Abstract
Objective To investigate a novel dual-port endonasal and subtemporal endoscopic approach targeting midline lesions with lateral extension beyond the intracavernous carotid artery anteriorly and the Dorello canal posteriorly.
Methods Ten dual-port approaches were performed on five cadaveric heads. All specimens underwent an endoscopic endonasal approach from the sella to middle clivus. The endonasal port was combined with an anterior or posterior endoscopic extradural subtemporal approach. The anterior subtemporal port was placed directly above the middle third of the zygomatic arch, and the posterior port was placed at its posterior root. The extradural space was explored using two-dimensional and three-dimensional endoscopes.
Results The anterior subtemporal port complemented the endonasal port with direct access to the Meckel cave, lateral sphenoid sinus, superior orbital fissure, and lateral and posterosuperior compartments of the cavernous sinus; the posterior subtemporal port enhanced access to the petrous apex. Endoscopic dissection and instrument maneuverability were feasible and performed without difficulty in both the anterior and posterior subtemporal ports.
Conclusion The anterior and posterior subtemporal ports enhanced exposure and control of the region lateral to the carotid artery and Dorello canal. Dual-port neuroendoscopy is still minimally invasive yet dramatically increases surgical maneuverability while enhancing visualization and control of anatomical structures.
Keywords: 3D, dual port, subtemporal, endoscopy, endonasal
Introduction
The development of the expanded endoscopic endonasal approach (EEA) and associated surgical tools have pushed the limits of endoscopic transnasal access to the ventral skull base. EEA provides direct access to pathologies while minimizing manipulation of neurovascular structures and avoiding brain retraction, ultimately decreasing morbidity.
Numerous studies have described the advantages of endoscopic approaches for midline skull base tumors.1 2 3 4 However, tumors with firm consistency, retrosellar extension, large suprasellar components, and/or lateral extension beyond the cavernous sinus and internal carotid artery (ICA) cannot be treated exclusively through an endonasal approach.5
The use of rigid endoscopes in lateral transcranial approaches have improved visualization of anatomical structures while minimizing brain retraction and reducing surgical morbidity. But these minimally invasive lateral approaches have limitations in maneuverability and in the use of surgical instrumentation reducing their clinical application in the treatment of multicompartmental tumors.6 7 8 9 10 11 12 13 14
Combined simultaneous approaches have been used to treat giant adenomas with multilobular extensions. Loyo et al in 1984 described a simultaneous transsphenoidal and transcranial frontotemporal craniotomy, and ensuing publications on various combinations of transsphenoidal (TS) and transcranial (TC) surgery using microscopy and endoscopy followed.15 16 17 18 19 20 21 22
The concept of approaching skull base lesions exclusively with “dual-port” or “multiport” endoscopy has not been widely studied. Dual-port neuroendoscopy may ease mobilization and removal of mass lesions, assist anatomical orientation, and provide circumferential visualization of neurovascular structures, especially when displaced by the tumor.
We propose a novel simultaneous dual-port endonasal and subtemporal endoscopic approach to the midline skull base for lesions with lateral extension beyond the cavernous carotid artery anteriorly and Dorello canal posteriorly. We investigate the microsurgical anatomy of the simultaneous endonasal and subtemporal endoscopic approaches, their versatility, and the feasibility of combining them into a single procedure.
Methods
Ten endonasal and subtemporal dual-port approaches, consisting of an endonasal port and an anterior or posterior subtemporal port, were performed on five preserved cadaveric heads injected with colored latex (red for arteries, blue for veins). For the anterior subtemporal (AST) port, a keyhole craniotomy was placed directly above the middle third of the zygomatic arch, and for the posterior subtemporal (PST) port, a keyhole craniotomy was placed at the posterior root of the zygomatic arch (Fig. 1).
Fig. 1.
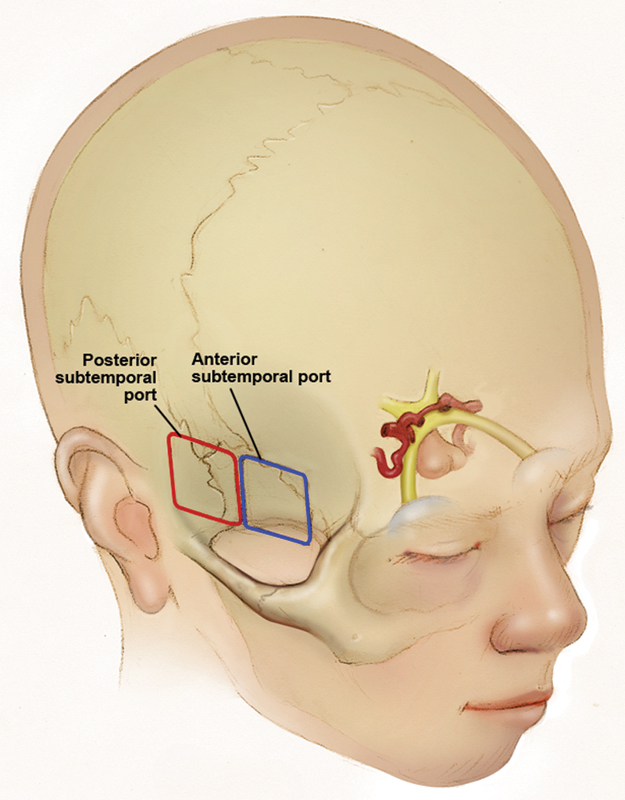
Position of the keyhole craniotomy at the anterior and posterior subtemporal ports.
All specimens underwent an EEA from the tuberculum sella to the middle clivus on the sagittal plane and exposure of the lateral recess and wall of the sphenoid sinus on the coronal plane through a transpterygoid approach. The EEA was followed by placement of an AST port on one side. After initial exposure, the dissection proceeded with two surgeons and two endoscopes (one in each port), in a simultaneous or alternating fashion to complete anterolateral exposure.
On the contralateral side, an endoscopic PST port was made. The dissection proceeded with two surgeons and two endoscopes (one in each port), in a simultaneous or alternating fashion to complete posterolateral exposure.
Dissections were performed using 0-degree and 30-degree two-dimensional optics (4-mm diameter, 18-cm length; Karl Storz, Tuttlingen, Germany) and 0-degree three-dimensional (3D) optics (4.9-mm diameter, 30-cm length; Visionsense, New York, USA). Images were recorded and stored using the Karl Storz AIDA system and the Visionsense software, respectively. An Anspach eMax 2 Plus (DePuy Synthes, Pennsylvania, USA) electric neurosurgical drill was used. An endoscope holder was used to perform bimanual dissection and drilling.
Anatomical Areas
Because the dual-port approach targeted midline lesions with lateral extensions, the areas of interest were lateral to the sagittal plane passing through the paraclival ICA, distal to petrolingual ligament, and proximal to the posterior bend of the cavernous ICA. To facilitate description and comprehension of the surgical anatomy of the proposed dual-port endoscopic approaches, we divided such anatomical area of interest into four anatomical areas (Areas A–D) based on a coronal plane and on their relationships to the abducens nerve (Fig. 2).
Fig. 2.
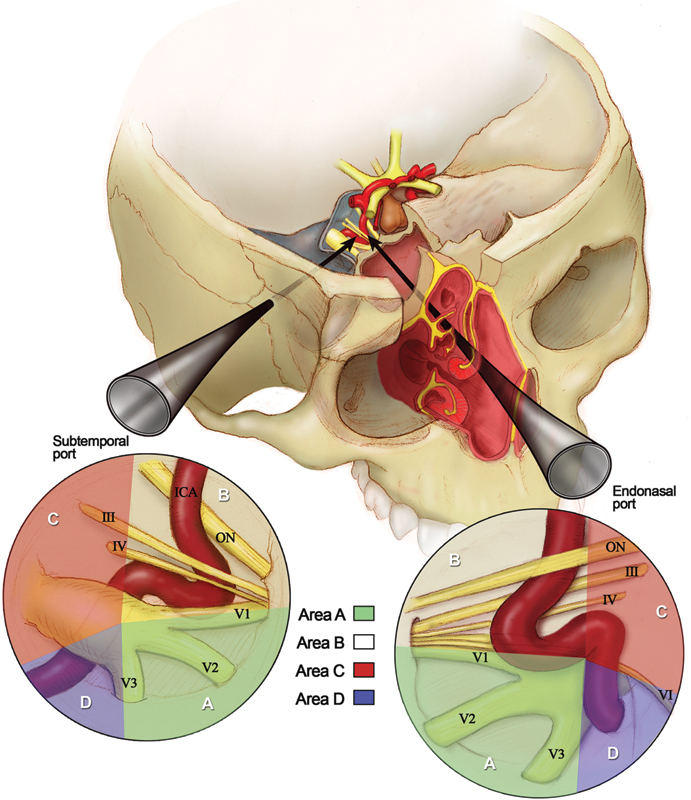
The anatomical areas of interest (areas A–D) seen through subtemporal and endonasal perspectives. ICA, interior carotid artery; ON, optic nerve.
The coronal plane passed through the posterior end of foramen ovale and divide areas A and B from areas C and D. We defined areas A and B as the regions anterior to the coronal plane up to the superior orbital fissure and the pterygopalatine fossa on the coronal axis. Areas C and D were located posterior to the coronal plane up to the porus trigeminus on the coronal axis.
The abducens nerve crossed the anatomical areas of interest from Dorello canal to the superior orbital fissure (SOF) in an oblique trajectory, and divided area A from area B and area C from area D.
As such, area A is inferior to and area B is superior to the abducens nerve, and both regions are lateral to the sagittal plane and anterior to the coronal plane. Area C is superior to and area D is inferior to the abducens nerve, and both regions are lateral to the sagittal plane and posterior to the coronal plane (Fig. 2).
Surgical Positioning
A three-point fixation was achieved using a Mayfield head holder. The head was positioned with 5-degrees of extension, with the vertex tilted slightly down and oriented 30 degrees to the ipsilateral side of the subtemporal port. Positioning was designed to give priority to the endonasal port as the subtemporal port took on an auxiliary role.
Endonasal Endoscopic Port
The endonasal approaches from the tuberculum sella to the middle clivus were all performed with a binostril approach, as previously described.2 23 24 25
After initial exposure of the sella, the bone overlying the medial opticocarotid recess, optic nerve, cavernous sinus, and ICA (parasellar and paraclival segments) were removed extradurally (Fig. 3). The dissection proceeded with drilling of the floor of the sphenoid sinus and the upper and middle clivus.26 27
Fig. 3.

The parasellar segment of the cavernous internal carotid artery and the optic nerves seen through the endoscopic port after initial bone removal while maintaining an extradural dissection.
Dissection of the coronal plane was extended with a posterior ethmoidectomy and partial removal of the medial pterygoid plate to allow for full access to the lateral recess and the lateral wall of the sphenoid sinus.28 29 30
The extradural dissection continued on the posteromedial side of the paraclival ICA where the remaining clivus and the petrous apex bone were drilled. Complete bone removal around the cavernous and paraclival segments of the ICA was completed to allow for lateral and medial mobilization of the vessel.31
Anterior Subtemporal Port
The positioning used, with the vertex tilted down, facilitated the steep caudal to cranial approaching angle. For the AST port, an ∼ 5-cm lazy S skin incision was made starting at the inferior rim of the zygomatic arch along the hairline. After the skin was retracted bilaterally, additional caution was needed for the interfacial dissection to preserve the frontal branch of the facial nerve. The fibers of the temporalis muscle were dissected and retracted laterally to expose the squamous bone. A rectangular-shaped craniotomy, ∼ 2 to 2.5 cm in width and 2 cm in height, was performed as close as possible to the cranial base with its center over the middle portion of the zygomatic arch (Fig. 1).
Bone work was required due to the restricted working corridor. Additional shaving of the superior rim of the zygomatic arch further facilitated the upward viewing angle. Remaining bone was removed for the craniotomy to become flush with the floor of the middle fossa floor. Following the craniotomy, the endoscope was introduced into the extradural space, and the dura mater was bluntly peeled from the floor of the middle cranial fossa using a dissector. Anatomical landmarks were the same as those used in microscopic subtemporal approaches.
Detachment of the dura mater was a crucial maneuver, allowing enough working space in the extradural compartment of the middle cranial fossa for the use of bimanual techniques under fixation of the endoscope, which served as a retractor (Fig. 4).
Fig. 4.
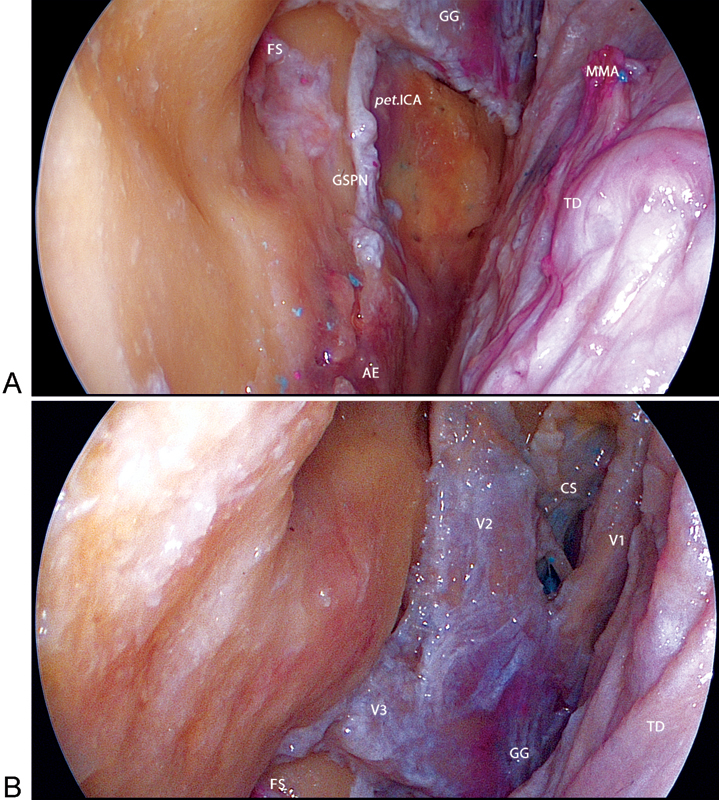
Left anterior subtemporal (AST) port. (A) Initial exposure of the AST port after the dissection of the dura mater overlying the trigeminal nerve. (B) Anatomical landmarks of the middle fossa. The greater superficial petrosal nerve (GSPN) and petrous internal carotid artery (ICA) run under the Gasserian ganglion. AE, arcuate eminence; CS, cavernous sinus; CN III, oculomotor nerve; CN IV, trochlear nerve; FS, foramen spinosum; GG, Gasserian ganglion; MMA, middle meningeal artery; pet.ICA, petrous internal carotid artery; TD, temporal dura mater; V1, ophthalmic nerve; V2, maxillary nerve; V3, mandibular nerve.
Posterior Subtemporal Port
For the PST port, a ∼ 5-cm vertical linear epifascial skin incision was made from the point at the inferior rim of the zygomatic arch to ∼ 1 cm anterior to the external auditory meatus. After the skin was retracted bilaterally, the temporal muscle fascia was incised in a reverse Y-shaped fashion, with the basal leaflet reflected caudally over the zygomatic arch. The temporal muscle was then retracted upward and anteriorly, exposing the squamous bone. If required, a small vertical temporal muscle incision at the posterior border of the skin incision allowed for increased bone exposure. A 2 to 2.5 cm (width) by 2 cm (height) rectangular craniotomy was performed close to the cranial base with its center over the posterior root of the zygomatic arch (Fig. 1).
Shaving of the superior rim of the zygomatic arch and middle fossa floor was performed, as previously described for the anterior port. Anatomical landmarks were the same as those used in microscopic subtemporal approaches. Using a dissector, the dura was bluntly peeled from the floor of the middle cranial fossa providing enough working space (Fig. 5).
Fig. 5.
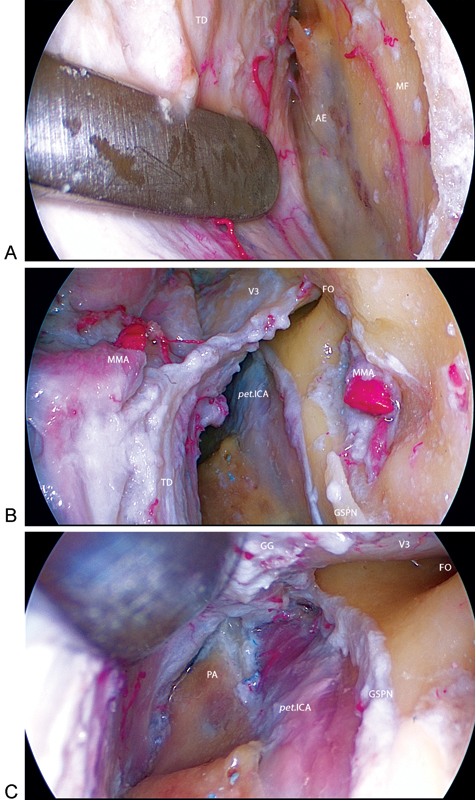
Right posterior subtemporal (PST) port. (A) The dura mater is bluntly peeled from the floor of the middle fossa where the arcuate eminence was identified. (B) Anatomical landmarks at the PST port. (C) View of the petrous apex, internal carotid artery (ICA), and greater superficial petrosal nerve (GSPN) under the Gasserian ganglion. AE, arcuate eminence; FO, foramen ovale; FR, foramen rotundum; GG, Gasserian ganglion; MF, middle fossa; MMA, middle meningeal artery; PA, petrous apex; pet.ICA, petrous internal carotid artery; TD, temporal dura mater; V2, maxillary nerve; V3, mandibular nerve.
Results
Endonasal and Anterior Subtemporal Ports
After initial exposures though the endonasal and AST ports were completed, the dissection continued with two surgeons and two endoscopes (one in each port) in a simultaneous or alternating fashion.
The endonasal port provided better visualization and understanding of the cavernous sinus compartments as they were identified in relation to the ICA (Fig. 6). The main venous spaces within the sinus were the medial, lateral, anteroinferior, and posterosuperior compartments.
Fig. 6.

Endonasal port. (A) The right side with an intact endosteal dura and the left side with exposure of the cavernous sinus contents. (B) Three-dimensional relationships of the neurovascular structures around the left cavernous sinus. cav.ICA, cavernous internal carotid artery; CN VI, abducens nerve; GG, Gasserian ganglion; lac., foramen lacerum; V1, ophthalmic nerve; V2, maxillary nerve; V3, mandibular nerve; vid., vidian nerve.
The cavernous sinus was initially opened at the medial compartment, and the endosteal dural layer was removed exposing the ICA from the clinoidal segment to the lacerum segment.
At this point, in a caudal to rostral order, we observed that the paraclival ICA had an extra- and intracavernous segment, followed by the parasellar segment of ICA that was C-shaped and located almost entirely inside the cavernous sinus. From a medial perspective, the convexity of the “C” was directed anterolaterally (Fig. 7A). Maintaining the caudal to rostral sequence, we subdivided the parasellar ICA into four parts: the posterior bend, the inferior horizontal segment, the anterior vertical segment, and the superior horizontal segment. The posterior bend of the ICA was located posteriorly to the floor of the sella. The inferior horizontal segment was a relatively long segment located at the level of the floor of the sella. The anterior vertical segment, or the anterior bend of the ICA, was located just lateral to the anterior wall of the sella and constituted to the most convex portion of the C-shaped ICA. The superior horizontal segment of the ICA was composed of the clinoidal segment of the ICA as well as the subarachnoid portion.29
Fig. 7.

Simultaneous visualization of the endonasal and left anterior subtemporal (AST) ports. (A) A 30-degree endoscope through the endonasal port viewing an instrument inserted through the anterolateral triangle of the middle fossa. (B) The AST port perspective of the same maneuver. (C, D) Three-dimensional relationships of the combined endonasal and AST ports. (E) The anterolateral, anteromedial, and infratrochlear triangles are also visualized as the working surgical corridors. The posterosuperior compartment of the cavernous sinus is exposed where the posterior bend, horizontal segment, and anterior bend of the cavernous carotid may be accessed. (F) Medial mobilization of the parasellar segment of the cavernous ICA, exposing the cranial nerves running toward the superior orbital fissure and the posterosuperior and lateral compartments of the cavernous sinus. (E) The posterior bend of the intracavernous ICA seen through the infratrochlear triangle of the cavernous sinus. ALT, anterolateral triangle of the middle fossa; AMT, anteromedial triangle of the middle fossa; cav.ICA, cavernous internal carotid artery; CN III, oculomotor nerve; CN IV, trochlear nerve; CN VI, abducens nerve; GG, Gasserian ganglion; lac., foramen lacerum; MF, middle fossa; PA, petrous apex; TD, temporal dura mater; V1, ophthalmic nerve; V2, maxillary nerve; V3, mandibular nerve; vid., vidian nerve.
Area A
Through the AST port, the following anatomical landmarks in area A were identified sequentially in a caudal to rostral direction: V3, the anterolateral triangle of the middle fossa; V2, the anteromedial triangle of the middle fossa; and V1. A ∼ 45-degree angle was found between the branches of the trigeminal nerve (Fig. 7B).
The lateral side of Meckel cave and the Gasserian ganglion were fully exposed. The endosteal dura was opened on the anterolateral (between V2 and V3) triangle of the middle fossa providing access to the sphenoid sinus immediately anterior to the paraclival ICA, where the vidian canal can be visualized in the lateral recess of the sinus. Opening the anteromedial (between V1 and V2) triangle of the middle fossa provided a greater window to the sphenoid sinus, where the anteroinferior compartment of the cavernous sinus and the anterior bend of the parasellar ICA were found superiorly. From this lateral perspective, the surgical access to the abducens nerve was restricted because it was hidden medially to V1 as both nerves run together to the SOF (Fig. 7E).
Through the endonasal port, the vidian canal and its neurovascular bundle were found inferolaterally to the lacerum ICA at the inferior region of area A. Foramen ovale and V3 were located laterally in the same coronal plane as the paraclival ICA. At a 45-degree angle to V3, the V2 and foramen rotundum were observed anteriorly. In this area, we found the previously described quadrangular space, which gives access to the medial side of Meckel cave and the Gasserian ganglion.32 The anterolateral (between V2 and V3) and anteromedial (between V1 and V2) triangles provided access to the middle fossa; however, endonasal maneuverability of the instruments through these windows was very difficult. The distal cavernous segment of the abducens nerve was clearly identified medial to V1 in its oblique trajectory to the SOF. Its proximal segment in area A was covered by the C-shaped parasellar ICA (Fig. 7).
Area B
In area B, the AST port provided a clear lateral view of the following anatomical landmarks, identified sequentially from caudal to rostral: V1, the infratrochlear (Parkinson) triangle, the trochlear nerve, the supratrochlear triangle of the cavernous sinus, and the oculomotor nerve.
After opening the endosteal dura of the infratrochlear (Parkinson) triangle of the cavernous sinus, the lateral compartment of the sinus was accessed where we observed the inferior horizontal segment of the C-shaped parasellar ICA. The abducens nerve was hidden inferiorly and medial to V1 in this surgical window (Figs. 6A, B, 7B, and 8B) and was only visualized with the aid of an angled endoscope (Fig. 7).
Fig. 8.
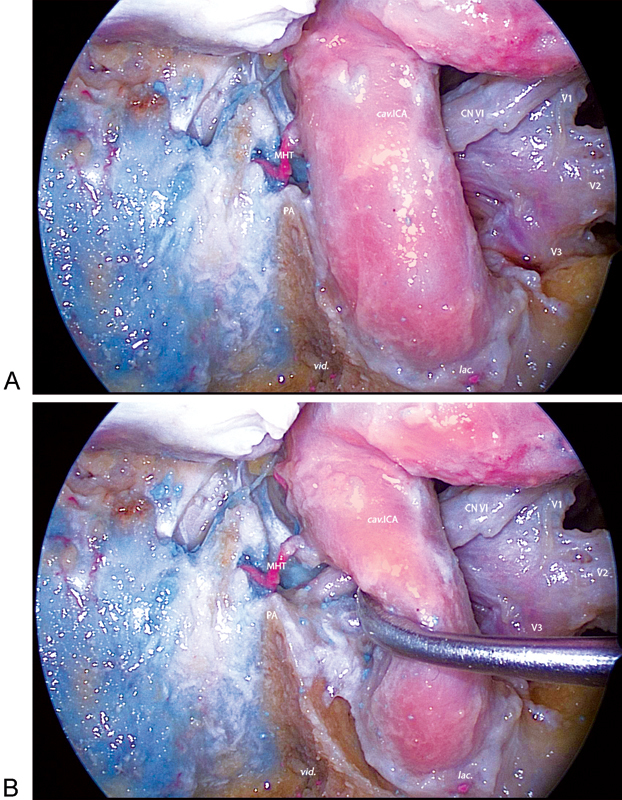
Endonasal perspective of the left side petrous apex region (areas C and D). (A) A 30-degree endoscope view of neurovascular structures lateral and posterior to the internal carotid artery (ICA). (B) After anterolateral mobilization of the ICA, the trajectory of the abducens nerve, the petrous apex, and venous gulf are exposed. The limitation of the endonasal approach to this region is the position of the ICA. cav.ICA, cavernous internal carotid artery; CN VI, abducens nerve; lac., foramen lacerum; MHT, meningohypophyseal trunk; PA, petrous apex; V1, ophthalmic nerve; V2, maxillary nerve; V3, mandibular nerve; vid., vidian nerve.
Although the supratrochlear triangle of the cavernous sinus can be opened by separating the trochlear and oculomotor nerves, this was a narrow and limited working window to the cavernous sinus.
Through the endonasal port, area B structures were covered by the C-shaped parasellar ICA. Its horizontal segment was identified from an anterior view. It was found to be a relatively long segment of the cavernous ICA; however, it may be mistaken for being short when viewed through the endonasal perspective.
The parasellar ICA had to be mobilized medially to expose the lateral compartment of the cavernous sinus (Fig. 7F). The infratrochlear (Parkinson) triangle window was identified above V1 and the abducens nerve. The trochlear nerve was found with difficulty from this anteromedial perspective, as opposed to the oculomotor nerve that was easily identified at the superior area and SOF (Fig. 7F).
Area C
Area C was located immediately posterior to area B, so that the lateral anatomical landmarks seen through the AST port were the same but in its proximal region. The posterosuperior compartment was accessed through the infratrochlear (Parkinson) triangle. This was one of the largest compartments of the cavernous sinus, where the meningohypophyseal artery arises and the superior petrosal sinus, basilar plexus, and inferior petrosal sinus come together. This complex venous confluence has been labeled the sphenopetroclival venous confluence or gulf.33 34 35 The posterior bend of the ICA was observed posteriorly to the floor of the sella. Angled endoscopic probes allowed for visualization of the proximal cavernous (gulfar) segment of the abducens nerve between the ICA and V1.
This area cannot readily be seen through the anteroinferior endonasal approach, requiring medial mobilization of the ICA (Fig. 7F). After this maneuver, the abducens nerve was followed proximally into the posterosuperior compartment until its gulfar segment. Area C was also viewed from a medial perspective with an angled endoscope, after opening of the medial compartment of the cavernous sinus to allow anterolateral mobilization of the posterior bend of the ICA (Fig. 8B). Even though area C was visualized with ICA mobilization, surgical access was restricted and the posterior bend of the parasellar ICA was often hidden when approached through the nasal cavity.
Area D
The posterolateral (Glasscock) and the posteromedial (Kawase) triangles of the middle fossa were visualized through the AST port. This portion of the posterolateral triangle contains the middle meningeal artery (MMA) as it passed through foramen spinosum, and it provides access to the infratemporal fossa. The portion of the posteromedial triangle contains the petrous ICA as it crosses its anterior margins and the cochlea below the floor of the middle fossa on its lateral apex. Removal of the bone on the medial part of the Kawase triangle exposed the clivus and the inferior petrosal sinus; however, it was difficult to perform and the petrous apex could not be accessed through the AST port due to the interposition of V3 and the Gasserian ganglion.
Through the endonasal port, area D was visualized through the medial or lateral sides of the paraclival ICA. On the lateral side, part of the petrous apex was visualized along with the petrolingual ligament over the distal petrous segment of the ICA, but no surgical maneuvers could be accomplished through this surgical corridor. On the medial side, the clivus was removed and the petrous apex was identified posterior to the paraclival ICA. The extent of petrous bone removal depended on individual anatomy that would permit drilling behind the ICA. The abducens nerve was viewed superior to the petrous apex as it exited Dorello canal into the venous gulf (Fig. 8).
Endonasal and Posterior Subtemporal Ports
After the initial exposure of the endonasal and PST ports were completed, the dissection was continued with two surgeons and two endoscopes (one in each port), in a simultaneous or alternating fashion. Additional lateralization of the head was sometimes needed to improve surgical ergonomics when dissecting the petrous apex.
The PST port permitted an extradural approach to the petrous apex along the petrous ridge. An anterior petrosectomy was initiated at Kawase triangle and extended to the petrous apex where it may be tailored through the endonasal port (Fig. 9).
Fig. 9.
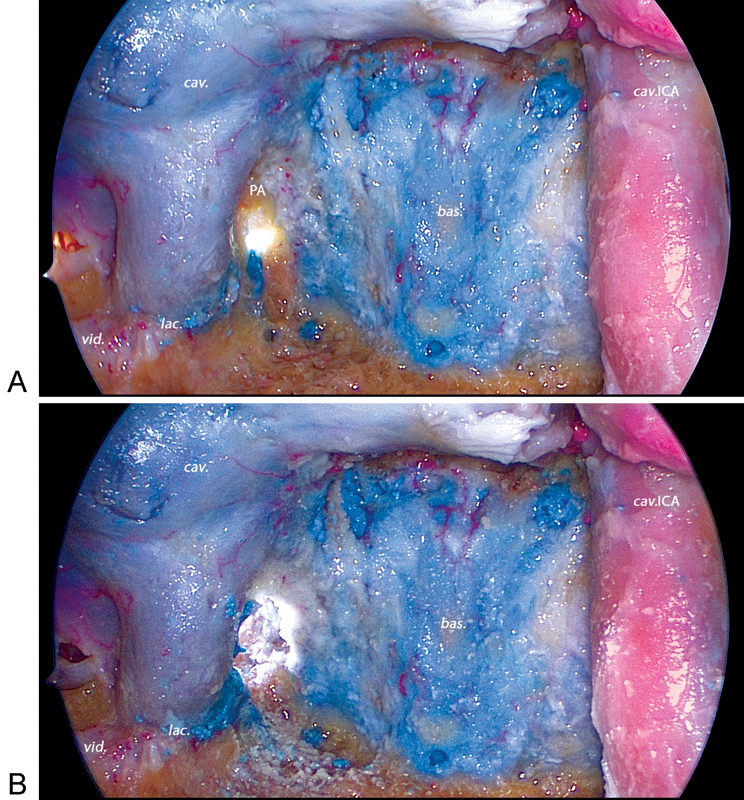
Extradural drilling of the right petrous apex performed through a right posterior subtemporal (PST) port viewed with a 30-degree endoscope through the endonasal port. (A) Further drilling of the petrous apex through the endonasal port is limited by the internal carotid artery. (B) Bone removal significantly increases the working area from the PST port. bas., basilar sinus; cav., cavernous sinus; cav.ICA, cavernous internal carotid artery; lac., foramen lacerum; PA, petrous apex; vid., vidian nerve.
The cavernous sinus was then opened with removal of the endosteal dural layer as described previously.
Area A
Through the PST port, the following anatomical landmarks were identified sequentially in area A in a caudal to rostral direction: V3, the anterolateral triangle of the middle fossa, V2, and the anteromedial triangle of the middle fossa.
The lateral side of Meckel cave and the Gasserian ganglion were exposed. The endosteal dura was opened on the anterolateral (between V2 and V3) triangle of the middle fossa providing access to the sphenoid sinus. Although the inferior region of area A was visualized, the keyhole craniotomy of the PST port restricted maneuverability of the instruments. Because area A was located in an anteromedial position relative the PST port, the working angle was limited because the endoscope and instruments had to be in almost the same direction to reach the surgical target.
The endonasal perspective of area A was described previously, and the PST port added little advantage.
Areas B and C
The PST port does not provide access to areas B and C due to the difficulty of dissection above V2.
Area D
The posterolateral (Glasscock) and the posteromedial (Kawase) triangles of the middle fossa were fully observed through the PST port. Anterior petrosectomy, under the fixation of the endoscope and with limited brain retraction, was possible because of the surgical corridor along the petrous ridge. This procedure exposed the lateral area of the middle clivus that had been removed through the endonasal port as well as the inferior petrosal sinus.
At the endonasal port, the region posterior to the paraclival ICA gained posterolateral working space with the extended removal of the petrous bone. Area D visualization, from the medial and lateral sides of the paraclival ICA, improved significantly due to enhanced ICA mobilization. The extradural dissection helped maintain the abducens nerve intact, and it was identified superiorly to the region of the petrous apex that had been removed (Fig. 10).
Fig. 10.
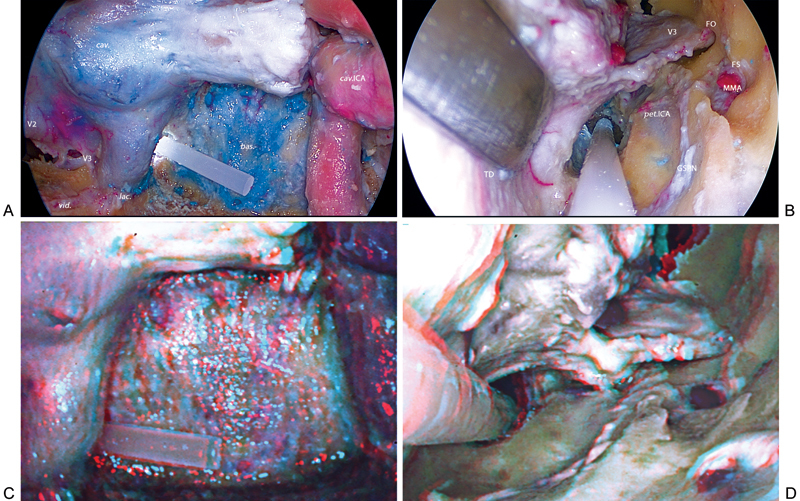
(A–D) Two- and three-dimensional visualization of the simultaneous dissection of endonasal and right posterior subtemporal (PST) ports. bas., basilar sinus; cav., cavernous sinus; cav.ICA, cavernous internal carotid artery; GSPN, greater superficial petrosal nerve; FO, foramen ovale; FS, foramen spinosum; lac., foramen lacerum; MMA, middle meningeal artery; pet.ICA, petrous internal carotid artery; TD, temporal dura mater; V2, maxillary nerve; V3, mandibular nerve; vid., vidian nerve.
Discussion
There is no doubt about the value of endonasal endoscopic approaches in the treatment of midline skull base lesions and, in recent years, more lateral skull base lesions. However, large tumors with firm consistency and lateral extension beyond the cavernous sinus and ICA may need more than one procedure to achieve gross total resection.5
Since the report of a transsphenoidal and transcranial fronto-temporal craniotomy by Loyo et al in 1984, various combinations of TS and TC approaches have been reported including the microscopic TS and TC approach,15 16 17 18 the endoscopic TS and microscopic TC approach,19 and the TS and transventricular endoscopic approach.20 21 22
Simultaneous approaches have several advantages including the possibility of extensive tumor removal in a single operation, eased mobilization and removal of mass lesions from different directions, facilitated anatomical orientation, and circumferential visualization of neurovascular structures, especially when displaced by the tumor.15 16 17 18 19 20 21 22
Approaching skull base lesions using exclusively endoscopy in a dual-port fashion has yet to be studied in depth and as shown, can provide significant logistical advantages when compared with combined approaches using microscopy.
In 1996 Fries and Reisch studied different dual-port neuroendoscopic approaches to the subarachnoid basal cisterns in cadavers and found that biportal combinations of subfrontal supraorbital (epi- or intradural), anterior and posterior subtemporal, and frontal interhemispheric approaches were effective and safe enough for clinical application.36
In a 2010 multiport cadaveric study, Ciporen et al demonstrated that the supraorbital subfrontal and precaruncular transorbital approaches can be used as adjunct ports in endoscopic transnasal approaches to enhance visualization of and access to the pituitary gland, clivus, cavernous sinus, and suprasellar region.37
Endoscopic dual-port approaches can improve the safety of endoscopic dissection, increase gross total resection rates, and improve surgical reconstruction due to better visualization and instrumental access, and they may be the next progression in the evolution of skull base surgery.36 37
In this study, we performed an endoscopic extradural subtemporal approach (anterior or posterior) as an auxiliary port to the EEA. Some authors have selected the subtemporal keyhole approach as the treatment of choice for tumors confined to the petroclival and suprasellar regions that do not extend beyond the carotid artery anteriorly and the internal auditory canal posterolaterally.6 Despite the benefit of a short distance to the suprasellar and petroclival regions, the main criticism of this approach is the use of temporal lobe retraction.38 However, the extradural subtemporal approach has been considered a safe alternative, avoiding any infraction of the subdural space and its associated complications.39 40 41 42
We used an endoscopic extradural subtemporal port because, aside from the minimally invasive advantages, the endoscope provides better illumination and visualization of microanatomical structures with less brain retraction, and thus decreased surgical morbidity.6 7 8 9 10 11 12 13 14
Our goal was not only to describe the anatomy of the areas of interest, as they have been described previously,28 29 43 44 but to highlight the differences of anatomical exposure of two different keyhole subtemporal approaches, provide a different perspective of the anatomical relationships, the versatility and limitations of the approaches, and the feasibility of combining them into a single procedure.
The risk-benefit profile of the minimally invasive subtemporal ports, when used in conjunction with the EEA, will provide enhanced safety and decreased time when dissecting the associated neurovascular structures in midline skull base lesions with lateral extension beyond the cavernous carotid artery anteriorly and Dorello canal posteriorly. The concept of “not crossing nerves,” a limitation of the lateral reach in endoscopic endonasal surgery, was overcome by the subtemporal ports that provided views and working space “beyond” the nerves.
The difference in the location of the subtemporal craniotomy, anterior or posterior, was also evaluated considering the importance of adequate anatomical exposure when working through a keyhole approach.
The AST port was a valuable adjunct to the endonasal port for dissection of areas A, B, and C. The AST port added an increased level of safety when dissecting the lateral and posterosuperior compartments of the cavernous sinus (areas B and C) through the Parkinson triangle due to better anatomical orientation and increased working space provided by the lateral approach. The same regions may not be safely approached through the endonasal port because of the need to mobilize the ICA and the difficulty in dissecting the cranial nerves inside the cavernous sinus. In a clinical setting, this is of particular importance for conserving cranial nerve function when dealing with patients with lesions of the cavernous sinus.
The PST port was only beneficial when dealing with area D through the surgical corridor along the petrous ridge. It provided the added advantage of eased anatomical orientation and control of the paraclival ICA, petrous ICA, petrous apex, and anterior petrous bone. Clinically, the PST port has the potential to reduce the extent of bone removal and increase the speed of dissection of endoscopic endonasal approaches to the petrous apex. In addition, it could reduce the risk of injury to the ICA, brainstem, V2, V3, the abducens nerve, the vidian nerve, and the eustachian tube.31
The potential limitations of surgical maneuverability related to the transcranial endoscopic approaches were minimized when the keyhole craniotomy was placed based on the target area of dissection (anterior for areas A, B, and C and posterior for area D).
Recent advances in 3D endoscopy provided high-resolution images that detailed the depth of the surgical field and critical spatial relationships of vital neurovascular structures. The 3D view helped facilitate dissection and aided in depth-oriented tasks while improving understanding of the advantages and limitations provided by each port. This combined approach highlights the utility of 3D endoscopy in appreciating anatomical relationships, which is useful not only for educational purposes but also in assisting in-depth perception and manual dexterity during surgery.45 46 47 48
In the future, dual-port or multiport approaches will likely become increasingly popular with the refinement of robotic neurosurgery.
Conclusion
A dual-port endoscopic approach is feasible when targeting midline skull base lesions with lateral extension beyond the cavernous carotid artery anteriorly and Dorello canal posteriorly. The subtemporal ports maintain minimally invasiveness and dramatically increase the working limits and control of anatomical structures well beyond what is attainable through single-port neuroendoscopy.
References
- 1.Jho H D, Carrau R L. Endoscopic endonasal transsphenoidal surgery: experience with 50 patients. J Neurosurg. 1997;87(1):44–51. doi: 10.3171/jns.1997.87.1.0044. [DOI] [PubMed] [Google Scholar]
- 2.Cavallo L M, Messina A, Cappabianca P. et al. Endoscopic endonasal surgery of the midline skull base: anatomical study and clinical considerations. Neurosurg Focus. 2005;19(1):E2. [PubMed] [Google Scholar]
- 3.Cappabianca P, Cavallo L M, Esposito F, De Divitiis O, Messina A, De Divitiis E. Extended endoscopic endonasal approach to the midline skull base: the evolving role of transsphenoidal surgery. Adv Tech Stand Neurosurg. 2008;33:151–199. doi: 10.1007/978-3-211-72283-1_4. [DOI] [PubMed] [Google Scholar]
- 4.Zhao B, Wei Y K, Li G L. et al. Extended transsphenoidal approach for pituitary adenomas invading the anterior cranial base, cavernous sinus, and clivus: a single-center experience with 126 consecutive cases. J Neurosurg. 2010;112(1):108–117. doi: 10.3171/2009.3.JNS0929. [DOI] [PubMed] [Google Scholar]
- 5.Zada G, Du R, Laws E R Jr. Defining the “edge of the envelope”: patient selection in treating complex sellar-based neoplasms via transsphenoidal versus open craniotomy. J Neurosurg. 2011;114(2):286–300. doi: 10.3171/2010.8.JNS10520. [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi M, Perneczky A. Subtemporal keyhole approach to the suprasellar and petroclival region: microanatomic considerations and clinical application. Neurosurgery. 1997;41(3):592–601. doi: 10.1097/00006123-199709000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Perneczky A Fries G Endoscope-assisted brain surgery: part 1—evolution, basic concept, and current technique Neurosurgery 1998422219–224.; discussion 224–225 [DOI] [PubMed] [Google Scholar]
- 8.Kocaoğullar Y, Avci E, Fossett D, Caputy A. The extradural subtemporal keyhole approach to the sphenocavernous region: anatomic considerations. Minim Invasive Neurosurg. 2003;46(2):100–105. doi: 10.1055/s-2003-39345. [DOI] [PubMed] [Google Scholar]
- 9.Mourgela S, Sakellaropoulos A, Anagnostopoulou S. Middle cranial fossa endoscopy using a rigid endoscope. Minim Invasive Ther Allied Technol. 2007;16(6):355–359. doi: 10.1080/13645700701705328. [DOI] [PubMed] [Google Scholar]
- 10.Pichierri A D'Avella E Ruggeri A Tschabitscher M Delfini R Endoscopic assistance in the epidural subtemporal approach and Kawase approach: anatomic study Neurosurgery 201067(3, Suppl Operative):ons29–ons37.; discussion ons37 [DOI] [PubMed] [Google Scholar]
- 11.Salma A Wang S Ammirati M Extradural endoscope-assisted subtemporal posterior clinoidectomy: a cadaver investigation study Neurosurgery 201067(3, Suppl Operative):ons43–ons48.; discussion ons48 [DOI] [PubMed] [Google Scholar]
- 12.Sun J Q, Han D M, Li Y X, Gong S S, Zan H R, Wang T. Combined endoscope-assisted translabyrinthine subtemporal keyhole approach for vestibular Schwannoma and auditory midbrain implantation: cadaveric study. Acta Otolaryngol. 2010;130(10):1125–1129. doi: 10.3109/00016481003699674. [DOI] [PubMed] [Google Scholar]
- 13.Gagliardi F, Boari N, Roberti F. et al. Extradural subtemporal transzygomatic approach to the clival and paraclival region with endoscopic assist. J Craniofac Surg. 2012;23(5):1468–1475. doi: 10.1097/SCS.0b013e31825a6497. [DOI] [PubMed] [Google Scholar]
- 14.Komatsu F, Komatsu M, Di Ieva A, Tschabitscher M. Endoscopic extradural subtemporal approach to lateral and central skull base: a cadaveric study. World Neurosurg. 2013;80(5):591–597. doi: 10.1016/j.wneu.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Loyo M, Kleriga E, Mateos H, de Leo R, Delgado A. Combined supra-infrasellar approach for large pituitary tumors. Neurosurgery. 1984;14(4):485–488. [PubMed] [Google Scholar]
- 16.Alleyne C H Jr Barrow D L Oyesiku N M Combined transsphenoidal and pterional craniotomy approach to giant pituitary tumors Surg Neurol 2002576380–390.; discussion 390 [DOI] [PubMed] [Google Scholar]
- 17.D'Ambrosio A L, Syed O N, Grobelny B T, Freda P U, Wardlaw S, Bruce J N. Simultaneous above and below approach to giant pituitary adenomas: surgical strategies and long-term follow-up. Pituitary. 2009;12(3):217–225. doi: 10.1007/s11102-009-0171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishioka H, Hara T, Usui M, Fukuhara N, Yamada S. Simultaneous combined supra-infrasellar approach for giant/large multilobulated pituitary adenomas. World Neurosurg. 2012;77(3–4):533–539. doi: 10.1016/j.wneu.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Umemura A, Suzuka T, Isomura K, Ito M, Mukodaka H. Simultaneous transcranial and endoscopic transnasal approach for recurrent huge pituitary adenoma. Acta Neurochir (Wien) 1999;141(12):1359–1360. doi: 10.1007/s007010050443. [DOI] [PubMed] [Google Scholar]
- 20.Greenfield J P, Leng L Z, Chaudhry U. et al. Combined simultaneous endoscopic transsphenoidal and endoscopic transventricular resection of a giant pituitary macroadenoma. Minim Invasive Neurosurg. 2008;51(5):306–309. doi: 10.1055/s-0028-1082323. [DOI] [PubMed] [Google Scholar]
- 21.Ojha B K Husain M Rastogi M Chandra A Chugh A Husain N Combined trans-sphenoidal and simultaneous trans-ventricular-endoscopic decompression of a giant pituitary adenoma: case report Acta Neurochir (Wien) 20091517843–847.; discussion 847 [DOI] [PubMed] [Google Scholar]
- 22.Romano A, Chibbaro S, Marsella M. et al. Combined endoscopic transsphenoidal-transventricular approach for resection of a giant pituitary macroadenoma. World Neurosurg. 2010;74(1):161–164. doi: 10.1016/j.wneu.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 23.Kassam A, Snyderman C H, Mintz A, Gardner P, Carrau R L. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus. 2005;19(1):E3. [PubMed] [Google Scholar]
- 24.Kassam A, Snyderman C H, Mintz A, Gardner P, Carrau R L. Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus. 2005;19(1):E4. [PubMed] [Google Scholar]
- 25.Schwartz T H Fraser J F Brown S Tabaee A Kacker A Anand V K Endoscopic cranial base surgery: classification of operative approaches Neurosurgery 2008625991–1002.; discussion 1002–1005 [DOI] [PubMed] [Google Scholar]
- 26.Kassam A B Prevedello D M Thomas A et al. Endoscopic endonasal pituitary transposition for a transdorsum sellae approach to the interpeduncular cistern Neurosurgery 20086230157–72.; discussion 72–74 [DOI] [PubMed] [Google Scholar]
- 27.Silva D, Attia M, Kandasamy J, Alimi M, Anand V K, Schwartz T H. Endoscopic endonasal posterior clinoidectomy. Surg Neurol Int. 2012;3:64. doi: 10.4103/2152-7806.97008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavallo L M Cappabianca P Galzio R Iaconetta G de Divitiis E Tschabitscher M Endoscopic transnasal approach to the cavernous sinus versus transcranial route: anatomic study Neurosurgery 200556(2, Suppl):379–389.; discussion 379–389 [DOI] [PubMed] [Google Scholar]
- 29.Alfieri A Jho H D Endoscopic endonasal cavernous sinus surgery: an anatomic study Neurosurgery 2001484827–836.; discussion 836–837 [PubMed] [Google Scholar]
- 30.Kassam A B, Vescan A D, Carrau R L. et al. Expanded endonasal approach: vidian canal as a landmark to the petrous internal carotid artery. J Neurosurg. 2008;108(1):177–183. doi: 10.3171/JNS/2008/108/01/0177. [DOI] [PubMed] [Google Scholar]
- 31.Zanation A M, Snyderman C H, Carrau R L, Gardner P A, Prevedello D M, Kassam A B. Endoscopic endonasal surgery for petrous apex lesions. Laryngoscope. 2009;119(1):19–25. doi: 10.1002/lary.20027. [DOI] [PubMed] [Google Scholar]
- 32.Kassam A B Prevedello D M Carrau R L et al. The front door to Meckel's cave: an anteromedial corridor via expanded endoscopic endonasal approach—technical considerations and clinical series Neurosurgery 200964(3, Suppl):ons71–ons82.; discussion ons82–ons83 [DOI] [PubMed] [Google Scholar]
- 33.Destrieux C, Velut S, Kakou M K, Lefrancq T, Arbeille B, Santini J J. A new concept in Dorello's canal microanatomy: the petroclival venous confluence. J Neurosurg. 1997;87(1):67–72. doi: 10.3171/jns.1997.87.1.0067. [DOI] [PubMed] [Google Scholar]
- 34.Iaconetta G, Fusco M, Samii M. The sphenopetroclival venous gulf: a microanatomical study. J Neurosurg. 2003;99(2):366–375. doi: 10.3171/jns.2003.99.2.0366. [DOI] [PubMed] [Google Scholar]
- 35.Rhoton A L Jr The cavernous sinus, the cavernous venous plexus, and the carotid collar Neurosurgery 200251(4, Suppl):S375–S410. [PubMed] [Google Scholar]
- 36.Fries G, Reisch R. Biportal neuroendoscopic microsurgical approaches to the subarachnoid cisterns. A cadaver study. Minim Invasive Neurosurg. 1996;39(4):99–104. doi: 10.1055/s-2008-1052226. [DOI] [PubMed] [Google Scholar]
- 37.Ciporen J N, Moe K S, Ramanathan D. et al. Multiportal endoscopic approaches to the central skull base: a cadaveric study. World Neurosurg. 2010;73(6):705–712. doi: 10.1016/j.wneu.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 38.Koos W TH Spetzler R F Lang J Tumors of the base of the skull New York, NY: Thieme; 1993275–283.. Color Atlas of Microneurosurgery; vol 1 [Google Scholar]
- 39.Sekhar L N, Pomeranz S, Sen C N. Extradural petrous bone and petroclival neoplasms. Acta Neurochir Suppl (Wien) 1991;53:183–192. doi: 10.1007/978-3-7091-9183-5_29. [DOI] [PubMed] [Google Scholar]
- 40.Bambakidis N C Kakarla U K Kim L J et al. Evolution of surgical approaches in the treatment of petroclival meningiomas: a retrospective review Neurosurgery 2007615, Suppl 02202–209.; discussion 209–211 [DOI] [PubMed] [Google Scholar]
- 41.Taha J M, Tew J M Jr, van Loveren H R, Keller J T, el-Kalliny M. Comparison of conventional and skull base surgical approaches for the excision of trigeminal neurinomas. J Neurosurg. 1995;82(5):719–725. doi: 10.3171/jns.1995.82.5.0719. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida K, Kawase T. Trigeminal neurinomas extending into multiple fossae: surgical methods and review of the literature. J Neurosurg. 1999;91(2):202–211. doi: 10.3171/jns.1999.91.2.0202. [DOI] [PubMed] [Google Scholar]
- 43.Doglietto F Lauretti L Frank G et al. Microscopic and endoscopic extracranial approaches to the cavernous sinus: anatomic study Neurosurgery 200964502413–421.; discussion 421–422 [DOI] [PubMed] [Google Scholar]
- 44.Jho H D, Ha H G. Endoscopic endonasal skull base surgery: Part 2—The cavernous sinus. Minim Invasive Neurosurg. 2004;47(1):9–15. doi: 10.1055/s-2004-818346. [DOI] [PubMed] [Google Scholar]
- 45.Fraser J F, Allen B, Anand V K, Schwartz T H. Three-dimensional neurostereoendoscopy: subjective and objective comparison to 2D. Minim Invasive Neurosurg. 2009;52(1):25–31. doi: 10.1055/s-0028-1104567. [DOI] [PubMed] [Google Scholar]
- 46.Brown S M, Tabaee A, Singh A, Schwartz T H, Anand V K. Three-dimensional endoscopic sinus surgery: feasibility and technical aspects. Otolaryngol Head Neck Surg. 2008;138(3):400–402. doi: 10.1016/j.otohns.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 47.Tabaee A Anand V K Fraser J F Brown S M Singh A Schwartz T H Three-dimensional endoscopic pituitary surgery Neurosurgery 200964502288–293.; discussion 294–295 [DOI] [PubMed] [Google Scholar]
- 48.Roth J Singh A Nyquist G et al. Three-dimensional and 2-dimensional endoscopic exposure of midline cranial base targets using expanded endonasal and transcranial approaches Neurosurgery 20096561116–1128.; discussion 1128–1130 [DOI] [PubMed] [Google Scholar]


