Abstract
Thermal ablative technologies have evolved considerably in the recent past and are now an important component of current clinical guidelines for the treatment of small renal masses. Both radiofrequency ablation and cryoablation have intermediate-term oncologic control that rivals surgical options, with favorable complication profiles. Studies comparing cryoablation and radiofrequency ablation show no significant difference in oncologic control or complication profile between the two modalities. Early data from small series with microwave ablation have shown similar promising results. Newer technologies including irreversible electroporation and high-intensity–focused ultrasound have theoretical advantages, but will require further research before becoming a routine part of the ablation armamentarium. The purpose of this review article is to discuss the current ablative technologies available, briefly review their mechanisms of action, discuss technical aspects of each, and provide current data supporting their use.
Keywords: small renal mass, cryoablation, radiofrequency, microwave, irreversible electroporation, interventional radiology
Objectives: Upon completion of this article, the reader will be able to describe the mechanism of action of available ablation modalities and data supporting their use.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Renal malignancy is among the most frequent cancers in both men and women, with approximately 65,150 new cases diagnosed in the United States in 2013.1 Ninety percent of these cases represent renal cell carcinoma (RCC), with transitional cell carcinoma of the renal pelvis comprising the remainder.2 Before the advent of modern cross-sectional imaging, kidney cancer was usually detected in the advanced stages, when tumor size and aggressive characteristics necessitated treatment with radical nephrectomy. However, in the early 1990s, the incidence of RCC demonstrated a steady rise, due in part to detection of stage I disease on increasingly used cross-sectional abdominal imaging studies.3 Recently, the incidence of new RCC has plateaued in most western societies4; however, the rise in detection of small renal masses (SRMs) has created a treatment predicament. Because of the heterogeneous tumor biology of SRMs, several different treatment options exist, ranging from active surveillance to ablation to partial or radical nephrectomy.5 This large range of treatment options poses a problem to practitioners and patients alike. As a result, the American Urologic Association (AUA)6 updated their guidelines for the treatment of stage I disease in 2009.
Radical nephrectomy still represents the most commonly used treatment for RCC4; however, there are data suggesting that surgically induced chronic kidney disease may increase cardiovascular and metabolic risk factors.7 In addition, there has been a gradual shift toward nephron-sparing treatment options, including partial nephrectomy and thermal ablation. While partial nephrectomy shows similar oncologic control to radical nephrectomy, it does so with an increased complication profile.8 Active surveillance has been listed as an appropriate initial strategy in the AUA guidelines for SRMs; however, this choice is usually unpopular with patients, especially younger patients who would require lifelong monitoring.
Ablative modalities use energy tissue interactions, rather than excision, to destroy tumors and a surrounding margin of normal tissue while sparing renal parenchyma. Ablative technologies have had steadily increasing usage for the treatment of RCC and have the potential of becoming standard therapy for stage I renal tumors. While long-term oncologic data supporting their use are limited, their use has a special role in the treatment algorithm of stage I RCC. Ablative procedures are best suited for patients with SRMs who are advanced in age and have significant comorbidities, or unable to undergo open or laparoscopic surgical intervention. Current AUA guidelines suggest use of tumor ablation for patients with substantial comorbidities and in patients with a solitary kidney who are high risk for complete loss of renal function after nephron-sparing surgery.6
Cryoablation
The use of cold to treat tumors is first credited to James Arnott, who in the 19th century used topical cold temperatures to treat tumors of the cervix and breast.9 In the 20th century, the use of liquid nitrogen-based cryotherapy became an important element in the treatment of benign and malignant prostatic conditions.10 While the first renal cryosurgery was reported in 1968 by Lutzeyer and Lymberopoulos,11 it was not until 1995 that Uchida et al reported the first use of percutaneous cryoablation in a canine model using a nitrogen-based system.11 12 Subsequently, the introduction of argon-based cryotherapy, first studied by Torre in 1975,13 then put into clinical practice in the 1990s,14 has allowed for reduction in probe size, thus facilitating percutaneous applications for treating solid organ tumors.
Cryoablation is based on the Joule–Thomson effect, which describes the cooling of a high-pressure gas as it travels through a pinhole valve into a region of lower pressure. Most gases, including nitrogen and argon, cool in such a system. Thus, high-pressure argon, passing through a valve in the tip of a cryoprobe, expands and cools to temperatures as low as −185°C.15 Importantly, helium is one of the few gases that warm when allowed to expand in such a fashion; therefore, it is used to warm the system and facilitate probe removal.
Cryoablation causes tissue destruction by two major mechanisms: direct cell injury and vascular injury.15 16 17 Initially, as the temperature in the tissue drops into the 0 to −20°C range, extracellular ice crystals form creating an osmotic gradient and pulling water from the cells, resulting in cellular dehydration. The increased intracellular electrolyte concentration is often sufficient to destroy the cell15; however, this is augmented by additional cellular damage from ice crystal formation, cell shrinkage, and direct damage to membranes.17 As the temperatures drop further to below −20°C, intracellular ice crystals form, destroying the cell by disruption of organelles and cell membranes, making cell death almost certain.17 Ice crystal formation, both intracellular and extracellular, results in loss of water from the system and leads to cell desiccation and death.16 17 It is interesting to note that intracellular ice formation is most associated with rapid cooling rates that are seen close to the cryoprobe, while cellular dehydration is associated with slower rates of cooling.15 Upon warming, ice crystals coalesce to form larger crystals that disrupt cell membranes, a process known as recrystallization.17 As the ice melts, briefly creating an extracellular hypotonic environment, free extracellular water can enter the damaged cells, increase the cell volume, and potentially lead to cell rupture.15 Apoptosis, or gene-regulated programmed cell death, has also been found to be a mechanism of direct cellular injury, particularly at the periphery of the lesion.16 18 19 In sum, the formation of intra- and extracellular ice crystals leads to a cascade of events that directly kills cells.15 16 17
While the direct cellular injury described earlier is fairly immediate, a more delayed vascular injury occurs secondary to failure of the microcirculation, vascular stasis, thrombosis, and ultimately necrosis.15 17 Vasoconstriction is the first vascular response to the initial freeze cycle, with an associated decrease in the flow of blood that ceases completely upon freezing. With rewarming, vasodilation ensues briefly followed by increased vascular permeability, edema, platelet aggregation, and microthrombus formation.17 Free radical propagation, neutrophil activation, and the release of toxic enzymes may play a role in endothelial injury during the thaw phase as well.18 The resultant ischemia renders the tissue necrotic, except at the periphery of the target volume.15 16 17
Keys to successful cryoablation include proper monitoring of the ablation, rapid cooling to lethal temperature, slow thawing, and repetition of the freeze–thaw cycle.15 What temperature is lethal remains a topic of debate, as it depends on the water and electrolyte content of the tissue, and the normal or malignant nature of the tissue.17 While temperatures of −40 to −50°C are thought to be critical for absolute cell death after a single freeze–thaw cycle,15 17 temperatures of −20 to −40°C are thought to be sufficient to cause cellular death based on the makeup of most solid parenchymal tumors.18 20 Recently, an animal model was used to identify that the distance from the outer margin of the visualized ice ball to the conservative (−40°C) lethal isotherm for renal cell cancer is approximately 6 mm.21 This corresponds to the clinical recommendation of creating a volume of ice which encompasses the tumor plus a 5- to 10-mm margin.22 Typically, three 1.7 mm cryoprobes placed 1.5 cm apart are sufficient to ablate a 2-cm lesion, and five probes for a 3-cm tumor.22 Ideally, the cryoprobes are placed parallel to one another, but at times anatomy may prohibit this and other geometries must be employed (Fig. 1). Increasing the number of freeze–thaw cycles also has a beneficial effect on death, as with each successive cycle, tissue cooling is quicker, the frozen tissue volume is increased, and the zone of necrosis is brought closer to the edge of the frozen volume.15 Typically, two freeze–thaw cycles are used clinically.22 23
Figure 1.
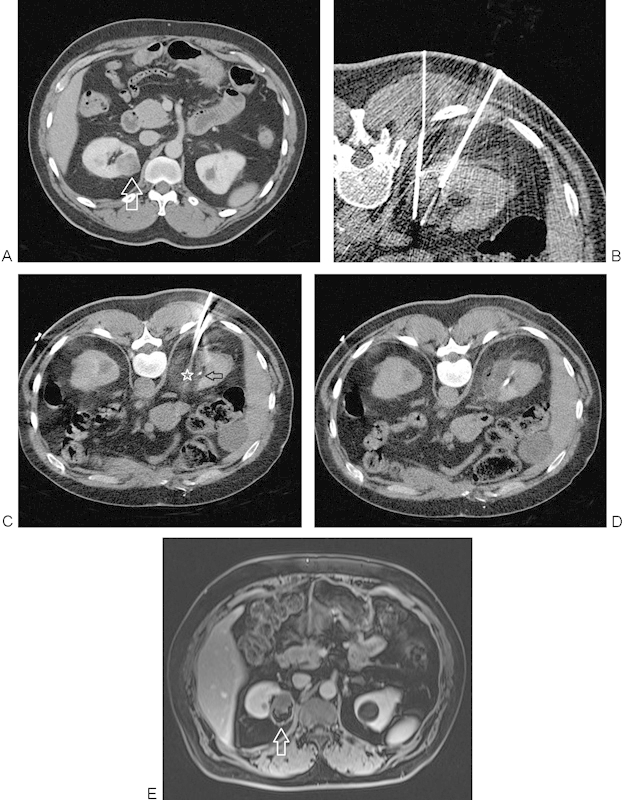
(A) Contrast-enhanced CT image shows an enhancing lesion (arrow) in the upper pole of the right kidney. (B) CT image during placement of cryoprobes using CT fluoroscopy. Ideally, cryoprobes are placed parallel to one another; however, in this case, the rib prevented ideal geometry. Therefore, the two probes inserted cephalad to this image were placed parallel, while these probes converged. Care was taken not to directly puncture the collecting system. (C) Images just cephalad to B show nearly parallel cryoprobes, with the hypodense ice ball (star) converging upon the contrast within the collecting system (arrow). (D) Hypodense region of cryoablation after removal of the probes seen with no contrast extravasation. (E) Follow-up MRI at 3 years shows no enhancement of the successfully ablated lesion (arrow). CT, computed tomography; MRI, magnetic resonance imaging.
Cryoablation can be performed both surgically (open or laparoscopic) and via a percutaneous approach. Several series comparing surgical and percutaneous approaches have been performed. One recent single center, retrospective study compared 54 surgically treated tumors with 154 percutaneously treated lesions, showing similar oncologic control between the two groups, but a reduced length in hospital stay in the percutaneous group.24 Other series have shown similar results with oncologic control comparable between surgical and percutaneous groups, but with lower complication profile, reduced cost, and reduced hospital stay in the percutaneous group.25 26 27 28 29
Excluding direct puncture by the probe, cryoablation has been reported to be less likely to injure the urothelium than heat-based systems, as the cryodamaged collecting system heals by secondary intention in a watertight fashion.30 In a retrospective review of 67 cases that involved the ice ball overlapping the renal sinus, none of the cases were complicated by collecting system injury.31 However, stenosis or complete occlusion of the ureter is still a possible complication.32 Therefore, if the ureter appears to be in close proximity to the planned area of ablation (common with lower pole lesions), protective maneuvers such as warming the collecting system via a previously placed ureteral stent,22 displacement of the ureter by hydrodissection with sterile saline,33 or probe retraction34 should be considered. If a ureteral stenosis or occlusion occurs as a complication of thermal ablation, then placement of a ureteral or nephroureteral stent is indicated.
Either general anesthesia or moderate sedation can be used for percutaneous cryoablation. Tumor location plays a role in the selection of approach.35 The majority of tumors can be treated via a percutaneous approach. Anteriorly located tumors are the most technically challenging; however, even difficult anteriorly located tumors can be treated using a percutaneous approach.36 Hydrodissection can displace adjacent structures and allow for a clear window to the tumor of interest, as well as a margin of safety for adjacent structures at risk for thermal injury (Fig. 2).22 33 Central tumors remain a challenging treatment location due to the increased risk of hemorrhage and potential injury to the renal collecting system by inadvertent direct puncture.
Figure 2.
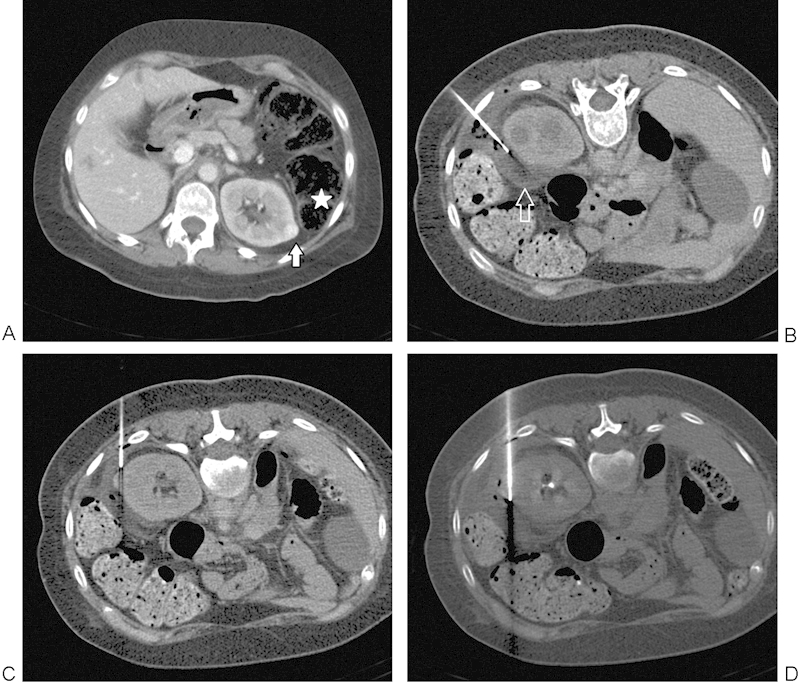
(A) A 38-year-old woman with tuberous sclerosis and prior right nephrectomy for renal cell carcinoma found to have a 1.1-cm enhancing left renal mass (arrow). Note the immediately adjacent colon (star). (B) Insertion of 21-gauge needle allows hydrodissection to be performed with injection of sterile water (arrow) to displace the adjacent colon away from the targeted lesion. (C) RFA electrode positioned under CT guidance, just prior to final position across the lesion. (D) RFA electrode in final position across the lesion with colon successfully displaced. CT, computed tomography; RFA, radiofrequency ablation.
Percutaneous probe placement is performed under image guidance, with the modality of choice dependent on the institution and the operator skill and preference.22 While magnetic resonance imaging (MRI) can been used to guide probe placement,37 38 computed tomography (CT) is used at the authors' institution, occasionally augmented by ultrasound depending on the technical situation. CT is used to monitor ice ball formation during freeze–thaw cycles, as ultrasound is limited by the acoustic shadow created by ice ball formation. Either contrast-enhanced CT or MRI can be used for follow-up imaging, although MRI offers the benefit of not using ionizing radiation. Imaging follow-up is traditionally done at 3, 6, 9, 12, and 18 months, and then annually until 5 years.22 While specific imaging follow-up intervals may vary slightly from institution to institution, 5-year imaging follow-up should always be completed.
Most long-term oncologic data are limited to single institution reports with restricted patient numbers and follow-up periods. The available series suggests that cryoablation has high efficacy and low morbidity, and that oncologic control is promising.39 From several prior studies, cryoablation of small RCC has achieved 96.9 to 100% treatment effectiveness rate, 1.3 to 5.2% local tumor progression rate, and 94.1 to 100% overall survival rate.25 40 41 42 A recent prospective single arm study following 134 consecutive patients treated using percutaneous cryoablation for biopsy-proven RCC was published.43 Patients were followed up for 5 years. The 5-year efficacy of percutaneous cryoablation reached 97%, comparable to that of partial nephrectomy. Overall 5-year survival was 97.8% and cancer-specific 5-year survival was 100%.43 These data suggest that in select patients, percutaneous cryoablation offers similar oncologic efficacy as the gold standard surgical options of radical or partial nephrectomy.
Cryoablation is a minimally invasive modality that is reasonably safe, but that has some inherent risk. Data reported from a multicenter retrospective study show major and minor complication rates of 1.8 and 9.2%, respectively. Major complications included postprocedure ileus, life-threatening hemorrhage, conversion to open surgery, urinary leakage, and scarring of the ureteropelvic junction with stenosis or obstruction.44 A meta-analysis comparing percutaneous to surgical tumor ablation reported a major complication rate of 3% in the percutaneous group, compared with a complication rate of 7% in the surgical group. Major complications after percutaneous ablation included hematuria with blood loss, ureteric injury, cutaneous fistula, colonic injury, and seeding of the needle tract.28 A recent retrospective review of complications from percutaneous renal ablation using both radiofrequency ablation (RFA) and cryoablation reported a major complication rate of 7.4% for cryoablation. The major complications were hemorrhage and hematuria, occurring 4.8 and 2.6%, respectively.45
Radiofrequency Ablation
Heat used as a medical tool dates back to prehistoric times when hot stones were used to achieve hemostasis.46 Around 3,000 bc, the Egyptians used cautery to treat tumors, while Hippocrates later described using it for a variety of common conditions, including shoulder dislocations and hemorrhoids.47 Much later, around the turn of the 20th century, electricity was used to treat a wide array of conditions ranging from insomnia to circulatory ailments to skin cancers, and a variety of terms were used to describe these remedies, including diathermy, fulguration, electrocoagulation, and desiccation.46 Perhaps the best known application of electrical current in medicine was when Harvey Cushing and William T. Bovie47 combined forces to use Bovie's electrosurgical device to provide hemostasis during the resection of a vascular brain tumor in 1926. Since then, alternating high-frequency current has been adapted for a multitude of uses, including the ablation of solid tumors. The first hepatic ablations48 49 were described in 1990, and the first renal ablation50 in 1997.
RFA employs a generator to create an alternating current, typically with a frequency of approximately 500 kHz,51 via an electrode that is placed in the targeted tumor. Grounding pads applied to the patient complete the circuit. This alternating current creates agitation of ions in the tissues near the tip of the probe, which results in intense frictional heat that spreads outward by conduction into the adjacent tissues. Coagulative necrosis then occurs in the tumor and surrounding margins once sufficient heating occurs.52 The zone of ablation is limited to the area of active heating surrounding the electrode. When tissue is heated to above 49°C, cell death occurs within minutes.53 Temperatures of 60 to 100°C result in immediate cell death by denaturation of proteins, loss of enzymatic function, melting of cell membranes, and destruction of cytoplasm.53 54 Desiccation or charring of tissues can occur with rapid or excessive heating to greater than 100°C, when tissue vaporization and carbonization occurs, inhibiting conduction of heat.54 55 Several mechanisms are used to control and limit the heat produced by the probe, including internal liquid cooling of the electrode, and pulsed energy delivery.53 Several RFA systems are currently available using either temperature or impedance-based algorithms.54 Temperature-based systems determine endpoint by monitoring probe temperature, which once reached is maintained for a prescribed time based on desired size of the ablation zone. Impedance-based systems determine the treatment endpoint by monitoring tissue impedance to the emitted radiofrequency current. Depending on the manufacturer, once the impedance in the tissue rises significantly above baseline, the system either shuts off temporarily to facilitate gradual heating of the tissue before restarting or shuts down entirely signaling completion of the ablation.54
Investigators have compared surgical and percutaneous introduction of RFA probes. Typically, CT or ultrasound guidance is used to percutaneously introduce electrodes, while CT is best for intermittently monitoring of the ablation zone during the procedure, as it has the advantage of easily imaging adjacent structures (bowel, ureter, and pancreatic tail) to avoid nontarget injury. One study describes a retrospective follow-up of 115 patients (51 patients undergoing RFA, 54 patients undergoing radical nephrectomy, and 10 patients undergoing partial nephrectomy).56 While overall survival differed in this study, cancer-related survival following RFA was 100% at 5 years, the same as both partial and radical nephrectomy, and disease-free survival was 98% at 5 years, also similar to surgery. The percent decrease in glomerular filtration rate was significantly lower in the RFA group (7.9%) compared with radical nephrectomy (29.0%, p < 0.001), and similar to the partial nephrectomy group (11.5%, p = 0.73).56 A separate 5-year retrospective study comparing RFA (both percutaneous [n = 25] and laparoscopic [n = 12]) and partial nephrectomy (both laparoscopic [n = 28] and open [n = 9]) with 37 patients in each arm also reported no significant difference between 5-year oncologic survival cancer-specific survival, and disease-free survival.57 Of note, there was no statistically significant difference in oncologic outcomes between the two RFA groups.57
Similar to cryoablation, sedation or general anesthesia can be used to perform RFA. General anesthesia allows for complete respiratory control during probe placement and has been shown to optimize patient tolerance during ablation.58 59 Tumor size and location are both predictors of success of RFA.60 RFA is less successful for centrally located hilar tumors, due to the heat sink effect.61 Anterior lesions, as with any percutaneous ablation, are technically difficult tumors to treat. Hydrodissection with sterile water has been described as a technique to assist in thermal protection of adjacent structures.62 Other thermal protective measures, including CO2 instillation and the use of balloons, have been described as well.63 Recent series suggests that transhepatic RFA may also be safe for the treatment of difficult to access right-sided tumors.64 Follow-up imaging with either CT or MRI is performed as described earlier for cryoablation.
Long-term oncologic control data are maturing for RFA. Zagoria et al showed durable oncologic control for RCCs smaller than 4 cm, with no local recurrences in this group during a median follow-up of 61 months.59 The local recurrence-free, disease-free, and overall 5-year survival after initial RFA were 88, 83, and 66%, respectively. Another investigation of renal RFA, with an average tumor size of 2.4 cm, found 5-year actuarial metastasis-free and cancer-specific survival rates of 95 and 99%, respectively.65 A recent study of 58 healthy adults undergoing RFA for treatment of T1a tumors showed excellent long-term outcomes. Mean tumor size in this study was 2.2 cm. Five- and 10-year recurrence-free survival rates were 94.2%, and 5- and 10-year overall survival rates were 95.7 and 91.1%, respectively.66 Wah et al recently published a case series of 200 patients undergoing RFA for RCC, with a mean tumor size of 2.9 cm. Overall technical success rate was 98.5%. Five-year overall cancer survival rate was 75.8%, cancer-specific survival rate was 97.9%, and metastasis-free survival rate was 87.7%.67 A second smaller series of 62 patients published by Balageas et al reported a primary technical success rate of 95.2%; disease-free survival rate of 88.3 and 61.9% at 3 and 5 years, respectively; and a major complication rate of 5.9%.68
In a meta-analysis performed by Hui et al, the major complication rate of percutaneous RFA was 3.1%, versus 7.4% associated with open surgical approaches. In this analysis, percutaneous ablation also conferred a shorter hospital stay.28 Peripheral and exophytic lesions are more safely treated than centrally located lesions, while hemorrhage is the most commonly reported complication following renal RFA. Minor perinephric hematomas are common, but life-threatening hemorrhage is rare. Theoretically, renal infarction should be an expected complication following renal RFA; however, this complication is exceedingly rare.69 Collecting system injury is also possible, with the proximal ureter being the most commonly injured segment. When this results in ureteral stricture and hydronephrosis, stent placement is necessary. Again, this can be avoided by various techniques including cooling the collecting system via a previously placed ureteral stent.22 Thermal injury to adjacent structures is also described, including injury to adjacent small bowel. If necessary, hydrodissection with sterile water, CO2 insufflation, or balloon placement can protect adjacent structures. Injury to nerves, such as the ilioinguinal, iliohypogastric, or genitofemoral nerves that are in close proximity to the psoas muscle, has also been described.70
Cryoablation versus Radiofrequency Ablation
A meta-analysis published by El Dib et al71 evaluated 31 case series of cryoablation and RFA. The pooled data demonstrated clinical efficacy of 89% for cryoablation and 90% for RFA, with no significant difference in complication outcomes.71 Pirasteh et al conducted a retrospective comparison between cryoablation and RFA comparing recurrence rates between the two modalities. Reported recurrence rates based on imaging were 11% for RFA and 7% for cryoablation, which did not reach statistical significance.72 It should be kept in mind that any difference in recurrence-free survival between cryoablation and RFA is likely small (< 5%), and that it would require a multi-institutional, randomized trial to confidently detect this difference.73
Truesdale et al compared the dose of sedation medication used for patients undergoing either cryoablation or RFA of renal tumors, and found that cryoablation was performed with fewer medications than RF.74 The less painful nature of cryoablation and the ability to visualize the ice ball during the ablation are advantages of cryoablation. Conversely, RFA allows one to cauterize the ablation tract, an advantage that may diminish the risk of significant hemorrhage and tract seeding. Operator preferences and experience will likely continue to guide the selection of ablation devices, while 10-year recurrence rates are identified through registries.73
Microwave Ablation
Microwave ablation has been studied extensively for the treatment of liver masses. Microwave energy causes cell death by agitation of water molecules, resulting in frictional heat. An oscillating electromagnetic field in the 900- to 2,450-MHz frequency range is generated by a probe inserted into the tumor bed using image guidance. Dipolar water molecules attempt to orient within the induced electromagnetic field. As the field oscillates, water molecules attempt to realign with the field creating frictional kinetic energy transformed to heat sufficient to cause cell death via coagulative necrosis.75 Unlike RFA, microwaves propagate through all forms of tissue, including charred and desiccated tissues, resulting in higher temperatures achieved in a shorter time frame and allowing for more uniform tissue necrosis.76 Because of the thermal profile, the problem with heat sink occurring in RFA when tumors are adjacent to large vessels is minimized.
A recent intermediate-term follow-up of 46 patients who underwent microwave ablation for treatment of RCC showed promising outcomes up to 3 years, with technical effectiveness of microwave of 98%, 3-year local tumor progression rate of 7.7%, and cancer-specific survival at 3 years of 97.8%.77 This is comparable to outcomes for cryoablation and RFA. However, a recent study showed disappointing results for microwave ablation of tumors with a mean size of 3.65 cm.78 In this study, the tumor recurrence rate was 38% at 18-month follow-up. In addition, the complication rate was 20% intraoperatively and up to 40% postoperatively.78 Tumor characteristics and small sample size may have played a role in these outcomes. A separate, recent, single-center prospective randomized study compared microwave ablation to partial nephrectomy.79 This study reported local recurrence-free survival rates at 3 years of 91.3% for microwave versus 96% for partial nephrectomy (p = 0.5414). Microwave demonstrated significantly reduced blood loss, lower complication rates, but worsening postoperative renal function compared with partial nephrectomy. Surgical and hospitalization times were comparable.79
Irreversible Electroporation
Irreversible electroporation (IRE) is a nonthermal ablation process by which microscopic pores are created within cell membranes from the application of rapid electrical pulses, resulting in cell death.80 81 This permeabilization of the cell membrane can be reversible or irreversible. Reversible electroporation allows the delivery of a variety of materials such as fluorescent dyes, chemotherapeutic agents, antibodies, enzymes, and DNA, while IRE renders the cell necrotic by induction of instabilities in the lipid bilayer of the cell membrane.80 81 By modulating electricity across the cell, IRE impacts the cellular membranes of the target tissues but may spare adjacent connective tissue. This potentially offers advantages over thermal ablation modalities, which carry the risk of injury to the collecting system.82 83 84 85 86 While both animal and human studies have demonstrated a relative sparing of the urothelium with IRE, additional studies are warranted.82 83 85 86 In addition, because IRE is a nonthermal modality, it is not susceptible to the “heat sink” effect of adjacent vessels.82 86 While attractive for these reasons, IRE has several drawbacks. The potential for inducing cardiac arrhythmias and muscle contractions requires electrocardiographic monitoring and synchronization, and general anesthesia with deep neuromuscular blockade.20 85 87 Moreover, the inability to cauterize the entry tract potentially increases the risk of bleeding and tract seeding.20 85
IRE has been investigated in several different organs, including the liver, pancreas, lung, and kidney.81 83 84 85 86 Multiple animal studies have demonstrated safety in treating renal tumors.81 82 83 86 A phase I clinical study in humans was performed in patients who were undergoing surgical resection of their RCCs (IRE followed by immediate resection), and this demonstrated that IRE for RCC was feasible and safe.88 While histopathologic analysis was performed, this only demonstrated that the cells had begun to swell, as the tissue was resected within 15 minutes of electroporation.88 Safety of IRE was again demonstrated in 2011 by Thomson et al who investigated ablation of liver, kidney, and lung lesions in 38 patients.85 Recently, a retrospective study also found IRE to be safe and the learning curve to be acceptable.87 Given that IRE is not susceptible to the thermal impact of adjacent vessels and that there is potential for relative urothelial protection, further investigation is warranted in the kidney for this promising ablation modality.
High-Intensity-Focused Ultrasound
High-intensity-focused ultrasound (HIFU) is based on the physical effects of ultrasound energy on tissue. A focused ultrasound beam is progressively absorbed and its mechanical energy converted to heat.89 90 The thermal effect induces rapid temperature rise at the ultrasound focus point, resulting in protein denaturing and coagulative necrosis, while sparing the superficial tissues and surrounding parenchyma. A secondary cavitation effect also occurs, where microbubble formation results in mechanical tissue lysis from high pressure.89 Both intra- and extracorporeal systems exist; intracorporeal systems are used in open and laparoscopic surgery, while extracorporeal systems are image guided using ultrasound or MRI.89 90
Results of clinical trials using HIFU for treating renal masses have been disappointing.89 91 92 93 Seven tumors (average size of 22 mm) in seven patients treated with laparoscopic, intracorporeal HIFU with “curative intent” before partial nephrectomy showed complete ablation in only four of the seven patients. An eighth patient who did not undergo partial nephrectomy was followed up with imaging and had no evidence of disease at 6 months.91 In another study, 16 patients were treated with HIFU, 2 for curative intent and 14 before surgical removal. Histopathologic review of the explanted tumors showed only 9 of 14 showing therapeutic necrosis, and the histologically damaged tissue comprised only 15 to 35% of the targeted tissue.92 In a similar study, 19 patients were treated before tumor explantation. Thermal damages of the tissues were variable and poor, partially seen in 15 of 19 cases.93
These trials show that extracorporeal HIFU has several technical limitations at present, including variable target tissue depth, respiratory motion, and limitations secondary to tissue variability.89 92 93 A trial using laparoscopic intracorporeal approach appears to have mitigated some of these effects; however, further data are necessary to validate this new technique.91
Conclusion
Several ablative modalities are available that use thermal and nonthermal means to achieve therapeutic tissue necrosis. While data regarding their long-term oncologic efficacy are still evolving, some have been incorporated into clinical guidelines as treatment alternatives for select patients. Cryoablation and RFA have been studied the most, demonstrating favorable safety and efficacy profiles compared with surgical options. Differences between RFA and cryoablation seem to be negligible with respect to intermediate-term oncologic outcomes and complications. Microwave ablation could potentially offer an advantage for central tumors because of its ability to overcome the heat sink effect. IRE offers the potential advantage of targeted therapy to tumor cells while sparing tissue surrounding the urothelium; however, requirements for general anesthesia and electrocardiographic synchronization make its use logistically challenging. HIFU is a relatively new modality, and requires further evaluation to determine its treatment efficacy.
References
- 1. American Cancer Society. 2014. What Are the Key Statistics about Kidney Cancer? Available at: http://www.cancer.org/cancer/kidneycancer/detailedguide/kidney-cancer-adult-key-statistics. Accessed January 27, 2014
- 2.Rojas I Rodríguez T Pierotic M Le Cerf P Histological evaluation of cryosurgery in high grade intraepithelial neoplasia (CIN-III)] of the uterine cervix [in Spanish] Rev Chil Obstet Ginecol 1993583200–204., discussion 204–205 [PubMed] [Google Scholar]
- 3.Homma Y, Kawabe K, Kitamura T. et al. Increased incidental detection and reduced mortality in renal cancer—recent retrospective analysis at eight institutions. Int J Urol. 1995;2(2):77–80. doi: 10.1111/j.1442-2042.1995.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 4.Woldrich J M, Palazzi K, Stroup S P. et al. Trends in the surgical management of localized renal masses: thermal ablation, partial and radical nephrectomy in the USA, 1998-2008. BJU Int. 2013;111(8):1261–1268. doi: 10.1111/j.1464-410X.2012.11497.x. [DOI] [PubMed] [Google Scholar]
- 5.Ljungberg B, Campbell S C, Choi H Y. et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60(4):615–621. doi: 10.1016/j.eururo.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 6.Campbell S C, Novick A C, Belldegrun A. et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182(4):1271–1279. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Weight C J, Larson B T, Fergany A F. et al. Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol. 2010;183(4):1317–1323. doi: 10.1016/j.juro.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 8.Stephenson A J, Hakimi A A, Snyder M E, Russo P. Complications of radical and partial nephrectomy in a large contemporary cohort. J Urol. 2004;171(1):130–134. doi: 10.1097/01.ju.0000101281.04634.13. [DOI] [PubMed] [Google Scholar]
- 9.Weber S M, Lee F T. , Jr. New York: Springer Science + Business Media; 2005. Cryoablation: history, mechanism of action and guidance modalities; pp. 250–266. [Google Scholar]
- 10.Ortved W E, O'Kelly F M, Todd I A, Maxwell J B, Sutton M R. Cryosurgical prostatectomy: a report of 100 cases. Br J Urol. 1967;39(5):577–583. doi: 10.1111/j.1464-410x.1967.tb11797.x. [DOI] [PubMed] [Google Scholar]
- 11.Permpongkosol S Link R E Kavoussi L R Solomon S B Percutaneous computerized tomography guided cryoablation for localized renal cell carcinoma: factors influencing success J Urol 200617651963–1968., discussion 1968 [DOI] [PubMed] [Google Scholar]
- 12.Uchida M Imaide Y Sugimoto K Uehara H Watanabe H Percutaneous cryosurgery for renal tumours Br J Urol 1995752132–136., discussion 136–137 [DOI] [PubMed] [Google Scholar]
- 13.Torre D. Alternate cryogens for cryosurgery. J Dermatol Surg. 1975;1(2):56–58. doi: 10.1111/j.1524-4725.1975.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 14.Hewitt P M, Zhao J, Akhter J, Morris D L. A comparative laboratory study of liquid nitrogen and argon gas cryosurgery systems. Cryobiology. 1997;35(4):303–308. doi: 10.1006/cryo.1997.2039. [DOI] [PubMed] [Google Scholar]
- 15.Theodorescu D. Cancer cryotherapy: evolution and biology. Rev Urol. 2004;6 04:S9–S19. [PMC free article] [PubMed] [Google Scholar]
- 16.Gage A A, Baust J M, Baust J G. Experimental cryosurgery investigations in vivo. Cryobiology. 2009;59(3):229–243. doi: 10.1016/j.cryobiol.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gage A A, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37(3):171–186. doi: 10.1006/cryo.1998.2115. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann N E Bischof J C The cryobiology of cryosurgical injury Urology 200260(2, Suppl 1)40–49. [DOI] [PubMed] [Google Scholar]
- 19.Lagerveld B W. Cryosurgical induced injury of human cancerous tissues—How it works? Br J Med Surg Urol. 2012;5S:S24–S27. [Google Scholar]
- 20.Ahmed M, Brace C L, Lee F T Jr, Goldberg S N. Principles of and advances in percutaneous ablation. Radiology. 2011;258(2):351–369. doi: 10.1148/radiol.10081634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Georgiades C, Rodriguez R, Azene E. et al. Determination of the nonlethal margin inside the visible “ice-ball” during percutaneous cryoablation of renal tissue. Cardiovasc Intervent Radiol. 2013;36(3):783–790. doi: 10.1007/s00270-012-0470-5. [DOI] [PubMed] [Google Scholar]
- 22.Uppot R N, Silverman S G, Zagoria R J, Tuncali K, Childs D D, Gervais D A. Imaging-guided percutaneous ablation of renal cell carcinoma: a primer of how we do it. AJR Am J Roentgenol. 2009;192(6):1558–1570. doi: 10.2214/AJR.09.2582. [DOI] [PubMed] [Google Scholar]
- 23.Woolley M L, Schulsinger D A, Durand D B, Zeltser I S, Waltzer W C. Effect of freezing parameters (freeze cycle and thaw process) on tissue destruction following renal cryoablation. J Endourol. 2002;16(7):519–522. doi: 10.1089/089277902760367494. [DOI] [PubMed] [Google Scholar]
- 24.Goyal J, Verma P, Sidana A, Georgiades C S, Rodriguez R. Single-center comparative oncologic outcomes of surgical and percutaneous cryoablation for treatment of renal tumors. J Endourol. 2012;26(11):1413–1419. doi: 10.1089/end.2012.0244. [DOI] [PubMed] [Google Scholar]
- 25.Mues A C, Okhunov Z, Haramis G, D'Agostino H, Shingleton B W, Landman J. Comparison of percutaneous and laparoscopic renal cryoablation for small (<3.0 cm) renal masses. J Endourol. 2010;24(7):1097–1100. doi: 10.1089/end.2010.0067. [DOI] [PubMed] [Google Scholar]
- 26.Derweesh I H, Malcolm J B, Diblasio C J. et al. Single center comparison of laparoscopic cryoablation and CT-guided percutaneous cryoablation for renal tumors. J Endourol. 2008;22(11):2461–2467. doi: 10.1089/end.2008.0196. [DOI] [PubMed] [Google Scholar]
- 27.Hinshaw J L, Shadid A M, Nakada S Y, Hedican S P, Winter T C III, Lee F T Jr. Comparison of percutaneous and laparoscopic cryoablation for the treatment of solid renal masses. AJR Am J Roentgenol. 2008;191(4):1159–1168. doi: 10.2214/AJR.07.3706. [DOI] [PubMed] [Google Scholar]
- 28.Hui G C, Tuncali K, Tatli S, Morrison P R, Silverman S G. Comparison of percutaneous and surgical approaches to renal tumor ablation: metaanalysis of effectiveness and complication rates. J Vasc Interv Radiol. 2008;19(9):1311–1320. doi: 10.1016/j.jvir.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Bandi G, Hedican S, Moon T, Lee F T, Nakada S Y. Comparison of postoperative pain, convalescence, and patient satisfaction after laparoscopic and percutaneous ablation of small renal masses. J Endourol. 2008;22(5):963–967. doi: 10.1089/end.2007.0261. [DOI] [PubMed] [Google Scholar]
- 30.Sung G T, Gill I S, Hsu T H. et al. Effect of intentional cryo-injury to the renal collecting system. J Urol. 2003;170(2, Pt 1):619–622. doi: 10.1097/01.ju.0000068722.22186.66. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg M D, Kim C Y, Tsivian M. et al. Percutaneous cryoablation of renal lesions with radiographic ice ball involvement of the renal sinus: analysis of hemorrhagic and collecting system complications. AJR Am J Roentgenol. 2011;196(4):935–939. doi: 10.2214/AJR.10.5182. [DOI] [PubMed] [Google Scholar]
- 32.Littrup P J, Ahmed A, Aoun H D. et al. CT-guided percutaneous cryotherapy of renal masses. J Vasc Interv Radiol. 2007;18(3):383–392. doi: 10.1016/j.jvir.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Bodily K D, Atwell T D, Mandrekar J N. et al. Hydrodisplacement in the percutaneous cryoablation of 50 renal tumors. AJR Am J Roentgenol. 2010;194(3):779–783. doi: 10.2214/AJR.08.1570. [DOI] [PubMed] [Google Scholar]
- 34.Froemming A, Atwell T, Farrell M, Callstrom M, Leibovich B, Charboneau W. Probe retraction during renal tumor cryoablation: a technique to minimize direct ureteral injury. J Vasc Interv Radiol. 2010;21(1):148–151. doi: 10.1016/j.jvir.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Long C J, Canter D J, Smaldone M C. et al. Role of tumor location in selecting patients for percutaneous versus surgical cryoablation of renal masses. Can J Urol. 2012;19(5):6417–6422. [PMC free article] [PubMed] [Google Scholar]
- 36.Breen D J, Bryant T J, Abbas A. et al. Percutaneous cryoablation of renal tumours: outcomes from 171 tumours in 147 patients. BJU Int. 2013;112(6):758–765. doi: 10.1111/bju.12122. [DOI] [PubMed] [Google Scholar]
- 37.Ahrar K, Ahrar J U, Javadi S. et al. Real-time magnetic resonance imaging-guided cryoablation of small renal tumors at 1.5 T. Invest Radiol. 2013;48(6):437–444. doi: 10.1097/RLI.0b013e31828027c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverman S G, Tuncali K, vanSonnenberg E. et al. Renal tumors: MR imaging-guided percutaneous cryotherapy—initial experience in 23 patients. Radiology. 2005;236(2):716–724. doi: 10.1148/radiol.2362041107. [DOI] [PubMed] [Google Scholar]
- 39.Khan F, Sriprasad S, Keeley F X Jr. Cryosurgical ablation for small renal masses, current status and future prospects. Br J Med Surg Urol. 2012;5S:S28–S34. [Google Scholar]
- 40.Rodriguez R, Cizman Z, Hong K, Koliatsos A, Georgiades C. Prospective analysis of the safety and efficacy of percutaneous cryoablation for pT1NxMx biopsy-proven renal cell carcinoma. Cardiovasc Intervent Radiol. 2011;34(3):573–578. doi: 10.1007/s00270-010-9934-7. [DOI] [PubMed] [Google Scholar]
- 41.Kunkle D A, Uzzo R G. Cryoablation or radiofrequency ablation of the small renal mass : a meta-analysis. Cancer. 2008;113(10):2671–2680. doi: 10.1002/cncr.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chalasani V, Martinez C H, Lim D, Abdelhady M, Chin J L. Surgical cryoablation as an option for small renal masses in patients who are not ideal partial nephrectomy candidates: intermediate-term outcomes. Can Urol Assoc J. 2010;4(6):399–402. doi: 10.5489/cuaj.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Georgiades C S, Rodriguez R. Efficacy and safety of percutaneous cryoablation for stage 1a/b renal cell carcinoma: results of a prospective, single-arm, 5-year study. Cardiovasc Intervent Radiol. 2014 doi: 10.1007/s00270-013-0831-8. [DOI] [PubMed] [Google Scholar]
- 44.Johnson D B, Solomon S B, Su L M. et al. Defining the complications of cryoablation and radio frequency ablation of small renal tumors: a multi-institutional review. J Urol. 2004;172(3):874–877. doi: 10.1097/01.ju.0000135833.67906.ec. [DOI] [PubMed] [Google Scholar]
- 45.Atwell T D, Carter R E, Schmit G D. et al. Complications following 573 percutaneous renal radiofrequency and cryoablation procedures. J Vasc Interv Radiol. 2012;23(1):48–54. doi: 10.1016/j.jvir.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Massarweh N N, Cosgriff N, Slakey D P. Electrosurgery: history, principles, and current and future uses. J Am Coll Surg. 2006;202(3):520–530. doi: 10.1016/j.jamcollsurg.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 47.O'Connor J L, Bloom D A, William T. Bovie and electrosurgery. Surgery. 1996;119(4):390–396. doi: 10.1016/s0039-6060(96)80137-1. [DOI] [PubMed] [Google Scholar]
- 48.McGahan J P, Browning P D, Brock J M, Tesluk H. Hepatic ablation using radiofrequency electrocautery. Invest Radiol. 1990;25(3):267–270. doi: 10.1097/00004424-199003000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Rossi S, Fornari F, Pathies C, Buscarini L. Thermal lesions induced by 480 KHz localized current field in guinea pig and pig liver. Tumori. 1990;76(1):54–57. doi: 10.1177/030089169007600114. [DOI] [PubMed] [Google Scholar]
- 50.Zlotta A R, Wildschutz T, Raviv G. et al. Radiofrequency interstitial tumor ablation (RITA) is a possible new modality for treatment of renal cancer: ex vivo and in vivo experience. J Endourol. 1997;11(4):251–258. doi: 10.1089/end.1997.11.251. [DOI] [PubMed] [Google Scholar]
- 51.Lui K W, Gervais D A, Arellano R A, Mueller P R. Radiofrequency ablation of renal cell carcinoma. Clin Radiol. 2003;58(12):905–913. doi: 10.1016/s0009-9260(03)00222-8. [DOI] [PubMed] [Google Scholar]
- 52.Hsu T H, Fidler M E, Gill I S. Radiofrequency ablation of the kidney: acute and chronic histology in porcine model. Urology. 2000;56(5):872–875. doi: 10.1016/s0090-4295(00)00737-8. [DOI] [PubMed] [Google Scholar]
- 53.Zagoria R J. Imaging-guided radiofrequency ablation of renal masses. Radiographics. 2004;24 01:S59–S71. doi: 10.1148/rg.24si045512. [DOI] [PubMed] [Google Scholar]
- 54.Salas N, Castle S M, Leveillee R J. Radiofrequency ablation for treatment of renal tumors: technological principles and outcomes. Expert Rev Med Devices. 2011;8(6):695–707. doi: 10.1586/erd.11.51. [DOI] [PubMed] [Google Scholar]
- 55.McGhana J P, Dodd G D III. Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001;176(1):3–16. doi: 10.2214/ajr.176.1.1760003. [DOI] [PubMed] [Google Scholar]
- 56.Takaki H, Yamakado K, Soga N. et al. Midterm results of radiofrequency ablation versus nephrectomy for T1a renal cell carcinoma. Jpn J Radiol. 2010;28(6):460–468. doi: 10.1007/s11604-010-0451-z. [DOI] [PubMed] [Google Scholar]
- 57.Olweny E O, Park S K, Tan Y K, Best S L, Trimmer C, Cadeddu J A. Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur Urol. 2012;61(6):1156–1161. doi: 10.1016/j.eururo.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 58.Gupta A, Raman J D, Leveillee R J. et al. General anesthesia and contrast-enhanced computed tomography to optimize renal percutaneous radiofrequency ablation: multi-institutional intermediate-term results. J Endourol. 2009;23(7):1099–1105. doi: 10.1089/end.2008.0499. [DOI] [PubMed] [Google Scholar]
- 59.Zagoria R J, Pettus J A, Rogers M, Werle D M, Childs D, Leyendecker J R. Long-term outcomes after percutaneous radiofrequency ablation for renal cell carcinoma. Urology. 2011;77(6):1393–1397. doi: 10.1016/j.urology.2010.12.077. [DOI] [PubMed] [Google Scholar]
- 60.Gervais D A, McGovern F J, Arellano R S, McDougal W S, Mueller P R. Radiofrequency ablation of renal cell carcinoma: part 1, Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol. 2005;185(1):64–71. doi: 10.2214/ajr.185.1.01850064. [DOI] [PubMed] [Google Scholar]
- 61.Gervais D A, Arellano R S, Mueller P. Percutaneous ablation of kidney tumors in nonsurgical candidates. Oncology (Williston Park) 2005;19(11) 04:6–11. [PubMed] [Google Scholar]
- 62.Lee S J, Choyke L T, Locklin J K, Wood B J. Use of hydrodissection to prevent nerve and muscular damage during radiofrequency ablation of kidney tumors. J Vasc Interv Radiol. 2006;17(12):1967–1969. doi: 10.1097/01.RVI.0000248829.49442.0E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kam A W, Littrup P J, Walther M M, Hvizda J, Wood B J. Thermal protection during percutaneous thermal ablation of renal cell carcinoma. J Vasc Interv Radiol. 2004;15(7):753–758. doi: 10.1097/01.rvi.0000133535.16753.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hegg R M, Schmit G D, Kurup A N, Weisbrod A J, Boorjian S A, Atwell T D. Ultrasound-guided transhepatic radiofrequency ablation of renal tumors: a safe and effective approach. Cardiovasc Intervent Radiol. 2014;37(2):508–512. doi: 10.1007/s00270-013-0716-x. [DOI] [PubMed] [Google Scholar]
- 65.Tracy C R, Raman J D, Donnally C, Trimmer C K, Cadeddu J A. Durable oncologic outcomes after radiofrequency ablation: experience from treating 243 small renal masses over 7.5 years. Cancer. 2010;116(13):3135–3142. doi: 10.1002/cncr.25002. [DOI] [PubMed] [Google Scholar]
- 66.Ma Y, Bedir S, Cadeddu J A, Gahan J C. Long-term outcomes in healthy adults after radiofrequency ablation of T1a renal tumours. BJU Int. 2014;113(1):51–55. doi: 10.1111/bju.12366. [DOI] [PubMed] [Google Scholar]
- 67.Wah T M, Irving H C, Gregory W, Cartledge J, Joyce A D, Selby P J. Radiofrequency ablation (RFA) of renal cell carcinoma (RCC): experience in 200 tumours. BJU Int. 2014;113(3):416–428. doi: 10.1111/bju.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Balageas P, Cornelis F, Le Bras Y. et al. Ten-year experience of percutaneous image-guided radiofrequency ablation of malignant renal tumours in high-risk patients. Eur Radiol. 2013;23(7):1925–1932. doi: 10.1007/s00330-013-2784-3. [DOI] [PubMed] [Google Scholar]
- 69.Dib R E, Touma N J, Kapoor A. Review of the efficacy and safety of radiofrequency ablation for the treatment of small renal masses. Can Urol Assoc J. 2009;3(2):143–149. doi: 10.5489/cuaj.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rhim H, Dodd G D III, Chintapalli K N. et al. Radiofrequency thermal ablation of abdominal tumors: lessons learned from complications. . Radiographics. 2004;24(1):41–52. doi: 10.1148/rg.241025144. [DOI] [PubMed] [Google Scholar]
- 71.El Dib R, Touma N J, Kapoor A. Cryoablation vs radiofrequency ablation for the treatment of renal cell carcinoma: a meta-analysis of case series studies. BJU Int. 2012;110(4):510–516. doi: 10.1111/j.1464-410X.2011.10885.x. [DOI] [PubMed] [Google Scholar]
- 72.Pirasteh A, Snyder L, Boncher N, Passalacqua M, Rosenblum D, Prologo J D. Cryoablation vs. radiofrequency ablation for small renal masses. Acad Radiol. 2011;18(1):97–100. doi: 10.1016/j.acra.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 73.Gervais D A. Cryoablation versus radiofrequency ablation for renal tumor ablation: time to reassess? J Vasc Interv Radiol. 2013;24(8):1135–1138. doi: 10.1016/j.jvir.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 74.Truesdale C M, Soulen M C, Clark T W. et al. Percutaneous computed tomography-guided renal mass radiofrequency ablation versus cryoablation: doses of sedation medication used. J Vasc Interv Radiol. 2013;24(3):347–350. doi: 10.1016/j.jvir.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 75.Simon C J, Dupuy D E, Mayo-Smith W W. Microwave ablation: principles and applications. . Radiographics. 2005;25 01:S69–S83. doi: 10.1148/rg.25si055501. [DOI] [PubMed] [Google Scholar]
- 76.Brace C L. Microwave tissue ablation: biophysics, technology, and applications. Crit Rev Biomed Eng. 2010;38(1):65–78. doi: 10.1615/critrevbiomedeng.v38.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu J, Liang P, Yu X L. et al. US-guided percutaneous microwave ablation of renal cell carcinoma: intermediate-term results. Radiology. 2012;263(3):900–908. doi: 10.1148/radiol.12111209. [DOI] [PubMed] [Google Scholar]
- 78.Castle S M, Salas N, Leveillee R J. Initial experience using microwave ablation therapy for renal tumor treatment: 18-month follow-up. Urology. 2011;77(4):792–797. doi: 10.1016/j.urology.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 79.Guan W, Bai J, Liu J. et al. Microwave ablation versus partial nephrectomy for small renal tumors: intermediate-term results. J Surg Oncol. 2012;106(3):316–321. doi: 10.1002/jso.23071. [DOI] [PubMed] [Google Scholar]
- 80.Davalos R V, Mir I L, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33(2):223–231. doi: 10.1007/s10439-005-8981-8. [DOI] [PubMed] [Google Scholar]
- 81.Edd J F, Horowitz L, Davalos R V, Mir L M, Rubinsky B. In vivo results of a new focal tissue ablation technique: irreversible electroporation. IEEE Trans Biomed Eng. 2006;53(7):1409–1415. doi: 10.1109/TBME.2006.873745. [DOI] [PubMed] [Google Scholar]
- 82.Deodhar A, Monette S, Single G W Jr. et al. Renal tissue ablation with irreversible electroporation: preliminary results in a porcine model. Urology. 2011;77(3):754–760. doi: 10.1016/j.urology.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 83.Wendler J J, Porsch M, Hühne S. et al. Short- and mid-term effects of irreversible electroporation on normal renal tissue: an animal model. Cardiovasc Intervent Radiol. 2013;36(2):512–520. doi: 10.1007/s00270-012-0452-7. [DOI] [PubMed] [Google Scholar]
- 84.Silk M T, Wimmer T, Lee K S. et al. Percutaneous ablation of peribiliary tumors with irreversible electroporation. J Vasc Interv Radiol. 2014;25(1):112–118. doi: 10.1016/j.jvir.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 85.Thomson K R, Cheung W, Ellis S J. et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol. 2011;22(5):611–621. doi: 10.1016/j.jvir.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 86.Tracy C R, Kabbani W, Cadeddu J A. Irreversible electroporation (IRE): a novel method for renal tissue ablation. BJU Int. 2011;107(12):1982–1987. doi: 10.1111/j.1464-410X.2010.09797.x. [DOI] [PubMed] [Google Scholar]
- 87.Philips P, Hays D, Martin R C. Irreversible electroporation ablation (IRE) of unresectable soft tissue tumors: learning curve evaluation in the first 150 patients treated. PLoS ONE. 2013;8(11):e76260. doi: 10.1371/journal.pone.0076260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pech M, Janitzky A, Wendler J J. et al. Irreversible electroporation of renal cell carcinoma: a first-in-man phase I clinical study. Cardiovasc Intervent Radiol. 2011;34(1):132–138. doi: 10.1007/s00270-010-9964-1. [DOI] [PubMed] [Google Scholar]
- 89.Nabi G, Goodman C, Melzer A. High intensity focused ultrasound treatment of small renal masses: clinical effectiveness and technological advances. Indian J Urol. 2010;26(3):331–337. doi: 10.4103/0970-1591.70561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dubinsky T J, Cuevas C, Dighe M K, Kolokythas O, Hwang J H. High-intensity focused ultrasound: current potential and oncologic applications. AJR Am J Roentgenol. 2008;190(1):191–199. doi: 10.2214/AJR.07.2671. [DOI] [PubMed] [Google Scholar]
- 91.Klingler H C Susani M Seip R Mauermann J Sanghvi N Marberger M J A novel approach to energy ablative therapy of small renal tumours: laparoscopic high-intensity focused ultrasound Eur Urol 2008534810–816., discussion 817–818 [DOI] [PubMed] [Google Scholar]
- 92.Marberger M, Schatzl G, Cranston D, Kennedy J E. Extracorporeal ablation of renal tumours with high-intensity focused ultrasound. BJU Int. 2005;95 02:52–55. doi: 10.1111/j.1464-410X.2005.05200.x. [DOI] [PubMed] [Google Scholar]
- 93.Häcker A, Michel M S, Marlinghaus E, Köhrmann K U, Alken P. Extracorporeally induced ablation of renal tissue by high-intensity focused ultrasound. BJU Int. 2006;97(4):779–785. doi: 10.1111/j.1464-410X.2006.06037.x. [DOI] [PubMed] [Google Scholar]


