Abstract
Context:
Like other tissues, the prostate is an admixture of many different cell types that can be segregated into components of the epithelium or stroma. Reciprocal interactions between these 2 types of cells are critical for maintaining prostate homeostasis, whereas aberrant stromal cell proliferation can disrupt this balance and result in diseases such as benign prostatic hyperplasia. Although the androgen and estrogen receptors are relatively well studied for their functions in controlling stromal cell proliferation and differentiation, the role of the progesterone receptor (PR) remains unclear.
Objective:
The aim of the study was to investigate the expression and function of the PR in the prostate.
Design and Setting:
Human prostate biopsies, renal capsule xenografts, and prostate stromal cells were used. Immunohistochemistry, Western blotting, real-time quantitative PCR, cell proliferation, flow cytometry, and gene microarray analyses were performed.
Results:
Two PR isoforms, PRA and PRB, are expressed in prostate stromal fibroblasts and smooth muscle cells, but not in epithelial cells. Both PR isoforms suppress prostate stromal cell proliferation through inhibition of the expression of cyclinA, cyclinB, and cdc25c, thus delaying cell cycling through S and M phases. Gene microarray analyses further demonstrated that PRA and PRB regulated different transcriptomes. However, one of the major gene groups commonly regulated by both PR isoforms was the one associated with regulation of cell proliferation.
Conclusion:
PR plays an inhibitory role in prostate stromal cell proliferation.
The prostate gland consists of not only the epithelium but also supporting stroma hosting smooth muscle cells, fibroblasts, endothelial cells, and infiltrated inflammatory cells (1, 2). Reciprocal interactions between the epithelium and stroma are critical for maintaining prostate homeostasis and normal prostate function (2). Aberrantly increased proliferation of smooth muscle cells and fibroblasts disrupt this balance, and this is thought to result in abnormal benign prostate growth referred to as benign prostatic hyperplasia (BPH). Therefore, it is important to understand the homeostatic mechanisms that control the growth of prostate stromal cells if we want to develop better treatments to prevent or suppress BPH.
The androgen receptor (AR) and estrogen receptor (ER) are expressed in prostate stromal cells and play important roles for stromal cell proliferation and differentiation (3–5). Belonging to the same steroid receptor family as AR and ER, the progesterone receptor (PR) was also reported to be expressed in the prostate (6–11), but there is little known regarding its role or function in normal or abnormal prostate growth. PR shares extensive homology with the AR protein, including 83% identity in the DNA binding domain (DBD) (12). Progesterone (P4) is synthesized by testes and adrenal glands and is used as a precursor for androgen biosynthesis in men. Its concentration in systemic circulation is in the range of 0.64–4.45 nmol/L (http://cclnprod.cc.nih.gov/dlm/testguide.nsf), high enough to activate prostatic PR. Several reports attempt to localize PR protein expression to the various cells of the prostate (7, 9, 10), but these reports are contradictory because some claim that PR is specific to stromal cells (9, 10), whereas the other (7) finds PR protein in all prostatic cell populations. Radio-ligand binding assays have established that PR is only present in the cytosol of stromal cells (10). Moreover, efforts to describe PR expression and/or function in prostate cells are complicated by the fact that there are 2 distinct PR isoforms: the full-length (PRB), and the N-terminal truncated isoform (PRA), which lacks the activation domain 3 unique to PRB (13). PRA and PRB differentially regulate gene expression (14), and this further confounds efforts to determine PR functions in the prostate gland. Among various functions is the regulatory role of PR in cell proliferation. P4 treatment of breast cancer cells has both stimulatory and inhibitory effects on their growth (15). These actions are mediated by PR through modulating the expression of cyclin D, cyclin-dependent kinase inhibitors, as well as the kinase activities of CDK2 and CDK4 (16–19). Multiple mechanisms are involved that either regulate gene promoters directly or regulate them indirectly through signaling via c-src, epidermal growth factor receptor, and MAPKs (20, 21). Here, we describe our efforts to better characterize PR expression in the various cell compartments of the human prostate gland through using immunohistochemistry (IHC) and to determine the impact of PRA or PRB on prostate stromal cell growth and gene expression.
Patients and Methods
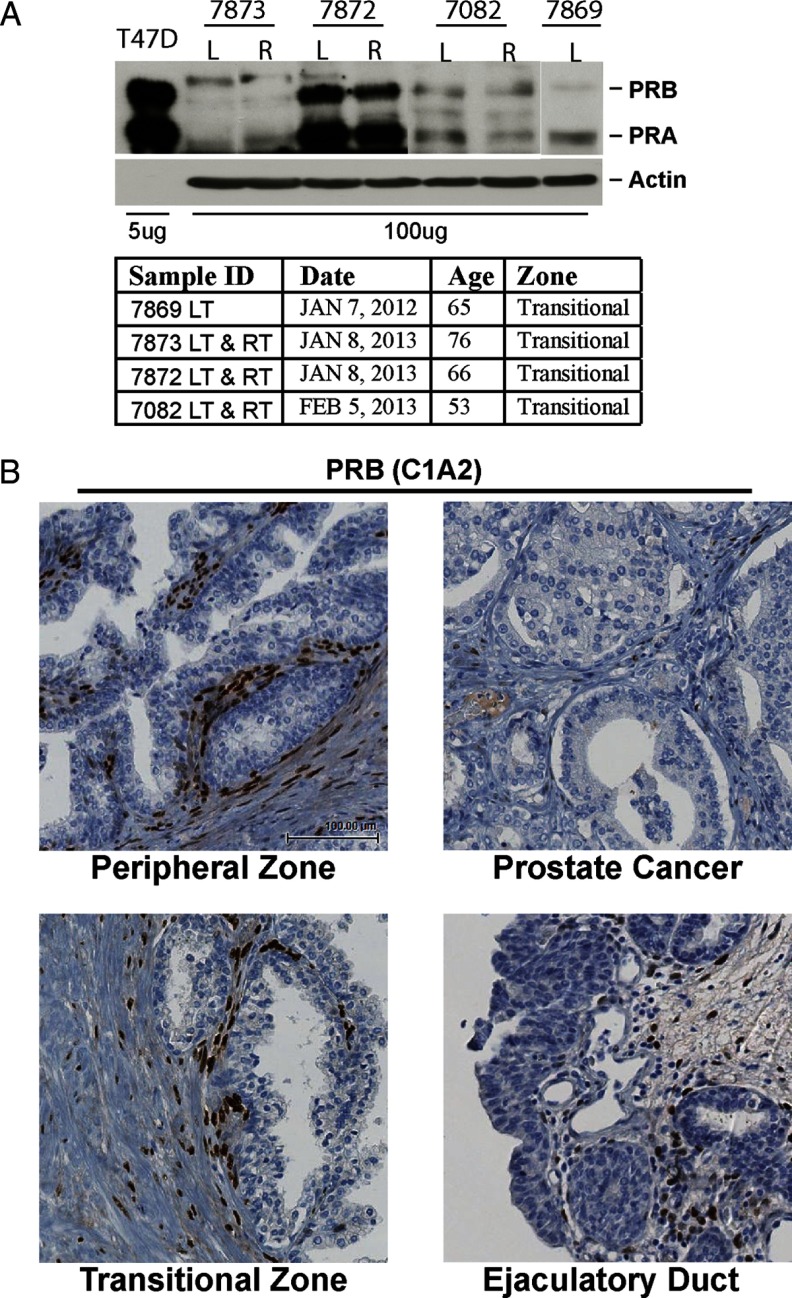
Radical prostatectomy specimens from 27 patients were obtained from the prostate tissue bank of the Vancouver Prostate Centre, University of British Columbia. Ten samples were from benign prostates, and 17 samples were from tissues with well-differentiated untreated prostate cancers (Gleason pattern 3). Four additional prostate tissue samples were freshly collected from prostate cancer patients undergoing radical prostatectomy (see Figure 2A). Tissue (1-cm3 samples) from the transitional zones (left side or right side) were homogenized by Precellys 24 homogenizer (Bertin Corp., Rockville, Maryland) to extract protein lyses. Detailed information on each tissue sample was shown in Figure 2A. All patients signed an informed consent to a protocol that was reviewed and approved by the University of British Columbia Clinical Research Ethics Board (certificate no. H09-01628).
Figure 2.
A, Four prostate tissue samples from transition zones were collected to extract whole cell proteins. Protein extracts (100 μg) were separated on a protein gel together with 5 μg of protein lysates from T47D cells and Western blotted with PR antibody. Information on these 4 tissue samples is also described. B, Whole-mount sections of human prostate biopsies (n = 27) were stained with PRB-specific antibody (C1A2). Four regions within the section (ID no. 7219) were amplified: peripheral zone (benign prostate), peripheral zone (cancer prostate), transitional zone, and ejaculatory duct. LT, left side; RT, right side.
Detailed information on renal capsule xenograft model, prostate stromal cell lines, and experimental procedures including lentiviral transduction, cell proliferation assay, Western blotting, real-time quantitative PCR (qPCR), flow cytometry, and gene microarray analyses are described in the Supplemental Data (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Results
PR is expressed in subset populations of prostate stromal cells
IHC with the PR antibody (SP2; AbCam, Toronto, Ontario, Canada) was performed on human prostate biopsy samples (n = 27) from radical prostatectomy specimens containing either benign or well-differentiated prostate cancer tissues. Morphological analyses showed that among all these regions, PR was expressed only in the nuclei of a subset of prostate stromal cells (Figure 1). The luminal and basal epithelium as well as inflammatory and endothelial cells in the prostate stroma were PR negative. This observation was consistent in all specimens. This selective IHC signal was blocked by the presence of a PR-derived peptide corresponding to the immunogen used to derive the antibody, but not by a control peptide (Supplemental Figure 1A). Additionally, 3 other commercially available anti-PR antibodies all showed the same stromal-specific localization of PR (Supplemental Figure 1B). Finally, the anti-PR SP2 antibody detected different PR levels on a human tissue microarray containing tumors with differing PR protein levels (Supplemental Figure 2). Western blots showed the presence of both PR isoforms in homogenates of various prostate tissue samples (Figure 2A). PR expression varied among patient samples, possibly due to different ratios of stroma:epithelium or the amounts of extracellular matrix in each specimen. Because the PR antibody detected both PRA and PRB isoforms, we also performed IHC with the PRB-specific antibody (clone C1A2; Cell Signaling Technology, Danvers, Massachusetts). Similarly to total PR IHC signals, PRB was also expressed only in the nuclei of a subset of prostate stromal cells (Figure 2B). Currently, there is no PRA-specific antibody available.
Figure 1.
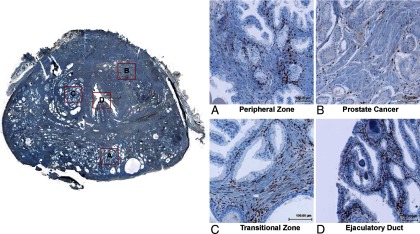
Whole-mount sections of human prostate biopsies (n = 27) were stained with the PR (SP2) antibody by IHC. Four regions within 1 section (ID no. 7219) were amplified: peripheral zone (benign prostate), peripheral zone (cancer prostate), transitional zone, and ejaculatory duct.
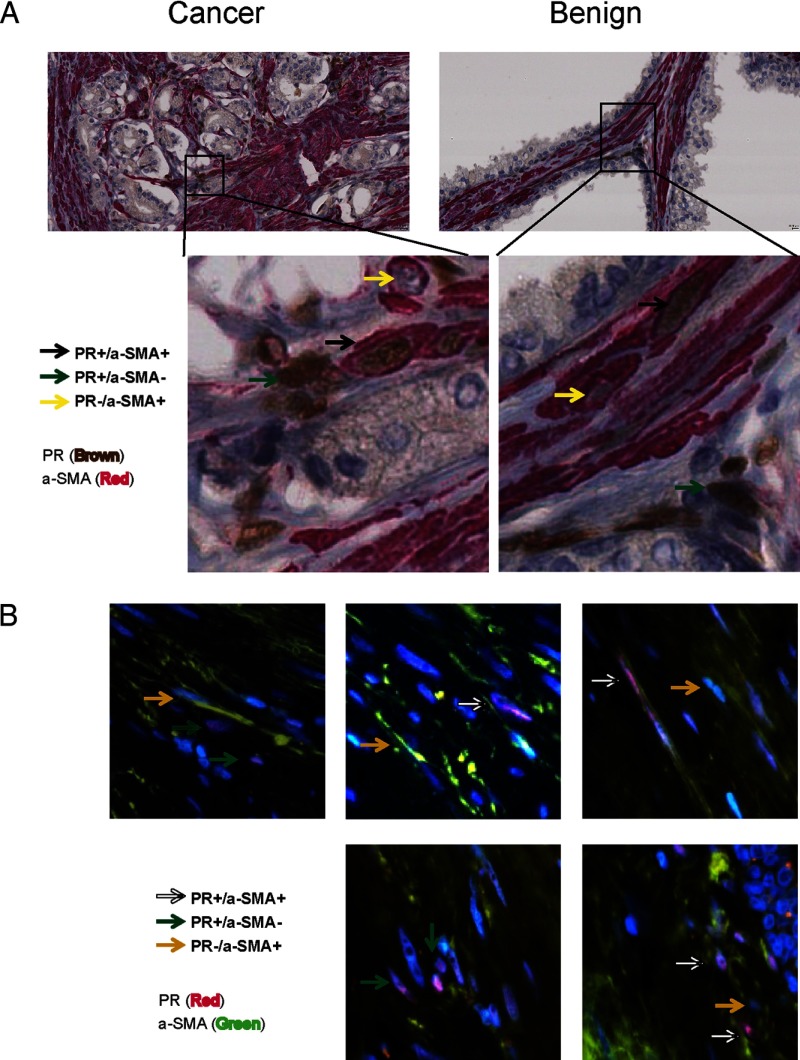
Because only a subset of prostate stromal cells were PR positive, we costained prostate tissue slides with PR and smooth muscle α-actin (α-SMA) antibodies to further characterize the phenotype of PR-positive stromal cells by IHC and immunofluorescence (Figure 3 and Supplemental Figure 3). α-SMA is a protein marker for cells with smooth muscle phenotype. We observed 3 distinct groups of stromal cells expressing: 1) both PR and α-SMA; 2) PR only; and 3) α-SMA only. These observations were consistent in both cancer and benign stroma in our specimens, suggesting that PR was expressed in stromal cells with fibroblast and or smooth muscle phenotypes. Similar observations were also obtained in tissues costained with PRB and α-SMA antibodies (Supplemental Figure 4).
Figure 3.
A whole-mount section of human prostate tissue (ID no. 7219) was costained with PR and α-SMA antibodies by IHC (A) or by immunofluorescence (B). In IHC studies, PR antibody was detected by UltraMap Anti-Rb HRP, whereas α-SMA antibody was detected by UltraMap Anti-Ms Alk Phos (Ventana Medical Systems, Oro Valley, Arizona). In immunofluorescence studies, PR antibody was detected by Alexa Fluor 568 (Invitrogen, San Diego, California), whereas α-SMA antibody was detected by Alexa Fluor 488 (Invitrogen).
Both PR isoforms inhibit prostate stromal cell proliferation
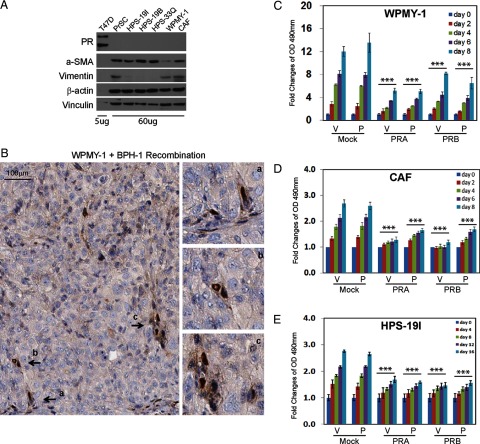
To study PR function, we collected primary cultured human prostate stromal cells from various donors, including commercially available PrSC cells (Lonza, Walkersville, Maryland) and laboratory original HPS-19I, HPS-19B, and HPS-33F cells (22). Two other immortalized human prostate stromal cells are WPMY-1 (American Type Culture Collection, Manassas, Virginia) and CAF (23). PR protein was not detected in any of these cells (Figure 4A), although various levels of α-SMA and vimentin were expressed. However, when WPMY-1, CAF, or HPS-19I cells were combined with BPH-1 cells and grafted under the renal capsule of immunodeficient mice, PR protein expression was restored, as was demonstrated by IHC (Figure 4B and Supplemental Figure 5). Additionally, rat urogenital sinus mesenchyme/BPH-1 cell tissue recombinants retrieved from subrenal xenografts of immunodeficient mice also showed that many cells in the stroma of the tissue recombinant were PR positive (Supplemental Figure 6). Based on our ability to observe restoration of PR expression in stromal cells of cell/tissue recombinants, we then attempted to introduce exogenous PR to our PR-negative prostate stromal cell lines to identify the effects of each PR isoform on cell growth and gene expression.
Figure 4.
A, Whole cell lysates from human prostate stromal cells (60 μg) and T47D breast cancer cells (5 μg) were separated on a protein gel and Western blotted with antibodies against PR, α-SMA, and vimentin. Vinculin and β-actin levels were used as loading controls. B, One million WPMY-1 cells were recombined with 0.5 million BPH-1 cells and grafted under renal capsule in SCID mice for 2 or 4 weeks (n = 3/time point). Xenograft tissues were fixed and immunostained with PR (SP2) antibody. Note that SP2 antibody only recognizes PR protein from human or rat. C and D, WPMY-1 and CAF cells were infected with lentivirus to express mock, PRA, or PRB. Cells were maintained in phenol red free medium containing 10% charcoal stripped serum for 48 hours and then seeded in 96-well culture plates for proliferation assay over 0–8 days. E, Human primary cultured HPS-19I cells were transiently infected with lentivirus encoding mock, PRA, or PRB. Cells were then seeded in 96-well culture plates in the presence of −/+ 10 nm P4. Cell proliferation rates were measured over 0–16 days.
We chose WPMY-1, CAF, and HPS-19I cells to further characterize PR function because they expressed different levels of α-SMA and vimentin. WPMY-1 has the lowest level of α-SMA, HPS-19I expresses no vimentin, and CAF has relatively high levels of α-SMA and vimentin. To study the function of each PR isoform among these different prostate stromal cells, we exogenously expressed PRA or PRB through transduction using lentiviral expression vectors. Stably transduced WPMY-1 and CAF cells were obtained after antibiotic expression, whereas HPS-19I cells were transiently transduced with PRA or PRB lentivirus. PR isoform expression after transduction was confirmed by Western blotting (Supplemental Figure 7). Transcriptional activities and cellular localization of each PR isoform in the presence of with or without P4 were also tested in these cells. Cell proliferation assays showed that both PR isoforms repressed cell proliferation rates in all 3 types of stromal cells, indicating the general inhibitory impacts of PR to stromal cell growth (Figure 4, C–E). These PR actions were not affected by P4 treatment.
PR regulates prostate stromal cell cycling
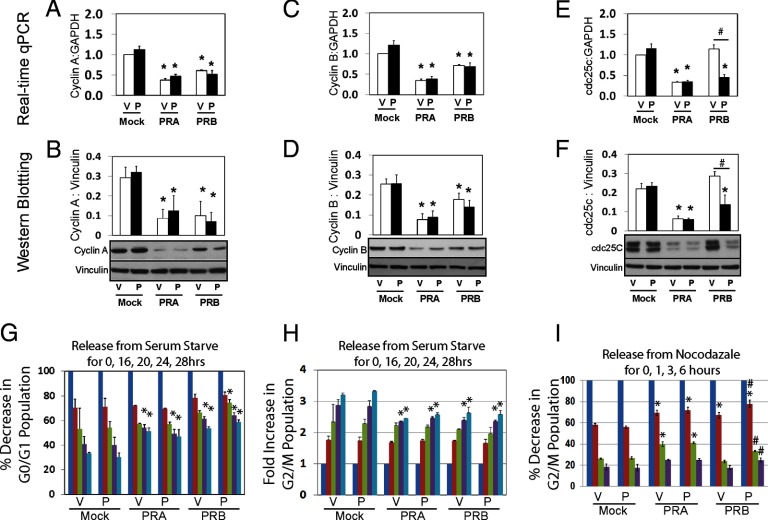
Next, we applied Western blotting and real-time qPCR techniques to measure expression levels of cyclins and cell division cycle 25 (cdc25) family members. PRA inhibited cyclinA, cyclinB, and cdc25c in both protein and mRNA levels in WPMY-1 cells independent of P4 treatment (Figure 5, A–F). Similar observations were also noted from CAF cells (Supplemental Figure 8A). PRB inhibited cyclinA and cyclinB in both mRNA and protein levels ligand-independently but only suppressed cdc25c in the presence of P4. Overexpression of PRA and PRB had no impact on cyclinE, cdc25a, and cdc25b protein levels (Supplemental Figure 8, B–E). Interestingly, PRA dramatically increased cyclinD expression in these cells, but did not affect cell populations at the G1 phase of the cell cycle, possibly representing a compensative response by stromal cells.
Figure 5.
A–F, Lentivirus infected WPMY-1 cells expressing mock, PRA, or PRB were maintained in phenol red free medium containing 10% charcoal stripped serum for 48 hours. Cells were treated with either vehicle or 10 nm P4 for 24 hours. Real-time qPCR and Western blotting assays measured mRNA and protein levels of cyclinA, cyclinB, and cdc25c. G and H, Cells were synchronized by serum starvation for 48 hours and then were supplemented with 10% charcoal stripped serum plus −/+10 nm P4 for 0–28 hours. Cells were collected at each time point and subjected to FACS assays. G0/G1 and G2/M cell populations were counted and plotted over the time course. I, Cells were synchronized by 0.1 μg/ml nocodazole for overnight and then released for 0–6 hours. G2/M cell population was counted and plotted over the time course. *One-way ANOVA analyses compared among cells expressing mock, PRA, and PRB with P < .05. #Comparisons between −/+P4 treatments with P < .05.
Fluorescence-activated cell sorting (FACS) analyses were used to determine the effects of PR expression on cell cycle progression of these cells. WPMY-1 cells (mock transduced or PRA or PRB expressing) were synchronized at G0/G1 by serum starvation for 48 hours. Cells were then replenished with 10% charcoal stripped serum in the presence of −/+ 10 nm P4 for 0–28 hours. FACS assays measured the cell populations at each cell cycle phase, and data were plotted over the time course. Decreased G0/G1 cell population represented the G1-S cell cycle transition, whereas increased G2/M population represented the S-G2 phase transition (Figure 5G). Expression of PRA and PRB did not alter the G0/G1 cell population until 24 hours in the absence of P4. PRB expression delayed the G1-S transition starting at 20 hours after P4 treatment. Consistently, PRA and PRB also delayed the S-G2 phase transition at both the 24- and 28-hour time points (Figure 5H). These PR effects were not altered by P4, consistent with repression of cyclinA expression by both isoforms ligand-independently. These same cells were also synchronized at G2/M phase by nocodazole treatment and then released for an additional 0 to 6 hours. FACS assays also measured G2/M cell populations and were plotted over the time course. Decreased cell population at G2/M phases represented the M-G1 phase transition. PRA expression delayed M-G1 transition at 1 and 3 hours, but by 6 hours there was no significant difference compared to mock cells (Figure 5I). PRB expression also inhibited the M-G1 phase transition, but only at the 1-hour time point. Those PR impacts were consistent with the effects of PR expression on cyclinB levels in these cells. Treatment with P4 further delayed the M-G1 phase transition at all time points in PRB-expressing cells, supporting PRB inhibition of cdc25c expression. Similar inhibitory effects of PR expression on cell cycling were also observed in CAF and HPS-19I cells (Supplemental Figure 9). Together, these results showed that both PR isoforms targeted the S and M phases to inhibit prostate stromal cell cycling.
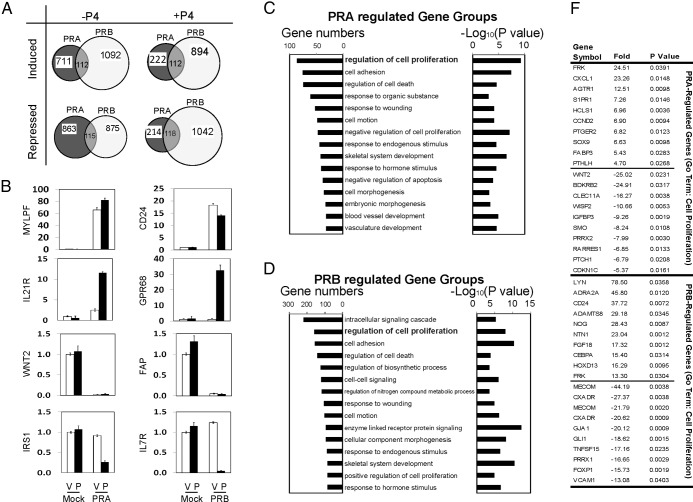
PRA and PRB regulate different gene transcription in prostate stromal cells
Next, we applied gene microarray technology to profile the transcriptome regulated by each PR isoform in prostate stromal cells. WPMY-1 cells expressing mock, PRA, and PRB were treated with −/+P4 for 24 hours. Global gene expression profiles compared PRA- or PRB-expressing cells with mock infected cells using the GeneSpring microarray expression analysis software. P4 treatment did not significantly affect gene expression in mock transduced WPMY-1 cells, supporting the PR negativity in these cells. Compared to mock transduced cells, 5456 genes were significantly affected by the expression of PRA or PRB in the presence or absence of P4. A Venn diagram (Figure 6A) shows that PRA and PRB regulate different transcriptomes in the WPMY-1 cells, with less than 10% of the genes commonly regulated by both PR isoforms. Real-time qPCR measured 8 representative genes regulated by each PR isoform in the presence of −/+P4, and these outcomes confirmed the results predicted by our microarray studies (Figure 6B).
Figure 6.
A, Venn diagram showed gene transcription regulated by either PRA or PRB in the presence of −/+ 10 nm P4 from gene microarray analyses. Unpaired t test with P value < 0.05, a fold change > 2.0, and a Benjamini-Hochberg multiple testing correction were applied. B, Validation of 8 representative genes regulated by either PRA or PRB in the presence of −/+ 10 nm P4 was performed by real-time qPCR. C and D, GO analysis on the 5654 genes regulated by either PRA or PRB was performed by using the online tool from DAVID Bioinformatics Resources. Both gene numbers and P values of specific gene groups were listed. F, Within the gene group of regulation of cell proliferation, top-ranked 15 genes regulated by PRA or PRB in the presence of −/+ 10 nm P4 were listed.
Gene ontology (GO) analysis further characterized the key cellular processes regulated by each PR isoform. Among the 5456 PR-regulated genes, 32 GO annotation terms were enriched in PRA-regulated genes (P < .001), and 93 GO annotation terms were enriched in PRB-regulated genes (P < .001). All GO terms represented at least 20 genes in each gene group. Top-ranked 15 GO annotated gene groups were listed in Figure 6, C and D. Interestingly, although PRA and PRB regulated different transcriptomes, one of the most top-ranked gene groups affected by both PR isoforms is involved in regulation of cell proliferation (GO:0042127). Within this gene group, 87 genes were regulated by PRA (P = 1.5 × 10−6), and 160 genes were regulated by PRB (P = 1.2 × 10−8). Top 20 gene information is listed in Figure 6F. Other gene groups commonly regulated by both PR isoforms were those involved in the regulation of cell death (GO:0010941), cell adhesion (GO:0007155), cell motion (GO:0006928), response to wounding (GO:0009611), and response to hormone stimulus (GO:0009725). These affected gene groups suggest that both PR isoforms modulate prostate stroma growth and structural remolding in responses to external stimuli. In addition, we also observed distinct gene signatures that were not shared by the 2 PR isoforms. Genes involved in vascular development were preferentially regulated by PRA, whereas genes involved in intracellular signaling and nitrogen compound metabolism were differentially regulated only by PRB.
Discussion
Our studies confirm that both PR isoforms are expressed in a subset of prostate stromal cells. These PR-positive stromal cells can be further grouped by their phenotypes of smooth muscle and/or fibroblast. AR and ER were also shown to be expressed in a subset of prostate stromal cells and to play important roles in prostate development and disease processes. We are now trying to determine whether the subset of PR-positive prostate stromal cells also expresses AR or ER. However, based upon our evidence that PR expression has functional consequences on prostate stromal cell proliferation and gene expression, we propose that PR also likely functions through its effects on the mixed stromal cell compartment of the tissue and/or the cross talk between these cells and prostate epithelial cells.
When exogenous PR was introduced into the cultured prostate stromal cells, PR-expressing cells did not proliferate as robustly as cells that do not express PR. Further studies manifested the suppressive effects of PR on stromal cell cycle S and M phase transition and the expression of cyclinA, cyclinB, and cdc25c in the cells. In contrast, prolonged P4 treatment also decreases breast cancer cell proliferation, but through modulating cyclinD and CDK2 inhibitors, resulting in G1 cell cycle arrest (24). The possible explanation for the different PR signaling could be that prostate stroma is differentiated from the mesoderm, whereas breast epithelium is developed from endoderm during embryo development. Moreover, PRA inhibited the expression of cyclinA, cyclinB, and cdc25c in a ligand-independent manner, whereas PRB inhibited the levels of these proteins in both ligand-dependent and ligand-independent fashions. Together with gene microarray data, our results suggest that PRA and PRB expression delays prostate stromal cell cycle progression through different mechanisms. PR-suppressive effects on prostate stromal cell proliferation may also explain why none of our cultured prostate stromal cells express PR protein. Culture conditions may select for the most robustly growing stromal cells that lack PR expression. The evidence that tissue recombinants (under the renal capsule) created from prostate stromal cells admixed with BPH-1 cells showed that the stromal cells reacquire PR expression. It suggests that the epithelium-stroma interactions are important for maintaining PR expression in the stromal cells. Collectively, our results support the idea that the presence of functional PR in prostate stromal cells may be involved in homeostatic regulation of adult prostate tissue.
Exogenous expression of PRA or PRB also has differential effects on the transcriptomes of prostate stromal cells. Our microarray gene profiling results showed that less than 10% of PR-targeted genes were commonly regulated by both PR isoforms (Figure 6A). But it is of further interest that, although the overwhelming majority of genes changed by PR expression are different between the A and B isoforms, one of the main gene groups commonly regulated by both PR isoforms is the one associated with the control of cell proliferation (Figure 6, C and D). Differential activities of PRA and PRB were reported in several other cell contexts and knockout mouse models (14, 25). These differences were previously shown to be related to the presence of the 2 leucine motifs within the C-terminal activation domain 3 domain of PRB that changes its affinity for coactivator binding, resulting in different transcription activities when compared to PRA (26–28).
PR actions in uterine mesenchymal cells were relatively well studied, when compared with that in prostate stroma. P4/PR signaling stimulates leiomyoma cell proliferation under estradiol-primed conditions (29). Antiprogestins effectively reduce leiomyoma tumor sizes, emphasizing the mitotic effects of PR to aberrant myometrial cell growth (30). In normal endometrium stroma, PR was shown to inhibit DNA synthesis in endometrial epithelial cells in the presence of estradiol (31). These observations support the hypothesis that PR is in coordination with ER to maintain uterine epithelium-stroma homeostasis. Our study also showed mutual regulations between ERα and PR signaling in prostate stromal cells (Supplemental Figure 10) and suggested that both receptors may participate in balancing epithelium and stroma growth in the prostate. This hypothesis is further supported by the gene microarray data that identify PR-regulated genes involved in cell proliferation, cell death, and remodeling. PR may also regulate expression of secretory factors by stromal cells and affect both stromal and epithelial cell proliferation through paracrine pathways. The AR is also expressed in prostate stroma. Due to the high homology in the DBDs of AR and PR, it is hypothesized that AR and PR commonly regulate downstream gene transcription. However, dihydrotestosterone-stimulated AR did not affect mRNA levels of genes that were strongly regulated by PR in WPMY-1 cells (Supplemental Figure 11, A and B), nor did proliferation rates of WPMY-1 cells (Supplemental Figure 11C). Furthermore, when comparing gene microarray data between AR- and PR-regulated genes in the same WPMY-1 cell context, there was only partial overlap between AR-and PR-regulated genes (Supplemental Table 1). In fact, PRA and PRB regulated different transcriptomes in prostate stromal cells in the current study as well as in studies in other cell contexts from previous reports, although they have the identical protein sequences in DBDs and ligand-binding domains. These results together led us to hypothesize that AR and PR mediate different functions in prostate stroma. The possible explanations could be that the expression levels of steroid receptor coregulators in certain prostate stromal cell populations may be favorable to coactivating one receptor over the other. In addition, AR or PR may regulate gene transcription through protein associations with other transcriptional factors, such as activator protein-1 and nuclear factor κB, rather than receptor-DNA interactions on targeted gene promoters.
In summary, we defined the expression of PR isoforms in the human prostate and provided new insights into PR functions in prostate stromal cell proliferation. The P4/PR signaling was well known for its wide range of other functions including differentiation, anti-inflammatory action, and prevention of uterine smooth muscle (25, 32, 33). Whether those PR functions are also involved in the prostate and have any association with prostate diseases such as BPH and prostate cancer warrants further investigation. Fully characterizing the complicated PR signaling will require multiple animal knockout models, tissue recombination grafts, and in vitro cell models.
Acknowledgments
The authors greatly appreciate Robert Bell, Anne Haegert, and Shawn Anderson from Vancouver Prostate Centre for the microarray and statistical analyses; Estelle Li and Rebecca Wu for immunohistochemistry; Antonio Hurtado-Coll for human tissue collection; and former lab member Tingting Wang for participation in this project. We thank Drs David Rowley and Simon Hayward for providing the human prostate stromal.
This work was supported by Canadian Institutes of Health Research (CIHR) Operating Grant (MOP-97934; to X.D.), Rising Star Award from Prostate Cancer Canada (RS2013-58; to X.D.), and PNW Prostate Cancer SPORE pilot grant, National Cancer Institute (P50CA097186; to X.D.), CIHR operating grant (MOP-102904; to Y.W.), and Terry Fox New Frontiers Program Grant/CIHR (to M.G.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- androgen receptor
- BPH
- benign prostatic hyperplasia
- cdc25
- cell division cycle 25
- DBD
- DNA binding domain
- ER
- estrogen receptor
- FACS
- fluorescence-activated cell sorting
- GO
- gene ontology
- IHC
- immunohistochemistry
- P4
- progesterone
- PR
- progesterone receptor
- qPCR
- quantitative PCR
- α-SMA
- smooth muscle α-actin.
References
- 1. Chung LW, Davies R. Prostate epithelial differentiation is dictated by its surrounding stroma. Mol Biol Rep. 1996;23:13–19 [DOI] [PubMed] [Google Scholar]
- 2. Cunha GR. Mesenchymal-epithelial interactions: past, present, and future. Differentiation. 2008;76:578–586 [DOI] [PubMed] [Google Scholar]
- 3. Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nat Rev Urol. 2011;8:29–41 [DOI] [PubMed] [Google Scholar]
- 4. Yu S, Yeh CR, Niu Y, et al. Altered prostate epithelial development in mice lacking the androgen receptor in stromal fibroblasts. Prostate. 2012;72:437–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ho CK, Nanda J, Chapman KE, Habib FK. Oestrogen and benign prostatic hyperplasia: effects on stromal cell proliferation and local formation from androgen. J Endocrinol. 2008;197:483–491 [DOI] [PubMed] [Google Scholar]
- 6. Latil A, Bieche I, Vidaud D, et al. Evaluation of androgen, estrogen (ER α and ER β), and progesterone receptor expression in human prostate cancer by real-time quantitative reverse transcription-polymerase chain reaction assays. Cancer Res. 2001;61:1919–1926 [PubMed] [Google Scholar]
- 7. Bonkhoff H, Fixemer T, Hunsicker I, Remberger K. Progesterone receptor expression in human prostate cancer: correlation with tumor progression. Prostate. 2001;48:285–291 [DOI] [PubMed] [Google Scholar]
- 8. Luetjens CM, Didolkar A, Kliesch S, et al. Tissue expression of the nuclear progesterone receptor in male non-human primates and men. J Endocrinol. 2006;189:529–539 [DOI] [PubMed] [Google Scholar]
- 9. Mobbs BG, Liu Y. Immunohistochemical localization of progesterone receptor in benign and malignant human prostate. Prostate. 1990;16:245–251 [DOI] [PubMed] [Google Scholar]
- 10. Brolin J, Skoog L, Ekman P. Immunohistochemistry and biochemistry in detection of androgen, progesterone, and estrogen receptors in benign and malignant human prostatic tissue. Prostate. 1992;20:281–295 [DOI] [PubMed] [Google Scholar]
- 11. Wernert N, Gerdes J, Loy V, Seitz G, Scherr O, Dhom G. Investigations of the estrogen (ER-ICA-test) and the progesterone receptor in the prostate and prostatic carcinoma on immunohistochemical basis. Virchows Arch A Pathol Anat Histopathol. 1988;412:387–391 [DOI] [PubMed] [Google Scholar]
- 12. Zilliacus J, Wright AP, Carlstedt-Duke J, Gustafsson JA. Structural determinants of DNA-binding specificity by steroid receptors. Mol Endocrinol. 1995;9:389–400 [DOI] [PubMed] [Google Scholar]
- 13. Kastner P, Krust A, Turcotte B, et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9:1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277:5209–5218 [DOI] [PubMed] [Google Scholar]
- 15. Dressing GE, Lange CA. Integrated actions of progesterone receptor and cell cycle machinery regulate breast cancer cell proliferation. Steroids. 2009;74:573–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Musgrove EA, Hamilton JA, Lee CS, Sweeney KJ, Watts CK, Sutherland RL. Growth factor, steroid, and steroid antagonist regulation of cyclin gene expression associated with changes in T-47D human breast cancer cell cycle progression. Mol Cell Biol. 1993;13:3577–3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Owen GI, Richer JK, Tung L, Takimoto G, Horwitz KB. Progesterone regulates transcription of the p21(WAF1) cyclin- dependent kinase inhibitor gene through Sp1 and CBP/p300. J Biol Chem. 1998;273:10696–10701 [DOI] [PubMed] [Google Scholar]
- 18. Gizard F, Robillard R, Gervois P, et al. Progesterone inhibits human breast cancer cell growth through transcriptional upregulation of the cyclin-dependent kinase inhibitor p27Kip1 gene. FEBS Lett. 2005;579:5535–5541 [DOI] [PubMed] [Google Scholar]
- 19. Musgrove EA, Swarbrick A, Lee CS, Cornish AL, Sutherland RL. Mechanisms of cyclin-dependent kinase inactivation by progestins. Mol Cell Biol. 1998;18:1812–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boonyaratanakornkit V, Bi Y, Rudd M, Edwards DP. The role and mechanism of progesterone receptor activation of extra-nuclear signaling pathways in regulating gene transcription and cell cycle progression. Steroids. 2008;73:922–928 [DOI] [PubMed] [Google Scholar]
- 21. Faivre E, Skildum A, Pierson-Mullany L, Lange CA. Integration of progesterone receptor mediated rapid signaling and nuclear actions in breast cancer cell models: role of mitogen-activated protein kinases and cell cycle regulators. Steroids. 2005;70:418–426 [DOI] [PubMed] [Google Scholar]
- 22. Tuxhorn JA, McAlhany SJ, Yang F, Dang TD, Rowley DR. Inhibition of transforming growth factor-β activity decreases angiogenesis in a human prostate cancer-reactive stroma xenograft model. Cancer Res. 2002;62:6021–6025 [PubMed] [Google Scholar]
- 23. Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Groshong SD, Owen GI, Grimison B, et al. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27(Kip1). Mol Endocrinol. 1997;11:1593–1607 [DOI] [PubMed] [Google Scholar]
- 25. Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–1754 [DOI] [PubMed] [Google Scholar]
- 26. Dong X, Challis JR, Lye SJ. Intramolecular interactions between the AF3 domain and the C-terminus of the human progesterone receptor are mediated through two LXXLL motifs. J Mol Endocrinol. 2004;32:843–857 [DOI] [PubMed] [Google Scholar]
- 27. Tung L, Shen T, Abel MG, et al. Mapping the unique activation function 3 in the progesterone B-receptor upstream segment. Two LXXLL motifs and a tryptophan residue are required for activity. J Biol Chem. 2001;276:39843–39851 [DOI] [PubMed] [Google Scholar]
- 28. Giangrande PH, Kimbrel EA, Edwards DP, McDonnell DP. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol. 2000;20:3102–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151:2433–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366:409–420 [DOI] [PubMed] [Google Scholar]
- 31. Kurita T, Young P, Brody JR, Lydon JP, O'Malley BW, Cunha GR. Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology. 1998;139:4708–4713 [DOI] [PubMed] [Google Scholar]
- 32. Hardy DB, Janowski BA, Chen CC, Mendelson CR. Progesterone receptor inhibits aromatase and inflammatory response pathways in breast cancer cells via ligand-dependent and ligand-independent mechanisms. Mol Endocrinol. 2008;22:1812–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Challis JR, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21:514–550 [DOI] [PubMed] [Google Scholar]