Abstract
A new ablation modality, irreversible electroporation (IRE), has been of increasing interest in interventional radiology. Its nonthermal mechanism of action of killing tumor cells allows physicians the ability to ablate tumors in areas previously contraindicated for thermal ablation. This article reviews the current published clinical outcomes, imaging follow-up, and the current knowledge gaps in the procedure for patients treated with IRE.
Keywords: irreversible electroporation, interventional oncology, ablation, kidney, liver, lung, pancreas, interventional radiology
Objectives: Upon completion of this article, the reader will be able to discuss the presumed advantages and disadvantages of irreversible electroporation compared with thermal ablative modalities, and have an appreciation of its current clinical use.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Irreversible electroporation (IRE) is a new ablation modality that uses pulsed electric fields to induce cell death. The technology behind IRE, electroporation, has been in use since the 1960s.1 At a specific threshold of electric potential, a cell membrane lipid bilayer becomes inundated with pores, changes that are reversible at low current. Increasing the electric field strength results in permanent pore formation (hence “irreversible”) and results in cell death. In 2005, Davalos et al2 first demonstrated the usefulness of IRE as an ablation modality, describing significant ablation of liver tissue without thermal effects.
The mostly nonthermal3 nature of IRE has some advantages compared with thermal ablation. Since IRE does not depend on heating or cooling of tissues, ablation of tumors close to major blood vessels is not limited by the heat sink effect.4 IRE also does not seem to have a significant effect on connective tissue, such that ablation of tumors adjacent to sensitive structures such as nerves5 6 and bile ducts4 7 is possible without serious long-term effects.
Clinical Use
There is currently one IRE commercially available device (Nanoknife [AngioDynamics, Latham, NY]), approved for clinical use (CE mark and FDA 501k approval) (Fig. 1). The device can deliver up to 3,000 V and 50 A through either unipolar or bipolar needle electrodes. Although any number of electrodes can be used to enclose a target fully with IRE, pulses are delivered through only one cathode and one anode at a time (i.e., one bipolar electrode or one pair of unipolar electrodes). In addition, if unipolar electrodes are used, the electrodes need to be as parallel as possible to achieve a predictable ablation zone. Ablation zone size can be influenced by length of the active tip (0.5–4 cm), pulse number (typically 70–90), duration of pulses (typically 90–100 µs), distance between probes, and voltage applied.
Figure 1.
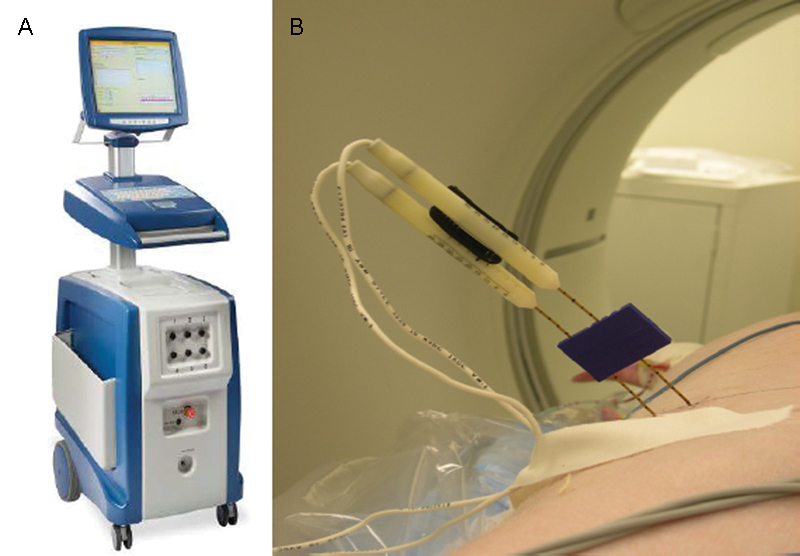
Nanoknife ablation device and needle electrodes. (A) Nanoknife device by AngioDynamics (Latham, NY). (B) Placement of two monopolar electrodes and a blue plastic spacer used to keep the needles parallel.
The procedure is performed under general anesthesia with complete muscle blockade. The patient's electrocardiogram should be synchronized to the IRE device such that pulses are delivered during the refractory cardiac period to prevent dysrhythmias. IRE has been used clinically mainly in the liver, pancreas, kidney, and lung, while new applications are emerging for the prostate,8 9 eye,10 and brain.11 12 13
Liver
Thermal ablative techniques are highly effective in treating liver tumors14; however, there is still a significant population of patients whose tumors are either adjacent to large (> 3 mm) blood vessels or major bile ducts such that thermal ablation would be ineffective or dangerous. IRE may potentially be an ideal ablation modality for those tumors where thermal techniques are contraindicated (Fig. 2).
Figure 2.
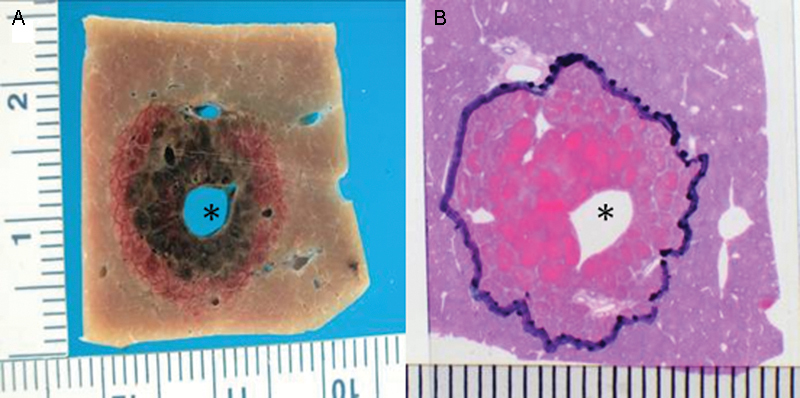
Irreversible electroporation ablation of normal swine liver. Gross pathologic (A) and histopathologic (B) images show complete tissue necrosis adjacent to a large blood vessel (asterisk).
In a single-center prospective nonrandomized cohort, Thomson et al15 studied the safety of liver IRE in 25 patients. Computed tomography (CT) scans at 1 and 3 months were evaluated for treatment efficacy, and laboratory values 24 hours, 1 month, and 3 months after the procedure were evaluated for treatment safety. The authors reported a tumor response rate of 50%, and the failure of IRE to have any effect on tumors larger than 5 cm in any dimension. There was no evidence of liver damage in any of the patients treated even with electrode placement in critical areas.
Kingham et al16 retrospectively evaluated the safety and efficacy of open and percutaneous IRE of perivascular liver tumors in 28 patients. There was a median follow-up of 6 months; four patients (14%) demonstrated recurrence or persistent disease, and one patient had new onset segment 6 portal vein thrombosis.
Cannon et al17 retrospectively reviewed an Institutional Review Board-approved prospective registry of patients undergoing IRE for liver tumors over a 2-year period. In the 44 patients included in the study, the authors found a 1-year local recurrence-free survival rate of 60% and a trend toward higher recurrence rates for tumors over 4 cm (hazard ratio, 3.236; 95% confidence interval, 0.585–17.891; p = 0.178). There were three adverse events recorded in the 90 days following IRE that were possibly procedure related (neurogenic bladder, abdominal pain, and flank pain).
Silk et al7 retrospectively reviewed all percutaneous IRE ablations at a single institution of hepatic tumors within 1 cm of the common, left, or right hepatic ducts, with average follow-up of 9 months. The authors reported a local recurrence rate of 55%, and one patient had new onset bile duct prominence as a result of the thermal energy deposited to tissues in direct contact with the electrode.
From the aforementioned studies, the safety of IRE in the liver has been well documented; however, local control following IRE is relatively low in comparison to thermal techniques. Although this may be due to patient selection and learning curve, IRE should only be used in the liver when thermal ablative techniques are contraindicated.
Pancreas
Percutaneous thermal ablation of the pancreas was described in 1999,18 however, a systematic review concluded that radiofrequency (RF) ablation in this setting, while feasible, has an unacceptably high complication rate without a clear benefit in survival.19 The mechanism of cell death from IRE overcomes the thermal complications, and studies in porcine models20 have demonstrated safety and feasibility of the procedure.
Narayanan et al21 evaluated the safety of percutaneous IRE in 14 patients with pancreatic adenocarcinoma. Twenty-four hours postablation scans showed patent vasculature in the treatment zone. The 6-month overall survival was 70%. There were no severe complications following the procedure, although one patient had grade 2 pancreatitis that resolved without medical intervention.
Martin et al22 evaluated the overall survival in 54 patients with local pancreatic adenocarcinoma and compared their IRE-treated cohort to a matched stage III patients treated with standard therapy. They found an increase in local progression-free survival (14 vs. 6 months, p = 0.01), distant progression-free survival (15 vs. 9 months, p − 0.02), and overall survival (20 vs. 13 months, p − 0.03).
Preliminary results of IRE for primary pancreatic tumors have had very promising results. Current phase 2 trials are underway: “PANFIRE—Pilot-study: Irreversible Electroporation (IRE) to Treat Locally Advanced Pancreatic Carcinoma” (NCT01369420) and “Outcomes of Ablation of Unresectable Pancreatic Cancer Using the NanoKnife Irreversible Electroporation (IRE) System” (NCT02041936), which should be completed by 2015 and 2018, respectively.
Kidney
A major consideration in the treatment of kidney tumors is the preservation of renal function. Thermal ablative techniques are effective for tumors smaller than 4 cm,23 and the majority of studies demonstrate no significant24 to minimal25 decrease in estimated glomerular filtration rate. The preservation of renal tubules and structural proteins following IRE would suggest its role as a favorable modality for treating kidney tumors, especially if the collecting system, renal pelvis, or ureter is in the ablation zone.
Pech et al,26 in a phase 1 ablate and resect clinical study, evaluated the safety and immediate histology following intraoperative IRE of six patients with renal cell carcinoma. They reported no treatment complications; however, due to the time window in which cell histology was examined, they could not make a conclusion toward the effectiveness of IRE.
Thomson et al,15 in a single-center prospective nonrandomized cohort studied the safety of kidney IRE in seven patients with 10 tumors when the treatment zone included portion of the ureter. These authors reported two cases of transient hematuria (< 24 hours) in two patients with IRE treatment including the central portion of the kidney. One ureter previously damaged by RF ablation required a ureteric stent after IRE, and one patient had unplanned insertion of an electrode tip into the adrenal gland. The procedure produced transient hypertension and the patient reported postural hypotension for 2 months following the procedure. CT follow-up at 3 months demonstrated complete ablation of 5 of 10 tumors, with IRE failing to ablate the entire tumor if larger than 3.5 cm.
Kidney IRE has a reasonable safety profile but further studies are required to assess its efficacy compared with thermal techniques. Preliminary results seem promising but long-term efficacy has not been established.
Lung
A limitation with thermal ablation in lung parenchyma is the difficult assessment of treatment efficacy postablation. Often, thermal ablation creates a spiculated hyperdensity that is difficult if not impossible to truly differentiate from viable tumor.27 A proposed advantage of IRE in the lung is that it should be able to ablate tumors and not leave an ablation defect obscuring evaluation of treatment efficacy (Fig. 3).
Figure 3.
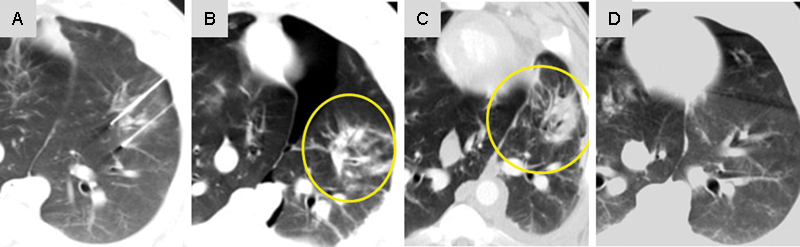
Irreversible electroporation of swine lung showing CT imaging (A) during treatment, (B) immediately after (yellow circle around ablation defect), (C) 1 week after treatment (yellow circle around ablation defect), and (D) 3 weeks after treatment demonstrating resolution of the ablation defect.
Thomson et al15 studied the safety of lung IRE in four patients. Two patients were treated for metastatic colorectal carcinoma, one for metastatic breast carcinoma and one for non-small cell lung carcinoma (NSCLC). Two of the four patients were found to have clinically insignificant pneumothoraces postablation, both of which resolved spontaneously. In their study, the postablation findings mimicked those found following thermal ablation. All four patients showed progression of disease at 3 months.
Usman et al28 described two cases of lung IRE for metastatic synovial cell sarcoma and NSCLC. Both patients tolerated the procedure without major adverse effects. Six-month follow-up imaging demonstrated enlargement of the treated tumor and were described as treatment failures.
Silk et al29 reported one case of lung IRE for metastatic papillary thyroid carcinoma. There were no procedure-related complications in this patient, and 3-month cross-sectional imaging showed a significant reduction in the size of the ablation zone and resolution of FDG positron emission tomography avidity.
One major drawback of IRE in the lung is the difficulty in positioning the electrodes. Probes must be placed in parallel fashion, and interposition of ribs makes for a difficult approach. In addition, mathematical modeling of IRE in the lung shows dramatic differences in ablation zones due to the differences in conductivity between tumor, lung parenchyma, and air.30 The benefits of IRE in the lung over thermal ablation modalities are less clear.
Anesthesia Use with Irreversible Electroporation
A major consideration when choosing IRE as an ablation modality is the requirement for general anesthesia31 with complete muscle blockade (paralysis) to reduce muscle stimulation from the treatment. Even with complete muscle blockade, local muscle stimulation in close proximity to IRE is common. An electrocardiogram synchronizer should be used to minimize the risk of dysrhythmias. Attention to the position of the arms is required to maximize CT scan quality but minimize brachial plexus strain. Simple postoperative analgesia is all that is required in most patients.
Imaging Findings following Irreversible Electroporation
Computed Tomography
CT imaging findings after IRE are similar to those following RF ablation (Fig. 4). Areas in the liver treated by IRE appear hypodense in comparison with normal liver parenchyma. In contrast-enhanced studies immediately following ablation, arterial enhancement is commonly seen and was not associated with tumor recurrence.32 In addition, gas densities are frequently also seen in ablation areas from electrolysis. Due to the reduced scarring after ablation, normal tissue is proposed to be able to heal faster, resulting in rapid involution of the ablation defect. An ablation defect that does not diminish over time has been associated with tumor recurrence,32 but guidelines for expected involution have not been validated.
Figure 4.
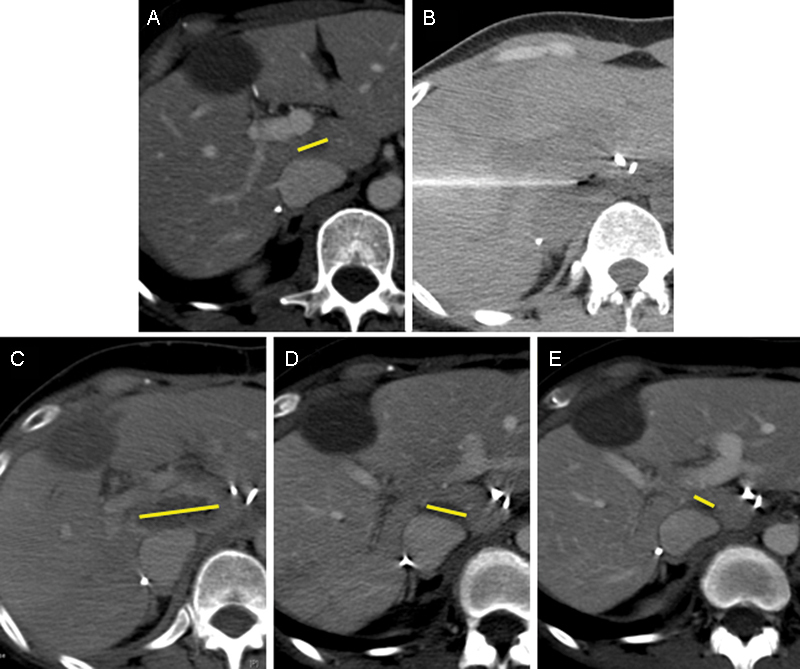
CT imaging of a 48-year-old woman with metastatic colon cancer to the liver. (A) Pretreatment tumor imaging (yellow bar shows major axis of tumor). (B) Treatment CT image after placement of two monopolar electrodes in the liver. (C) Immediate postprocedure imaging demonstrating hypodense ablation zone (yellow bar shows major axis of ablation zone). (D) One-month postablation imaging showing an involution of the ablation cavity (yellow bar shows major axis of ablation zone). (E) Four-month postablation imaging showing continued involution of the ablation cavity (yellow bar shows major axis of ablation zone). CT, computed tomography.
Ultrasound
Unlike thermal ablation where thermal artifacts interfere with margin measurements, ultrasound (US) has been shown to be an accurate method for measuring IRE ablation margins in porcine models33 34 (Fig. 5). Areas treated by IRE appear hyperechoic with increased stiffness on elastography. Pathologic correlation to US findings have strong correlation coefficients (Pearson = 0.7).
Figure 5.
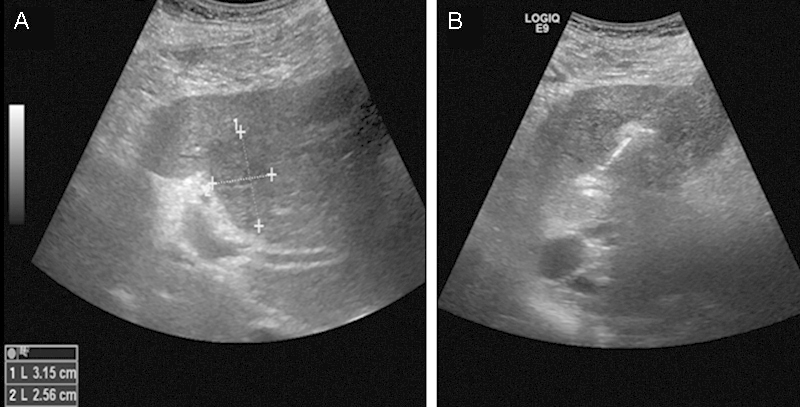
Ultrasound images during IRE of a 76-year-old man with metastatic colon cancer to the liver. (A) Targeted tumor dimensions. (B) Image obtained half way through the treatment. The area treated by IRE becomes hyperechoic compared with surrounding liver tissue. IRE, irreversible electroporation.
Magnetic Resonance Imaging
Any tissue treated by IRE has a zone of irreversibly treated tissue and a penumbra of reversibly electroporated tissue. Hyperintense regions on T1-weighted gradient recalled echo was shown to have a strong correlation (Pearson = 0.891) with necrotic areas in a rat model.35
Knowledge Gaps in Irreversible Electroporation
Incomplete Ablation within the Tumor and Optimal Device Settings
Recent evidence has surfaced questioning the ability of IRE to destroy tumor tissue in the same way it destroys normal tissue. The original report by Rubinsky et al36 had described the threshold for IRE of cells to be approximately 600 V/cm with 8 pulses, a pulse duration of 100 µs, and a frequency of 10 Hz. Recent reports have described varying voltage thresholds, pulse number, and pulse duration to effectively kill tumor cells.37 38 Qin et al found that even at 1,300 V/cm with 99 pulses, a pulse duration of 100 µs, and 10 Hz, there were still islands of viable tumor cells seen.38 This brings into question a potential flaw in the assumption that tumor tissue will have the same response to IRE as normal tissue. The mechanism of cell death following IRE relies on cell apoptotic responses to loss of homeostasis from pore formation. Tumor cells, known to be resistant to apoptotic pathways, may require higher thresholds to be adequately treated, analogous to increased chemotherapy levels required for tumor cell death.
Local Environment Effects Ablation Zone
Electric fields are strongly influenced by the conductivity of the local environment, and thus IRE treatment zones can be altered dramatically if tissue conductance varies or if metal (e.g., biliary stents) are located in the ablation zone. The positioning of needles either parallel, perpendicular, or in plane with muscle striations have resulted in varying shapes of ablation zones.39 In the kidney, ablations of more central renal tissue resulted in irregularly shaped ablation zones with comma-shaped tails deviating into the collecting system due to increased conductivity of urine.39 Although IRE may be able to avoid heat-sink limitations, tissue composition and electrical characteristics drastically alter ablation shapes. Electrical attributes of tumors and surrounding normal tissue represent a challenge toward predictable IRE ablation zones.
Thermal versus Nonthermal Damage
The mechanism for cell death created by IRE is nonthermal; however, the pulsed electric fields still create ohmic heating.40 IRE has been shown to create thermal effects41 42 that could cause thermal damage if parameters are not chosen correctly. Faroja et al42 have pointed out that IRE can have a thermal effect, but only with device settings that are typically not used clinically (i.e., unusual in high pulse repetitions [> 270 pulses] and voltage settings [> 2,900 V]). However, tissues in immediate contact with electrodes will experience ohmic heating and direct contact with vital structures should be avoided.
Conclusion
IRE is an exciting new modality that can extend patient care to those with contraindications for thermal ablation. More studies are needed to optimize device settings, probe positioning, and treatment parameters before IRE becomes more mainstream. In scenarios where sensitive surrounding structures or major blood vessels are present, IRE should be considered a potential option.
References
- 1.Coster H G. A quantitative analysis of the voltage-current relationships of fixed charge membranes and the associated property of “punch-through”. Biophys J. 1965;5(5):669–686. doi: 10.1016/S0006-3495(65)86745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davalos R V, Mir I L, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33(2):223–231. doi: 10.1007/s10439-005-8981-8. [DOI] [PubMed] [Google Scholar]
- 3.Breton M, Mir L M. Microsecond and nanosecond electric pulses in cancer treatments. Bioelectromagnetics. 2012;33(2):106–123. doi: 10.1002/bem.20692. [DOI] [PubMed] [Google Scholar]
- 4.Charpentier K P, Wolf F, Noble L, Winn B, Resnick M, Dupuy D E. Irreversible electroporation of the liver and liver hilum in swine. HPB (Oxford) 2011;13(3):168–173. doi: 10.1111/j.1477-2574.2010.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoellnast H, Monette S, Ezell P C. et al. Acute and subacute effects of irreversible electroporation on nerves: experimental study in a pig model. Radiology. 2011;260(2):421–427. doi: 10.1148/radiol.11103505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoellnast H, Monette S, Ezell P C. et al. The delayed effects of irreversible electroporation ablation on nerves. Eur Radiol. 2013;23(2):375–380. doi: 10.1007/s00330-012-2610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silk M T, Wimmer T, Lee K S. et al. Percutaneous ablation of peribiliary tumors with irreversible electroporation. J Vasc Interv Radiol. 2014;25(1):112–118. doi: 10.1016/j.jvir.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Neal R E II, Smith R L, Kavnoudias H. et al. The effects of metallic implants on electroporation therapies: feasibility of irreversible electroporation for brachytherapy salvage. Cardiovasc Intervent Radiol. 2013;36(6):1638–1645. doi: 10.1007/s00270-013-0704-1. [DOI] [PubMed] [Google Scholar]
- 9.Onik G, Mikus P, Rubinsky B. Irreversible electroporation: implications for prostate ablation. Technol Cancer Res Treat. 2007;6(4):295–300. doi: 10.1177/153303460700600405. [DOI] [PubMed] [Google Scholar]
- 10.Mandel Y, Laufer S, Belkin M, Rubinsky B, Pe'er J, Frenkel S. Irreversible electroporation of human primary uveal melanoma in enucleated eyes. PLoS ONE. 2013;8(9):e71789. doi: 10.1371/journal.pone.0071789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia P A, Rossmeisl J H Jr, Davalos R V. Electrical conductivity changes during irreversible electroporation treatment of brain cancer. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:739–742. doi: 10.1109/IEMBS.2011.6090168. [DOI] [PubMed] [Google Scholar]
- 12.Hjouj M, Last D, Guez D. et al. MRI study on reversible and irreversible electroporation induced blood brain barrier disruption. PLoS ONE. 2012;7(8):e42817. doi: 10.1371/journal.pone.0042817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neal R E II, Rossmeisl J H Jr, D'Alfonso V. et al. In vitro and numerical support for combinatorial irreversible electroporation and electrochemotherapy glioma treatment. Ann Biomed Eng. 2013;42(3):475–487. doi: 10.1007/s10439-013-0923-2. [DOI] [PubMed] [Google Scholar]
- 14.Gervais D A Goldberg S N Brown D B Soulen M C Millward S F Rajan D K Society of Interventional Radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors J Vasc Interv Radiol 200920(7, Suppl):S342–S347. [DOI] [PubMed] [Google Scholar]
- 15.Thomson K R, Cheung W, Ellis S J. et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol. 2011;22(5):611–621. doi: 10.1016/j.jvir.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Kingham T P, Karkar A M, D'Angelica M I. et al. Ablation of perivascular hepatic malignant tumors with irreversible electroporation. J Am Coll Surg. 2012;215(3):379–387. doi: 10.1016/j.jamcollsurg.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 17.Cannon R, Ellis S, Hayes D, Narayanan G, Martin R C II. Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J Surg Oncol. 2013;107(5):544–549. doi: 10.1002/jso.23280. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg S N, Mallery S, Gazelle G S, Brugge W R. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc. 1999;50(3):392–401. doi: 10.1053/ge.1999.v50.98847. [DOI] [PubMed] [Google Scholar]
- 19.Pezzilli R, Serra C, Ricci C. et al. Radiofrequency ablation for advanced ductal pancreatic carcinoma: is this approach beneficial for our patients? A systematic review. Pancreas. 2011;40(1):163–165. doi: 10.1097/MPA.0b013e3181eab751. [DOI] [PubMed] [Google Scholar]
- 20.Bower M, Sherwood L, Li Y, Martin R. Irreversible electroporation of the pancreas: definitive local therapy without systemic effects. J Surg Oncol. 2011;104(1):22–28. doi: 10.1002/jso.21899. [DOI] [PubMed] [Google Scholar]
- 21.Narayanan G, Hosein P J, Arora G. et al. Percutaneous irreversible electroporation for downstaging and control of unresectable pancreatic adenocarcinoma. J Vasc Interv Radiol. 2012;23(12):1613–1621. doi: 10.1016/j.jvir.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Martin R C II, McFarland K, Ellis S, Velanovich V. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann Surg Oncol. 2013;20 03:S443–S449. doi: 10.1245/s10434-012-2736-1. [DOI] [PubMed] [Google Scholar]
- 23.Zagoria R J, Pettus J A, Rogers M, Werle D M, Childs D, Leyendecker J R. Long-term outcomes after percutaneous radiofrequency ablation for renal cell carcinoma. Urology. 2011;77(6):1393–1397. doi: 10.1016/j.urology.2010.12.077. [DOI] [PubMed] [Google Scholar]
- 24.Pettus J A, Werle D M, Saunders W. et al. Percutaneous radiofrequency ablation does not affect glomerular filtration rate. J Endourol. 2010;24(10):1687–1691. doi: 10.1089/end.2010.0029. [DOI] [PubMed] [Google Scholar]
- 25.Park S Y, Park B K, Kim C K. et al. Percutaneous radiofrequency ablation of renal cell carcinomas in patients with von Hippel Lindau disease previously undergoing a radical nephrectomy or repeated nephron-sparing surgery. Acta Radiol. 2011;52(6):680–685. doi: 10.1258/ar.2011.100435. [DOI] [PubMed] [Google Scholar]
- 26.Pech M, Janitzky A, Wendler J J. et al. Irreversible electroporation of renal cell carcinoma: a first-in-man phase I clinical study. Cardiovasc Intervent Radiol. 2011;34(1):132–138. doi: 10.1007/s00270-010-9964-1. [DOI] [PubMed] [Google Scholar]
- 27.Zhu J C, Yan T D, Glenn D, Morris D L. Radiofrequency ablation of lung tumors: feasibility and safety. Ann Thorac Surg. 2009;87(4):1023–1028. doi: 10.1016/j.athoracsur.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Usman M, Moore W, Talati R, Watkins K, Bilfinger T V. Irreversible electroporation of lung neoplasm: a case series. Med Sci Monit. 2012;18(6):CS43–CS47. doi: 10.12659/MSM.882888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silk M, Wimmer T, Getrajdman G. et al. Safety of irreversible electroporation (IRE) treatment for metastatic disease in humans. J Vasc Interv Radiol. 2013;24:S21–S22. [Google Scholar]
- 30.Srimathveeravalli G, Wimmer T, Silk M. et al. Treatment planning considerations for IRE in the lung: placement of needle electrodes is critical. J Vasc Interv Radiol. 2013;24:S22. [Google Scholar]
- 31.Ball C, Thomson K R, Kavnoudias H. Irreversible electroporation: a new challenge in “out of operating theater” anesthesia. Anesth Analg. 2010;110(5):1305–1309. doi: 10.1213/ANE.0b013e3181d27b30. [DOI] [PubMed] [Google Scholar]
- 32.Silk M T Lee H M Wimmer T et al. Evolution of the ablation zone following irreversible electroporation of liver tumors on computed tomography and possible predictors of recurrence Paper Presented at: World Conference of Interventional Oncology; May 17, 2013; New York, NY
- 33.Au J T, Kingham T P, Jun K. et al. Irreversible electroporation ablation of the liver can be detected with ultrasound B-mode and elastography. Surgery. 2013;153(6):787–793. doi: 10.1016/j.surg.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt C R, Shires P, Mootoo M. Real-time ultrasound imaging of irreversible electroporation in a porcine liver model adequately characterizes the zone of cellular necrosis. HPB (Oxford) 2012;14(2):98–102. doi: 10.1111/j.1477-2574.2011.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo Y, Zhang Y, Nijm G M. et al. Irreversible electroporation in the liver: contrast-enhanced inversion-recovery MR imaging approaches to differentiate reversibly electroporated penumbra from irreversibly electroporated ablation zones. Radiology. 2011;258(2):461–468. doi: 10.1148/radiol.10100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality—clinical implications. Technol Cancer Res Treat. 2007;6(1):37–48. doi: 10.1177/153303460700600106. [DOI] [PubMed] [Google Scholar]
- 37.Neal R E II, Singh R, Hatcher H C, Kock N D, Torti S V, Davalos R V. Treatment of breast cancer through the application of irreversible electroporation using a novel minimally invasive single needle electrode. Breast Cancer Res Treat. 2010;123(1):295–301. doi: 10.1007/s10549-010-0803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin Z, Jiang J, Long G, Lindgren B, Bischof J C. Irreversible electroporation: an in vivo study with dorsal skin fold chamber. Ann Biomed Eng. 2013;41(3):619–629. doi: 10.1007/s10439-012-0686-1. [DOI] [PubMed] [Google Scholar]
- 39.Ben-David E, Ahmed M, Faroja M. et al. Irreversible electroporation: treatment effect is susceptible to local environment and tissue properties. Radiology. 2013;269(3):738–747. doi: 10.1148/radiol.13122590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davalos R V, Rubinsky B. Temperature considerations during irreversible electroporation. Int J Heat Mass Transfer. 2008;51:5617–5622. [Google Scholar]
- 41.Olweny E O, Kapur P, Tan Y K, Park S K, Adibi M, Cadeddu J A. Irreversible electroporation: evaluation of nonthermal and thermal ablative capabilities in the porcine kidney. Urology. 2013;81(3):679–684. doi: 10.1016/j.urology.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 42.Faroja M, Ahmed M, Appelbaum L. et al. Irreversible electroporation ablation: is all the damage nonthermal? Radiology. 2013;266(2):462–470. doi: 10.1148/radiol.12120609. [DOI] [PubMed] [Google Scholar]


