Abstract
Study Design Retrospective cohort study.
Objective To evaluate whether the proposed one-stage biopsy, culture, debridement, and reconstruction with stabilization strategy is a viable option for pyogenic vertebral osteomyelitis (PVO). PVO is increasing in frequency globally, particularly in immunologically compromised individuals. Typically, biopsy and culture sensitivity followed by staged surgery and long-term antibiotic therapy is the mainstay of therapy.
Methods This is a study of a group of 32 consecutive cases of PVO (spondylodiskitis) treated in our institution from 2010 to 2012. All cases had one-stage biopsy, culture sensitivity, debridement, reconstruction with iliac bone graft, and stabilization with titanium implants. The mean age in this series was 51 years, and the male-to-female ratio was 2.2:1. Approximately 50% of the patients had impaired immunity status. The commonest organism isolated was Pseudomonas aeruginosa. Culture-specific antibiotics were given for a minimum of 6 weeks to all patients. The follow-up period ranged from 6 months to a maximum of 2 years. All patients were assessed for wound healing, recurrence of infection, deformity progression, pain, and healing by radiologic and biochemical parameters. No generic or disease-specific outcome tools were used for this study.
Results All patients had good wound healing, although there was one case of superficial infection that was resolved with debridement. There were two implant failures with pseudarthrosis and one localized kyphosis in this series.
Conclusions The one-stage technique of biopsy, debridement, bone grafting, and stabilization can be recommended for most cases of PVO.
Keywords: pyogenic vertebral osteomyelitis, biopsy, debridement, reconstruction, stabilization, spondylodiskitis, instrumentation
Introduction
Pyogenic vertebral osteomyelitis (PVO) and spondylodiskitis remain potentially life-threatening. The course of the disease is determined by several factors including the immune status of the host, the virulence of the organism, and the potency of newer antibiotics.1 2 3 The general objective of treatment is eradication of the infection, prevention of recurrence, relief of pain, prevention or reversal of neurologic defect, restoration of spinal stability and alignment, and correction of deformity, among others. The mainstay of the treatment is conservative with systemic antibiotics until control or eradication of infectious disease is achieved.2 It has been accepted that in the absence of life-threatening sepsis, antibiotics should not be started empirically without vigorous attempt to isolate the pathogen. Surgical indication typically includes failure of medical management (i.e., progressive sepsis, persistent local abscess, progressive neurologic loss, progressive instability, and deformity).1 2 3 4 5 6 Nonetheless, surgical treatment of a patient with PVO remains a challenge due to several factors. The care of this patient is often complicated by medical comorbidities like senility, diabetes mellitus, chronic renal failure, cirrhosis of liver, psychiatric illness, malnutrition, drug and alcohol abuse, and so on.2 Treatment tends to be prolonged with several interventions for biopsy and culture/sensitivity testing, prolonged antibiotic therapy, repeated debridements, reconstruction, and often prolonged bed rest and hospitalization. Despite the theoretical risk of reactivation associated with the use of metallic implants in the setting of active bacterial infection, instrumentation has been used without persistent or recurrent infection by several authors in the past.2 7
A one-stage biopsy, radical debridement, reconstruction, and stabilization for PVO procedure is conceptually an attractive option for these patients who have multiple comorbidities and are not ideal candidates for multiple anesthesia and surgeries. The rationale for this procedure is as follows:
Improvement of general condition after abscess drainage
Obtaining tissue sample for diagnosis and culture sensitivity
Disease eradication by appropriate chemotherapy
Total extirpation of infected focus to achieve clinical cure
Prevention of secondary deformity due to reconstruction
Less frequent late recurrence
Putative shorter hospital stay and early return to work/activity
Rapid pain relief due to disease eradication and stabilization
Less anesthesia-related morbidity
The aim of this study was to establish whether such one-stage radical surgical procedure does indeed have perceived advantages in terms of patient care and outcomes.
Materials and Methods
This retrospective cohort study included all patients with PVO who underwent surgery from July 2010 to June 2012 at Khoula Hospital, Muscat, Sultanate of Oman. Khoula Hospital is the only tertiary care orthopedic and neurosurgical center in Oman, where spinal disorders are managed. The data will therefore give an epidemiologic overview of PVO in the country as well. Only de novo PVO cases have been included in this review, and postinterventional (post–spinal surgery and post–spinal injection) spondylodiskitis has been excluded. Similarly, tuberculosis and brucellosis of the spine were excluded. Computed tomography (CT)-guided biopsy is no longer routinely used in our center because the yield has been consistently poor.
There were 32 patients in this study group. The age of the patients varied from 4 to 80 years with a mean age of 51 years. The 4-year-old child was the lone pediatric case in this series; all others were adults. The male-to-female ratio was 2.2:1. Three patients were positive for either human immunodeficiency virus (HIV) or hepatitis C virus (HCV) or both and were all intravenous drug abusers. Four had diabetes, two had liver cirrhosis (alcoholic), one had congenital immunodeficiency syndrome (the child), and five with chronic renal failure were on dialysis. All the patients were ethnic Omani nationals with no expatriate case in this study.
The level of the spine affected was as follows. There were 24 cases involving the lumbar spine, 5 involving the thoracic spine, and 3 affecting the cervical spine. All the cases had at least 2 levels or more of destruction on imaging studies. It appeared that many of the patients presented quite late to the spine service, and several of them had symptoms for 2 months or more. All patients presented with axial back pain as the main clinical feature. Seven of the 32 patients had varying degrees of neurologic compromise on admission, though no patient was completely paralyzed. Fever and systemic toxicity was seen only in 2 patients, one of whom was on dialysis for chronic renal disease. It was difficult to record an accurate history regarding the source of infection in most cases due to educational and sociocultural barriers. Similarly, a reliable history or documentation of antibiotic administration was not forthcoming.
The protocol at our center is to perform total and differential blood counts, erythrocyte sedimentation rate, and C-reactive protein in all patients along with blood cultures on admission. They also had blood sugars, liver function, and renal function tests besides HIV and HCV screening. X-rays and magnetic resonance imaging scans were done in all these patients. CT scans were used selectively to determine the extent of bony involvement and plan surgery. None of the patients in this series had an isotope bone scan.
The Surgical Technique
All the 32 patients in this cohort were treated surgically. The surgeries were performed through the posterior approach with the exception of the three cervical cases that were done anteriorly. The patients were positioned prone on bolsters. The skin was infiltrated with 1:500,000 adrenaline. A vertical midline incision was used in most cases in these regions. One side of the spine was instrumented with titanium pedicle screw instrumentation. The montage spanned two levels above to two below the affected segments in cases with extensive vertebral destruction and multilevel involvement and single motion segment in early disease with minimal bone loss (Figs. 1 and 2). On the opposite side, the transverse processes and the rib heads (in case of the thoracic spine) were removed across the affected segments, and the vertebral body was approached from the posterolateral extracavitary approach. The infected vertebral bodies and disks were radically debrided with angled curettes. The dura was decompressed by exposure through the neural foramen where indicated. The vertebral body space filled with autologous cancellous bone graft from the iliac crest or ribs mixed with 2 g of streptomycin power. Structural corticocancellous bone graft was used only in those cases with large defects where no cortical shell remained after debridement. The debrided material was saved for histopathology, culture sensitivity, and Gram staining as well as acid-fast bacilli staining, tuberculosis culture, and tuberculosis polymerase chain reaction examination.8 The pedicle screw implants on the second side of the spine were then mounted and tightened. Cross-links were added where appropriate. Postoperative pain was managed by epidural analgesia in all these cases; the cannula was placed by the surgeon at the end of the procedure through a puncture of the ligamentum flavum above the infected segment. In case of the cervical spine disease, the right sternomastoid approach was used and the infected vertebral bodies were debrided. Corticocancellous bone graft block was harvested from the iliac crest and impacted into the resultant space and self-locking titanium plate and screws used to stabilize the segment. All cases had suction drains installed. Depending on culture and sensitivity report, patient was put on parenteral antibiotics for 2 to 3 weeks followed by 4 to 6 weeks of oral antibiotics. In the cases where no culture could be obtained, a combination of two antibiotics was instituted as per the institutional infection control guidelines covering gram-positive and negative organisms. These patients were all mobilized by the second to third day postoperatively depending on their pain. External bracing was used only in a few cases of lumbar disease where the instrumentation was perceived to be less stable due to extensive vertebral destruction. No cages were used in this series of cases.
Fig. 1.
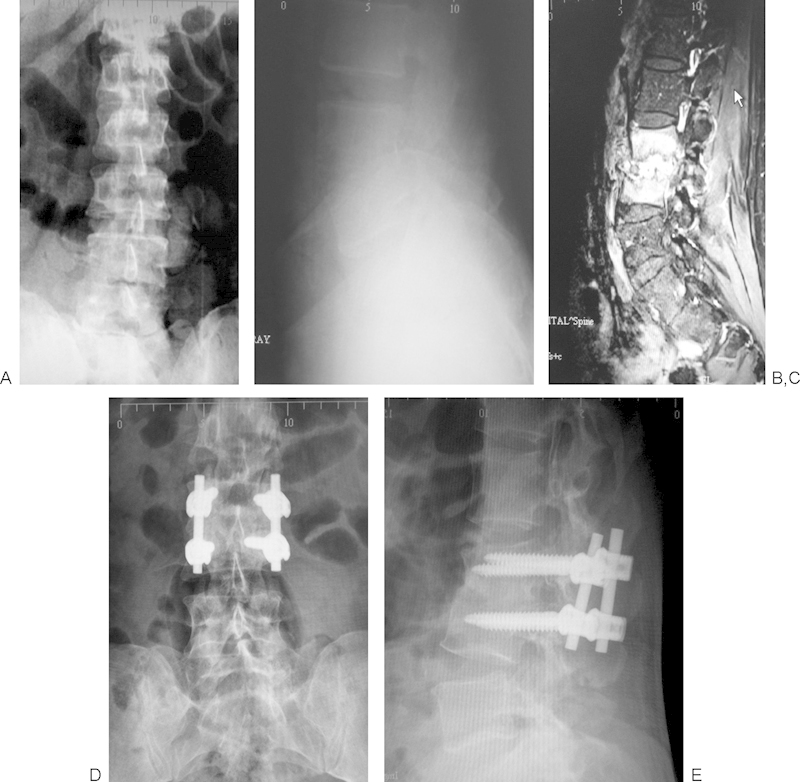
Case of pyogenic vertebral osteomyelitis (PVO) with minimal destruction. (A, B) Preoperative anteroposterior and lateral X-rays showing irregular reduction of disk space. (C) T1-weighted magnetic resonance image showing typical findings of PVO. (D, E) Postoperative X-rays at 6 months showing good fusion after the one-stage surgery with short segment stabilization.
Fig. 2.
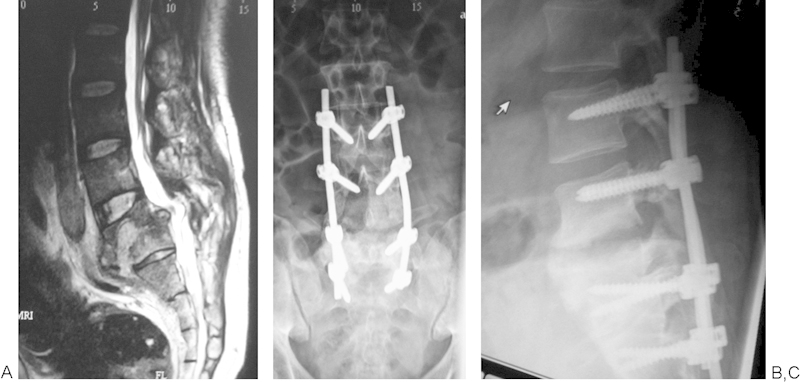
(A) Extensive vertebral column destruction and abscess formation seen on magnetic resonance imaging. (B, C) Postoperative images with long segment stabilization and good fusion after 6 months.
Microbiology
No organisms were isolated in 47% of the cases in this series. The commonest pathogen in this community appeared to be Pseudomonas aeruginosa, occurring in 7 of the 32 cases with Staphylococcus aureus coming closely behind in 6 cases. Interestingly, none of the patients with Pseudomonas were immunologically challenged. The only positive blood culture in this series was in a patient with S. aureus infection. There were two infections by Klebsiella and one each by Staphylococcus epidermidis and Streptococcus. All three patients with history of intravenous drug abuse were culture-negative.
Results
No disease-specific or generic outcome measures were used for this study. All the patients' surgical wounds successfully healed. The mean hospital stay was 20.2 days with a range of 5 to 64 days. Being a public health service with no uniform community nursing facility, and also due to cultural reasons, it is difficult to discharge patients early from inpatient care in our system. Therefore, these results may not reflect the true rate of progress of healing of the disease. The review was organized at 6 weeks, 3 months, 6 months, and 12 months and thereafter only if indicated. Again, for educational, cultural, and social reasons, the review was neither accurate nor regular. Nineteen patients completed regular follow-up until 1 year. Eight patients were followed regularly for 6 months and had radiologic healing and were then lost to follow-up. Another five patients were reviewed irregularly for a minimum of 6 months and a maximum of 2 years. In all 32 patients, the surgical site healed, and they returned to their preoperative mobility status on review. Two patients reported with implant failure and pseudarthrosis without recurrent infection. Interestingly, both the cases were instrumented using “soft stabilization systems” typically intended for degenerative disorders. One of the implant failures occurred at 6 months and was revised by implant removal alone, and it went on to heal well (Fig. 3). The second patient reported at 2 years' follow-up and is awaiting revision. There was one case of progressive collapse of one of the affected vertebrae and localized kyphosis in the lumbar spine with implant pullout, but this healed in situ and the patient refused intervention because he was pain-free (Fig. 4). There was one instance of donor site infection at the iliac crest, which subsided without additional intervention but with dressing and sensitive antibiotics alone. All the seven patients with neurologic deficits improved with minimal motor residue. One patient who was wheelchair bound preoperatively remained so despite her neurologic improvement. At 6 months, all blood parameters relating to infection were normal, and X-ray showed implants in situ with radiologic fusion progressing well.
Fig. 3.
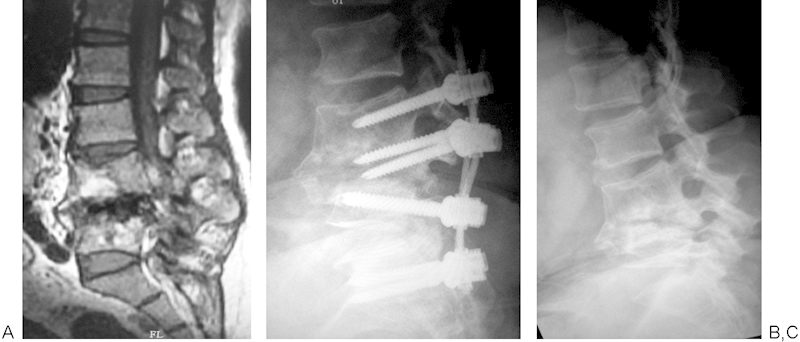
(A) L4–5 pyogenic vertebral osteomyelitis on magnetic resonance image. (B) Implant breakage and collapse of vertebrae. (C) Spontaneous fusion after implant removal.
Fig. 4.
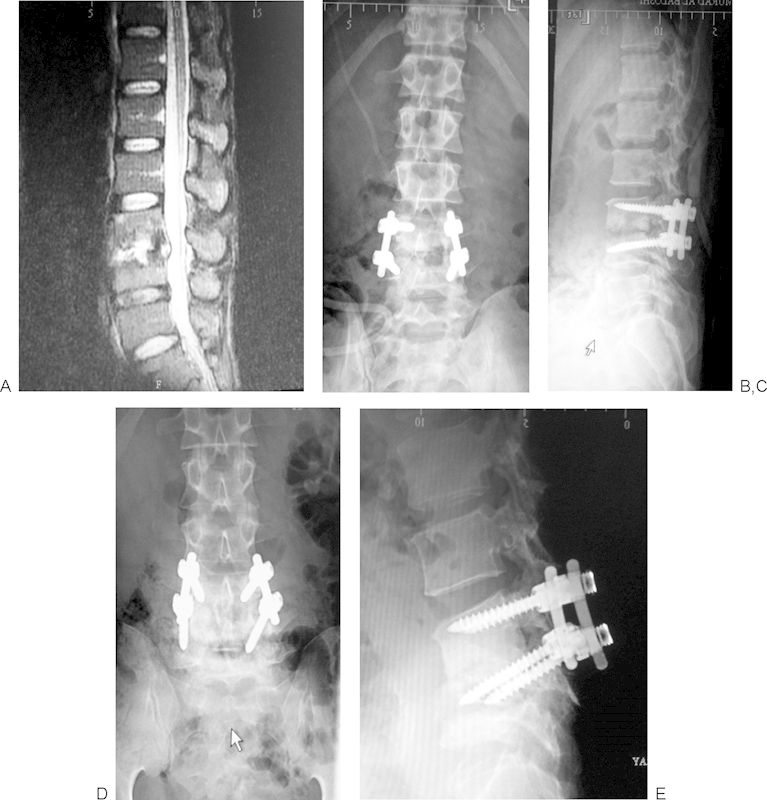
(A) Magnetic resonance image with spondylodiskitis at L3–4 level. (B, C) Early postoperative images showing good position of implants. (D, E) Asymptomatic, progressive destruction of L4 vertebra and kyphosis with implant cut out.
In the early postoperative period, one patient had superficial surgical site infection. Revision surgery was undertaken, the wound was debrided, and the infection responded to culture-sensitive antibiotics.
Discussion
The typical surgical plan for PVO would include three interventions: a diagnostic procedure consisting of CT-guided or open biopsy and culture sensitivity testing, an anterior debridement and reconstruction, followed by a posterior stabilization at the same sitting or sequentially.9 10 Khoula Hospital is the only tertiary care orthopedic spine center in Oman, and so waiting lists for elective surgery are rather long, particularly for patients who need multiple procedures. In this series, nearly all patients were admitted through the emergency services and worked up for early surgical treatment. Our past experience in this center with image-guided biopsy of the spine under local anesthetic has been unsatisfactory with very low yield; therefore, it is currently our policy to perform open transpedicular biopsy under general anesthesia whenever vertebral body biopsy is indicated. A single-stage surgery to combine diagnostics and therapy seemed to be the logical step forward. Additionally, the senior author's past experience with tuberculosis of the spine and pyogenic spondylodiskitis treated in a similar manner by one-stage posterior surgery seemed promising. While starting this therapeutic protocol, there were definite logistic and sociocultural issues that influenced the decision making, but as we moved forward with the strategy, it became abundantly clear that the advantages in terms of surgical morbidity, patient comfort, hospital stay, and clinical outcomes justified the adoption of a one-stage debridement and reconstruction policy for pyogenic infections of the spine.
Epidemiology
The incidence of PVO is reported to be 2 per 100,000 annually.2 3 4 The incidence is increasing due to aging of the society, abuse of intravenous drugs, immunosuppressant treatment, and increasing prevalence of diabetes and obesity.1 2 3 4 This disease most frequently affects the at-risk populations, namely, the elderly, the physically and psychologically challenged, and the immune-compromised due to afflictions like malnutrition, diabetes mellitus, chronic smoking, and intravenous drug abuse, polytrauma leading to long-term recumbency, and obesity.1 2 3 4 5 6
Several interesting observations were made from this study. Out of a population of 3.3 million (with approximately 39% expatriates), if one assumes that all cases of PVO in the country did actually report to Khoula Hospital, the annual prevalence would be 8 cases per million per year. (All the subjects studied were ethnic Omani nationals.) This is against a similarly calculated prevalence of seven to nine cases of spinal tuberculosis treated per year at this hospital. It appears that in this part of the globe, PVO exceeds tuberculosis of the spine in frequency. Several authors have reported increasing incidence of PVO in recent years, but this is predominantly in immune-compromised individuals, whereas in our study population only ∼47% were challenged by preexisting medical disorders.2 3 This implies that 53% cases had de novo community-acquired infection in immunologically normal individuals. As substantiated in the literature, PVO is largely an adult disease with male predominance even in our study cohort.2 3 4 5
Carragee and Dimar et al have reported that the causative organism in de novo infections is most often S. aureus, the frequency ranging from 48 to 67%.1 9 Interestingly in our study this was only 18.75%, with P. aeruginosa predominating, even in immunocompetent patients. This is a rather unusual observation. Consistent with other authors is our finding that the lumbar spine is the most frequently affected region of the spine and the sacrum rarely affected.1 3 Axial back pain is the commonest presentation, though systemic manifestations of infection were rare in this cohort. Neurologic manifestations were also unusual, as reported by other authors.1 3
Surgical Strategy
The surgical strategy for PVO is still evolving because most centers have small numbers of patients. The conventional technique is radical debridement through the anterior approach followed by reconstruction with corticocancellous bone blocks harvested from the iliac crest.9 10 11 12 Ruf et al, Liljenqvist et al, and Kuklo et al have demonstrated that titanium mesh cages can be used satisfactorily for reconstruction of the destroyed vertebral body.11 13 14 Other authors have suggested allograft.15 We have used radical debridement and reconstruction with cancellous chip graft only when the destruction is limited to part of a vertebra or when the bony shell is intact. The graft is most often harvested from the posterior superior iliac spine and mixed with 2 g of streptomycin powder before introduction. Despite the use of nonstructural graft and early mobilization of patients, we had only two implant failures with pseudarthrosis. The exact prevalence of pseudarthrosis after surgery for spinal infection is not known. This would seem to depend on several factors like extent of the disease and destruction, thoroughness of debridement, type and extent of grafting, instrumentation and approach, and a cluster of host-related factors. It is well documented that in spinal tuberculosis anterior debridement and grafting procedures result in better radiologic fusion rates than posterior procedures, but the data are less conclusive regarding pyogenic infections. Lee and colleagues have reported only 1 of 30 cases when instrumented surgery is performed for pyogenic infection of the spine.16 It is also known that pseudarthrosis in the setting of spinal infection is not always symptomatic. Significant late collapse was observed only in one case in our series, and this patient was asymptomatic with this protocol. We do not use antibiotic-loaded continuous suction irrigation in this category of patients as recommended by Jeanneret and Magerl.17 Halpen and colleagues have suggested an ingenious technique of spinal column shortening and reported their results in a small series of cases.18
The concept of single-stage surgery is not unique. Many authors have suggested one-stage debridement and reconstruction, but these have all been through the anterior approach or combined anterior and posterior approach.13 18 19 Titanium implants have been used quite safely in the spine in the presence of infection and abscess formation even in immunocompromised patients.7 Anterior debridement and reconstruction followed by posterior instrumentation is a viable option in PVO cases; nonetheless, in debilitated patients with multisystem disease, this might prove dangerous. The one-stage posterior instrumentation and debridement through the extracavitary approach allows adequate debridement and reconstruction of the body. Moreover, most surgeons are familiar with the posterior approach, and the surgical time and access-related morbidity are also reduced.
The potential for infections to remain dormant with bacterial colonies in biofilms on the surface of metallic implants has for decades determined our strategies for treating axial and appendicular skeletal infections. Bauer and colleagues in a recent review have reaffirmed the accepted role of bacterial adhesion and biofilm formation and the factors that modify this behavior of microorganisms in relation to implant-related infections.20 Titanium and its alloys tend to form less bacterial biofilm than stainless steel, more so when the surface is coated with nitride or oxide alloy and the microtexturing of the surface is less conducive to such adhesion. It is also known that Mycobacterium tuberculosis forms less biofilm that S. aureus and epidermidis.
Instrumentation in infections of the spine has several merits such as restoration and maintenance of the sagittal alignment of the spine.2 7 9 10 Stabilization reduces pain and hospital stay and facilitates faster rehabilitation. Many researchers believe that instability breeds infection and stability suppresses infection and facilitates fusion. Chen et al compared recurrence rates in noninstrumented surgery with instrumented surgery.12 They found no persistence of infection or increase in recurrence in the instrumented surgery group. In the present series, there were no recurrent infections, chronic pain, or mechanical instability in any of the patients except the two who had implant failure.
Conclusion
One-stage biopsy, debridement, reconstruction, and stabilization is a safe and effective strategy for PVO and infective spondylodiskitis. The use of titanium implants is safe and does not lead to persistence or recurrence of infection. Cancellous bone graft in the morselized form is adequate when the affected vertebral shell is preserved and late collapse is infrequent. It is advisable not to use any soft stabilization systems for this indication.
Limitations
This study has the limitations of all retrospective studies—small numbers and short follow-up periods. Moreover, it is a single-center study with two consultant-level spine surgeons leading the planning and execution of the procedure. Further, double blind, randomized, multicentric studies are required to draw more definitive conclusions.
Footnotes
Disclosures No financial or other support has been received or will be received by any of the authors involved in this study.
References
- 1.Carragee E J. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1997;79(6):874–880. doi: 10.2106/00004623-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Boos N. Heidelberg, Germany: Springer; 2008. Infections of the spine; pp. 1021–1039. [Google Scholar]
- 3.Butler J S, Shelly M J, Timlin M, Powderly W G, O'Byrne J M. Nontuberculous pyogenic spinal infection in adults: a 12-year experience from a tertiary referral center. Spine (Phila Pa 1976) 2006;31(23):2695–2700. doi: 10.1097/01.brs.0000244662.78725.37. [DOI] [PubMed] [Google Scholar]
- 4.Calderone R R, Larsen J M. Overview and classification of spinal infections. Orthop Clin North Am. 1996;27(1):1–8. [PubMed] [Google Scholar]
- 5.Hadjipavlou A G, Mader J T, Necessary J T, Muffoletto A J. Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976) 2000;25(13):1668–1679. doi: 10.1097/00007632-200007010-00010. [DOI] [PubMed] [Google Scholar]
- 6.Tay B K, Deckey J, Hu S S. Spinal infections. J Am Acad Orthop Surg. 2002;10(3):188–197. doi: 10.5435/00124635-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Carragee E, Iezza A. Does Acute placement of instrumentation in the treatment of vertebral osteomyelitis predispose to recurrent infection: long-term follow-up in immune-suppressed patients. Spine (Phila Pa 1976) 2008;33(19):2089–2093. doi: 10.1097/BRS.0b013e3181839b9c. [DOI] [PubMed] [Google Scholar]
- 8.Yoon S H, Chung S K, Kim K J, Kim H J, Jin Y J, Kim H B. Pyogenic vertebral osteomyelitis: identification of microorganism and laboratory markers used to predict clinical outcome. Eur Spine J. 2010;19(4):575–582. doi: 10.1007/s00586-009-1216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimar J R Carreon L Y Glassman S D Campbell M J Hartman M J Johnson J R Treatment of pyogenic vertebral osteomyelitis with anterior debridement and fusion followed by delayed posterior spinal fusion Spine (Phila Pa 1976) 2004293326–332., discussion 332 [DOI] [PubMed] [Google Scholar]
- 10.Eysel P, Hopf C, Vogel I, Rompe J D. Primary stable anterior instrumentation or dorsoventral spondylodesis in spondylodiscitis? Results of a comparative study. Eur Spine J. 1997;6(3):152–157. doi: 10.1007/BF01301428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruf M, Stoltze D, Merk H R, Ames M, Harms J. Treatment of vertebral osteomyelitis by radical debridement and stabilization using titanium mesh cages. Spine (Phila Pa 1976) 2007;32(9):E275–E280. doi: 10.1097/01.brs.0000261034.83395.7f. [DOI] [PubMed] [Google Scholar]
- 12.Chen W H, Jiang L S, Dai L Y. Surgical treatment of pyogenic vertebral osteomyelitis with spinal instrumentation. Eur Spine J. 2007;16(9):1307–1316. doi: 10.1007/s00586-006-0251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuklo T R, Potter B K, Bell R S, Moquin R R, Rosner M K. Single-stage treatment of pyogenic spinal infection with titanium mesh cages. J Spinal Disord Tech. 2006;19(5):376–382. doi: 10.1097/01.bsd.0000203945.03922.f6. [DOI] [PubMed] [Google Scholar]
- 14.Liljenqvist U, Lerner T, Bullmann V, Hackenberg L, Halm H, Winkelmann W. Titanium cages in the surgical treatment of severe vertebral osteomyelitis. Eur Spine J. 2003;12(6):606–612. doi: 10.1007/s00586-003-0614-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuster J M Avellino A M Mann F A et al. Use of structural allografts in spinal osteomyelitis: a review of 47 cases J Neurosurg 200093(1, Suppl)8–14. [DOI] [PubMed] [Google Scholar]
- 16.Lee M C, Wang M Y, Fessler R G, Liauw J, Kim D H. Instrumentation in patients with spinal infection. Neurosurg Focus. 2004;17(6):E7. doi: 10.3171/foc.2004.17.6.7. [DOI] [PubMed] [Google Scholar]
- 17.Jeanneret B, Magerl F. Treatment of osteomyelitis of the spine using percutaneous suction/irrigation and percutaneous external spinal fixation. J Spinal Disord. 1994;7(3):185–205. doi: 10.1097/00002517-199407030-00001. [DOI] [PubMed] [Google Scholar]
- 18.Halpern E M, Bacon S A, Kitagawa T, Lewis S J. Posterior transdiscal three-column shortening in the surgical treatment of vertebral discitis/osteomyelitis with collapse. Spine (Phila Pa 1976) 2010;35(13):1316–1322. doi: 10.1097/BRS.0b013e3181c0a158. [DOI] [PubMed] [Google Scholar]
- 19.Sundararaj G D, Babu N, Amritanand R. et al. Treatment of haematogenous pyogenic vertebral osteomyelitis by single-stage anterior debridement, grafting of the defect and posterior instrumentation. J Bone Joint Surg Br. 2007;89(9):1201–1205. doi: 10.1302/0301-620X.89B9.18776. [DOI] [PubMed] [Google Scholar]
- 20.Bauer T W, Parvizi J, Kobayashi N, Krebs V. Diagnosis of periprosthetic infection. J Bone Joint Surg Am. 2006;88(4):869–882. doi: 10.2106/JBJS.E.01149. [DOI] [PubMed] [Google Scholar]


