Abstract
Objectives During microvascular decompression (MVD) of the facial nerve for hemifacial spasm (HFS), an abnormal muscle response can be recorded upon stimulation of the facial nerve, also known as the lateral spread response. This response may vanish after MVD and has been associated with a successful outcome. The purpose of this study was to determine if resolution of lateral spread correlated with the elimination of HFS in a single surgeon's experience.
Design and Setting (1) Retrospective analysis of 38 patients undergoing MVD with intraoperative electromyography for HFS. (2) Meta-analysis of studies from the literature.
Main Outcome Measure Presence or absence of HFS and any complications.
Results Lateral spread response was seen in 36 patients; 20 patients had full resolution. Of these, 15 patients became HFS free, and 5 five patients still had some degree of HFS. Sixteen patients had a persistent lateral spread response despite a technically successful MVD; 11 of these became spasm free, and 5 still suffered from some degree of facial twitching. Analyzing 16 studies reporting a total of 1301 patients, a significant correlation (p < 0.0001) between response cessation and resolution of HFS was found.
Conclusion The role of monitoring lateral spread response as a predictor for clinical outcome is limited.
Keywords: hemifacial spasm, intraoperative monitoring, microvascular decompression, lateral spread response
Introduction
Hemifacial spasm (HFS) is a debilitating condition characterized by unilateral, uncontrollable, rhythmic facial twitching.1 Although almost never painful, it can be extremely embarrassing socially and interfere with vision due to complete blepharospasm causing disruption in the ability to read, drive, or work. The pathogenesis is not fully understood; however, the most commonly proposed etiology is chronic compression and irritation of the facial nerve as it exits the brainstem by one or more vessels of the vertebrobasilar system (Fig. 1). Microvascular decompression (MVD) to relieve the vascular compression was introduced in the late 1970s.2 3 The largest reported single-institution clinical series of > 1100 patients reports a 94.1% cure rate with an only 1.1% risk of hearing loss and a 0.7% risk of facial weakness.4
Fig. 1.
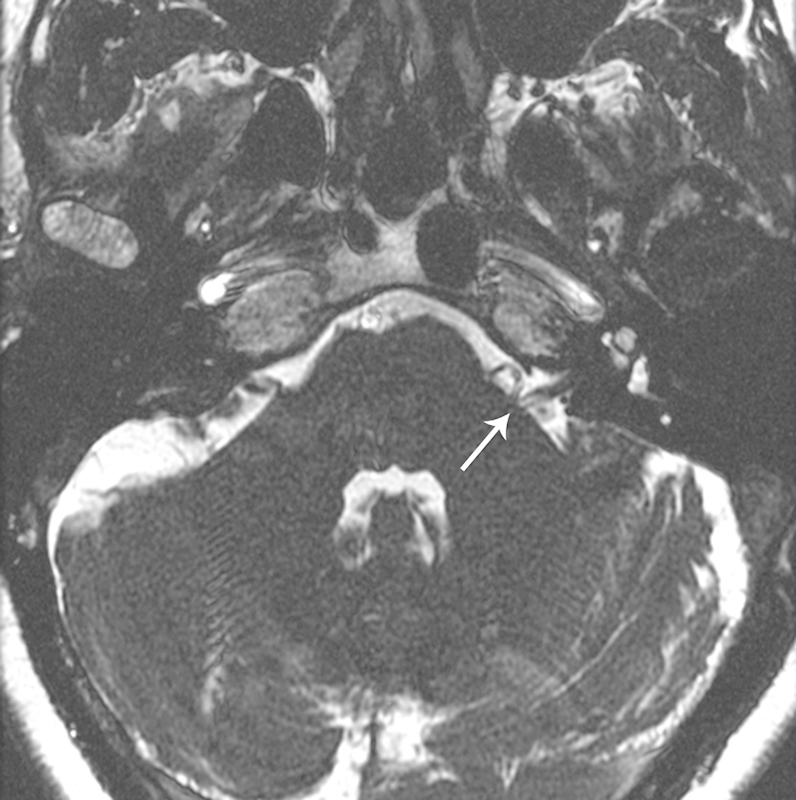
Axial magnetic resonance imaging fast imaging employing steady state acquisition image of the posterior fossa in a patient with left hemifacial spasm showing typical compression of the left facial nerve at the brainstem by the ipsilateral anterior inferior cerebellar artery (white arrow).
Intraoperative electrophysiologic monitoring of the facial and cochlear nerves is believed to decrease the risks of surgery.5 Additionally, in HFS, an abnormal reflex muscle response, the lateral spread response (LSR), can be elicited by electrical stimulation of the zygomatic branch of the facial nerve, recording from the mentalis muscle, or by stimulation of the mandibular branch while recording from the orbicularis muscle (Fig. 2).6 The LSR is likely produced by either ephaptic transmission between axons at the site of vascular compression7 or hyperexcitability of the facial nucleus.8 9 10
Fig. 2.
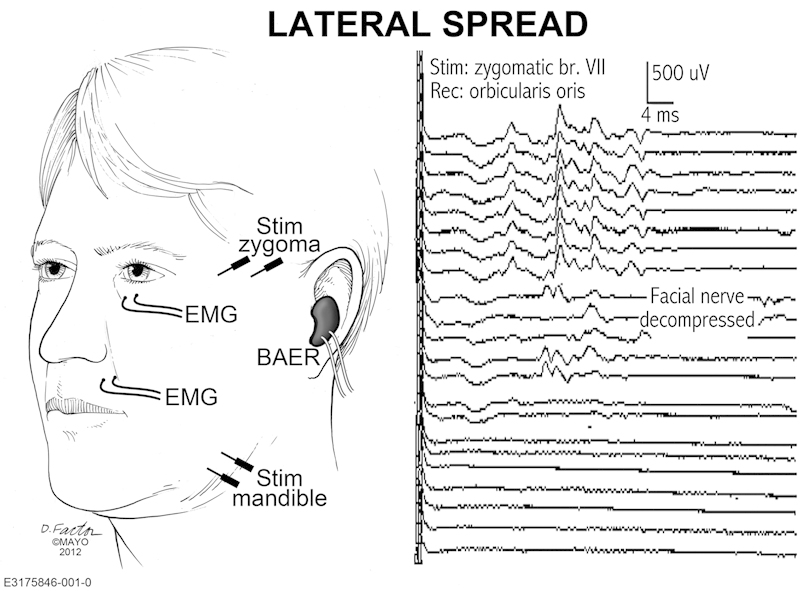
Electromyographic (EMG) recording of the lateral spread response (LSR) in microvascular decompression for hemifacial spams. Position of facial stimulation and recording electrodes (left) and typical recording with resolution of LSR after decompression (right). BAER, Brainstem Auditory Evoked Response.
Møller and Jannetta described the LSR in 1986,6 and 1 year later they published findings demonstrating a positive correlation between intraoperative resolution of LSR with clinical outcome in patients undergoing MVD for hemifacial spasm.11 Their finding that intraoperative improvement of LSR serves as a valid prognostic factor for resolution of clinical spasms have been supported by some12 13 14 15 16 and disputed by others.17 18 19 20 21 A meta-analysis from 2009 concluded that complete resolution of LSR was associated with a 4.2 times higher chance of clinical cure.22 As there have been further larger studies published after this analysis arguing against the prognostic value,19 21 the matter is still not settled.
We sought to review our own experience from 36 consecutive cases with LSR and integrate these numbers into the body of literature regarding this important aspect of intraoperative monitoring in MVD for HFS.
Material and Methods
We conducted a retrospective analysis of clinical outcomes in 38 consecutive patients undergoing MVD for HFS by a single surgeon (MJL) between December 2000 and December 2010 and correlated this with intraoperative electrophysiologic parameters. A complete clinical history and neurologic examination including MR imaging was obtained at the time of initial presentation. Outcome was assessed at regular follow-up visits by the operating surgeon and an independent neurologist not involved in interpreting the electromyograph (EMG) data. All patients underwent MVD of the seventh cranial nerve in the lateral decubitus position via a standard retrosigmoid craniotomy and an infrafloccular approach. Intraoperative EMG was performed to monitor the seventh cranial nerve and record the LSR. Auditory brainstem responses were also monitored in all patients to track cochlear nerve function. The study was approved by the institutional review board.
In addition, a query was performed, and English original articles were selected on the following criteria: Included studies (1) focused on idiopathic HFS exclusively, (2) had follow-up data of at least 3 months, and (3) reported absolute numbers of patients with both presence and resolution of LSR during surgery in comparison with clinical results or this was easily calculated from relative numbers. Care was taken to exclude studies that evidently included patients previously reported. Test statistics were performed with GraphPad Prism.
Results
A total of 38 patients were evaluated (23 women, 15 men) with a median age of 56 years (mean: 55 years; range: 19–76 years) at the time of surgery. The left side was involved more frequently (21 cases). One patient presented with bilateral HFS, but only one side was operated. The mean duration of symptoms prior to microvascular surgery was 6 years. At the time of surgery, 33 patients had a history of botulinum toxin injections for spasms in their past medical history. All but one patient were undergoing their first operative procedure for treatment of HFS. A single patient underwent MVD for the second time after failing to improve following the first MVD procedure. All patients had been evaluated preoperatively by a neurologist and had clinically diagnosed HFS. Only 6 of 38 patients (16%) had preoperative EMG evaluation, and all 6 had evidence of LSR on that diagnostic evaluation.
Intraoperatively, as expected, the anterior inferior cerebellar artery (AICA) was the most common compressing vessel (n = 10); the posterior inferior cerebellar artery (PICA) was less frequently involved (n = 7). In three cases a combined AICA-PICA vessel was the offending vessel. In the remainder of cases there was more than one vessel believed to be responsible for compression of the facial nerve at the brainstem. During five operations the ipsilateral vertebral artery, AICA, and PICA were felt to be responsible. In three cases, AICA and PICA were both compressing the facial nerve, and in an additional three cases the ipsilateral vertebral artery and AICA were thought to be responsible. No particular vessel or vessel combination was found to be more likely to result in failure of complete resolution of HFS. In seven cases, interestingly, no significant vascular compression was identified, although six of these patients with negative explorations at the time of surgery were free of HFS at follow-up.
During EMG monitoring of the facial nerve throughout surgery, the LSR was not detected in two patients (5%) and unchanged throughout the operation in nine patients (24%). In seven patients (18%), a reduction of LSR was noted without full resolution. In 20 procedures (53%), LSR resolved completely after decompression. In one patient with partial resolution of LSR, and two patients with complete elimination of LSR, this occurred even before the actual MVD of the nerve took place. Just by opening the dura and draining cerebrospinal fluid, the LSR monitored during intraoperative EMG improved or resolved. All of these patients with very early resolution of LSR had resolution of HFS.
There was one case of permanent hearing loss and ipsilateral facial weakness postoperatively, improving to House Brackmann grade III23 at 3-month follow-up, but unfortunately no improvement of ipsilateral hearing. One patient developed a pseudomeningocele that resolved spontaneously.
Considering the entire cohort of 38 patients, 22 (58%) showed an immediate sustained relief of symptoms, an additional five patients (13%) achieved complete relief of HFS of delayed onset 5 days to 9 months, postoperatively. Another five patients (13%) reported a pronounced improvement of symptoms of >90% but still had occasional twitches. Four patients (11%) rated the improvement at 50 to 90%; whereas only two patients (5%) had no lasting effect of MVD surgery. Both of these patients had initial relief of HFS but then experienced recurrence of symptoms at 2 and 12 months, respectively. To date, both patients have declined repeat exploration. Overall, we conclude that 27 patients (71%) had a successful outcome, nine patients improved (24%), and two patients (5%) did not benefit from surgery.
Analyzing the data just described, we did not find sufficient evidence that resolution of LSR during intraoperative facial nerve monitoring predicts outcome in patients undergoing MVD of cranial nerve VII for HFS (Fisher two-tailed test, p = 0.7225; Table 1).
Table 1. Contingency table of our own patients.
| (Own patients, n = 36) | Clinical outcome | ||
|---|---|---|---|
| Complete resolution | Residual symptoms | ||
| Lateral spread intraoperatively | Complete resolution | 15 | 5 |
| Not resolved completely | 11 | 5 | |
Note: Fisher two-tailed test, p = 0.7225.
We were able to identify a total of 1301 similar cases for analysis including the previously published meta-analysis,22 plus 417 additional cases from the literature,13 19 21 24 and our 36 cases that had LSR reported here (Table 2). All studies included in the previous analysis22 were found to be relevant and have again been considered. In one study,25 however, the listed total number of cases had to be corrected from 226 to 132 because electrophysiologic studies were recorded and presented only for a limited number of patients. One large current study was not included in further analysis, since different resolution patterns of LSR were evaluated and no clinical data were given for patients with persistent LSR during surgery.26
Table 2. Studies specifying lateral spread response in patients undergoing microvascular decompression for hemifacial spasm.
| n a | Lateral spread resolvedb | Lateral spread persistentc | |||
|---|---|---|---|---|---|
| No HFSd | HFS ongoinge | No HFSd | HFS ongoinge | ||
| Møller and Jannetta11 | 67 | 42 | 2 | 17 | 6 |
| Haines and Torres24 | 8 | 7 | 0 | 1 | 0 |
| Isu et al16 | 40 | 36 | 2 | 0 | 2 |
| Shin et al25 | 132 | 65 | 5 | 44 | 18 |
| Hatem et al17 f | 33 | 20 | 3 | 7 | 3 |
| Kiya et al18 g | 38 | 21 | 0 | 17 | 0 |
| Mooij et al30 h | 74 | 61 | 8 | 4 | 1 |
| Yamashita et al14 | 60 | 50 | 3 | 6 | 1 |
| Kong et al15 i | 263 | 178 | 52 | 19 | 14 |
| Joo et al20 | 72 | 36 | 4 | 26 | 6 |
| Huang et al12 | 36 | 34 | 0 | 1 | 1 |
| Neves et al13 | 24 | 13 | 2 | 2 | 7 |
| Sekula et al22 | 69 | 48 | 5 | 15 | 1 |
| Thirumala et al19 | 259 | 193 | 14 | 49 | 3 |
| Li et al21 j | 90 | 74 | 6 | 8 | 2 |
| Present studyk | 36 | 15 | 5 | 11 | 5 |
| Σl | 1301 | 893 | 111 | 227 | 70 |
Abbreviations: AMR, abnormal muscle response; HFS, hemispatial spasm; LSR, lateral spread response.
Only patients are selected and analyzed who showed AMR prior to craniotomy; patients showing no AMR were excluded.
Including only patients with complete resolution of LSR.
Including patients with incomplete or no resolution of LSR.
Including only patients with absence of symptoms of HFS at 3- or 6-month follow-up.
Including patients with incomplete or no resolution of symptoms of HFS.
In late follow-up at 36 months, the authors report a cessation of symptoms in all patients with persistent LSR.
In the immediate postoperative period, two patients with resolution of LSR had twitches; of patients with persistent AMR, six were not asymptomatic. However, at 3 months, all patients were free of symptoms.
The authors used a different reporting scheme and specified the “guiding role” of monitoring. Only their “inconclusive” category was regarded as persistent LSR.
At 1-year follow-up, 90.4% of patients with resolution of LSR and 66.7% of patients with persistent LSR were free of symptoms, respectively.
Fourteen patients were lost to follow-up; only patients with follow-up were counted.
See Table 1.
See Table 3.
In considering the 1301 cases available for analysis, there is strong evidence that complete resolution of LSR is associated with a higher cure rate at 3- to 6-month follow-up (chi-square test = 29.96; p < 0.0001; odds ratio: 2.48; sensitivity: 0.80; specificity: 0.39; positive predictive value: 0.89; negative predictive value: 0.24; Table 3).
Table 3. Contingency table of pooled patients from the literature.
| (Pooled literature patients, n = 1301) | Clinical outcome | ||
|---|---|---|---|
| Complete resolution | Residual symptoms | ||
| Lateral spread intraoperatively | Complete resolution | 893 | 111 |
| Not resolved completely | 227 | 70 | |
Note: For selection criteria, please see text. χ2 = 29.96, p < 0.0001. Odds ratio 2.48; sensitivity 0.80 (95% confidence interval [CI], 0.77–0.82); specificity 0.39 (CI, 0.32–0.46), positive predictive value 0.89 (CI, 0.87–0.91), negative predictive value 0.24 (CI, 0.18–0.29).
Discussion
We have presented our own data and calculated pooled data from the literature on intraoperative cessation of LSR as a prognostic marker for clinical cure in cases of HFS treated with MVD. Different working groups have addressed this question since the first description of the LSR in patients with idiopathic HFS. As groups reported on samples as small as 8 and as powerful as 250 individuals, results and interpretations changed. A first meta-analysis was performed in 2009,22 concluding that clinical cure is more likely with an odds ratio of 4.2 with specific EMG changes during intraoperative monitoring.
We report our 36 patients, for whom we did not see a statistical correlation between intraoperative EMG monitoring and outcome. Of note, our complete cure rate (71%) is lower compared with other series. In case series published with > 1000 patients each, resolution of HFS was accomplished in 88.7%,27 90.5%,28 and 94.1%.4 This might be due to a difference in outcome reporting because objective criteria are not widely accepted, although available.29 In our assessment, improvement > 90% was not considered “cured” when patients still experienced mild occasional symptoms. If we included this group of patients, our cure rate would be 84%. Utilizing the most liberal definition of improvement following MVD, our “success rate” could be considered as high as 95%; however ,we feel <90% improvement of the HFS is not a truly satisfactory result. For further evaluation of prognostic factors, a wide and consistent use of validated scales is necessary, making comparison and statistical grouping of patients possible.
Another limitation in comparing our results with the results of others, and in analyzing pooled data, is the possible inconsistency in reporting the results of intraoperative EMG monitoring. Some authors only describe the presence or absence of LSR at the beginning and conclusion of the operation,11 15 20 whereas others note the timing of the changes in LSR pattern and discuss this in the context of clinical outcome.26 For instance, Kim et al evaluated 273 patients with LSR and found a different clinical outcome in patients in whom LSR resolved only after MVD (183 patients), compared with cases in which LSR resolved with simply opening the dura and drainage of cerebrospinal fluid (90 patients), as we also saw in three of our patients.26 In the former group, 92.9% of patients were spasm free at 1-year follow-up, but in the latter group only 75.6% were asymptomatic at 1 year. Another recent study categorized LSR pattern regarding how it influenced the surgeon's intraoperative decision making (“guiding,” “confirming,” “indirect confirming,” “inconclusive role,” see also annotation in Table 2).30 Additionally, depending on how aggressively the EMG technician tries to elicit LSR can influence whether it is “present” or “absent” during the operation. We often observe a significant increase in the stimulus threshold for elicitation of the LSR or a change in the complexity and configuration of the LSR after MVD. The LSR was still recorded as “present” in these cases. It is unclear from the review of most of the available literature if this is the case at other centers performing MVD for HFS.
There has been some concern that previous treatment with local injection with botulinum toxin might alter detection and assessment of LSR. Evaluating 282 patients undergoing MVD for HFS and comparing intraoperative findings and clinical outcome in patients with previous botulinum toxin injections, the Pittsburgh group found higher LSR baseline amplitudes, attributed to poly-reinnervation after recovery from repeated botulinum toxin use, but they did not see any significant differences in LSR changes and clinical outcome.31 In our series a clear majority of patients have had previous botulinum toxin treatment although usually not within 3 months prior to undergoing MVD; in most other larger series prior treatment with botulinum toxin is not discussed.15 22 30
We have updated the previously mentioned meta-analysis from 200922 and have added four additional studies and our own for a total of 1301 patients. We have pooled the data from the original publications and, in some cases, recalculated the figures to have a uniform set of data: (1) Exclusion of patients without a LSR, (2) clear-cut differentiation between full recovery from all symptoms and only partial or no improvement at 3- to 6-month follow-up, and (3) clear-cut differentiation between full cessation of the LSR and only partial or no improvement. Utilizing these criteria, we found a significant correlation between cessation of LSR intraoperatively and clinical outcome. Patients who have intraoperative resolution of the LSR will be free of HFS symptoms at 3 months a little more than twice as often as patients with only partial or no improvement of these specific EMG findings. However, the positive predictive value of cessation of LSR is only marginal (0.89), and the negative predictive value of persistent LSR is poor (0.24). That is, there is an 89% chance of the patient being cured of HFS if the LSR completely goes away intraoperatively after MVD. This is somewhat reassuring that any further exploration and/or decompression is likely not necessary in those cases. Even more importantly, if the LSR persists despite vascular decompression, there is only a 24% chance that the patient will continue to experience HFS. Although it may be worth another look at the nerve in such cases before concluding the operation, we do not recommend aggressive additional exploration that might, for instance, put the eighth nerve and hearing at risk.
Conclusion
We believe that the role of monitoring LSR in patients with HFS as a predictor of clinical outcome is somewhat limited. In particular, disappearance of the LSR during and absence of the LSR at the end of the MVD procedure predicts clinical resolution of HFS in ∼ 90% of cases; however, persistence of a response does not predict a poor outcome. We primarily use LSR in guiding the surgeon to assess for possible remaining compression.12 24 30 If the LSR response does not change at all, we perform additional intraoperative investigation and/or decompression to make sure all vascular compression of the facial nerve is relieved. If we are satisfied we have identified and decompressed the vascular compression at the brainstem but the LSR has not completely resolved, we do not perform any further exploration so as to not put the adjacent neural and vascular structures at undue risk.
References
- 1.Wang A, Jankovic J. Hemifacial spasm: clinical findings and treatment. Muscle Nerve. 1998;21(12):1740–1747. doi: 10.1002/(sici)1097-4598(199812)21:12<1740::aid-mus17>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Jannetta P J, Abbasy M, Maroon J C, Ramos F M, Albin M S. Etiology and definitive microsurgical treatment of hemifacial spasm. Operative techniques and results in 47 patients. J Neurosurg. 1977;47(3):321–328. doi: 10.3171/jns.1977.47.3.0321. [DOI] [PubMed] [Google Scholar]
- 3.Auger R G, Piepgras D G, Laws E R Jr, Miller R H. Microvascular decompression of the facial nerve for hemifacial spasm: clinical and electrophysiologic observations. Neurology. 1981;31(3):346–350. doi: 10.1212/wnl.31.3.346. [DOI] [PubMed] [Google Scholar]
- 4.Hyun S J Kong D S Park K Microvascular decompression for treating hemifacial spasm: lessons learned from a prospective study of 1,174 operations Neurosurg Rev 2010333325–334.; discussion 334 [DOI] [PubMed] [Google Scholar]
- 5.Shin J C, Kim Y C, Park C I, Chung U H. Intraoperative monitoring of microvascular decompression in hemifacial spasm. Yonsei Med J. 1996;37(3):209–213. doi: 10.3349/ymj.1996.37.3.209. [DOI] [PubMed] [Google Scholar]
- 6.Møller A R, Jannetta P J. Physiological abnormalities in hemifacial spasm studied during microvascular decompression operations. Exp Neurol. 1986;93(3):584–600. doi: 10.1016/0014-4886(86)90178-0. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita S, Kawaguchi T, Fukuda M. et al. Lateral spread response elicited by double stimulation in patients with hemifacial spasm. Muscle Nerve. 2002;25(6):845–849. doi: 10.1002/mus.10123. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa M, Namiki J, Takase M, Ohira T, Nakamura A, Toya S. Effect of repetitive stimulation on lateral spreads and F-waves in hemifacial spasm. J Neurol Sci. 1996;142(1–2):99–106. doi: 10.1016/0022-510x(96)00137-2. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa M, Ohira T, Namiki J, Gotoh K, Takase M, Toya S. Electrophysiological investigation of hemifacial spasm: F-waves of the facial muscles. Acta Neurochir (Wien) 1996;138(1):24–32. doi: 10.1007/BF01411719. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson M F, Kaufmann A M. Monitoring of facial muscle motor evoked potentials during microvascular decompression for hemifacial spasm: evidence of changes in motor neuron excitability. J Neurosurg. 2005;103(1):64–69. doi: 10.3171/jns.2005.103.1.0064. [DOI] [PubMed] [Google Scholar]
- 11.Møller A R, Jannetta P J. Monitoring facial EMG responses during microvascular decompression operations for hemifacial spasm. J Neurosurg. 1987;66(5):681–685. doi: 10.3171/jns.1987.66.5.0681. [DOI] [PubMed] [Google Scholar]
- 12.Huang B R, Chang C N, Hsu J C. Intraoperative electrophysiological monitoring in microvascular decompression for hemifacial spasm. J Clin Neurosci. 2009;16(2):209–213. doi: 10.1016/j.jocn.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Neves D O, Lefaucheur J P, de Andrade D C. et al. A reappraisal of the value of lateral spread response monitoring in the treatment of hemifacial spasm by microvascular decompression. J Neurol Neurosurg Psychiatry. 2009;80(12):1375–1380. doi: 10.1136/jnnp.2009.172197. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita S Kawaguchi T Fukuda M Watanabe M Tanaka R Kameyama S Abnormal muscle response monitoring during microvascular decompression for hemifacial spasm Acta Neurochir (Wien) 20051479933–937.; discussion 937–938 [DOI] [PubMed] [Google Scholar]
- 15.Kong D S, Park K, Shin B G, Lee J A, Eum D O. Prognostic value of the lateral spread response for intraoperative electromyography monitoring of the facial musculature during microvascular decompression for hemifacial spasm. J Neurosurg. 2007;106(3):384–387. doi: 10.3171/jns.2007.106.3.384. [DOI] [PubMed] [Google Scholar]
- 16.Isu T Kamada K Mabuchi S et al. Intra-operative monitoring by facial electromyographic responses during microvascular decompressive surgery for hemifacial spasm Acta Neurochir (Wien) 1996138119–23.; discussion 23 [DOI] [PubMed] [Google Scholar]
- 17.Hatem J, Sindou M, Vial C. Intraoperative monitoring of facial EMG responses during microvascular decompression for hemifacial spasm. Prognostic value for long-term outcome: a study in a 33-patient series. Br J Neurosurg. 2001;15(6):496–499. doi: 10.1080/02688690120105101. [DOI] [PubMed] [Google Scholar]
- 18.Kiya N, Bannur U, Yamauchi A, Yoshida K, Kato Y, Kanno T. Monitoring of facial evoked EMG for hemifacial spasm: a critical analysis of its prognostic value. Acta Neurochir (Wien) 2001;143(4):365–368. doi: 10.1007/s007010170091. [DOI] [PubMed] [Google Scholar]
- 19.Thirumala P D, Shah A C, Nikonow T N. et al. Microvascular decompression for hemifacial spasm: evaluating outcome prognosticators including the value of intraoperative lateral spread response monitoring and clinical characteristics in 293 patients. J Clin Neurophysiol. 2011;28(1):56–66. doi: 10.1097/WNP.0b013e3182051300. [DOI] [PubMed] [Google Scholar]
- 20.Joo W I, Lee K J, Park H K, Chough C K, Rha H K. Prognostic value of intra-operative lateral spread response monitoring during microvascular decompression in patients with hemifacial spasm. J Clin Neurosci. 2008;15(12):1335–1339. doi: 10.1016/j.jocn.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Zhang Y, Zhu H, Li Y. Prognostic value of intra-operative abnormal muscle response monitoring during microvascular decompression for long-term outcome of hemifacial spasm. J Clin Neurosci. 2012;19(1):44–48. doi: 10.1016/j.jocn.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 22.Sekula R F Jr, Bhatia S, Frederickson A M. et al. Utility of intraoperative electromyography in microvascular decompression for hemifacial spasm: a meta-analysis. Neurosurg Focus. 2009;27(4):E10. doi: 10.3171/2009.8.focus09142. [DOI] [PubMed] [Google Scholar]
- 23.House J W, Brackmann D E. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2):146–147. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- 24.Haines S J, Torres F. Intraoperative monitoring of the facial nerve during decompressive surgery for hemifacial spasm. J Neurosurg. 1991;74(2):254–257. doi: 10.3171/jns.1991.74.2.0254. [DOI] [PubMed] [Google Scholar]
- 25.Shin J C Chung U H Kim Y C Park C I Prospective study of microvascular decompression in hemifacial spasm Neurosurgery 1997404730–734.; discussion 734–735 [DOI] [PubMed] [Google Scholar]
- 26.Kim C H Kong D S Lee J A, Kwan-Park. The potential value of the disappearance of the lateral spread response during microvascular decompression for predicting the clinical outcome of hemifacial spasms: a prospective study Neurosurgery 20106761581–1587.; discussion 1587–1588 [DOI] [PubMed] [Google Scholar]
- 27.Yuan Y, Wang Y, Zhang S X, Zhang L, Li R, Guo J. Microvascular decompression in patients with hemifacial spasm: report of 1200 cases. Chin Med J (Engl) 2005;118(10):833–836. [PubMed] [Google Scholar]
- 28.Chung S S, Chang J H, Choi J Y, Chang J W, Park Y G. Microvascular decompression for hemifacial spasm: a long-term follow-up of 1,169 consecutive cases. Stereotact Funct Neurosurg. 2001;77(1-4):190–193. doi: 10.1159/000064620. [DOI] [PubMed] [Google Scholar]
- 29.Tan E K, Fook-Chong S, Lum S Y, Thumboo J. Validation of a short disease specific quality of life scale for hemifacial spasm: correlation with SF-36. J Neurol Neurosurg Psychiatry. 2005;76(12):1707–1710. doi: 10.1136/jnnp.2005.065656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mooij J J Mustafa M K van Weerden T W Hemifacial spasm: intraoperative electromyographic monitoring as a guide for microvascular decompression Neurosurgery 20014961365–1370.; discussion 1370–1371 [DOI] [PubMed] [Google Scholar]
- 31.Habeych M E, Shah A C, Nikonow T N. et al. Effect of botulinum neurotoxin treatment in the lateral spread monitoring of microvascular decompression for hemifacial spasm. Muscle Nerve. 2011;44(4):518–524. doi: 10.1002/mus.22104. [DOI] [PubMed] [Google Scholar]


