, Pant HC, Stenger DA, Barker JL. Acetylcholine stimulates cortical precursor cell proliferation in vitro via muscarinic receptor activation and MAP kinase phosphorylation. Eur J Neurosci. 2000;12:1227–1240. doi: 10.1046/j.1460-9568.2000.00010.x. [DOI] [PubMed] [Google Scholar]
- Maric D, Maric I, Barker JL. Developmental changes in cell calcium homeostasis during neurogenesis of the embryonic rat cerebral cortex. Cereb Cortex. 2000;10:561–573. doi: 10.1093/cercor/10.6.561. [DOI] [PubMed] [Google Scholar]
- Maric D, Maric I, Chang YH, Barker JL. Prospective cell sorting of embryonic rat neural stem cells and neuronal and glial progenitors reveals selective effects of basic fibroblast growth factor and epidermal growth factor on self-renewal and differentiation. J Neurosci. 2003;23:240–251. doi: 10.1523/JNEUROSCI.23-01-00240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally BA, Prakriya M. Permeation, selectivity and gating in store-operated CRAC channels. J Physiol. 2012;590:4179–4191. doi: 10.1113/jphysiol.2012.233098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally BA, Yamashita M, Engh A, Prakriya M. Structural determinants of ion permeation in CRAC channels. Proc Natl Acad Sci U S A. 2009;106:22516–22521. doi: 10.1073/pnas.0909574106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally BA, Somasundaram A, Yamashita M, Prakriya M. Gated regulation of CRAC channel ion selectivity by STIM1. Nature. 2012;482:241–245. doi: 10.1038/nature10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Alvarez-Buylla A. Neural stem cells in mammalian development. Curr Opin Cell Biol. 2006;18:704–709. doi: 10.1016/j.ceb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Braun N, Shukla V, Füllgrabe M, Schomerus C, Korf HW, Gachet C, Ikehara Y, Sévigny J, Robson SC, Zimmermann H. Extracellular nucleotide signaling in adult neural stem cells: synergism with growth factor-mediated cellular proliferation. Development. 2006;133:675–684. doi: 10.1242/dev.02233. [DOI] [PubMed] [Google Scholar]
- Moe MC, Varghese M, Danilov AI, Westerlund U, Ramm-Pettersen J, Brundin L, Svensson M, Berg-Johnsen J, Langmoen IA. Multipotent progenitor cells from the adult human brain: neurophysiological differentiation to mature neurons. Brain. 2005;128:2189–2199. doi: 10.1093/brain/awh574. [DOI] [PubMed] [Google Scholar]
- Morte MI, Carreira BP, Machado V, Carmo A, Nunes-Correia I, Carvalho CM, Araujo IM. Evaluation of proliferation of neural stem cells in vitro and in vivo. Curr Protoc Stem Cell Biol Chapter. 2013;2 doi: 10.1002/9780470151808.sc02d14s24. Unit 2D.14. [DOI] [PubMed] [Google Scholar]
- Motiani RK, Hyzinski-García MC, Zhang X, Henkel MM, Abdullaev IF, Kuo YH, Matrougui K, Mongin AA, Trebak M. STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion. Pflugers Arch. 2013;465:1249–1260. doi: 10.1007/s00424-013-1254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munaron L, Antoniotti S, Lovisolo D. Intracellular calcium signals and control of cell proliferation: how many mechanisms? J Cell Mol Med. 2004;8:161–168. doi: 10.1111/j.1582-4934.2004.tb00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S, Okazawa M. Membrane potential-regulated Ca2+ signalling in development and maturation of mammalian cerebellar granule cells. J Physiol. 2006;575:389–395. doi: 10.1113/jphysiol.2006.113340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Borelly L, Somasundaram A, Yamashita M, Ren D, Miller RJ, Prakriya M. STIM1-Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy. J Physiol. 2008;586:5383–5401. doi: 10.1113/jphysiol.2008.162503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Concentration profiles of intracellular calcium in the presence of a diffusible chelator. Experimental Brain Research Series. 1986;14:80–96. [Google Scholar]
- Nguyen T, Di Giovanni S. NFAT signaling in neural development and axon growth. Int J Dev Neurosci. 2008;26:141–145. doi: 10.1016/j.ijdevneu.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveria SF, Dell'Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Patterns of intracellular calcium fluctuation in precursor cells of the neocortical ventricular zone. J Neurosci. 1998;18:5374–5388. doi: 10.1523/JNEUROSCI.18-14-05374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana E, Silva-Vargas V, Doetsch F. Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell. 2011;8:486–498. doi: 10.1016/j.stem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathania M, Yan LD, Bordey A. A symphony of signals conducts early and late stages of adult neurogenesis. Neuropharmacology. 2010;58:865–876. doi: 10.1016/j.neuropharm.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel JC, Dave KA, Bordey A. Control of neuroblast production and migration by converging GABA and glutamate signals in the postnatal forebrain. J Physiol. 2008;586:3739–3743. doi: 10.1113/jphysiol.2008.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M. The molecular physiology of CRAC channels. Immunol Rev. 2009;231:88–98. doi: 10.1111/j.1600-065X.2009.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP3 receptors. J Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Lingle CJ. Activation of BK channels in rat chromaffin cells requires summation of Ca2+ influx from multiple Ca2+ channels. J Neurophysiol. 2000;84:1123–1135. doi: 10.1152/jn.2000.84.3.1123. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Solaro CR, Lingle CJ. [Ca2+]i elevations detected by BK channels during Ca2+ influx and muscarine-mediated release of Ca2+ from intracellular stores in rat chromaffin cells. J Neurosci. 1996;16:4344–4359. doi: 10.1523/JNEUROSCI.16-14-04344.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A. The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr Relat Cancer. 2001;8:11–31. doi: 10.1677/erc.0.0080011. [DOI] [PubMed] [Google Scholar]
- Resende RR, Adhikari A. Cholinergic receptor pathways involved in apoptosis, cell proliferation and neuronal differentiation. Cell Commun Signal. 2009;7:20. doi: 10.1186/1478-811X-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Rietze RL. Neural stem cells and neurospheres–re-evaluating the relationship. Nat Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WM, Jacobs RA, Hudspeth AJ. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990;10:3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozov A, Burnashev N, Sakmann B, Neher E. Transmitter release modulation by intracellular Ca2+ buffers in facilitating and depressing nerve terminals of pyramidal cells in layer 2/3 of the rat neocortex indicates a target cell-specific difference in presynaptic calcium dynamics. J Physiol. 2001;531:807–826. doi: 10.1111/j.1469-7793.2001.0807h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki Y, Sugioka M, Fukuda Y, Yamashita M. Capacitative Ca2+ influx in the neural retina of chick embryo. J Neurobiol. 1997;32:62–68. doi: 10.1002/(SICI)1097-4695(199701)32:1<62::AID-NEU6>3.0.CO%3B2-C. [DOI] [PubMed] [Google Scholar]
- Sée V, Rajala NK, Spiller DG, White MR. Calcium-dependent regulation of the cell cycle via a novel MAPK–NF-kappaB pathway in Swiss 3T3 cells. J Cell Biol. 2004;166:661–672. doi: 10.1083/jcb.200402136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singaravelu K, Lohr C, Deitmer JW. Calcium-independent phospholipase A2 mediates store-operated calcium entry in rat cerebellar granule cells. Cerebellum. 2008;7:467–481. doi: 10.1007/s12311-008-0050-z. [DOI] [PubMed] [Google Scholar]
- Singh G, Singh SK, König A, Reutlinger K, Nye MD, Adhikary T, Eilers M, Gress TM, Fernandez-Zapico ME, Ellenrieder V. Sequential activation of NFAT and c-Myc transcription factors mediates the TGF-beta switch from a suppressor to a promoter of cancer cell proliferation. J Biol Chem. 2010;285:27241–27250. doi: 10.1074/jbc.M110.100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibinska-Kijek A, Wisniewska MB, Gruszczynska-Biegala J, Methner A, Kuznicki J. Immunolocalization of STIM1 in the mouse brain. Acta Neurobiol Exp (Warsz) 2009;69:413–428. doi: 10.55782/ane-2009-1753. [DOI] [PubMed] [Google Scholar]
- Spitzer NC, Root CM, Borodinsky LN. Orchestrating neuronal differentiation: patterns of Ca2+ spikes specify transmitter choice. Trends Neurosci. 2004;27:415–421. doi: 10.1016/j.tins.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Spitzer NC, Borodinsky LN, Root CM. Homeostatic activity-dependent paradigm for neurotransmitter specification. Cell Calcium. 2005;37:417–423. doi: 10.1016/j.ceca.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Steinbeck JA, Henke N, Opatz J, Gruszczynska-Biegala J, Schneider L, Theiss S, Hamacher N, Steinfarz B, Golz S, Brüstle O, Kuznicki J, Methner A. Store-operated calcium entry modulates neuronal network activity in a model of chronic epilepsy. Exp Neurol. 2011;232:185–194. doi: 10.1016/j.expneurol.2011.08.022. [DOI] [PubMed] [Google Scholar]
- Stern MD. Buffering of calcium in the vicinity of a channel pore. Cell Calcium. 1992;13:183–192. doi: 10.1016/0143-4160(92)90046-U. [DOI] [PubMed] [Google Scholar]
- Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-K. [DOI] [PubMed] [Google Scholar]
- Venkiteswaran G, Hasan G. Intracellular Ca2+ signaling and store-operated Ca2+ entry are required in Drosophila neurons for flight. Proc Natl Acad Sci U S A. 2009;106:10326–10331. doi: 10.1073/pnas.0902982106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Wissenbach U, Philipp SE, Gross SA, Cavalié A, Flockerzi V. Primary structure, chromosomal localization and expression in immune cells of the murine ORAI and STIM genes. Cell Calcium. 2007;42:439–446. doi: 10.1016/j.ceca.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Adams DJ. Physiological roles of ion channels in adult neural stem cells and their progeny. J Neurochem. 2010;114:946–959. doi: 10.1111/j.1471-4159.2010.06822.x. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Bartlett PF, Adams DJ. K(ir) and K(v) channels regulate electrical properties and proliferation of adult neural precursor cells. Mol Cell Neurosci. 2008;37:284–297. doi: 10.1016/j.mcn.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Zhang W, Couldwell WT, Song H, Takano T, Lin JH, Nedergaard M. Tamoxifen-induced enhancement of calcium signaling in glioma and MCF-7 breast cancer cells. Cancer Res. 2000;60:5395–5400. [PubMed] [Google Scholar]
- Zheng JQ, Poo MM. Calcium signaling in neuronal motility. Annu Rev Cell Dev Biol. 2007;23:375–404. doi: 10.1146/annurev.cellbio.23.090506.123221. [DOI] [PubMed] [Google Scholar]
- Zitt C, Strauss B, Schwarz EC, Spaeth N, Rast G, Hatzelmann A, Hoth M. Potent inhibition of Ca2+ release-activated Ca2+ channels and T-lymphocyte activation by the pyrazole derivative BTP2. J Biol Chem. 2004;279:12427–12437. doi: 10.1074/jbc.M309297200. [DOI] [PubMed] [Google Scholar]