Abstract
Study Design Retrospective cohort study.
Objective To identify the incidence of adjacent segment pathology (ASP) after thoracolumbar fusion of three or more levels, the risk factors for the development of ASP, and the need for further surgical intervention in this particular patient population.
Methods A retrospective analysis of a prospective surgical database identified 217 patients receiving polysegmental (≥ 3 levels) spinal fusion with minimum 5-year follow-up. Risk factors were evaluated, and the following data were obtained from the review of radiographs and charts: radiographic measures—levels fused, fusion status, presence of ASP; clinical measures—patient assessment, Oswestry Disability Index (ODI), and the need for further surgery.
Results The incidence of radiographic ASP (RASP) was 29%; clinical or symptomatic ASP (CASP), 18%; and those requiring surgery, 9%. Correlation was observed between ODI and ASP, symptomatic ASP, and need for revision surgery. Age, preoperative degenerative diagnosis, and absence of fusion demonstrated significant association to ASP.
Conclusions ASP was observed in a significant number of patients receiving polysegmental fusion of three or more levels. ODI scores correlated to RASP, CASP, and the need for revision surgery.
Keywords: adjacent segment pathology, adjacent segment breakdown, adjacent segment disease, adjacent segment degeneration, posterolateral fusion, spine
Introduction
Although surgical techniques may vary, posterior spinal fusion, often in conjunction with decompression, remains the primary surgical modality to manage degenerative, deforming, traumatic, and malignant pathologies of the lumbar spine.1 Clinical success following lumbar fusion has been well documented,2 3 4 5 6 and according to a recent report, greater than 300,000 procedures are performed annually in the United States,7 with the surgical volumes continuing to increase both in the United States and in Canada.8 Although this surgical procedure is successful for managing many spinal pathologies, adjacent segment pathology (ASP) may complicate long-term outcomes of spinal fusion surgery.
Estimates of the incidence of ASP vary drastically in the literature.9 This may be due in part to disease definitions, length of observation, surgical intervention, and other clinical and research factors. A recent systematic review has calculated a radiographic incidence of 34% (adjacent segment degeneration, now referred to as radiographic adjacent segment pathology [RASP]) and a clinical incidence of only 14% (adjacent segment disease, now referred to as clinical adjacent segment pathology [CASP]).10 11 The data are limited at present as most evidence of ASP has been based on single-level surgery.9
There is much debate in the literature concerning the etiology of ASP.9 Some believe that it is a natural progression of a pre-existing disease process,12 13 14 and others suggest that it is a problem resulting from the biomechanical alteration of the lumbar spine or surgical injury to the adjacent level.15 Despite controversy regarding the etiology, medium- to long-term observation has revealed that following posterior lumbar fusion, the spine is prone to persistent deterioration at the adjacent nonfused motion segment, regardless of pathology or surgical technique.9 11 With the increasing volume of lumbar spinal fusions, there is a need to determine the causes and risk factors associated with ASP.
At our institution, it was clinically identified that the incidence of ASP appeared higher in polysegmental fusions of three or more levels, thus triggering the need for a retrospective review of patients in this cohort. Given the uncertain nature of the influence of multiple fusion levels on the development of ASP, the purposes of this study were: (1) to determine the incidence of RASP and CASP in the cohort of patients who have undergone polysegmental (≥ 3 levels) spinal fusion and (2) to identify the risk factors for the development of ASP and the need for further surgical intervention in this particular patient population.
Methods
Following Research Ethics Board approval, a retrospective review of all patients undergoing a posterior instrumented thoracolumbar spinal fusion of three or more levels between 1988 and 2000 was conducted. All patients included in the cohort were those of the principal investigator (E.P.A.), a fellowship-trained spinal surgeon from a single institution. All deformity, traumatic, and degenerative conditions of the spine were included in the review, and a minimum 5-year postoperative follow-up was required for inclusion in the study.
For the purpose of this article, ASP encompasses the entities of RASP and CASP. RASP is the radiographic deterioration observed at the motion level, either cephalad or caudad, to the site of spinal fusion. CASP is the presence of clinical symptoms secondary to this junctional deterioration.11 Radiographs exhibiting no ASP were distinguished from radiographs demonstrating findings such as degenerative disk disease, listhesis, instability, stenosis, and/or deformity. The clinical evaluation identified those patients with RASP who were reporting recurrent back or leg pain, neurologic dysfunction, and/or diminished mobility. Therefore, patients clinically diagnosed with symptoms such as neurogenic claudication and/or radiculopathy (CASP) via patient history and physical examination were distinguished from those who did not subjectively report symptoms (RASP). In this manner, the patients can be divided into three groups: those patients exhibiting no ASP, those with nonsymptomatic RASP, and those with symptomatic RASP (CASP).
The primary outcome measure was defined as the presence or absence of ASP upon radiographic analysis. Radiographic evaluation was completed by authors E.A.P. and N.A.M., fellowship-trained spinal surgeons. The evaluation compared standing anteroposterior and lateral lumbar radiographs performed preoperatively to standing anteroposterior and lateral lumbar radiographs obtained at most recent follow-up. Further follow-up included myelography, computed tomography (CT), and/or magnetic resonance imaging (MRI) as indicated based on patient clinical presentation. The incidence of ASP was determined based on the following radiologic parameters16:
Complete collapse of the disk space with end plate sclerosis
Sagittal or coronal translation of more than 3 mm
Five degrees wedging of the disk space on the coronal view
Angular instability of more 10 degrees on dynamic radiographs
Significant spinal canal compression, as seen on MRI
Secondary outcome measures evaluated patient symptoms and disability. Subjective complaints and physician assessment identified issues such as back pain, radiculopathy, and neurogenic claudication that were correlated to radiographic findings to confirm symptomatic ASP. Oswestry Disability Index (ODI) was administered at a minimum 5 years post–index operation. Clinical assessment and ODI allowed comparison of patients without ASP, patients with nonsymptomatic ASP (RASP), and patients with symptomatic ASP (CASP) in an effort to determine if there were differences in disability between these groups. Further analysis evaluated (1) patient demographics, obtained from the patient's medical records; (2) fusion status, measured via plain radiography1 or CT if available17; and (3) revision surgery, the details of which were obtained from the patient's medical records.
Patients requiring revision surgery underwent posterior approach to the previous surgical site and affected motion level. In situ instrumentation was identified, set screws and rods were removed, and pedicle screws were assessed for stability and revised if considered unstable. Posterolateral fusion mass was inspected for arthrodesis and previous decompression was inspected as patient symptoms or imaging demanded. The fusion was extended to incorporate the level of ASP. Instrumented stabilization techniques for the affected motion level were dependent on the nature of the degeneration. Posterolateral decortication and placement of autologous bone graft created the fusion bed. Standard wound closure was performed, no drains were placed, and patients were mobilized on postoperative day 1 with a brace.
To determine the relationship between patients' medical and demographic characteristics and the likelihood of ASP, proportions of patients experiencing ASP were compared across different subsamples. A difference of proportions was used to determine whether different patient groups experienced a higher incidence of ASP. The ability of specific patient characteristics to predict incidences of ASP were not tested due to small subsample sizes for different diagnostic groups. However, mean differences in ODI scores were compared between patients without ASP and patients with RASP and CASP using a one-way analysis of variance to determine if these groups differed significantly in disability. A post hoc Tukey test was used to determine which group differed significantly from the other.
Results
In all, 425 consecutive patients receiving posterior spinal fusion of three or more levels between 1988 and 2000 were identified in the database. Of the 425, 11 patients were deceased, 127 were untraceable (relocated or unable to contact), and 70 had incomplete data and absence of preoperative radiograph access. Thus, 217 patients were available for the study with complete pre- and postoperative data. Minimum 5-year follow-up was achieved, with an average of 9.5 years and a range of 5 to 12 years. See Table 1 for patient demographics.
Table 1. Patient demographics.
| Total (n = 217) | No ASP (n = 154) | ASP (n = 63) | |
|---|---|---|---|
| Age | 51.0 (± 22.4 SD) | 47.0 (± 23.2 SD) | 59.4 (± 17.7 SD) |
| Sex | |||
| Male | 77 (35.5%) | 55 (71.4%) | 22 (28.6%) |
| Female | 140 (64.5%) | 99 (70.7%) | 41 (29.3%) |
| ODI at follow-up | 31.1 (± 18.0 SD) | 26.1 (± 16.4 SD) | 43.5 (± 15.6 SD) |
| Initial pathology | |||
| Degenerative | 153 (70.5%) | 96 (62.3%) | 57 (90.5%) |
| Nondegenerative | 64 (29.5%) | 58 (37.7%) | 6 (9.4%) |
| Fusion status | |||
| Fused | 200 (92.2%) | 144 (93.5%) | 56 (88.9%) |
| Nonfused | 17 (7.8%) | 10 (6.5%) | 7 (11.1%) |
| Radicular symptoms | 39 (18.0%) | 0 | 39 (18.0%) |
| Revision required | 19 (8.8%) | 0 | 19 (8.8%) |
Abbreviations: ASP, adjacent segment pathology; ODI, Oswestry Disability Index; SD, standard deviation.
The majority (150 patients) of the cohort received initial treatment for spinal stenosis with the possibility of associated spinal instability or deformity. A small subgroup (24 cases) of the cohort consisted of pediatric scoliosis, a group that displayed no signs of ASP and displayed a final postoperative ODI score of 6.
Of the 217 patients, 35.5% (77) were male and 64.5% (140) were female; 30% (64) did not have degenerative diagnosis at first assessment and 70% (153) did have a degenerative diagnosis. In addition, 71% (154) had no ASP and 29% (63) had RASP. Radiographic diagnosis of ASP is categorized in Fig. 1. Of the 63 patients who had RASP, 11% (24) had no symptoms and 18% (39) experienced symptoms (CASP). Nineteen patients (9%) with ASP required revision surgery. Of the 19 patients who underwent revision surgery, all but 1 (95%) had a positive fusion status as determined radiographically.
Fig. 1.

Radiographic diagnosis of patients with adjacent segment pathology. Patients may have more than one radiographic diagnosis. Abbreviation: DDD, degenerative disk disease.
ASP was observed in 29% of both male (22/77) and female (41/140) patients. Gender did not correlate to presence of ASP with and without symptoms, diagnosis, fusion status, or revision surgery. There was significant difference between the mean age of patients without ASP (47 ± 23.2 years) and those with ASP (61 ± 16.7 years; t(149) = − 4.869, p < 0.001).
However, of the 63 patients with ASP, 89% (56) had a positive fusion status and 11% (7) had a negative fusion status. Negative fusion status was observed in 3 of 24 patients with RASP, 3 of 20 with CASP not requiring revision surgery, and 1 of 19 requiring revision surgery. Surgeon interpretation of symptom reporting was used to differentiate symptoms felt to be related to ASP rather than pseudarthrosis. No patients received surgery for a diagnosis of symptomatic pseudarthrosis. Based on calculations of relative risk, a negative fusion status demonstrated a 1.5 times greater risk of ASP as compared with those with a positive fusion status.
On average, patients with RASP received fusion to 4.4 levels with 83% ending at the S1 level. Average fusion length was 4.8 levels with 90% ending at the S1 level in the CASP cohort. Average fusion length was 5.1 levels with 89% ending at the S1 level in the patients requiring revision surgery. The groups did not differ statistically in this regard.
Overall, the mean ODI score at final follow-up for all patients was 31 ± 18.0. The mean ODI scores for patients with no ASP (26 ± 16.5), patients with RASP (35 ± 16.6), and patients with CASP (49 ± 12.3) were significantly different (F(2.214) = 33.020, p < 0.001), reported in Fig. 2. Post hoc testing indicated that the group means of patients without ASP differed from patients with RASP (p < 0.05) and CASP (p < 0.001). Also, the group means of RASP differed from CASP (p < 0.01).
Fig. 2.
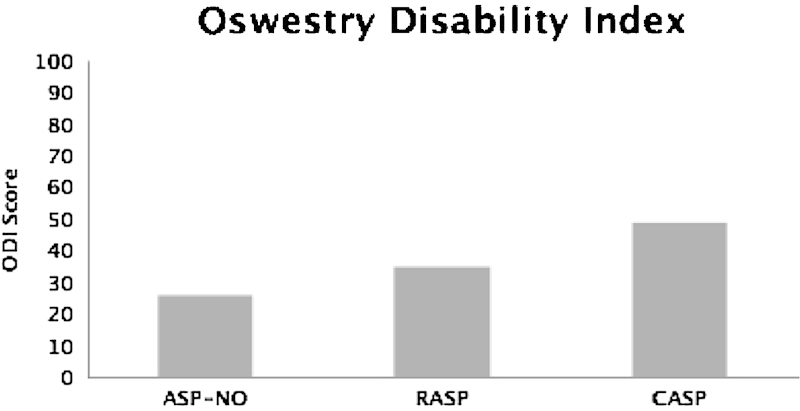
Oswestry Disability Index (ODI) scores for patients without adjacent segment pathology (ASP-NO), patients with nonsymptomatic adjacent segment pathology (RASP), patients with symptomatic adjacent segment pathology (CASP) and scores for patients who did and did not undergo revision surgery. All scores are statistically different between groups (p < 0.05).
The mean ODI scores for patients with an initial nondegenerative diagnosis (21 ± 15.4) and for patients with an initial degenerative diagnosis (35 ± 17.5) were statistically different (t(215) = −5.529, p < 0.001). The mean ODI scores for patients who did not have revision surgery (30 ± 17.5) and for patients who did have revision surgery (49 ± 13.65) were statistically different (t(215) = −4.684, p < 0.001). The mean ODI scores for patients with a negative fusion status (39 ± 15.8) and for patients with a positive fusion status (31 ± 18.1) approached statistical difference (t(215) = 1.957, p = 0.052). The mean ODI scores for female patients (33 ± 8.3) and for male patients (30 ± 17.9) did not differ significantly.
Discussion
The occurrence of ASP has been attributed to multiple variables. Patient factors, the nature of the surgical pathology, the surgical intervention performed, and the type and length of follow-up contribute to the observation of ASP. Thus, surgeon and patient decision making remains challenging due to lack of specific correlations and recommendations.
To date, extensive literature is available regarding ASP, and these findings have been synthesized in several recent systematic reviews.9 11 18 19 The disease occurrence appears to demonstrate reasonable consistency, although the etiology has yet to be confirmed. The incidence of ASP for single-level fusion has been reported to range from 5 to 30% at a 2-year follow-up.9 20 The results from the current study indicate that the radiographic incidence of ASP is 29% and the clinical incidence is 18% with multiple fusion levels. These results are in line with a recent review that calculated radiographic incidence at 34% and clinical incidence at 14%.11 Although the incidence rate from the current study is comparable to those calculated in the review by Harrop et al,11 there are some differences that, as noted above, may be due in part to numerous factors, including length of fusion and length of observation. Fusion extension to multiple segments has demonstrated increasing biomechanical alterations with greater fusion lengths in human cadaveric models.21 22
Several authors have implicated polysegmental fusion with ASP.1 20 23 24 25 Cheh et al found a difference in the incidence of CASP when patients with a single level fused were compared with those with four levels fused (27% versus 67%), an average of 7.8 years following posterior fusion with pedicle screw fixation.1 Yang and colleagues observed that with longer fusions more severe radiographic progression of degeneration was seen.20 A correlation was found between larger changes in degeneration to poorer clinical outcomes.20 Also noted was an increase in the incidence of radiographically defined ASP from 11.6 to 14.5 to 16.3% when one, two, or more than two segments were fused, respectively; however, this relationship was not statistically significant.20 As well, Rahm and Hall observed an ASP incidence of 35% with an average fusion length of 2.5 levels in an average of 5.1 years after intertransverse fusion.23 None of their patients with a single level fused developed ASP, and they found a significant correlation between the number of levels fused and the development of ASP. In the present study, polysegmental (≥ 3) fusion was assessed at a mean 9.5 years and a minimum 5-year follow-up. The incidence of RASP was 29%, the incidence of CASP was 18%, and the overall incidence of patients requiring surgery was 9%.
Numerous authors have attempted to identify risk factors for ASP. Examined factors include the use of instrumentation, posterior lumbar interbody fusion, facet joint injury during surgery, sagittal alignment, pre-existing degenerated disk at the adjacent level, lumbar stenosis, age, osteoporosis, female sex, postmenopausal state, pedicle screw placement, and polysegmental fusions; none of these factors have yet to be confirmed as a definitive cause of ASP, and therefore a multifactorial etiology is likely.9 18 19 26 The results from our current study suggest that these factors may include increased age, degenerative diagnosis, and fusion status. The results of our current study did not indicate support for female sex as a risk factor for ASP as was also noted in a systematic review conducted by Lawrence et al,19 where the strength of evidence for sex was found to be “low.”
To subjectively assess disability in patients with and without ASP, ODI was administered once at the follow-up appointment in the present study. Cautious interpretation of these values is required in the absence of comparative preoperative baseline scores. However, when ODI scores were averaged for patients with and without ASP, the average score indicated a statistically higher level of disability in the ASP group. When the ASP group was divided into asymptomatic (RASP) and symptomatic (CASP) patients, these two groups also differed significantly in ODI scores. Patients who required revision surgery scored significantly higher on the ODI as compared with those who did not need revision surgery. These results suggest that ODI scores may be an important indicator of ASP clinical significance and could be useful in treatment decision making.
Protective effects of additional anterior spinal procedures or incorporation of the degenerative adjacent level in the original surgical procedure may influence the occurrence of ASP.12 14 Natural progression of the original preexisting disease will likely contribute to ASP.13 27 Revision surgery may be considered the ultimate manifestation of adjacent segment failure and the end of the progression from surgery to radiographic deterioration through clinical deterioration. Revision surgery remains an important end point for patients receiving posterior spinal fusion, and thus, its occurrence requires quantification.
Conclusion
This study provides insight into the occurrence of both radiographic and clinical breakdown around polysegmental fusions and the subsequent need for further surgery in this patient population. In thoracolumbar fusions of three or more levels, ASP was identified in 29% of cases, was clinically symptomatic in 18%, and required revision surgery in 9%. Possible risk factors include fusion status and age of patient, and ODI scores may help identify patients with asymptomatic ASP.
As previously noted, ASP continues to be a well-debated postsurgical problem. Despite its etiology, understanding the occurrence and management of degeneration adjacent to a previously fused spinal region is paramount for spine care clinicians. Continued effort to understand the pathomechanics of the breakdown and to minimize the occurrence is necessary. Although new treatment options may be available, fusion remains the mainstay of surgical management for certain spinal pathologies; thus, awareness of the frailties of this treatment is crucial.
Acknowledgment
We would like to thank Denise LeBlanc-Duchin, PhD, of the University of New Brunswick and Horizon Health Network, for her assistance with manuscript editing and statistical methods.
Footnotes
Disclosures This project was funded by Medtronic Canada. Unrestricted research funding provided support for research salary and administration. We have no conflict of interest to declare.
References
- 1.Cheh G, Bridwell K H, Lenke L G. et al. Adjacent segment disease following lumbar/thoracolumbar fusion with pedicle screw instrumentation: a minimum 5-year follow-up. Spine (Phila Pa 1976) 2007;32(20):2253–2257. doi: 10.1097/BRS.0b013e31814b2d8e. [DOI] [PubMed] [Google Scholar]
- 2.Lehman R A Jr, Lenke L G, Keeler K A. et al. Operative treatment of adolescent idiopathic scoliosis with posterior pedicle screw-only constructs: minimum three-year follow-up of one hundred fourteen cases. Spine (Phila Pa 1976) 2008;33(14):1598–1604. doi: 10.1097/BRS.0b013e318178872a. [DOI] [PubMed] [Google Scholar]
- 3.Rampersaud Y R Annand N Dekutoski M B Use of minimally invasive surgical techniques in the management of thoracolumbar trauma: current concepts Spine (Phila Pa 1976) 200631(11, Suppl):S96–S102., discussion S104 [DOI] [PubMed] [Google Scholar]
- 4.Rampersaud Y R, Ravi B, Lewis S J. et al. Assessment of health-related quality of life after surgical treatment of focal symptomatic spinal stenosis compared with osteoarthritis of the hip or knee. Spine J. 2008;8(2):296–304. doi: 10.1016/j.spinee.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Weinstein J N, Lurie J D, Tosteson T D. et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356(22):2257–2270. doi: 10.1056/NEJMoa070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rampersaud Y R, Wai E K, Fisher C G. et al. Postoperative improvement in health-related quality of life: a national comparison of surgical treatment for focal (one- to two-level) lumbar spinal stenosis compared with total joint arthroplasty for osteoarthritis. Spine J. 2011;11(11):1033–1041. doi: 10.1016/j.spinee.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Deyo R A, Nachemson A, Mirza S K. Spinal-fusion surgery—the case for restraint. N Engl J Med. 2004;350(7):722–726. doi: 10.1056/NEJMsb031771. [DOI] [PubMed] [Google Scholar]
- 8.Vellet A D, Lee D. Anecdote or science? CMAJ. 1998;158(1):63–64. [PMC free article] [PubMed] [Google Scholar]
- 9.Park P, Garton H J, Gala V C, Hoff J T, McGillicuddy J E. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine (Phila Pa 1976) 2004;29(17):1938–1944. doi: 10.1097/01.brs.0000137069.88904.03. [DOI] [PubMed] [Google Scholar]
- 10.Anderson P A Andersson G B Arnold P M et al. Terminology Spine (Phila Pa 1976) 201237(22, Suppl):S8–S9. [DOI] [PubMed] [Google Scholar]
- 11.Harrop J S, Youssef J A, Maltenfort M. et al. Lumbar adjacent segment degeneration and disease after arthrodesis and total disc arthroplasty. Spine (Phila Pa 1976) 2008;33(15):1701–1707. doi: 10.1097/BRS.0b013e31817bb956. [DOI] [PubMed] [Google Scholar]
- 12.Penta M, Sandhu A, Fraser R D. Magnetic resonance imaging assessment of disc degeneration 10 years after anterior lumbar interbody fusion. Spine (Phila Pa 1976) 1995;20(6):743–747. doi: 10.1097/00007632-199503150-00018. [DOI] [PubMed] [Google Scholar]
- 13.Pellisé F, Hernández A, Vidal X, Minguell J, Martínez C, Villanueva C. Radiologic assessment of all unfused lumbar segments 7.5 years after instrumented posterior spinal fusion. Spine (Phila Pa 1976) 2007;32(5):574–579. doi: 10.1097/01.brs.0000256875.17765.e6. [DOI] [PubMed] [Google Scholar]
- 14.Wai E K, Santos E R, Morcom R A, Fraser R D. Magnetic resonance imaging 20 years after anterior lumbar interbody fusion. Spine (Phila Pa 1976) 2006;31(17):1952–1956. doi: 10.1097/01.brs.0000228849.37321.a8. [DOI] [PubMed] [Google Scholar]
- 15.Rao R D, David K S, Wang M. Biomechanical changes at adjacent segments following anterior lumbar interbody fusion using tapered cages. Spine (Phila Pa 1976) 2005;30(24):2772–2776. doi: 10.1097/01.brs.0000190813.27468.2d. [DOI] [PubMed] [Google Scholar]
- 16.Anandjiwala J, Seo J Y, Ha K Y, Oh I S, Shin D C. Adjacent segment degeneration after instrumented posterolateral lumbar fusion: a prospective cohort study with a minimum five-year follow-up. Eur Spine J. 2011;20(11):1951–1960. doi: 10.1007/s00586-011-1917-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glassman S D, Dimar J R, Carreon L Y, Campbell M J, Puno R M, Johnson J R. Initial fusion rates with recombinant human bone morphogenetic protein-2/compression resistant matrix and a hydroxyapatite and tricalcium phosphate/collagen carrier in posterolateral spinal fusion. Spine (Phila Pa 1976) 2005;30(15):1694–1698. doi: 10.1097/01.brs.0000172157.39513.80. [DOI] [PubMed] [Google Scholar]
- 18.Xia X P, Chen H L, Cheng H B. Prevalence of adjacent segment degeneration after spine surgery: a systematic review and meta-analysis. Spine (Phila Pa 1976) 2013;38(7):597–608. doi: 10.1097/BRS.0b013e318273a2ea. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence B D Wang J Arnold P M Hermsmeyer J Norvell D C Brodke D S Predicting the risk of adjacent segment pathology after lumbar fusion: a systematic review Spine (Phila Pa 1976) 201237(22, Suppl):S123–S132. [DOI] [PubMed] [Google Scholar]
- 20.Yang J Y, Lee J K, Song H S. The impact of adjacent segment degeneration on the clinical outcome after lumbar spinal fusion. Spine (Phila Pa 1976) 2008;33(5):503–507. doi: 10.1097/BRS.0b013e3181657dc3. [DOI] [PubMed] [Google Scholar]
- 21.Chow D H, Luk K D, Evans J H, Leong J C. Effects of short anterior lumbar interbody fusion on biomechanics of neighboring unfused segments. Spine (Phila Pa 1976) 1996;21(5):549–555. doi: 10.1097/00007632-199603010-00004. [DOI] [PubMed] [Google Scholar]
- 22.Weinhoffer S L, Guyer R D, Herbert M, Griffith S L. Intradiscal pressure measurements above an instrumented fusion. A cadaveric study. Spine (Phila Pa 1976) 1995;20(5):526–531. doi: 10.1097/00007632-199503010-00004. [DOI] [PubMed] [Google Scholar]
- 23.Rahm M D, Hall B B. Adjacent-segment degeneration after lumbar fusion with instrumentation: a retrospective study. J Spinal Disord. 1996;9(5):392–400. [PubMed] [Google Scholar]
- 24.Ahn D K, Park H S, Choi D J, Kim K S, Yang S J. Survival and prognostic analysis of adjacent segments after spinal fusion. Clin Orthop Surg. 2010;2(3):140–147. doi: 10.4055/cios.2010.2.3.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sears W R, Sergides I G, Kazemi N, Smith M, White G J, Osburg B. Incidence and prevalence of surgery at segments adjacent to a previous posterior lumbar arthrodesis. Spine J. 2011;11(1):11–20. doi: 10.1016/j.spinee.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 26.Dickerman R D Reynolds A S Rashbaum R Hochschuler S Adjacent segment degeneration: time is not as important as facet preservation! Acta Orthop 2008793452–453., author reply 452–453 [DOI] [PubMed] [Google Scholar]
- 27.Lee M J Dettori J R Standaert C J Brodt E D Chapman J R The natural history of degeneration of the lumbar and cervical spines: a systematic review Spine (Phila Pa 1976) 201237(22, Suppl):S18–S30. [DOI] [PubMed] [Google Scholar]


