Abstract
Objective The purpose of this article is to review and integrate the available literature in different fields to gain a better understanding of the basic physiology and optimize vascular delay as a reconstructive surgery technique.
Methods A broad search of the literature was performed using the Medline database. Two queries were performed using “vascular delay,” a search expected to yield perspectives from the field of plastic and reconstructive surgery, and “ischemic preconditioning,” (IPC) which was expected to yield research on the same topic in other fields.
Results The combined searches yielded a total of 1824 abstracts. The “vascular delay” query yielded 76 articles from 1984 to 2011. The “ischemic preconditioning” query yielded 6534 articles, ranging from 1980 to 2012. The abstracts were screened for those from other specialties in addition to reconstructive surgery, analyzed potential or current uses of vascular delay in practice, or provided developments in understanding the pathophysiology of vascular delay. 70 articles were identified that met inclusion criteria and were applicable to vascular delay or ischemic preconditioning.
Conclusion An understanding of IPC's implementation and mechanisms in other fields has beneficial implications for the field of reconstructive surgery in the context of the delay phenomenon. Despite an incomplete model of IPC's pathways, the anti-oxidative, anti-apoptotic and anti-inflammatory benefits of IPC are well recognized. The activation of angiogenic genes through IPC could allow for complex flap design, even in poorly vascularized regions. IPC's promotion of angiogenesis and reduction of endothelial dysfunction remain most applicable to reconstructive surgery in reducing graft-related complications and flap failure.
Keywords: Ischemic preconditioning, vascular delay, delay phenomenon
The delay phenomenon, also referred to as vascular delay or ischemic preconditioning, describes the observation that a tissue rendered partially ischemic will undergo neovascularization and enhance its vascularity. The field of reconstructive surgery uses this phenomenon to facilitate the survival of flaps. When employed, the delay phenomenon has been shown to promote flap survival, increase the length-to-breadth ratio in random pattern flaps, and ensure the reliable transfer of larger volumes in axial pattern flaps.1 2 3
Though there were earlier reports of attempts at vascular delay in nasal reconstruction,3 4 5 the Renaissance surgeon Gaspar Tagliacozzi is commonly accredited with the discovery as he popularized vascular delay in upper arm flaps in his 1597 work De Curtorum Chirurgia per insitionem. 6 7 S. H. Milton was the first to design experiments to study the delay phenomenon in 1969. He demonstrated in a porcine model that a 2-week delay optimized flap survival, and that survival is dependent on vascular supply instead of the length-to-width ratio as previously thought.8 9 Simultaneously, M. B. Myers isolated the optimal delay time to 8–10 days in rabbits undergoing bipedicled skin flaps.3
More recently, the delay phenomenon has become a topic of increasing interest in other medical fields under the term “ischemic preconditioning” (IPC). Heart and vascular-related fields in particular have contributed much of the recent literature devoted to unraveling the pathways involved. Although the mechanism remains unclear, significant research efforts have focused on revealing the underlying basic science at the cellular and metabolic levels. The purpose of this article is to review and integrate the available literature in different fields to gain a better understanding of the basic physiology and optimize vascular delay as a reconstructive surgery technique.
Methods
A broad search of the literature was performed using the MEDLINE database. Searches cutoff date was January 4, 2012. The MEDLINE database was initially searched using the query “vascular delay,” a search expected to yield perspectives from the field of reconstructive surgery. A second query was performed in the Medline database using the keyword “ischemic preconditioning,” which was expected to yield research on the same topic in other fields.
Results
The combined searches yielded a total of 1824 abstracts. The “vascular delay” query yielded 76 articles from 1984 to 2011. The “ischemic preconditioning” query yielded considerably more results. Ultimately, 6534 articles were produced by this search, ranging from 1980 to 2012. The search results were restricted to recent literature to assess for modern applications of the delay phenomenon across disciplines. The first 200 articles from the second query in addition to the 76 yielded from the first were assessed. The abstracts were screened for those written in English which provided perspectives across specialties in addition to reconstructive surgery, analyzed potential or current uses of vascular delay in practice, or provided developments in understanding the pathophysiology of vascular delay. 70 articles were thereby identified that met inclusion criteria. All articles included were applicable to vascular delay or ischemic preconditioning, depending on specialty.
Discussion
Vascular Delay – Reconstructive Surgery Perspective
Vascular delay is a technique widely used to enhance the vascularity of an existing flap. It takes advantage of the tissue's ability to neovascularize under ischemic conditions, and manipulates the vascular supply to fit the appropriate pattern for flap design. It is especially useful in creating a strong axial blood supply where none previously existed. Clinical research demonstrated that vascular delay increases flap survival significantly and allows for earlier divisions of pedicle.10 One study demonstrated that vascular delay in flaps improved survival 2.5–3 times over the control.11
Biomechanics of Vascular Delay
Vascular delay affects the target tissue in two phases – early and late. Early effects derive predominantly from transection of sympathetic fibers leading to dilation and reorientation of choke vessels. The late phase effects lead to enhanced flap vascularity due to new vessel growth.
The early phase is characterized by three findings: changes in sympathetic tone, dilatation and reorientation of choke vessels, and alterations of metabolic pathways (Fig. 1).12 During the initial flap dissection, the transected sympathetic nerves release norepinephrine into the tissue, leading to a hyperadrenergic state. This sudden catecholamine infusion can constrict the blood vessels up to 30 hours, amplifying the early ischemic state.13 When the severed nerve endings are depleted of these neurotransmitters, the hyperadrenergic state resolves leading to a reactive vasodilation and increased blood flow.14 15 For unclear reasons, vascular delay also leads to hyperplasia and hypertrophy of cells making up the vessel walls.16 This enlargement occurs most rapidly within the first 48 to 72 hours of ischemia, and is entirely independent of the vascular tone.17 Concurrently, the choke vessels reorient along the long axis of the flap, thereby enhancing blood supply to regions of the flap most prone for necrosis.18
Figure 1.
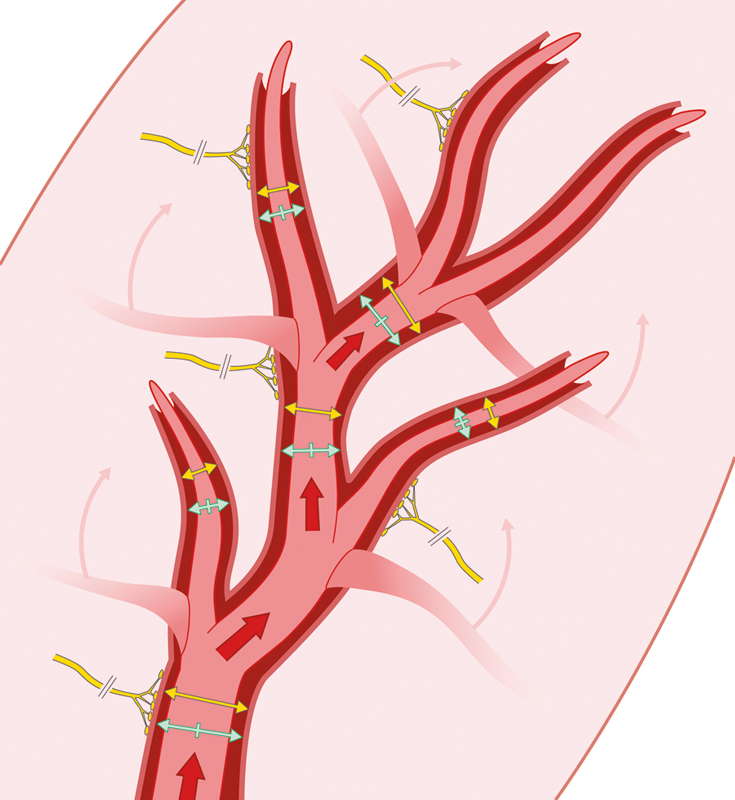
The early phase of vascular delay. In the early phase, choke vessels reorient themselves, changes occur in sympathetic tone to promote vasodilation, and the velocity of blood flow increases.
The early metabolic effects of vascular delay were first demonstrated in canine models which showed a 70% reduction of infarcted myocardium in dogs who underwent ischemic preconditioning via temporary clamping of the vascular supply.19 Later studies revealed that this protective effect is biphasic – a strong protection 2–3 hours after the original ischemic stimulus followed by a weaker protection lasting 48–96 hours.20 Wan et al's murine model demonstrated similar effects in latissimus dorsi flaps.21 Preconditioned tissues exhibited reduced energy requirements,19 fewer reactive oxygen species,22 improved electrolyte homeostasis,23 and reduced apoptosis.24
The late phase effects involve prolonged changes in tissue metabolism and neovascularization (Fig. 2). Vascular delay creates an imbalance between vasodilating (prostaglandin E2) and vasoconstricting (prostaglandin F2, thromboxane) metabolites.12 Murry found that subsequent elevation of a delayed flap leads to a blunted vasoconstrictor response, and an elevated vasodilator response, when compared with an undelayed flap. This is thought to minimize ischemic injury and enhance flap survival.19
Figure 2.
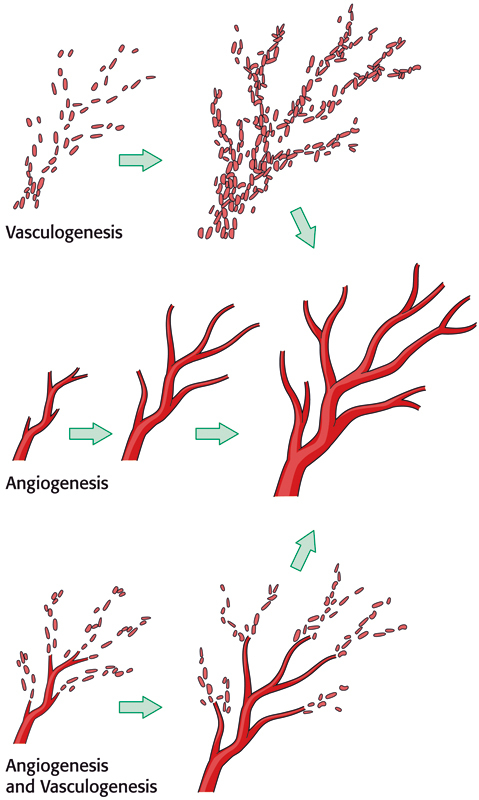
The late phase of vascular delay. The late phase is characterized by neovascularization in the form of both angiogenesis and vasculogenesis.
Most of the long-term benefits of vascular delay, however, derive from neovascularization of the flap. Although the debate regarding mechanisms of neovascularization is ongoing, most agree that new vessels grow by two methods: angiogenesis and vasculogenesis.12 Angiogenesis refers to the sprouting of new vessels from a preexisting vascular network. Vasculogenesis refers to in situ formation from bone-marrow-derived endothelial progenitor cells.12 Vascular delay induces neovascularization by both methods: angiogenesis via increased production of angiogenic growth factors,25 and vasculogenesis via recruitment of bone-marrow derived endothelial stem cells.12 In addition, vascular delay is found to have anti-inflammatory effects by altering function of neutrophils.26
Indications and Applications
The benefits of vascular delay are currently achieved with either the surgical or chemical decrease of blood flow to the flap. Surgically, the major vessels supplying the proposed flap are ligated to increase peripheral vascularity or the perimeter of the flap is divided to bolster flow from the pedicle's base.12 The transverse rectus abdominis musculocutaneous (TRAM) flap serves as a common example where the delay phenomenon is employed. One surgical technique involves ligation of the superficial and deep inferior epigastric vessels two weeks prior to raising the flap for the proposed reconstruction, resulting in increased arterial pressure and decreased venous congestion in the flap.27 Blood flow restrictions can also be achieved via the selective embolization of the deep inferior epigastric arteries to similar improve perfusion of the TRAM flap.28 Chemical delay has also been described. The administration of human VEGF has been demonstrated to similarly induce angiogenesis in TRAM flaps prior to use in reconstruction.29 Regardless of the method of delay, the delayed flaps experience a decrease in fat necrosis and partial flap loss compared with flaps which were not delayed.
Historically, these tissue transfers have been performed with low rates of flap loss and complications. Vascular delay can further decrease these complications and also increase the number of candidates with vascular co-morbidities which would otherwise be restricted from surgery or be higher-risk patients. Patients with varying degrees of vascular compromise include those with radiated tissue, crush injuries, burns or otherwise traumatized tissues. Similarly, vascular delay benefits smokers, obese patients, and diabetics.30 Patients who had previously undergone surgery to the proposed flap region with scarred tissue and disrupted vessels are also candidates for vascular delay to increase the vascularity of the tissue.
Beyond addressing vascular insufficiencies, the delay phenomenon has also been used in patients with insufficiently available tissue such as small children or burn patients.31 32 By delaying and expanding free flaps prior to transfer, the delay phenomenon increases the amount of well-perfused, viable tissue available as well as to improve the tissue's condition at the donor site to facilitate primary closure.
Ischemic Preconditioning – other Perspectives
The delay phenomenon is commonly known as “ischemic preconditioning” (IPC) in fields outside of plastic and reconstructive surgery. It is a topic of great interest as its protective effect has therapeutic potentials for various organs of the body. Recent research in cardiology and hepatology contributed most of the current knowledge in the metabolic influence of IPC. Major signaling pathways, including MAPK,25 PKC,33 Akt-eNOS12 and JAK-STAT,34 have been linked to the anti-oxidative, anti-inflammatory and angiogenic effects of IPC, which originate at a molecular level by altering gene transcription. An extensive literature review was conducted aiming to integrate the research findings across medical specialties. This review consistently revealed two major benefits – diminished ischemia/reperfusion injury, and enhanced tissue regeneration.
Diminished Ischemia/Reperfusion Injury
The primary pathogenesis of ischemia/reperfusion injury involves an overactive inflammatory response35 production of reactive oxygen species,36 and subsequent activation of the apoptotic pathway.37 Cardiology is one of the earliest specialties to recognize the potentially therapeutic effects of ischemic preconditioning. As early as 1986, Murray demonstrated the cardioprotective effects of IPC in canine models.19 A later study revealed that IPC preserves mitochondrial structure in cardiac myocytes, thereby minimizing oxidative injury.38 Specifically, this factor activates signaling pathways that affect the mitochondrial permeability transition pores (mPTP) and the ATP-dependent K channels (mitoKATP).39 Research in neurology found that IPC opens mitochondrial ATP-dependent K+ channels thereby inhibiting the formation of the mPTP – a key step leading to cell death. In addition, animals models showed that IPC may directly downregulate apoptotic gene activation.40
IPC reduces the levels of proliferating cell nuclear antigen labeling index (PCNA-LI) and immunoreactivities to protein kinase B (Akt) and caspase-9, which regulate the mitochondrial apoptosis pathways.41 This protection may be related to transcription levels of c-fos and c-jun. These two proteins in combination form activator protein-1 which regulates differentiation, proliferation and apoptosis.42
IPC modulates systemic immune reaction by inhibiting leukocyte activation with suppression of myeloperoxidase levels and nitric oxide-related oxidative-inflammatory pathway.43 Studies supporting this theory indicate that IPC increases serum concentrations of IL-6, IL-8, IL-10, TNF-α, LDH, CK and TnI, decreases cytokine release and heart enzyme leakage, increases expression of heat shock protein 70, and lowers malondialdehyde (MDA) and superoxide dismutase (SOD).44 Lowering MDA accumulation advances the expressive phase of surviving protein in hepatic tissue and improves cellular function, particularly in the liver.45
Other implicated pathways include phosphorylation of ser727-STAT3,46 HSP-70 and HDj1 (heat shock proteins), reduction of ubiquitin levels after IPC,47 and TRIF-IRF3 signaling.48 Indeed, IPC has been shown to downregulate the pro-inflammatory TLR (toll-like receptor) signaling pathway, reducing inflammation that causes brain injury.49 IPC reduces neutrophil activation through reduced expression of neutrophil CD11b and platelet-neutrophil complexes.50 51 (Fig. 3)
Figure 3.
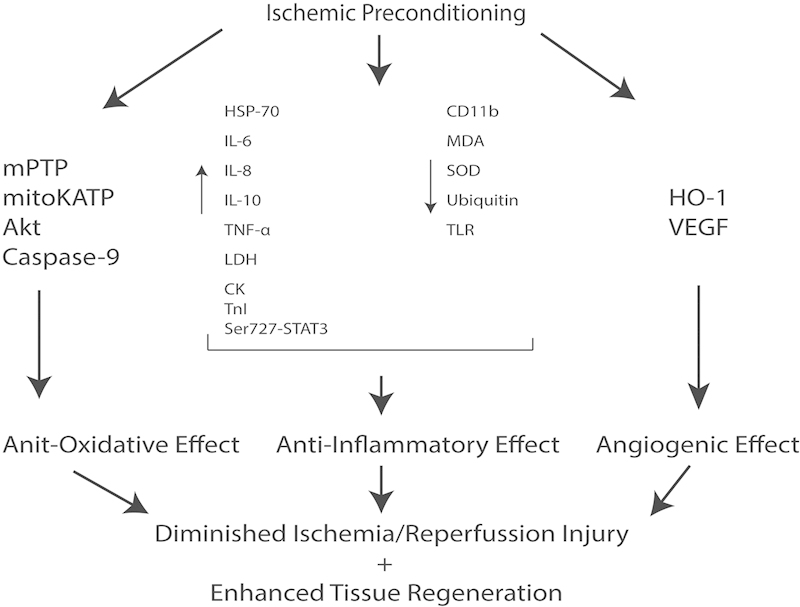
The interplay of major signaling pathways in ischemic preconditioning. Multiple major signaling pathways are implicated on a molecular level in ischemic preconditioning to ultimately produce an anti-oxidative, anti-inflammatory and angiogenic effect. These features promote the rapid regeneration of affected tissues and diminish ischemia/reperfusion injury.
Enhanced Tissue Regeneration
IPC is also found to encourage tissue regeneration. In addition to diminishing oxidative injury, stabilization of mitochondria also ensures continuous availability of ATP and sustained aerobic metabolism.52 IPC doubles the levels of myocardial ATP as compared with controls after aortic clamping procedures.53 Hepatology studies revealed that IPC increase levels of cyclin D1 during early ischemic reperfusion and may enhance hepatocyte proliferation.54 55 IPC also appears to be a potent activator of angiogenic genes and promote stem cell survival.56 57 It induces overexpression of VEGF – crucial for angiogenesis – in cholangiocytes after liver autotransplantation in rats.44 Remote IPC modulates hepatic microcirculation and endothelial function through heme oxygenase-1 (HO-1).58 59 60 On a larger scale, IPC prevents post-ischemic flow reduction of the portal vein and increases arterial perfusion.61 These findings are consistent with late phase benefits seen in muscle flaps as discussed earlier. (Fig. 3)
Other Organ Specific Effects
Heart
Shimizu et al uncovered a <15 kDa hydrophobic circulating factor (or factors) induced by IPC with characteristics of the “cardioprotective factor.”62 Some implicated factors include cardiac enkephalins,63 p38 MAP-kinase,64 65 and glycogen synthase kinase (GSK-3).66
Guo et al demonstrated the dual role of cytokine-inducible, nitric oxide synthase (iNOS) as a mediator of myocardial ischemia/reperfusion (I/R) injury. It is found to be protective in cardiomyocytes but deleterious in peripheral red blood cells.67 The implications of this finding in the IPC setting remain to be seen. Similarly, endothelial nitric oxide synthase (eNOS) appears to bind to heat shock protein 9 (Hsp9) to induce the cardioprotective IPC effect.68
Clinically, Gao et al showed that the ideal sequence for maximizing the benefits of IPC is one day of remote IPC followed by a one-day interval before surgery.69 Research from cardiothoracic surgery determined that IPC must last at least two minutes to confer a benefit to the patient.70 IPC reduces the release of CK-MB following CABG and stimulates left ventricular HSP-72 protein expression.71 The ischemia must be sustained continuously as intermittent ischemia does not provide cardioprotection nor does it incite changes in cytokine levels.72
There are conflicting reports regarding IPC's ability to reduce procedure-related cardiac troponin I release or to protect the lungs.73 Lin et al demonstrated that by reducing lipid peroxidation and systemic inflammatory responses, IPC is protective of the lungs.74 Another study indicated that in children, remote IPC significantly lowered postoperative airway resistance.75
Gastrointestinal System
Despite its protective effects in the liver, IPC demonstrated inconsistent results in delayed intestinal procedures. One study demonstrated no protective effects in colonic anastomosis.76 Another study showed a reduced injury risk by mesenteric ischemia through a mechanism involving PKC inhibition.77 Remote IPC is preferential to local IPC in many cases – remote IPC eliminates the anastomotic instability seen in small bowel surgeries with local IPC.78 IPC directly prior to performing a small bowel anastomosis has a time-dependent beneficial effect on anastomotic stability.79
Urinary System
The research on IPC in urology and nephrology mirrors that found in other fields. The proposed key players are adenosine, bradykinin and cannabinoids as potential humoral mediators in IPC's over anti-inflammatory effects. As in cardiology, the ATP-sensitive K channel appears to be critical.80 Unlike in the intestine, effects of IPC are not related to heme oxygenase-1 induction or neural transmission in the kidney. Renal IPC is associated with local and systemic protection.81 Cardiothoracic research notes IPC effect on endothelial function, as in other fields. In rats, IPC reduced fibrosis and α-smooth muscle active expression in kidneys 15 days after reperfusion.82 However, IPC did not provide endothelial protection in the kidneys of children.33
Conclusion
The field of reconstructive surgery can benefit greatly from an understanding of the discoveries in ischemic preconditioning in other fields in the context of the delay phenomenon. While the current understanding of ischemic preconditioning leaves many questions unanswered, a model of IPC's pathways and effects are becoming clearer with the collective knowledge from other medical fields. IPC is well established in its promotion of anti-oxidative, anti-apoptotic and anti-inflammatory effects. Similarly, IPC's angiogenic potency and humoral mediation of the immune system via biochemical mediators are consistently recognized.
The angiogenic potentiating effect seen across the fields in IPC, of course, remains a hallmark of reconstructive surgery's interest in this phenomenon. Promoting angiogenesis via IPC is a useful tool to promote flap survival and reduce graft-related complications. IPC's activation of angiogenic genes has extensive implications for flap design, particularly in poorly vascularized regions. Furthermore, IPC's ability to reduce endothelial dysfunction is useful in enhancing and protecting the microcirculation of flaps. Interestingly, IPC's ability to reduce rejection rates in liver grafts may have applications in non-autologous implants and transplants. The implementation of IPC stands to leave a major mark on reconstructive surgery, opening the door for previously unfeasible flap designs secondary to improved vascularity and enhanced clinical outcomes.
Acknowledgments
Financial Disclosures / Commercial Associations: none
Products / Devices / Drugs: none
References
- 1.Myers M B, Cherry G. Mechanism of the delay phenomenon. Plast Reconstr Surg. 1969;44(1):52–57. doi: 10.1097/00006534-196907000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Myers M B, Cherry G. Differences in the delay phenomenon in the rabbit, rat, and pig. Plast Reconstr Surg. 1971;47(1):73–78. doi: 10.1097/00006534-197101000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Myers M B, Cherry G. Augmentation of tissue survival by delay: an experimental study in rabbits. Plast Reconstr Surg. 1967;39(4):397–401. doi: 10.1097/00006534-196704000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Gurunluoglu R, Gurunluoglu A. Giulio Cesare Arantius (1530-1589): a surgeon and anatomist: his role in nasal reconstruction and influence on Gaspare Tagliacozzi. Ann Plast Surg. 2008;60(6):717–722. doi: 10.1097/SAP.0b013e31815888f5. [DOI] [PubMed] [Google Scholar]
- 5.Santoni-Rugiu P, Sykers P A. Berlin: Springer-Veriag; 2007. History of Plastic Surgery. [Google Scholar]
- 6.Zimbler M S. Gaspare Tagliacozzi (1545-1599): renaissance surgeon. Arch Facial Plast Surg. 2001;3(4):283–284. doi: 10.1001/archfaci.3.4.283. [DOI] [PubMed] [Google Scholar]
- 7.Cormack G, Lamberry B. New York: Churchill Livingstone; 1994. The Arterial Anatomy of Skin Flaps, 2nd Ed. [Google Scholar]
- 8.Milton S H. The effects of “delay” on the survival of experimental pedicled skin flaps. Br J Plast Surg. 1969;22(3):244–252. doi: 10.1016/s0007-1226(69)80113-x. [DOI] [PubMed] [Google Scholar]
- 9.Milton S H. Pedicled skin-flaps: the fallacy of the length: width ratio. Br J Surg. 1970;57(7):502–508. doi: 10.1002/bjs.1800570705. [DOI] [PubMed] [Google Scholar]
- 10.Civelek B, Selcuk T, Bilgen E, Demirbag E, Celebioglu S. Intermittent ischaemia of skin flaps shortens time taken to divide pedicles: an experimental study in rats. Scand J Plast Reconstr Surg Hand Surg. 2009;43(5):241–244. doi: 10.3109/02844310903138906. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Li Z, Liu X. Effects of various protocols of ischemic preconditioning on rat tram flaps. Microsurgery. 2008;28(1):37–43. doi: 10.1002/micr.20436. [DOI] [PubMed] [Google Scholar]
- 12.Ghali S, Butler P EM, Tepper O M, Gurtner G C. Vascular delay revisited. Plast Reconstr Surg. 2007;119(6):1735–1744. doi: 10.1097/01.prs.0000246384.14593.6e. [DOI] [PubMed] [Google Scholar]
- 13.Pearl R M. A unifying theory of the delay phenomenon—recovery from the hyperadrenergic state. Ann Plast Surg. 1981;7(2):102–112. doi: 10.1097/00000637-198108000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Finseth F, Cutting C. An experimental neurovascular island skin flap for the study of the delay phenomenon. Plast Reconstr Surg. 1978;61(3):412–420. doi: 10.1097/00006534-197803000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Cutting C B, Bardach J, Finseth F. Haemodynamics of the delayed skin flap: a total blood-flow study. Br J Plast Surg. 1981;34(2):133–135. doi: 10.1016/s0007-1226(81)80078-1. [DOI] [PubMed] [Google Scholar]
- 16.Dhar S C, Taylor G I. The delay phenomenon: the story unfolds. Plast Reconstr Surg. 1999;104(7):2079–2091. doi: 10.1097/00006534-199912000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Morris S F, Taylor G I. The time sequence of the delay phenomenon: when is a surgical delay effective? An experimental study. Plast Reconstr Surg. 1995;95(3):526–533. doi: 10.1097/00006534-199503000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Callegari P R Taylor G I Caddy C M Minabe T An anatomic review of the delay phenomenon: I. Experimental studies Plast Reconstr Surg 1992893397–407., discussion 417–418 [PubMed] [Google Scholar]
- 19.Murry C E, Jennings R B, Reimer K A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 20.Marber M S, Latchman D S, Walker J M, Yellon D M. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88(3):1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- 21.Wan C, Maldonado C, Papanicolaou G. et al. Reducing the vascular delay period in latissimus dorsi muscle flaps for use in cardiomyoplasty. Plast Reconstr Surg. 2002;109(5):1630–1637. doi: 10.1097/00006534-200204150-00021. [DOI] [PubMed] [Google Scholar]
- 22.Chen H C, Kuo Y R, Hwang T L, Chen H H, Chang C H, Tang Y B. Microvascular prefabricated free skin flaps for esophageal reconstruction in difficult patients. Ann Thorac Surg. 1999;67(4):911–916. doi: 10.1016/s0003-4975(99)00152-6. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Hernandez F, Pederiva F. et al. Ischemic preconditioning of the graft for intestinal transplantation in rats. Pediatr Transplant. 2011;15(1):65–69. doi: 10.1111/j.1399-3046.2010.01362.x. [DOI] [PubMed] [Google Scholar]
- 24.Yadav S S, Sindram D, Perry D K, Clavien P A. Ischemic preconditioning protects the mouse liver by inhibition of apoptosis through a caspase-dependent pathway. Hepatology. 1999;30(5):1223–1231. doi: 10.1002/hep.510300513. [DOI] [PubMed] [Google Scholar]
- 25.Wong M S, Erdmann D, Sweis R. et al. Basic fibroblast growth factor expression following surgical delay of rat transverse rectus abdominis myocutaneous flaps. Plast Reconstr Surg. 2004;113(7):2030–2036. doi: 10.1097/01.prs.0000122217.16985.52. [DOI] [PubMed] [Google Scholar]
- 26.Lee T M, Lin M S, Tsai C H, Chang N C. Effect of ischaemic preconditioning on regional release of inflammatory markers. Clin Sci (Lond) 2005;109(3):267–276. doi: 10.1042/CS20050046. [DOI] [PubMed] [Google Scholar]
- 27.Codner M A, Bostwick J III, Nahai F, Bried J T, Eaves F F. TRAM flap vascular delay for high-risk breast reconstruction. Plast Reconstr Surg. 1995;96(7):1615–1622. doi: 10.1097/00006534-199512000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Scheufler O, Andresen R, Kirsch A, Banzer D, Vaubel E. Clinical results of TRAM flap delay by selective embolization of the deep inferior epigastric arteries. Plast Reconstr Surg. 2000;105(4):1320–1329. doi: 10.1097/00006534-200004040-00010. [DOI] [PubMed] [Google Scholar]
- 29.Seify H, Bilkay U, Jones G. Improvement of TRAM flap viability using human VEGF-induced angiogenesis: a comparative study of delay techniques. Plast Reconstr Surg. 2003;112(4):1032–1039. doi: 10.1097/01.PRS.0000076186.97093.92. [DOI] [PubMed] [Google Scholar]
- 30.Restifo R J, Ward B A, Scoutt L M, Brown J M, Taylor K J. Timing, magnitude, and utility of surgical delay in the TRAM flap: II. Clinical studies. Plast Reconstr Surg. 1997;99(5):1217–1223. doi: 10.1097/00006534-199704001-00002. [DOI] [PubMed] [Google Scholar]
- 31.Moghari A, Emami A, Sheen R, O'Brien B M. Lower limb reconstruction in children using expanded free flaps. Br J Plast Surg. 1989;42(6):649–652. doi: 10.1016/0007-1226(89)90076-3. [DOI] [PubMed] [Google Scholar]
- 32.Acarturk T O Glaser D P Newton E D Reconstruction of difficult wounds with tissue-expanded free flaps Ann Plast Surg 2004525493–499., discussion 500 [DOI] [PubMed] [Google Scholar]
- 33.Pedersen K R, Ravn H B, Povlsen J V, Schmidt M R, Erlandsen E J, Hjortdal V E. Failure of remote ischemic preconditioning to reduce the risk of postoperative acute kidney injury in children undergoing operation for complex congenital heart disease: a randomized single-center study. J Thorac Cardiovasc Surg. 2012;143(3):576–583. doi: 10.1016/j.jtcvs.2011.08.044. [DOI] [PubMed] [Google Scholar]
- 34.Tapuria N, Kumar Y, Habib M M, Abu Amara M, Seifalian A M, Davidson B R. Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury—a review. J Surg Res. 2008;150(2):304–330. doi: 10.1016/j.jss.2007.12.747. [DOI] [PubMed] [Google Scholar]
- 35.Farhood A, McGuire G M, Manning A M, Miyasaka M, Smith C W, Jaeschke H. Intercellular adhesion molecule 1 (ICAM-1) expression and its role in neutrophil-induced ischemia-reperfusion injury in rat liver. J Leukoc Biol. 1995;57(3):368–374. [PubMed] [Google Scholar]
- 36.Zimmerman B J, Granger D N. Oxygen free radicals and the gastrointestinal tract: role in ischemia-reperfusion injury. Hepatogastroenterology. 1994;41(4):337–342. [PubMed] [Google Scholar]
- 37.Lazarus B, Messina A, Barker J E. et al. The role of mast cells in ischaemia-reperfusion injury in murine skeletal muscle. J Pathol. 2000;191(4):443–448. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH666>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Oka N, Tropak M. et al. Remote ischemic preconditioning elaborates a transferable blood-borne effector that protects mitochondrial structure and function and preserves myocardial performance after neonatal cardioplegic arrest. J Thorac Cardiovasc Surg. 2008;136(2):335–342. doi: 10.1016/j.jtcvs.2007.12.055. [DOI] [PubMed] [Google Scholar]
- 39.Fantinelli J C, Pérez Núñez I A, González Arbeláez L F. et al. Participation of mitochondrial permeability transition pore in the effects of ischemic preconditioning in hypertrophied hearts: Role of NO and mitoK ATP. Int J Cardiol. 2013;166(1):173–180. doi: 10.1016/j.ijcard.2011.10.103. [DOI] [PubMed] [Google Scholar]
- 40.Park H K, Seol I J, Kim K S. Protective effect of hypoxic preconditioning on hypoxic-ischemic injured newborn rats. J Korean Med Sci. 2011;26(11):1495–1500. doi: 10.3346/jkms.2011.26.11.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamada F, Saito T, Abe T. et al. Ischemic preconditioning enhances regenerative capacity of hepatocytes in long-term ischemically damaged rat livers. J Gastroenterol Hepatol. 2007;22(11):1971–1977. doi: 10.1111/j.1440-1746.2006.04711.x. [DOI] [PubMed] [Google Scholar]
- 42.Xiao J S, Cai F G, Niu Y, Zhang Y, Xu X L, Ye Q F. Preconditioning effects on expression of proto-oncogenes c-fos and c-jun after hepatic ischemia/reperfusion in rats. Hepatobiliary Pancreat Dis Int. 2005;4(2):197–202. [PubMed] [Google Scholar]
- 43.Aban N, Cinel L, Tamer L, Aktas A, Aban M. Ischemic preconditioning reduces caspase-related intestinal apoptosis. Surg Today. 2005;35(3):228–234. doi: 10.1007/s00595-004-2918-y. [DOI] [PubMed] [Google Scholar]
- 44.Zhou B, Zhang P J, Tian T. et al. Role of vascular endothelial growth factor in protection of intrahepatic cholangiocytes mediated by hypoxic preconditioning after liver transplantation in rats. Transplant Proc. 2010;42(7):2457–2462. doi: 10.1016/j.transproceed.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 45.Li J Y, Gu X, Yin H Z, Zhou Y, Zhang W H, Qin Y M. Protective effect of ischemic preconditioning on hepatic ischemia-reperfusion injury by advancing the expressive phase of survivin in rats. Hepatobiliary Pancreat Dis Int. 2008;7(6):615–620. [PubMed] [Google Scholar]
- 46.Yagi T, Yoshioka H, Wakai T, Kato T, Horikoshi T, Kinouchi H. Activation of signal transducers and activators of transcription 3 in the hippocampal CA1 region in a rat model of global cerebral ischemic preconditioning. Brain Res. 2011;1422:39–45. doi: 10.1016/j.brainres.2011.08.076. [DOI] [PubMed] [Google Scholar]
- 47.Liu C, Chen S, Kamme F, Hu B R. Ischemic preconditioning prevents protein aggregation after transient cerebral ischemia. Neuroscience. 2005;134(1):69–80. doi: 10.1016/j.neuroscience.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vartanian K B, Stevens S L, Marsh B J, Williams-Karnesky R, Lessov N S, Stenzel-Poore M P. LPS preconditioning redirects TLR signaling following stroke: TRIF-IRF3 plays a seminal role in mediating tolerance to ischemic injury. J Neuroinflammation. 2011;8:140. doi: 10.1186/1742-2094-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jensen H A, Loukogeorgakis S, Yannopoulos F. et al. Remote ischemic preconditioning protects the brain against injury after hypothermic circulatory arrest. Circulation. 2011;123(7):714–721. doi: 10.1161/CIRCULATIONAHA.110.986497. [DOI] [PubMed] [Google Scholar]
- 50.Moskowitz M A, Waeber C. Remote ischemic preconditioning: making the brain more tolerant, safely and inexpensively. Circulation. 2011;123(7):709–711. doi: 10.1161/CIRCULATIONAHA.110.009688. [DOI] [PubMed] [Google Scholar]
- 51.Kim E, Raval A P, Defazio R A, Perez-Pinzon M A. Ischemic preconditioning via epsilon protein kinase C activation requires cyclooxygenase-2 activation in vitro. Neuroscience. 2007;145(3):931–941. doi: 10.1016/j.neuroscience.2006.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winbladh A, Björnsson B, Trulsson L, Offenbartl K, Gullstrand P, Sandström P. Ischemic preconditioning prior to intermittent Pringle maneuver in liver resections. J Hepatobiliary Pancreat Sci. 2012;19(2):159–170. doi: 10.1007/s00534-011-0402-9. [DOI] [PubMed] [Google Scholar]
- 53.Morita S. Remote ischemic preconditioning. -Is it time to introduce it in clinical practice?- Circ J. 2011;75(8):1821–1822. doi: 10.1253/circj.cj-11-0638. [DOI] [PubMed] [Google Scholar]
- 54.Abu-Amara M, Yang S Y, Quaglia A. et al. Effect of remote ischemic preconditioning on liver ischemia/reperfusion injury using a new mouse model. Liver Transpl. 2011;17(1):70–82. doi: 10.1002/lt.22204. [DOI] [PubMed] [Google Scholar]
- 55.Cai F G, Xiao J S, Ye Q F. Effects of ischemic preconditioning on cyclinD1 expression during early ischemic reperfusion in rats. World J Gastroenterol. 2006;12(18):2936–2940. doi: 10.3748/wjg.v12.i18.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stubbs S L, Hsiao S T, Peshavariya H M, Lim S Y, Dusting G J, Dilley R J. Hypoxic preconditioning enhances survival of human adipose-derived stem cells and conditions endothelial cells in vitro. Stem Cells Dev. 2012;21(11):1887–1896. doi: 10.1089/scd.2011.0289. [DOI] [PubMed] [Google Scholar]
- 57.Knudsen A R, Kannerup A S, Dich R. et al. Expression of genes involved in rat liver angiogenesis after ischaemia and reperfusion: effects of ischaemic pre- and post-conditioning. HPB (Oxford) 2010;12(8):554–560. doi: 10.1111/j.1477-2574.2010.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tapuria N, Junnarkar S P, Dutt N. et al. Effect of remote ischemic preconditioning on hepatic microcirculation and function in a rat model of hepatic ischemia reperfusion injury. HPB (Oxford) 2009;11(2):108–117. doi: 10.1111/j.1477-2574.2009.00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saeki I, Matsuura T, Hayashida M, Taguchi T. Ischemic preconditioning and remote ischemic preconditioning have protective effect against cold ischemia-reperfusion injury of rat small intestine. Pediatr Surg Int. 2011;27(8):857–862. doi: 10.1007/s00383-010-2810-3. [DOI] [PubMed] [Google Scholar]
- 60.Franco-Gou R, Roselló-Catafau J, Casillas-Ramirez A. et al. How ischaemic preconditioning protects small liver grafts. J Pathol. 2006;208(1):62–73. doi: 10.1002/path.1859. [DOI] [PubMed] [Google Scholar]
- 61.Heizmann O, Meimarakis G, Volk A, Matz D, Oertli D, Schauer R J. Ischemic preconditioning-induced hyperperfusion correlates with hepatoprotection after liver resection. World J Gastroenterol. 2010;16(15):1871–1878. doi: 10.3748/wjg.v16.i15.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shimizu M, Tropak M, Diaz R J. et al. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci (Lond) 2009;117(5):191–200. doi: 10.1042/CS20080523. [DOI] [PubMed] [Google Scholar]
- 63.Younès A, Pepe S, Yoshishige D, Caffrey J L, Lakatta E G. Ischemic preconditioning increases the bioavailability of cardiac enkephalins. Am J Physiol Heart Circ Physiol. 2005;289(4):H1652–H1661. doi: 10.1152/ajpheart.01110.2004. [DOI] [PubMed] [Google Scholar]
- 64.Hochhauser E, Leshem D, Kaminski O, Cheporko Y, Vidne B A, Shainberg A. The protective effect of prior ischemia reperfusion adenosine A1 or A3 receptor activation in the normal and hypertrophied heart. Interact Cardiovasc Thorac Surg. 2007;6(3):363–368. doi: 10.1510/icvts.2006.136317. [DOI] [PubMed] [Google Scholar]
- 65.Claytor R B, Aranson N J, Ignotz R A, Lalikos J F, Dunn R M. Remote ischemic preconditioning modulates p38 MAP kinase in rat adipocutaneous flaps. J Reconstr Microsurg. 2007;23(2):93–98. doi: 10.1055/s-2007-970189. [DOI] [PubMed] [Google Scholar]
- 66.Barandon L, Dufourcq P, Costet P. et al. Involvement of FrzA/sFRP-1 and the Wnt/frizzled pathway in ischemic preconditioning. Circ Res. 2005;96(12):1299–1306. doi: 10.1161/01.RES.0000171895.06914.2c. [DOI] [PubMed] [Google Scholar]
- 67.Guo Y, Sanganalmath S K, Wu W. et al. Identification of inducible nitric oxide synthase in peripheral blood cells as a mediator of myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2012;107(2):253. doi: 10.1007/s00395-012-0253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vladic N, Ge Z D, Leucker T. et al. Decreased tetrahydrobiopterin and disrupted association of Hsp90 with eNOS by hyperglycemia impair myocardial ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2011;301(5):H2130–H2139. doi: 10.1152/ajpheart.01078.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao J, Kang Y, Lou J. The optimal strategy of noninvasive limb ischemic preconditioning for protecting heart against ischemia-reperfusion injury in rats. J Surg Res. 2012;174(2):e47–e54. doi: 10.1016/j.jss.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 70.Jebeli M, Esmaili H R, Mandegar M H. et al. Evaluation of the effects of ischemic preconditioning with a short reperfusion phase on patients undergoing a coronary artery bypass graft. Ann Thorac Cardiovasc Surg. 2010;16(4):248–252. [PubMed] [Google Scholar]
- 71.Vahlhaus C, Neumann J, Lüss H. et al. Ischemic preconditioning by unstable angina reduces the release of CK-MB following CABG and stimulates left ventricular HSP-72 protein expression. J Card Surg. 2005;20(5):412–419. doi: 10.1111/j.1540-8191.2005.2004107.x. [DOI] [PubMed] [Google Scholar]
- 72.Karuppasamy P, Chaubey S, Dew T. et al. Remote intermittent ischemia before coronary artery bypass graft surgery: a strategy to reduce injury and inflammation? Basic Res Cardiol. 2011;106(4):511–519. doi: 10.1007/s00395-011-0185-9. [DOI] [PubMed] [Google Scholar]
- 73.Hoole S P, Heck P M, Sharples L. et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP stent) study: A prospective, randomized control trial. Circulation. 2009;119(6):820–827. doi: 10.1161/CIRCULATIONAHA.108.809723. [DOI] [PubMed] [Google Scholar]
- 74.Lin L N, Wang L R, Wang W T. et al. Ischemic preconditioning attenuates pulmonary dysfunction after unilateral thigh tourniquet-induced ischemia-reperfusion. Anesth Analg. 2010;111(2):539–543. doi: 10.1213/ANE.0b013e3181e368d2. [DOI] [PubMed] [Google Scholar]
- 75.Cheung M MH, Kharbanda R K, Konstantinov I E. et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006;47(11):2277–2282. doi: 10.1016/j.jacc.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 76.Colak T, Turkmenoglu O, Dag A. et al. The effect of remote ischemic preconditioning on healing of colonic anastomoses. J Surg Res. 2007;143(2):200–205. doi: 10.1016/j.jss.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 77.Um J W, Matthews J B, Song J C, Mun E C. Role of protein kinase C in intestinal ischemic preconditioning. J Surg Res. 2005;124(2):289–296. doi: 10.1016/j.jss.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 78.Holzner P A, Kulemann B, Kuesters S. et al. Impact of remote ischemic preconditioning on wound healing in small bowel anastomoses. World J Gastroenterol. 2011;17(10):1308–1316. doi: 10.3748/wjg.v17.i10.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marjanovic G, Jüttner E, zur Hausen A, Theodor Hopt U, Obermaier R. Ischemic preconditioning improves stability of intestinal anastomoses in rats. Int J Colorectal Dis. 2009;24(8):975–981. doi: 10.1007/s00384-009-0696-0. [DOI] [PubMed] [Google Scholar]
- 80.Zimmerman R F. Remote ischemic preconditioning: is the groove in the heart? Am J Kidney Dis. 2010;56(6):1019–1022. doi: 10.1053/j.ajkd.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 81.Reutzel-Selke A, Pratschke J, Martins P N. et al. Ischemic preconditioning produces systemic protective and adoptively transferable effects. Kidney Int. 2008;74(5):622–630. doi: 10.1038/ki.2008.208. [DOI] [PubMed] [Google Scholar]
- 82.Timsit M O, Gadet R, Ben Abdennebi H, Codas R, Petruzzo P, Badet L. Renal ischemic preconditioning improves recovery of kidney function and decreases α-smooth muscle actin expression in a rat model. J Urol. 2008;180(1):388–391. doi: 10.1016/j.juro.2008.02.043. [DOI] [PubMed] [Google Scholar]


