Abstract
Study Design Review.
Objective To describe a decision framework that incorporates key factors to be considered for optimal treatment of spinal metastasis and highlight how this system incorporates the Spinal Instability Neoplastic Score (SINS).
Methods We describe how treatment options for spinal metastasis have broadened in recent years with advancements in stereotactic radiosurgery, vertebral augmentation, and other minimally invasive techniques. We discuss classification-based approaches to the treatment of spinal metastasis versus principles-based approaches and argue that the latter may be more appropriate for optimal patient informed consent. Case examples are provided.
Results Scoring systems at best produce an estimate of life expectancy but fall short in incorporating all of the relevant factors that determine which treatment(s) may be indicated. We advocate a principle-based decision framework called LMNOP that considers: (L) location of disease with respect to the anterior and/or posterior columns of the spine and number of spinal levels involved (contiguous or non-contiguous); (M) mechanical instability as graded by SINS; (N) neurology (symptomatic epidural spinal cord compression); (O) oncology (histopathologic diagnosis), particularly with respect to radiosensitivity; and (P) patient fitness, patient wishes, prognosis (which is mostly dependent on tumor type), and response to prior therapy.
Conclusions LMNOP is the first systematic approach to spinal metastasis that incorporates SINS. It is easy to remember, it addresses clinical factors not directly addressed by other systems, and it is adaptable to changes in technology.
Keywords: spinal metastasis, spinal instability, cord compression, instrumented spine fusion, kyphoplasty, vertebroplasty, SINS, LMNOP
Introduction
Spinal metastasis is a complex condition requiring a multidisciplinary approach to therapy. Many variables reflecting the disease, patient, and treatment factors need to be taken into account for an optimal outcome. In general, the goals of therapy are palliative and include pain control, spinal stability, improving or maintaining neurologic function, and control of tumor growth. As systemic treatments improve, patients are living longer, and thus there is more emphasis on the need for durable functional outcomes and prevention of local tumor recurrence.
The surgical axiom to achieve maximum-quality clinical outcomes with the least invasive treatment remains at the core of the decision-making process. Treatment choices are complex because they depend on many interrelated clinical factors and patient-informed choice. Furthermore, several treatment options have developed in recent years, including stereotactic radiosurgery (SRS), minimally invasive spinal surgery (MIS), and vertebroplasty or kyphoplasty. The Spine Instability Neoplastic Score (SINS) is a relatively new tool that provides a reliable method to describe spinal instability in tumors, and will hopefully lead to a more consistent therapeutic approach to surgical decision making.1 2
We will review a decision framework that incorporates all of the key factors that need to be considered in the treatment of spinal metastasis. We will also highlight how this system incorporates the SINS as well as the latest technical advances.
Disease Factors
The thoracic spine is the most frequent site of spinal metastasis, followed by the lumbar, cervical, and sacral regions.3 The most common primary tumors metastasizing to the spine reside in the lung, breast, marrow, kidney, and prostate.4 5
Pain is the most common initial symptom, which is present in more that 95% of patients with spinal metastasis.6 7 Three types of pain have been described in the context of spinal metastasis: local (or “biologic”) pain, radicular pain, and mechanical pain. Local pain is described as constant, which usually does not worsen with movement and does not improve with recumbency. It can be localized close to the lesion site, and palpation or gentle percussion over the vertebral spinous process may assist in localization. This type of pain has been associated with the tumor itself. The exact etiology is unclear, but it may be partially attributed to stretching of the vertebral body periosteum by the tumor. Radicular pain is described as constant, which may be worsened or relieved with movement or changes in posture, and is usually associated with dysesthesia. It characteristically radiates in the dermatomal distribution and is related to nerve root compression by tumor or pathologic fracture. Mechanical pain worsens with movement and standing upright, and is characteristically relieved or significantly improved with recumbency. It is a significant cause of morbidity and is related to instability of the spinal column caused by the tumor.
Neurologic deficits may be present by the time of the diagnosis.7 8 Once neurologic symptoms appear, the natural history seems to be the progression of the deficits to complete paraplegia, albeit at variable rates.9
Treatment Factors
Treatment options for spinal metastasis have significantly evolved over the past 25 years.10 The earliest surgical approach, laminectomy, proved to be inadequate, leading to poor pain control and destabilization of the spine. With the development of instrumentation to effectively reconstruct and stabilize the spine, surgical methods have become more sophisticated, including corpectomy or spondylectomy via either anterior or extracavitary posterolateral approaches.11 12 In 2005, Patchell et al conclusively demonstrated that direct decompressive surgery plus postoperative radiation therapy was superior to radiotherapy alone in patients with symptomatic spinal cord compression due to metastasis.13
More recently, the development of MIS approaches has provided new ways of achieving surgical decompression and spinal stability. Approaches utilizing tubular retractors and percutaneous pedicle screws,14 as well as video-assisted thoracoscopic surgery,15 have been advocated, but rigorous studies comparing these new techniques to traditional open approaches have not been done yet.
Vertebral augmentation with vertebroplasty or kyphoplasty may be used to address mechanical pain from vertebral body fractures in the absence of severe instability or spinal cord compression. These techniques are particularly useful in patients with very limited life expectancy who hope to avoid open surgery and those with very poor bone quality (e.g., myeloma bone disease).16 The prospective randomized Cancer Fracture Evaluation (CAFÉ) trial showed superiority of kyphoplasty over nonoperative care in patients with cancer and vertebral compression fractures, with a similar rate of adverse events between treatment arms.17
Radiation therapy has also developed new treatment paradigms over the last several years. In patients with minimal epidural disease, SRS can be used to deliver radiation more precisely to the tumor, significantly reducing the dose to the spinal cord, and allowing greater dose delivery per fraction. This advance allows durable tumor control rates independent of tumor histology-specific radiosensitivity to conventional external beam radiation therapy (cEBRT). In other words, so-called radioresistant tumors such as renal cell carcinoma may be treated with SRS. As well, SRS may be used after cEBRT has failed. Both hypofractionated SRS (typically 18 to 36 Gy in five or six fractions) and more recently single-fraction SRS (24 Gy) have been advocated.18 Higher doses produce superior local control but are associated with vertebral compression fracture.19 Laufer et al have shown durable tumor control after only limited decompression of spinal metastasis (so-called separation surgery) to reconstitute the CSF space around the cord, combined with adjuvant SRS.18 This treatment paradigm nicely demonstrates the potential for MIS and SRS to work together to achieve the best outcomes.
Pharmacologic agents available for treatment of patients with spinal metastasis include hormonal agents, chemotherapeutic agents, bisphosphonates, and steroids.20 Hormonal agents have been used in patients with prostate cancer and breast cancer. Chemotherapeutic agents have a limited role in treating spinal metastasis, except in cases of Ewing's sarcoma and neuroblastoma, which are chemosensitive tumors. Bisphosphonates may be used to reduce the risk of pathologic fracture by inhibiting osteoclastic activity. Corticosteroids can be used to decrease tumor-associated inflammation and decrease spinal cord edema.
Patient Factors
Patients with spinal metastasis can present with wide range of comorbidities and length of expected survival.5 Also, in the Internet era, patients have become increasingly informed about the disease process and the current treatment options. Incorporating well-informed patients' wishes in the decision-making process is especially important in the palliative setting.21 One of the most difficult skills for the surgeon to learn is when disease has advanced to the point that surgery is likely to impart more harm than good. In the next section, we will discuss how classification systems can help determine the approach, based on an estimate of life expectancy.
Classification-Based Approaches to Decision Making
Classification systems, in particular those described by Tokuhashi et al22 23 and Tomita et al,24 are well-established methods to estimate life expectancy in patients with spinal metastasis. A major drawback is that the relative weighting of different categories may change in diverse patient populations. Some important aspects of care, such as the response to previous therapies, are not addressed by these systems. Life expectancy can change for certain tumor types with the development of systemic therapies, so systems need to be continuously updated.
Balain et al recently described the Oswestry Risk Index (OSRI), which condenses and combines the Tomita and Tokuhashi systems into one simplified score.21 The most predictive items were combined: the Primary Tumor Pathology score from the Tomita system and the General Condition score from the Tokuhashi system. Also, the Primary Tumor Pathology index from the Tomita system was expanded by adding a fourth category, “very rapid growth” with a score of 5, to include primary tumor of the lung. The OSRI score ranges from 1 to 7 and is grouped into five Risk Index categories, with associated median survival times and 85th percentile survival times. The OSRI system provides a useful tool for estimating survival times, taking into account disease (Primary Tumor) as well as patient factors (General Condition), and provides useful guidance in identifying patients who will not benefit from surgery: those with the shortest survival times. However, it does not provide any recommendation for the type of surgery for patients who may benefit from it.
In essence, this is the major limitation of all of these systems: at best, they provide guidance on who not to operate on (i.e., reduced life expectancy). However, they provide very little guidance with regards to which treatment(s) may be indicated. For example, the surgical strategies advocated by Tokuhashi et al include “conservative treatment,” “palliative surgery” and “excisional surgery.”23 Similarly, Tomita et al advocate a range of treatments including “supportive care,” “palliative surgery,” “marginal or intralesional surgery,” and “wide or marginal excision.”24 None incorporates newer treatment modalities such as kyphoplasty. Also, none consider the previous therapies the patient has failed, which may help predict response to other treatments and help determine risk (e.g., wound infection with surgery performed through an irradiated field).
Principle-Based Approach to Decision Making
The “principle-based” approach does not include an algorithm based solely on a score. Instead, decision making is based on a framework of key clinical considerations, recognizing that the care of each patient remains highly individualized for metastatic disease. Bilsky and Smith have described an approach called NOMS, which addresses neurologic (N) and oncologic (O) disease, mechanical instability (M), and systemic disease (S).25 The NOMS framework represents a comprehensive approach to spinal metastases, which takes into consideration patient-related (S) as well as disease-related (N, O, M) factors. However, mechanical instability is discussed only in the context of movement-related pain. Considering that spine instability is one of the major causes of morbidity in patients with spinal metastasis, a framework that addresses the instability question in greater detail is required.
LMNOP
We proposed a decision-making strategy for spinal metastasis called LMNOP, which incorporates: location and level(s) of spinal disease (L); mechanical instability (M); neurology (N); oncology (O); patient fitness, prognosis, patient wishes and prior therapy (P).26 LMNOP includes three key considerations that were only indirectly addressed by NOMS: (1) the location of disease with respect to the anterior and/or posterior columns of the spine; (2) the number of spinal levels involved with tumor, contiguous or noncontiguous; and (3) the response to previous therapies. In addition, it is the first surgical decision aid for spinal metastasis that is integrated with the SINS.
Location/Level (L)
Within individual vertebra, the well-vascularized vertebral body is the most common site of tumor involvement (80%).3 Therefore, epidural compression most commonly occurs from a ventral or ventrolateral direction. The location of disease compressing the spinal cord is obviously a key consideration in determining the approach to facilitate decompression. In addition, the level of the tumor involvement significantly influences the choice of surgical approach.
For lesions from C0 to C2, posterior stabilization and adjuvant radiation therapy is the preferred approach. Due to the large central canal at this region, symptomatic cord compression is relatively uncommon: most patients present with mechanical pain.27 Anterior approaches (transoral, transpharyngeal) are associated with significant morbidity and are therefore relatively contraindicated considering the palliative goals of the surgery.
For lesions from C3 to C6, both anterior and anterior/posterior approaches have been advocated. Anterior approaches are indicated in most cases undergoing surgical resection. However, combined anterior/posterior approaches are preferred in cases with multilevel disease, circumferential tumor involvement, severe instability, and poor bone quality.28 For lesions at the C7–T1 level, both anterior and posterior approaches can be employed; however, supplemental posterior stabilization is usually required.29
For lesions from T2 to T5, posterolateral approaches have been recommended.30 The location of great vessels and the heart complicate anterior approaches. For lesions from T6 to L5, a wide spectrum of approaches (anterior, posterior, and circumferential) have been described.11 30 The choice of approach depends on the goals of the surgery, and the experience and preference of the surgeon.
For lesions in the sacrum, surgical treatment is rarely indicated. The sacral canal has the capacity to accommodate large tumor volumes before developing neurologic symptoms. Also, the degree of bone destruction needed to cause the instability is substantial. Most often, sacral metastases do not cause significant instability, and palliative radiation therapy is indicated. If surgery is required, posterior approaches have been used most often.31 32 33
Multilevel involvement of the spine is not uncommon in metastatic spine disease. In some cases, two or more noncontiguous levels are involved.34 Usually, only one particular level is symptomatic, which needs to be identified accurately to provide optimum surgical intervention.
Mechanical Instability (M)
Mechanical instability is perhaps the most common indication for surgery in patients with metastatic spinal disease. It has been defined as the “loss of spinal integrity as a result of a neoplastic process that is associated with movement-related pain, symptomatic or progressive deformity and/or neural compromise under physiological loads.”1 Tumor related instability is graded by SINS using a combination of six clinical and radiologic criteria. Each component is assigned a score reflecting its contribution to the overall instability of the spinal segment.
The Spine Location score reflects the inherent stability of the various segments of the spine. The spine is divided into “junctional” (C0–C2, C7–T2, T11–L1, L5–S1), “mobile” (C3–C6, L2–L4), “semirigid” (T3–T10), and “rigid” (S2–S5) segments, and the scores of 3, 2, 1, and 0 are assigned, respectively.
The Mechanical Pain score reflects the presence of mechanical pain. Patients who have pain with movement, upright posture, or loading of the spine that is relieved or improved with recumbence receive a score of 3. Patients who present with local biologic pain without mechanical characteristics receive a score of 1. Nonpainful lesions are scored as 0.
The Bone Lesion Quality score reflects the presence of bone destruction. Lytic lesions receive a score of 2, blastic (sclerotic) lesions receive a score of 0, and mixed lesions are scored as 1.
The Spinal Alignment score can be assessed with serial radiographs, or by comparing supine and upright radiographs. Lesions with subluxation or translation receive a score of 4, those with a de novo deformity (kyphosis or scoliosis) without subluxation or translation receive a score of 2, and those with normal alignment receive a score of 0.
The Vertebral Body Collapse score reflects the extent of vertebral body involvement and collapse. Lesions with greater than 50% collapse receive 3 points, those with less than 50% collapse receive 2 points, those with no collapse but greater than 50% vertebral body involvement receive a score of 1, and those with no collapse and less than 50% vertebral body involvement receive a score of 0.
The Posterolateral Involvement of Spinal Elements score reflects the extent of involvement of the pedicles, facets, and/or costovertebral joints. Lesions with bilateral involvement receive a score of 3; those with unilateral involvement of these elements receive a score of 1; and those without posterolateral involvement receive a score of 0.
The total score of the six individual components (from 0 to 18) reflects the degree of spine instability. SINS of 0 to 6 denotes a “stable” lesion, 7 to 12 denotes a “potentially unstable” lesion, and 13 to 18 denotes an “unstable” lesion. Surgical consultation is recommended for patients with SINS of 7 to 18.
In terms of treatment decision making, patients in the “potentially unstable” group (SINS 7 to 12) who do not have spinal cord compression may be treated with less invasive procedures such as kyphoplasty or vertebroplasty, while supplemental pedicle screw fixation and multilevel constructs are more often indicted for those with more overtly unstable lesions (SINS 13 to 18).
Figs. 1 and 2 illustrate examples of scoring and demonstrate two patients with SINS score of 12, categorized as “potentially unstable.” Individualized surgical treatment options were used in these two cases, taking into account oncology, neurology, and patient factors. The younger patient in Fig. 1 with few comorbidities and with no history of previous radiation underwent a more aggressive surgery aiming for local tumor control, and the older patient in Fig. 2 with immunocompromise and previous radiation underwent less aggressive surgery with the goal of symptomatic management.
Fig. 1.
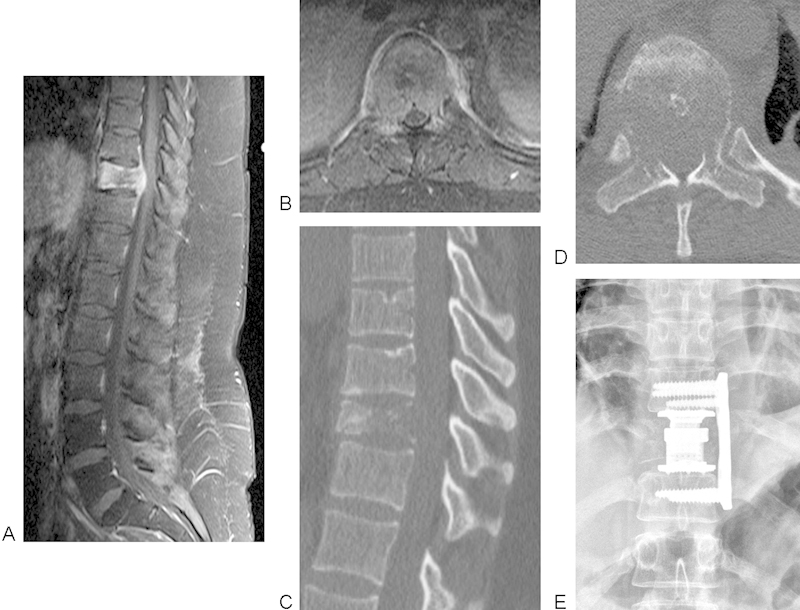
Sagittal (A) and axial (B) postcontrast magnetic resonance imaging and computed tomography scan (C, D) of a 24-year-old woman with a history of metastatic squamous cell carcinoma of the cervix who presented with back pain. Although there was radiologic spinal cord compression, she only had T10 radiculopathy (no symptoms or signs of myelopathy). Vertebrectomy was performed via left thoracotomy and reconstruction with a cage and plate as seen on X-ray film (E). LMNOP: L = solitary, anterior column, M = Spinal Instability Neoplastic Score (SINS) 12 (potentially unstable), N = symptomatic spinal cord compression, O = moderately radioresistant, P = good fitness, naïve to therapy but poor prognosis with limited systemic options. SINS: location T10 = 1, pain mechanical = 3, bone lesion lytic = 2, normal alignment = 0, vertebral body collapse > 50% = 3, posterolateral involvement of both pedicles = 3.
Fig. 2.
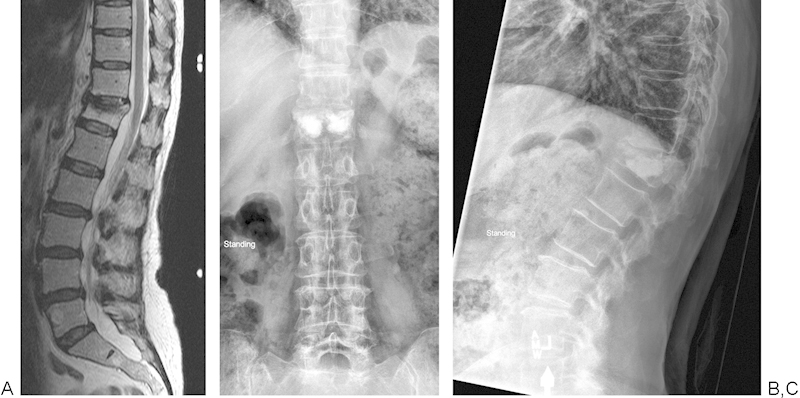
Sagittal T2-weighted magnetic resonance imaging (A) of a 63-year-old woman post–radiation therapy and autologous stem cell transplant for multiple myeloma with T12 pathologic fracture and persistent mechanical back pain. LMNOP: L = solitary, anterior column, M = Spinal Instability Neoplastic Score (SINS) 12 (potentially unstable), N = normal, O = highly radiosensitive, P = good fitness and prognosis. SINS: location = 3, pain mechanical = 3, bone lytic = 2, alignment kyphosis = 2, vertebral body collapse < 50% = 2, no posterolateral involvement. She received kyphoplasty as seen on X-ray films (B, C).
Neurology (N)
The randomized prospective study by Patchell et al conclusively demonstrated the superiority of surgical decompression plus postoperative radiotherapy compared with radiotherapy alone for the treatment of metastatic epidural spinal cord compression restricted to a single area with at least one neurologic symptom.13 Significantly more patients in the surgery group (42/50, 84%), compared with the patients in radiation group (29/51, 57%), were able to walk after the treatment (odds ratio 6.2 [95% confidence interval 2.0 to 19.8], p = 0.001); and surgery group patients maintained the ability to walk longer (median 122 days versus 13 days, p = 0.003). Also, surgically treated patients had a substantial reduction in the use of corticosteroids and analgesics. Surgery did not result in prolonged hospitalization, with the median length of a hospital stay being 10 days in both groups. In addition, 10 patients in the radiation group substantially declined in motor strength and needed to be operated on, three of whom developed wound infections, reflecting the high rate of wound complications related to surgery through the previously radiated area. Thus, decompressive surgery preceding radiotherapy is clearly the preferred approach. However, it is important to note that this study excluded patients with exquisitely radiosensitive lesions (e.g., lymphoma, myeloma).
Surgical decompression is unlikely to benefit when deficits are severe, especially if they have been present for more than 24 to 48 hours.35
Oncology (O)
Tumor biology affects the responsiveness to cEBRT, which has significant impact on treatment decision making. In this respect, tumors can be divided into three groups: highly radiosensitive, radiosensitive, and radioresistant. The highly radiosensitive group (which includes lymphomas, leukemias, multiple myeloma, and germ-cell tumors) generally respond well to urgent radiation therapy, regardless of the degree of spinal cord compression.25 The radiosensitive group, which includes most of the common solitary metastasis to the spine (e.g., breast, prostate, non–small cell lung), can be managed with cEBRT if there is no symptomatic cord compression or significant instability.36 SRS has also been advocated for this group by some authors because local disease control is improved over historic rates.18 So-called radioresistant tumors (e.g., renal carcinoma) are defined as such based on how they respond to cEBRT (e.g., 30 Gy in 20 fractions). Durable disease control for these tumors may now be achieved with SRS. As such, aggressive en bloc resection of solitary renal cell carcinoma may have become an obsolete strategy.37
Tumor biology, reflected in the growth rate, has a significant influence on the patients' survival, which has important implications in terms of management planning.21 Based on the growth rate, primary tumors have been divided into four groups: (1) tumors with slow growth (breast, thyroid, prostate, myeloma, hemangioma, endothelioma, non-Hodgkin lymphoma); (2) tumors with moderate growth (kidney, uterus, tonsil, epipharynx, synovial cell carcinoma, metastatic thymoma); 3) tumors with rapid growth (stomach, colon, liver, melanoma, teratoma, sigmoid colon, pancreas, rectum, unknown origin); and (4) tumors with very rapid growth (lung). With an increasing growth rate, the prognosis is much poorer, and aggressive surgery has less utility.
In recent years, an understanding of tumor biology at the molecular genetic has improved our ability to predict treatment response and survival time. For example, estrogen receptor positivity has been linked with longer median survival in patients undergoing spine surgery for metastatic breast disease.38
Patient Fitness, Prognosis, Patient Wishes, and Prior Response to Therapy (P)
Patients with significant medical risk and/or limited prognosis are most appropriately treated with nonsurgical or minimally invasive methods, such as cEBRT, SRS, and MIS approaches including kyphoplasty. However, there is no consensus regarding the life expectancy required to justify a more aggressive surgical intervention. A period of 3 to 6 months has been proposed; however, it is difficult to accurately determine life expectancy for an individual patient. As discussed earlier, the Oswestry Index Score can be useful in informing patients about their prognosis and eliciting an informed decision reflecting the patient's wishes.21
The response to previous treatments (such as radiation therapy, chemotherapy, and chronic corticosteroid use) needs to be closely reviewed and any adverse effects identified. In general, a patient with active systemic disease on fourth-line chemotherapy obviously has a poorer prognosis than a patient who is naïve to therapy. Cancer therapies may lead to bone marrow suppression with leukopenia and thrombocytopenia. There is a high rate of wound infection and dehiscence after surgery through previously irradiated tissues. In the past, failure of radiation therapy was a common indication for surgery; however, SRS may now be safely employed for local disease control in many circumstances.18
Summary
Spinal metastasis is a complex medical condition that poses a challenge to treatment decision making. Classification-based approaches may be helpful to predict life expectancy, but are inherently limited by the particular patient populations in which they were developed. A principles-based model allows a more individualized approach, which is most appropriate in the setting of advanced cancer. The LMNOP system is the first to incorporate the SINS as a reliable grading of spinal instability and includes factors not directly addressed by other principle-based mnemonics: the location and number of levels of spinal disease (L) as well as effects of prior therapy (P).
Footnotes
Disclosures None
References
- 1.Fisher C G, DiPaola C P, Ryken T C. et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976) 2010;35(22):E1221–E1229. doi: 10.1097/BRS.0b013e3181e16ae2. [DOI] [PubMed] [Google Scholar]
- 2.Fourney D R, Frangou E M, Ryken T C. et al. Spinal instability neoplastic score: an analysis of reliability and validity from the spine oncology study group. J Clin Oncol. 2011;29(22):3072–3077. doi: 10.1200/JCO.2010.34.3897. [DOI] [PubMed] [Google Scholar]
- 3.Perrin R G, McBroom R J. Anterior versus posterior decompression for symptomatic spinal metastasis. Can J Neurol Sci. 1987;14(1):75–80. doi: 10.1017/s0317167100026871. [DOI] [PubMed] [Google Scholar]
- 4.Feiz-Erfan I, Rhines L D, Weinberg J S. The role of surgery in the management of metastatic spinal tumors. Semin Oncol. 2008;35(2):108–117. doi: 10.1053/j.seminoncol.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Finkelstein J A, Zaveri G, Wai E, Vidmar M, Kreder H, Chow E. A population-based study of surgery for spinal metastases. Survival rates and complications. J Bone Joint Surg Br. 2003;85(7):1045–1050. doi: 10.1302/0301-620x.85b7.14201. [DOI] [PubMed] [Google Scholar]
- 6.York J E, Wildrick D M, Gokaslan Z L. St. Louis, MO: Quality Medical Publishing; 1999. Metastatic tumors; pp. 392–411. [Google Scholar]
- 7.Gokaslan Z L. Spine surgery for cancer. Curr Opin Oncol. 1996;8(3):178–181. doi: 10.1097/00001622-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Byrne T N. Spinal cord compression from epidural metastases. N Engl J Med. 1992;327(9):614–619. doi: 10.1056/NEJM199208273270907. [DOI] [PubMed] [Google Scholar]
- 9.Constans J P, de Divitiis E, Donzelli R, Spaziante R, Meder J F, Haye C. Spinal metastases with neurological manifestations. Review of 600 cases. J Neurosurg. 1983;59(1):111–118. doi: 10.3171/jns.1983.59.1.0111. [DOI] [PubMed] [Google Scholar]
- 10.Wu A S, Fourney D R. Evolution of treatment for metastatic spine disease. Neurosurg Clin N Am. 2004;15(4):401–411. doi: 10.1016/j.nec.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Fourney D R, Gokaslan Z L. Use of “MAPs” for determining the optimal surgical approach to metastatic disease of the thoracolumbar spine: anterior, posterior, or combined. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2005;2(1):40–49. doi: 10.3171/spi.2005.2.1.0040. [DOI] [PubMed] [Google Scholar]
- 12.Fourney D R Abi-Said D Lang F F McCutcheon I E Gokaslan Z L Use of pedicle screw fixation in the management of malignant spinal disease: experience in 100 consecutive procedures J Neurosurg 200194(1, Suppl):25–37. [DOI] [PubMed] [Google Scholar]
- 13.Patchell R A, Tibbs P A, Regine W F. et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 14.Lu D C, Chou D, Mummaneni P V. A comparison of mini-open and open approaches for resection of thoracolumbar intradural spinal tumors. J Neurosurg Spine. 2011;14(6):758–764. doi: 10.3171/2011.1.SPINE09860. [DOI] [PubMed] [Google Scholar]
- 15.Niazi T N, Sauri-Barraza J C, Schmidt M H. Minimally invasive treatment of spinal tumors. Semin Spine Surg. 2011;23(1):51–59. [Google Scholar]
- 16.Fourney D R Schomer D F Nader R et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients J Neurosurg 200398(1, Suppl):21–30. [DOI] [PubMed] [Google Scholar]
- 17.Berenson J, Pflugmacher R, Jarzem P. et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol. 2011;12(3):225–235. doi: 10.1016/S1470-2045(11)70008-0. [DOI] [PubMed] [Google Scholar]
- 18.Laufer I, Iorgulescu J B, Chapman T. et al. Local disease control for spinal metastases following “separation surgery” and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: outcome analysis in 186 patients. J Neurosurg Spine. 2013;18(3):207–214. doi: 10.3171/2012.11.SPINE12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunha M V, Al-Omair A, Atenafu E G. et al. Vertebral compression fracture (VCF) after spine stereotactic body radiation therapy (SBRT): analysis of predictive factors. Int J Radiat Oncol Biol Phys. 2012;84(3):e343–e349. doi: 10.1016/j.ijrobp.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 20.Sciubba D M, Gokaslan Z L. Diagnosis and management of metastatic spine disease. Surg Oncol. 2006;15(3):141–151. doi: 10.1016/j.suronc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Balain B, Jaiswal A, Trivedi J M, Eisenstein S M, Kuiper J H, Jaffray D C. The Oswestry Risk Index: an aid in the treatment of metastatic disease of the spine. Bone Joint J. 2013;95-B(2):210–216. doi: 10.1302/0301-620X.95B2.29323. [DOI] [PubMed] [Google Scholar]
- 22.Tokuhashi Y, Matsuzaki H, Toriyama S, Kawano H, Ohsaka S. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 1990;15(11):1110–1113. doi: 10.1097/00007632-199011010-00005. [DOI] [PubMed] [Google Scholar]
- 23.Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30(19):2186–2191. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 24.Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001;26(3):298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 25.Bilsky M, Smith M. Surgical approach to epidural spinal cord compression. Hematol Oncol Clin North Am. 2006;20(6):1307–1317. doi: 10.1016/j.hoc.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Paton G R, Frangou E, Fourney D R. Contemporary treatment strategy for spinal metastasis: the “LMNOP” system. Can J Neurol Sci. 2011;38(3):396–403. doi: 10.1017/s031716710001177x. [DOI] [PubMed] [Google Scholar]
- 27.Fourney D R York J E Cohen Z R Suki D Rhines L D Gokaslan Z L Management of atlantoaxial metastases with posterior occipitocervical stabilization J Neurosurg 200398(2, Suppl):165–170. [DOI] [PubMed] [Google Scholar]
- 28.Fehlings M G David K S Vialle L Vialle E Setzer M Vrionis F D Decision making in the surgical treatment of cervical spine metastases Spine (Phila Pa 1976) 200934(22, Suppl):S108–S117. [DOI] [PubMed] [Google Scholar]
- 29.Bilsky M H, Shannon F J, Sheppard S, Prabhu V, Boland P J. Diagnosis and management of a metastatic tumor in the atlantoaxial spine. Spine (Phila Pa 1976) 2002;27(10):1062–1069. doi: 10.1097/00007632-200205150-00011. [DOI] [PubMed] [Google Scholar]
- 30.Polly D W Jr Chou D Sembrano J N Ledonio C G Tomita K An analysis of decision making and treatment in thoracolumbar metastases Spine (Phila Pa 1976) 200934(22, Suppl):S118–S127. [DOI] [PubMed] [Google Scholar]
- 31.Huth J F, Dawson E G, Eilber F R. Abdominosacral resection for malignant tumors of the sacrum. Am J Surg. 1984;148(1):157–161. doi: 10.1016/0002-9610(84)90304-0. [DOI] [PubMed] [Google Scholar]
- 32.Nader R, Rhines L D, Mendel E. Metastatic sacral tumors. Neurosurg Clin N Am. 2004;15(4):453–457. doi: 10.1016/j.nec.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Raque G H Jr, Vitaz T W, Shields C B. Treatment of neoplastic diseases of the sacrum. J Surg Oncol. 2001;76(4):301–307. doi: 10.1002/jso.1050. [DOI] [PubMed] [Google Scholar]
- 34.Ruff R L, Lanska D J. Epidural metastases in prospectively evaluated veterans with cancer and back pain. Cancer. 1989;63(11):2234–2241. doi: 10.1002/1097-0142(19890601)63:11<2234::aid-cncr2820631130>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 35.Bach F, Larsen B H, Rohde K. et al. Metastatic spinal cord compression. Occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta Neurochir (Wien) 1990;107(1–2):37–43. doi: 10.1007/BF01402610. [DOI] [PubMed] [Google Scholar]
- 36.Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32(4):959–967. doi: 10.1016/0360-3016(95)00572-g. [DOI] [PubMed] [Google Scholar]
- 37.Sundaresan N, Rothman A, Manhart K, Kelliher K. Surgery for solitary metastases of the spine: rationale and results of treatment. Spine (Phila Pa 1976) 2002;27(16):1802–1806. doi: 10.1097/00007632-200208150-00021. [DOI] [PubMed] [Google Scholar]
- 38.Sciubba D M, Gokaslan Z L, Suk I. et al. Positive and negative prognostic variables for patients undergoing spine surgery for metastatic breast disease. Eur Spine J. 2007;16(10):1659–1667. doi: 10.1007/s00586-007-0380-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


