Abstract
Treatment with monoclonal antibodies (mabs) has become an established component of oncological therapy. The monoclonal antibodies available for this purpose are mainly administered intravenously in individually adapted doses according to body weight over longer treatment times. For other chronic diseases such as, for example, diabetes mellitus, the subcutaneous administration of drugs is an established therapy option. For the subcutaneous administration of larger volumes as needed for mab solutions the extracellular matrix of the subcutaneous tissue represents a problem. The co-formulation with recombinant human hyaluronidase makes the relatively pain-free administration of larger fluid volumes and thus the subcutaneous administration of monoclonal antibodies possible, as illustrated by the development of a subcutaneous formulation of trastuzumab. This constitutes a less invasive, time-optimised and flexible form of administration for patients with HER2-positive breast cancer that, with its fixed dosing possibilities, contributes to therapeutic safety. The example of trastuzumab shows that the subcutaneous administration of monoclonal antibodies can simplify oncological long-term therapy not only for the patients but also for the medical personnel.
Key words: monoclonal antibodies, subcutaneous therapy, breast cancer, trastuzumab, oncology
Abstract
Zusammenfassung
Die Behandlung mit monoklonalen Antikörpern (mAK) ist ein fester Bestandteil der onkologischen Therapie geworden. Die dafür zur Verfügung stehenden monoklonalen Antikörper werden überwiegend intravenös, individualisiert gewichtsadaptiert und über längere Behandlungszeiträume verabreicht. Bei anderen chronischen Erkrankungen wie beispielsweise Diabetes mellitus stellt die subkutane Applikation von Medikamenten, eine etablierte Therapieform dar. Für die subkutane Applikation größerer Volumina stellt die Physiologie der extrazellulären Matrix des subkutanen Gewebes ein Hindernis dar, wie sie für die Lösung mAK erforderlich ist. Die Koformulierung mit rekombinanter humaner Hyaluronidase ermöglicht die schmerzarme Gabe größerer Volumina und damit die subkutane Applikation von monoklonalen Antikörpern, wie die Entwicklung einer subkutanen Formulierung für Trastuzumab zeigt. Mit dieser steht Patientinnen mit HER2-positivem Mammakarzinom eine weniger invasive, zeitoptimierte und flexiblere Applikationsform zur Verfügung, die mit einer fixen Dosierungsmöglichkeit zur Therapiesicherheit beiträgt. Das Beispiel Trastuzumab zeigt, dass die subkutane Verabreichung monoklonaler Antikörper die onkologische Langzeittherapie sowohl für Patienten als auch für medizinisches Fachpersonal vereinfachen könnte.
Schlüsselwörter: monoklonale Antikörper, subkutane Therapie, Mammakarzinom, Trastuzumab, Onkologie
Introduction
In the advanced stages of numerous tumour entities, the optimisation of oncological therapy leads to a marked prolongation of survival. Thus, the chronic stage of the disease acquires a completely different relevance. According to data of the German Cancer Society (Deutsche Krebsgesellschaft) 81 % of the patients are still alive 5 years after the first diagnosis of breast cancer. Of those patients with advanced breast cancer, about one third are still alive 5 years after the first occurrence of metastases and one in ten of the afflicted patients survives for more than 10 years 1.
Monoclonal antibodies today are an established component of oncological therapy. They are mainly administered intravenously over longer treatment times of up to several years. For oncological therapy with mabs, a less invasive, appreciably less time-consuming and more flexible form of administration, as represented by subcutaneous administration, results in a significant improvement in quality of life for the patients who, in this stage of the disease, have often returned to their daily routine of life. They must spend less time as patients in treatment centres and can use the thus gained time for personal purposes and can benefit from a previously not known social independence. The subcutaneous route of administration simplifies the therapy with monoclonal antibodies not only for the patient but also for the medical personnel (Table 1). Ultimately, self-administration at home may become feasible.
Table 1 Advantages and disadvantages of subcutaneous and intravenous administration.
| Administration form | Advantages | Challenges |
|---|---|---|
| s. c. |
|
|
| i. v. |
|
|
Material and Methods
This article is based on literature searches on the use of monoclonal antibodies in oncological clinical entities in PubMed, MEDLINE, Clinicaltrials.gov and evaluation reports of the European Medical Agency (EMA) without limitation of the search period as well as a selective search of congress abstracts of the Annual Meetings of the American Society of Clinical Oncology (ASCO), Annual Meetings of the American Society of Hematology (ASH), the San Antonio Breast Cancer Symposia, the St. Gallen Breast Cancer Conferences up to and including 2009. In addition several review articles and their reference lists were evaluated.
Data on subcutaneous administration of monoclonal antibodies from oncological trials
The PubMed search (search item [antibody or antibodies] and monoclonal and cancer and subcutaneous, no limitation of search period) provided, after evaluation of the abstracts of all 945 hits and a subsequent targeted search for the previously identified antibodies, 70 publications in which the subcutaneous administration of monoclonal antibodies as oncological therapeutics in humans was reported (alemtuzumab × 33, denosumab × 30, trastuzumab × 1, rituximab × 1, veltuzumab × 1, abagovomab × 4).
Veltuzumab, an antibody directed against CD20, and abagovomab, a monoclonal murine antibody directed against CA125, are still undergoing clinical development.
For alemtuzumab (MabCampath®), an antibody directed against CD52, studies have pointed to a comparable efficacy for intravenous and subcutaneous administrations 2, 3. However, alemtuzumab up to its withdrawal from the market in 2012 by the manufacturer Genzyme on the basis of its clinical development as a drug for patients with multiple sclerosis was only approved for intravenous use in patients with chronic lymphatic leukaemia 4.
For rituximab (MabThera®), the first therapeutic antibody against the CD20 antigen on the surface of normal and malignant B cells, a subcutaneous administration form is currently under development 5, 6. The subcutaneous administration form of trastuzumab (Herceptin®), an antibody targeted against HER2 as therapy for HER2-positive metastatic and early breast cancer as well as HER2-positive metastatic gastric cancer was approved for use as therapy for patients with HER2-positive breast cancer by the European Drug Authority in August 2013 7.
Denosumab (Xgeva®), an antibody against RANK-L for the reduction of bone-related complications in patients with an osseous metastatic solid tumour, has been developed consequently as an antibody for subcutaneous administration. Its approval was based on the significant reduction of the risk for skeletal-related events in comparison with zolendronate therapy in three large phase III studies, one of which involved 2046 patients with advanced breast cancer 8, one with 1901 patients suffering from castration-resistant prostate cancer 9 and one with 1776 patients having various solid tumour entities (with the exception of breast and prostate cancer) and multiple myelomas 10. In a further placebo-controlled trial with 1432 patients suffering from castration-resistant prostate cancer a significant prolongation of the bone metastasis-free survival and a significant delay in the occurrence of bone metastases was achieved with denosumab 11.
The question arises as to why subcutaneous administration forms are scarcely available to patients (Table 2) although therapy with monoclonal antibodies over longer periods of time has developed to become a cornerstone of modern oncological therapy. The challenges for the development of alternative subcutaneous formulations can be nicely illustrated for the example of trastuzumab.
Table 2 Monoclonal antibodies with clinical data on subcutaneous administration as oncological therapeutic agents.
| Name | Antibody | Approval status as oncological therapeutic agent |
|---|---|---|
| Abagovomab | murine monoclonal antibody against CA125 | In clinical development for subcutaneous administration in ovarian cancer. |
| Veltuzumab | humanised monoclonal antibody against CD20 | In clinical development for subcutaneous administration in non-Hodgkinʼs lymphoma. |
| Alemtuzumab (MabCampath®) | humanised antibody against CD52 | Approved up to August 2012 as intravenous therapy for chronic lymphatic leukaemia, withdrawn from the market due to clinical development as therapy for multiple sclerosis. |
| Rituximab (MabThera®) | chimeric (murine/human) monoclonal antibody against CD20 | Approved as intravenous therapy for follicular lymphoma (after induction chemotherapy and refractory disease to or recurrence after chemotherapy), CD20-positive diffuse large-cell B-cell non-Hodgkin lymphoma, chronic lymphatic leukaemia (not pre-treated and recurrent/refractory). |
| Trastuzumab (Herceptin®) | humanised monoclonal antibody against HER2 | Approved as intravenous therapy for HER2-positive metastatic breast cancer, HER2-positive early breast cancer, HER2-positive metastatic gastric cancer.Approved as subcutaneous therapy for HER2-positive breast cancer. |
| Denosumab (Xgeva®) | human monoclonal antibody against RANK ligand | Approved as subcutaneous therapy to prevent bone-related complications of solid tumours and bone involvement. |
Therapy with trastuzumab for patients with HER2-positive breast cancer
Therapy with trastuzumab, a monoclonal antibody against the human epidermal growth factor receptor HER2, is nowadays the standard for treatment of HER2-positive breast cancer 12, 13, 14 and has appreciably improved the prognosis for patients not only in the early stages but also of those in a metastatic situation 15, 16.
Patients with early breast cancer receive trastuzumab as an adjuvant therapy every three weeks for a total of 12 months. Patients with metastatic breast cancer receive trastuzumab as a rule up to progression or even beyond as “treatment beyond progression”. For the add-on administration of trastuzumab to a standard chemotherapy, randomised phase III trials have reported median progression-free intervals of from 7.1 months 17 up to 11.1 months 18, and in randomised phase II trials of up to 18.6 months 19. According to the therapy recommendations of the Organ Commission Mamma of the “Arbeitsgemeinschaft Gynäkologische Onkologie (AGO)” and the European Society of Medical Oncology (ESMO) therapy with trastuzumab may also be continued beyond progression, i.e., altogether for a longer period of time 13, 14.
To date trastuzumab is exclusively administered by the intravenous route. According to the valid scientific information that is based on the results of the approval studies, the duration of infusion for the first dose amounts to 90 minutes with a follow-up observation time of 6 hours from the start of the infusion, the subsequent infusions then require 30 minutes with a follow-up observation period of 2 hours from the start of the infusion 20. In clinical routine, however, the actual infusion times will be longer or shorter than those mentioned above. As a rule intravenous therapy in the chronic disease phase is associated with a not inconsiderable expenditure of time in comparison with the actual duration of the therapy.
Injection volumes and the extracellular matrix of subcutaneous tissue
The monoclonal antibody denosumab is administered every 4 weeks as a subcutaneous injection at a dose of 120 mg in an injection volume of 1.7 mL 21. However, the dissolution of monoclonal antibodies usually requires larger injection volumes. Accordingly, the injection volumes of reconstituted solutions of trastuzumab at a dose of 6 mg/kg for a patient with a body weight of 70 kg amount to 20 mL for intravenous administration. A higher concentration of the solution and thus a reduction of the injection volume are only possible to a minor extent. Even at the maximum possible concentration for the injection solution of trastuzumab of 120 mg/mL 22 instead of the usual 21 mg/mL the required injection volume is still markedly in excess of 2 mL
The extracellular matrix of the subcutaneous tissue limits the injection of larger volumes (> 1–2 mL). The EM is a structural network of fibre proteins such as collagen and elastin in a viscoelastic gel consisting of hyaluronic acid and other glycosaminoglycans, complex linear polysaccharides. This network is important for the specific tissue architecture and controls the diffusion and flow of molecules. Collagen and hyaluronic acid form the decisive barrier to larger volumes (Fig. 1) 23. The injection of volumes larger than 2 mL is painful for the patient. In addition, larger molecules such as, e.g., proteins can be held back in the tissue due to the viscosity of the extracellular matrix. This can result in the local degradation of these substances, local reactions at the injection site and a lower bioavailability 23. The physiology of the extracellular matrix of subcutaneous tissue thus constitutes a barrier especially for the subcutaneous administration of monoclonal antibodies.
Fig. 1 a.
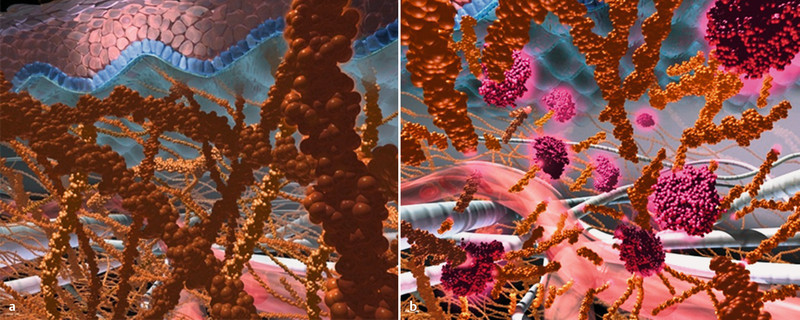
and b Principle of subcutaneous administration using recombinant human hyaluronidase as carrier. a The subcutaneous tissue with its matrix of hyaluronic acid and collagen fibres limits the subcutaneous administration to volumes < 2 mL. b The temporary and local degradation of hyaluronic acid by recombinant human hyaluronidase leads to a temporary increase in the subcutaneous dispersion surface and makes possible the administration of larger fluid volumes (source: Hoffmann-La Roche).
In the case of trastuzumab a problem-free and painless subcutaneous administration can be achieved with the ENHANZE™ technology that is described below.
Coformulation with recombinant hyaluronidase
The half-life of hyaluronic acid amounts to 15–20 hours. It is subject to hydrolytic degradation by the enzyme hyaluronidase. To overcome the volume limitations due to the extracellular matrix a coformulation of trastuzumab with recombinant hyaluronidase (rHuPH20) was chosen for subcutaneous administration. This enzyme effects a reversible hydrolysis of hyaluronic acid and thus reduces the viscosity of the gel-like extracellular matrix. This leads to an increase in permeability. The subcutaneous dispersion area increases and permits the administration of larger fluid volumes. On account of the half-life of hyaluronic acid these structural changes in the extracellular matrix are only temporary 23, 24. The human PH-20 gene codes for a hyaluronidase that is active at neutral pH, and degrades glycosaminoglycans under physiological conditions. Cloning of the cDNA of the soluble domain of this human PH-20 hyaluronidase makes possible the recombinant preparation of a glycosylated enzyme in a purified, homogeneous formulation with a high specific activity. Molecules with a size of up to 200 nm are uniformly distributed upon co-injection with rHuPH20 and exhibit a significantly higher bioavailability, similar to that achieved with intravenous administration 23, 25, 26.
Dose finding for subcutaneous trastuzumab
An alternative subcutaneous route for administration of a drug must provide a comparable efficacy, pharmacokinetics and safety in comparison with its established intravenous formulation. In a dose-finding trial with healthy male subjects and patients with HER2-positive breast cancer it was found that the dose of trastuzumab 8 mg/kg s. c. every 3 weeks provided a comparable exposure as the 3-weekly administration of 6 mg/kg trastuzumab i. v. (Fig. 2). Thus, the minimal or trough concentrations were reached immediately prior to the next administration of trastuzumab (Ctrough) which were at least as high as those after intravenous administration 27.
Fig. 2.
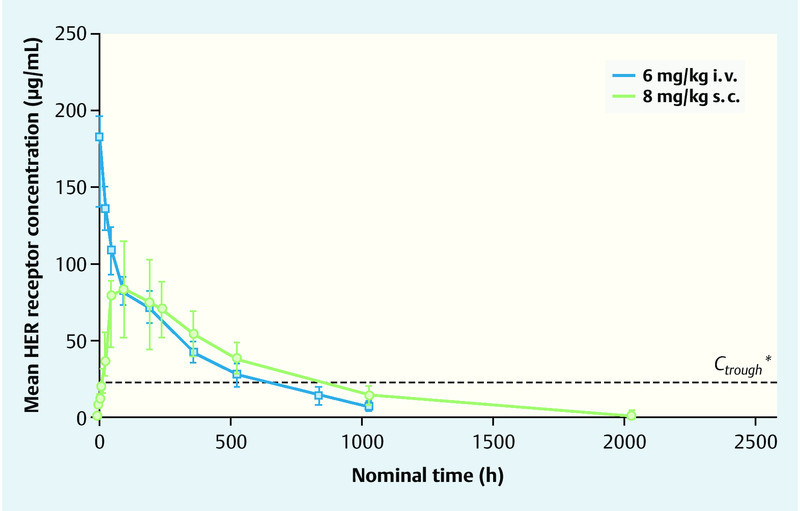
Trastuzumab exposure in patients with HER2-positive early breast cancer for the doses 8 mg/kg s. c. and 6 mg/kg i. v. (after Wynne et al. 27). * The Ctrough of 20 µg/mL reflects the target value established in preclinical xenograft models.
Development of a fixed dose for trastuzumab
In order to achieve the easiest possible handling and to assure a reduction in the sources of error such as wrong doses, a fixed dose irrespective of body weight for the subcutaneous administration of trastuzumab was sought after. The basic principle for the efficacy of trastuzumab is a saturation of the receptors which requires a minimum concentration of trastuzumab in serum. In order to maintain the efficacy of trastuzumab the serum concentration required for saturation of the receptors must be continuously held above a minimum level 22, 28. With the intravenous administration of trastuzumab this receptor saturation can be achieved more rapidly with higher initial doses 22. Also with a fixed dose a saturation of the receptors should be assured from the 1st cycle onwards. The pharmacokinetic parameters for the various subcutaneous doses of trastuzumab were calculated with the help of various model simulations in which the data from the previously described dose-finding trials were incorporated. In this way, with a fixed dose of 600 mg s. c., a similar Ctrough and an at least equivalent exposure to trastuzumab over the dosing interval (AUCtau) were achieved as with the usual 3-weekly and weekly i. v. usage of trastuzumab with higher initial doses. An adequate receptor saturation with a fixed dose of 600 mg s. c. is already reached even in the 1st cycle 22, 29.
Randomised comparison of pharmacokinetics and efficacy
The subcutaneous administration of the fixed dose of 600 mg trastuzumab in 5 mL injection volume (trastuzumab 120 mg/mL, rHuPH20 2000 U/mL) can be performed within 2–5 minutes. In the HannaH trial, a randomised open phase III study on 596 patients with HER2-positive early advanced or inflammatory breast cancer, this dose was compared with the established intravenous administration 30. The patients received a neoadjuvant chemotherapy of 4 cycles of docetaxel (75 mg/m2; q3w) and 4 cycles of FEC (fluorouracil 500 mg/m2, epirubicin 75 mg/m2, cyclophosphamide 500 mg/m2; q3w). In addition trastuzumab was administered every 3 weeks either in the fixed dose of 600 mg subcutaneous (n = 297) or intravenous (8 mg/kg initial dose, 6 mg/kg maintenance dose) (n = 299). The therapy with trastuzumab was continued after surgery through to completion of 18 cycles.
For both administration forms of trastuzumab, the study revealed similar pharmacokinetics and comparable efficacies. The geometric mean of the trough concentration Ctrough amounted to 69.0 µg/mL in the group with subcutaneous administration, and 51.8 µg/mL in the intravenous group. At 1.33 (90 % confidence interval 1.24–1.44) the ratio of the two values was above the previously defined non-inferiority limit of 0.80 (Fig. 3).
Fig. 3.
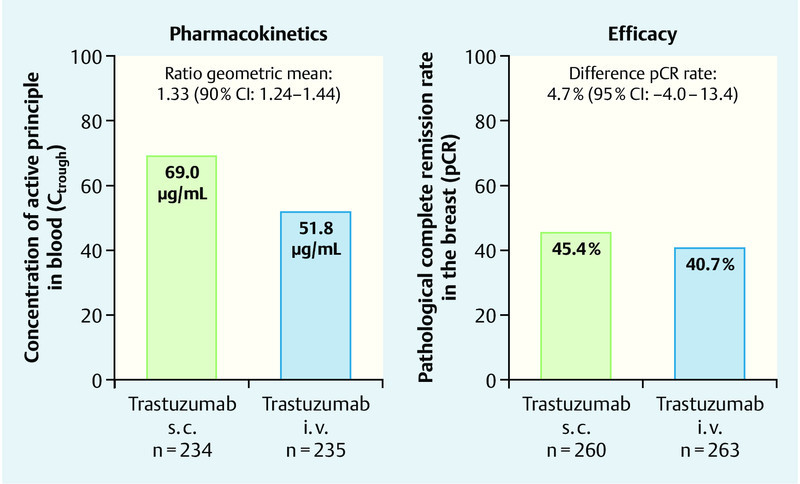
Results for the co-primary endpoints for trastuzumab s. c. vs. i. v. in the HannaH trial (after Ismael et al. 30).
45.4 % of the patients in the s. c. group and 40.7 % of those in the i. v. group had a pathological complete remission (pCR). The difference between the two groups of 4.7 % (95 % confidence interval − 4.0–13.4) in favour of trastuzumab s. c. was thus clearly above the non-inferiority limit of − 12.5 % (Fig. 3).
The incidence of adverse events of degrees 3–5 was similar in both groups. However, with 21 % there were more severe adverse events (SAEs) in the s. c. group as compared with 12 % in the i. v. group. This difference was mainly due to the differing frequencies of SAEs in the category infection: 8.1 % in the s. c. group versus 4.4 % in the i. v. group. The remaining SAEs showed similar distribution patterns and comparable frequencies over the different organ categories. The authors analysed these results and came to the conclusion that the trial physicians in this non-blinded study presumably followed a conservative policy with regard to the experimental subcutaneous administration and thus more frequently admitted these patients to hospital. Altogether, the safety profiles of the fixed subcutaneous dose and the established intravenous administration were comparable 30. For the fixed dose there was no correlation between body weight and efficacy or side effects 31.
Assessment of the preferences of patients and specialist medical personnel
Ultimately, the development of a subcutaneous formulation is only justifiable when in clinical routine it is considered by the patients to really be a simpler and a more comfortable alternative to intravenous administration. For the subcutaneous administration of trastuzumab this was prospectively examined in the PrefHer trial (PrefHer, registered on clinical.trials.gov under NCT01401166), which systematically evaluated not only the preferences of the patients but also the satisfaction of the medical personnel with the subcutaneous administration form in comparison with the intravenous administration. Administration with the help of disposable syringes as well as the expenditure of time and costs of the alternative administration form were also taken into account. The results demonstrated a convincing preference of the patients for the subcutaneous administration of trastuzumab. Accordingly, 216 (91.5 %) of the patients preferred the subcutaneous over the intravenous administration (95 % confidence interval 87.2–94.7 %; p < 0.0001). The most important reason mentioned was the time saving followed by less pain due to the subcutaneous administration 32. Indeed, the effective treatment time for the patients and the time expenditure by the medical personnel were lower for the subcutaneous administration (6.8 %, 95 % CI 3.9–10.8). The most frequent reasons mentioned were fewer reactions at the infusion site in the sense of less pain, haemorrhagic rashes and irritations. The treatment environment was mentioned as reason twice 32. During the crossover phase in this trial 6 (2.5 %) SAEs were reported. No relationship with the study medication was given for any of them 33. The safety of trastuzumab s. c. is currently being evaluated in a large global trial (SafeHer, registered on clinical.trials.gov under NCT01566721) with 2500 patients in parallel in 2 cohorts for injection by syringe and by disposable syringe by the patients themselves in the adjuvant treatment situation.
Classification of the data on subcutaneous administration of trastuzumab
For the example of trastuzumab it has been shown that, by means of a co-formulation with recombinant human hyaluronidase, a subcutaneous administration form that is also suitable for larger injection volumes with good bioavailability and comparable efficacy as for intravenous administration can be realised safely and effectively. The alternative subcutaneous administration of trastuzumab in a fixed dose of 600 mg s. c. over 5 minutes has shown, in a randomised comparison with the established intravenous administration, similar pharmacokinetics, efficacy and safety and was even preferred by the patients due to the resulting saving in time and its less invasive nature. This was also accompanied by a shorter time expenditure for the medical personnel.
The development of alternative subcutaneous administration formulations for long-term oncological therapy with monoclonal antibodies represents an as yet insufficiently exploited factor for cancer patients. In the patientʼs best interests, more intensive efforts should be undertaken to make further subcutaneous formulations of monoclonal antibodies available on the market. They could then make a valuable contribution to a higher quality of life for the longer lifetimes gained for oncological patients due to their less invasive nature and the significantly reduced time expenditure required for this more flexible form of administration. As a result, the oncological patients will have more time to follow their own personal interests. It is to be expected that this will lead to a more flexible time management for hospitals and the treating physicians. Ultimately, the subcutaneous form of administration will only become a real alternative when it does not lead to markedly higher health-care costs for the society. This needs to be carefully examined by means of correctly and appropriately performed pharmaco-economic trials. It should also be mentioned that there will still be patients and physicians who continue to favour the intravenous administration. For some patients the longer treatment times associated with the conventional intravenous administration of mabs also represent a more intensive health-care support and provide a greater sense of security for which they are prepared to accept the more invasive and longer treatments. Also the currently valid reimbursement regulations for the various forms of therapy may well provide a stimulus to favour use of the intravenous route over the subcutaneous administration.
Conclusion
The present article provides an overview on the subcutaneous administration of monoclonal antibodies in oncological therapy and the associated challenges. Up to now the desires of oncological patients for less invasive and more flexible forms of administration have not been sufficiently considered. The example of trastuzumab for patients with HER2-positive breast cancer has demonstrated that the development of alternative subcutaneous administration forms for monoclonal antibodies with at least comparable efficacy and tolerability and concomitantly lower and more effective utilization of medical resources is certainly possible in principle.
Footnotes
Conflict of Interest The authors declare that within the past 3 years they have been active or received support as follows: CJ consultancy fees from Roche, travel expenses from Roche and Amgen; VM fees for consultations and expertise from Roche, lectures for Roche, Colene, Pierre Fabre, travel expenses from GBG Forschungs GmbH; CM fees for the performance of contracted clinical studies from Roche; SH consultancy fees from Roche; BA fees for consultations, lectures and contracted clinical studies from Roche, travel expenses from Roche.
Supporting Information
German supporting informations for this article
References
- 1.Website of the Deutschen Krebsgesellschaft. Brustkrebs – ErkrankungsverlaufOnline:http://www.krebsgesellschaft.delast access: 15.10.2013
- 2.Faderl S, Ferrajoli A, Wierda W. et al. Alemtuzumab by continuous intravenous infusion followed by subcutaneous injection plus rituximab in the treatment of patients with chronic lymphocytic leukemia recurrence. Cancer. 2010;116:2360–2365. doi: 10.1002/cncr.24958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hale G, Rebello P, Brettman L R. et al. Blood concentrations of alemtuzumab and antiglobulin responses in patients with chronic lymphocytic leukemia after intravenous or subcutaneous routes of administration. Blood. 2004;104:948–955. doi: 10.1182/blood-2004-02-0593. [DOI] [PubMed] [Google Scholar]
- 4.MabCampath Alemtuzumab, Zusammenfassung des EPAR für die ÖffentlichkeitOnline:http://www.ema.europa.eu/docs/de_DE/document_library/EPAR_-_Summary_for_the_public/human/000353/WC500025261.pdflast access: 04.12.2013
- 5.Aue G, Lindorfer M A, Beum P V. et al. Fractionated subcutaneous rituximab is well-tolerated and preserves CD20 expression on tumor cells in patients with chronic lymphocytic leukemia. Haematologica. 2010;95:329–332. doi: 10.3324/haematol.2009.012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies A, Merli F, Mihaljevic B. et al. Pharmacokinetics, safety, and overall response rate achieved with subcutaneous administration of rituximab in combination with chemotherapy were comparable with those achieved with intravenous administration in patients with follicular lymphoma in the first-line setting: stage 1 results of the phase 3 SABRINA study (BO22334) Blood (ASH Annual Meeting Abstracts) 2012;120:1629. [Google Scholar]
- 7.Pressemitteilung F. Hoffmann-La Roche Ltd. vom 2. September 2013
- 8.Stopeck A T, Lipton A, Body J J. et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 9.Fizazi K, Carducci M, Smith M. et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henry D H, Costa L, Goldwasser F. et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 11.Smith M R, Saad F, Coleman R. et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DKG Interdisziplinäre S3-Leitlinie für die Diagnostik, Therapie und Nachsorge des Mammakarzinoms. Langversion 3.0, Aktualisierung 2012Online:http://www.krebsgesellschaft.de/download/S3_Brustkrebs_Update_2012_OL_Langversion.pdf
- 13.AGO Empfehlungen für die Diagnostik und Therapie von Patientinnen mit primärem und metastasiertem Brustkrebs, Version 2012.1DOnline:http://www.ago-online.de
- 14.Cardoso F, Harbeck N, Fallowfield L. et al. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23:vi11–vi19. doi: 10.1093/annonc/mds232. [DOI] [PubMed] [Google Scholar]
- 15.Dahabreh I J, Linardou H, Siannis F. et al. Trastuzumab in the adjuvant treatment of early-stage breast cancer: a systematic review and meta-analysis of randomized controlled trials. Oncologist. 2008;13:620–630. doi: 10.1634/theoncologist.2008-0001. [DOI] [PubMed] [Google Scholar]
- 16.Harris C A, Ward R L, Dobbins A. et al. The efficacy of HER2-targeted agents in metastatic breast cancer: a meta-analysis. Ann Oncol. 2011;22:1308–1317. doi: 10.1093/annonc/mdq593. [DOI] [PubMed] [Google Scholar]
- 17.Slamon D J, Leyland-Jones B, Shak S. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 18.Valero V, Forbes J, Pegram M D. et al. Multicenter phase III randomized trial comparing docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab as first-line chemotherapy for patients with HER2-gene-amplified metastatic breast cancer (BCIRG 007 study): two highly active therapeutic regimens. J Clin Oncol. 2011;29:149–156. doi: 10.1200/JCO.2010.28.6450. [DOI] [PubMed] [Google Scholar]
- 19.Wardley A M, Pivot X, Morales-Vasquez F. Randomized phase II trial of first-line trastuzumab plus docetaxel and capecitabine compared with trastuzumab plus docetaxel in HER2-positive metastatic breast cancer. J Clin Oncol. 2010;28:976–983. doi: 10.1200/JCO.2008.21.6531. [DOI] [PubMed] [Google Scholar]
- 20.Fachinformation Herceptin®, Fassung vom Januar 2013
- 21.Xgeva®, Zusammenfasung der Merkmale des Arzneimittels, Fassung vom 11.09.2012Online:http://www.ema.europa.eu/ema/index.jsp?curl=pages/includes/document/document_detail.jsp?webContentId=WC500110381&mid=WC0b01ac058009a3dc
- 22.Bittner B, Richter W F, Hourcade-Potelleret F. et al. Development of a subcutaneous formulation for trastuzumab – nonclinical and clinical bridging approach to the approved intravenous dosing regimen. Arzneimittelforschung. 2012;62:401–409. doi: 10.1055/s-0032-1321831. [DOI] [PubMed] [Google Scholar]
- 23.Haller F. Converting intravenous dosing to subcutaneous dosing with recombinant human hyaluronidase. Pharm Tech. 2007;10:861–864. [Google Scholar]
- 24.Watson D. Hyaluronidase. Br J Anaesth. 1993;71:422–425. doi: 10.1093/bja/71.3.422. [DOI] [PubMed] [Google Scholar]
- 25.Bookbinder L H, Hofer A, Haller M F. et al. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J Control Release. 2006;114:230–241. doi: 10.1016/j.jconrel.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 26.Frost G I. Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert Opin Drug Deliv. 2007;4:427–440. doi: 10.1517/17425247.4.4.427. [DOI] [PubMed] [Google Scholar]
- 27.Wynne C, Harvey V, Schwabe C. et al. Comparison of subcutaneous and intravenous administration of trastuzumab: A phase I/Ib trial in healthy male volunteers and patients with HER2-positive breast cancer. J Clin Pharmacol. 2013;53:192–201. doi: 10.1177/0091270012436560. [DOI] [PubMed] [Google Scholar]
- 28.Pegram M, Hsu S, Lewis G. et al. Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene. 1999;18:2241–2251. doi: 10.1038/sj.onc.1202526. [DOI] [PubMed] [Google Scholar]
- 29.Hourcade-Poterellet F, Lemenuel-Diot A, McIntyre C. et al. Use of a population pharmacokinetic approach for the clinical development of a fixed-dose subcutaneous formulation of trastuzumab. CPT Pharmacometrics Syst Pharmacol. 2014;3:e87. doi: 10.1038/psp.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ismael G, Hegg R, Muehlbauer S. et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I–III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012;13:869–878. doi: 10.1016/S1470-2045(12)70329-7. [DOI] [PubMed] [Google Scholar]
- 31.Melichar B, Stroyakovskiy D, Ahn J N, Pathological complete response to trastuzumab subcutaneous fixed-dose formulation in the HannaH study: Subgroup analysis of patient demographics and tumor characteristics and influence of body weight and serum trough concentration of trastuzumab. Präsentiert als Poster auf dem ESMO-Kongress in Wien 2012
- 32.Pivot X, Gligorov J, Müller V. et al. Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study. Lancet Oncol. 2013;14:962–970. doi: 10.1016/S1470-2045(13)70383-8. [DOI] [PubMed] [Google Scholar]
- 33.De Cock E, Semiglazov V, Lopez-Vivanco G, Time savings with trastuzumab subcutaneous vs. intravenous administration: a time-and-motion study. Präsentiert als Poster auf der International Breast Cancer Conference St. Gallen 2013, Abstract P209. 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
German supporting informations for this article


