Abstract
Mitochondrial DNA (mtDNA) contains higher steady-state levels of oxidative damage and mutates at rates significantly greater than nuclear DNA. Oxidative lesions in mtDNA are removed by a base excision repair (BER) pathway. All mtDNA repair proteins are nuclear encoded and imported. Most mtDNA repair proteins so far discovered are either identical to nuclear DNA repair proteins or isoforms of nuclear proteins arising from differential splicing. Regulation of mitochondrial BER is therefore not expected to be independent of nuclear BER, though the extent to which mitochondrial BER is regulated with respect to mtDNA amount or damage is largely unknown. Here we have measured DNA BER activities in lysates of mitochondria isolated from human 143B TK– osteosarcoma cells that had been depleted of mtDNA (ρ0) or not (wt). Despite the total absence of mtDNA in the ρ0 cells, a complete mitochondrial BER pathway was present, as demonstrated using an in vitro assay with synthetic oligonucleotides. Measurement of individual BER protein activities in mitochondrial lysates indicated that some BER activities are insensitive to the lack of mtDNA. Uracil and 8-oxoguanine DNA glycosylase activities were relatively insensitive to the absence of mtDNA, only about 25% reduced in ρ0 relative to wt cells. Apurinic/apyrimidinic (AP) endonuclease and polymerase γ activities were more affected, 65 and 45% lower, respectively, in ρ0 mitochondria. Overall BER activity in lysates was also about 65% reduced in ρ0 mitochondria. To identify the limiting deficiencies in BER of ρ0 mitochondria we supplemented the BER assay of mitochondrial lysates with pure uracil DNA glycosylase, AP endonuclease and/or the catalytic subunit of polymerase γ. BER activity was stimulated by addition of uracil DNA glycosylase and polymerase γ. However, no addition or combination of additions stimulated BER activity to wt levels. This suggests that an unknown activity, factor or interaction important in BER is deficient in ρ0 mitochondria. While nuclear BER protein levels and activities were generally not altered in ρ0 cells, AP endonuclease activity was substantially reduced in nuclear and in whole cell extracts. This appeared to be due to reduced endogenous reactive oxygen species (ROS) production in ρ0 cells, and not a general dysfunction of ρ0 cells, as exposure of cells to ROS rapidly stimulated increases in AP endonuclease activities and APE1 protein levels.
INTRODUCTION
Oxidative attack of DNA results in a wide range of chemical modifications, many of which are highly mutagenic. Genomic stability is maintained, in part, by reversing and/or repairing these lesions before mutations can occur. In the nucleus this task is accomplished primarily by the base excision repair (BER) pathway, which first recognizes and cleaves specific base lesions from DNA, followed by abasic site processing, gap-filling and ligation of the DNA strand. Mammalian mitochondria, which contain their own (16.5 kb) DNA molecule, have their own DNA repair systems (1). The mitochondrial BER proteins that have been identified are virtually all either identical to those found in the nucleus or are isoforms of nuclear BER proteins arising from variant RNA splicing. Little is known about the molecular organization of BER in mammalian mitochondria. Similarly, limited information is available regarding the regulation of mitochondrial DNA (mtDNA) repair, including the extent to which it is regulated independently of nuclear BER. Mitochondrial genomic stability is adversely affected in numerous human pathologies, including cancers, diabetes and neurodegenerative disorders (2), yet the mechanisms by which cells repair mtDNA damage and maintain the integrity of the mitochondrial genome remain incompletely understood.
Cultured cells lacking mtDNA have provided useful models for studying human mitochondrial diseases (3) and the regulation of proteins involved in mtDNA transactions (4,5). Many human cell lines can be rendered mtDNA-less (ρ0) in culture by growth in the presence of low concentrations of the intercalating agent ethidium bromide (6). These cells lose their ability to respire, but meet their energy needs via anaerobic glycolysis, even though cultured at atmospheric oxygen concentrations. The mitochondria of ρ0 cells maintain a membrane potential sufficient for import of nuclear-encoded proteins by coordinated action of the F1 ATPase and adenine nucleotide translocator, which together maintain the electrogenic exchange of ATP/ADP across the inner membrane (7,8). The gross morphology of mitochondria in ρ0 cells is similar to that of wild-type cells, though swelling is often apparent in the former. ρ0 cells maintain their responsiveness to many apoptotic stimuli (9), and divide at an only marginally slower rate (10). Thus, ρ0 cells are a viable model for studying the impact of mtDNA-deficiency on cell biology.
Interestingly, some proteins involved in mtDNA maintenance and replication are apparently insensitive to the absence of mtDNA. Polymerase γ, the sole mtDNA polymerase, continues to be expressed and localizes to mitochondria in ρ0 cells (9). In contrast, expression of mitochondrial transcription factor A (mtTFA), involved in mtDNA packaging and transcriptional activation, is profoundly depressed in ρ0 cells. Levels of this protein strongly correlate with mtDNA amount (5).
It is not known to what extent the proteins involved in maintaining mtDNA integrity are sensitive to mtDNA levels, damage, or indeed to the absence of mtDNA. Here, we have developed the use of ρ0 cells as an experimental model with which to study aspects of the organization and regulation of mitochondrial BER. Through these investigations, we have identified possible key regulatory steps in the BER pathway for repair of mtDNA, as well as aspects of mitochondrial BER regulation. We have also investigated the effect of ρ0 status on nuclear BER activities.
MATERIALS AND METHODS
Purified proteins
Human apurinic/apyrimidinic (AP) endonuclease 1 (APE1) was made as in Erzberger et al. (11). Human uracil DNA glycosylase (UDG) was purchased from Boehringer-Mannheim. Human oxoguanine DNA glycosylase (αOGG1) and bacterial T4 ligase were purchased from New England BioLabs. Human polymerase γ catalytic subunit was made as in Longley et al. (12).
Cell lines and growth conditions
mtDNA-less (ρ0) 143B TK– human osteosarcoma cells (ATCC CRL 8303) were a generous gift from Guiseppe Attardi (California Institute of Technology). Wild-type (wt) 143B TK– cells were acquired from the American Type Cell Collection (ATCC). wt and ρ0 cells were cultured under identical conditions, in a humidified 5% CO2 atmosphere in Dulbecco’s Modified Eagle’s Medium, supplemented with penicillin/streptomycin, 10% fetal bovine serum, 100 µg/ml pyruvate, 100 µg/ml BrdU and 50 µg/ml uridine.
PCR analysis of mtDNA
The mtDNA content of wt and ρ0 cells lines was determined essentially as in (4). Briefly, total DNA was prepared from ∼105 wt or ρ0 cells. PCR amplification of a portion of the Leu tRNA gene of the mitochondrial genome and the trinucleotide repeat region of the DNA polγ gene were done at the same time. The PCR mixture contained 200 µM dNTPs, 10 µCi [α-32P]dCTP (3000 Ci/mmol), 5 pmol primers (5′-AGCGACGGGCAGCGGCGGCGGCA-3′ and 5′-CCCTCCGAGGATAGCACTTGCGGC-3′ for nuclear encoded DNA pol γ gene and 5′-GATGGCAGAGCCCCGGTAATCGC-3′ and 5′-TAAGCATTAGGAATGCCATTGCG-3′ for mtDNA encoded tRNA Leu gene), 20 ng total DNA and 0.5 U Taq DNA polymerase. Reaction mixtures were incubated at 95°C for 3 min followed by 30 cycles of 95°C for 1 min, 50°C for 1 min and 72°C for 1 min. The reaction was completed by a final incubation at 72°C for 5 min. PCR products were loaded onto an 8% native polyacrylamide gel and electrophoresed at 200 V for 1 h 50 min, then visualized by Phosphor Imager.
Isolation of mitochondria
Approximately sixty 150 mm plates of wt and ρ0 143B TK– cells, grown to 80% confluence, were harvested. Cells were washed twice in PBS and then resuspended in 7 ml of homogenization buffer [MSHE; 210 mM mannitol, 70 mM sucrose, 10 mM HEPES (pH 7.4), 1 mM EGTA, 2 mM EDTA, 0.15 mM spermine, 0.75 mM spermidine, 1 mM dithiothreitol (DTT)], with the following protease inhibitors added immediately prior to use: 1 µg/ml chymostatin, 2 µg/ml leupeptin, 2 µM benzamide, 1 µM E-64 and 1 mM PMSF. Cells were homogenized with a Potter-Elvejhem glass/glass homogenizer. The homogenate was centrifuged at 500 g for 12 min. The supernatant was transferred to a new tube and centrifuged at 10 000 g for 10 min. The pellet was homogenized and centrifuged a second time. The 10 000 g pellets (crude mitochondrial fraction) were combined. The 500 g pellet (nuclear fraction) was frozen at –80°C. The combined 10 000 g pellets were layered onto a 1:1 Percoll/2× MSHE gradient and centrifuged at 50 000 g for 1 h 10 min. The mitochondrial layer, in the middle of the gradient, was collected and washed twice in homogenization buffer, then resuspended in 300 µl MSHE. Protein concentration was determined using the Bio-Rad assay (Bio-Rad Laboratories, Hercules, CA) using bovine serum albumin as standard, and mitochondrial fractions were stored at –80°C.
Citrate synthase assay
Citrate synthase (CS) activity was measured in isolated mitochondria. The assay buffer included 50 mM Tris (pH 8.0), 0.5 mM dithionitro blue tetrazolium (DTNB), 0.1 mM acetyl-CoA, 0.05% Triton X-100 and 20 µg mitochondrial protein. The assay was initiated by addition of 0.5 mM oxaloacetate and absorbance at 412 nm followed for several minutes at 30°C.
Preparation of nuclear and whole cell extracts
Nuclear extracts were prepared from frozen nuclear pellets. The pellets were resuspended in buffer containing 10 mM HEPES pH 7.9, 25% glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 10 mM KCl, 0.5 mM DTT and protease inhibitors. Triton X-100 was added to 0.5%, the suspension sonicated, and centrifuged at 15 000 g for 10 min. The supernatants were concentrated using Centricon protein concentrators (Millipore). Protein concentrations were determined by Bio-Rad assay and stored as for mitochondrial extracts.
Whole cell extracts (WCEs) were prepared essentially as described (13) from three to six 150 mm plates of wt or ρ0 cells. Cells were scraped from dishes, washed twice in PBS, then incubated in lysis buffer on ice for 1 h. Lysis buffer contained 10 mM Tris (pH 8.0), 150 mM NaCl, 2 mM EDTA, 2 mM DTT, 0.4 mM PMSF, 40% glycerol and 0.2% NP40. Following incubation, the cell lysate was centrifuged for 10 min at 13 000 g. Determination of protein concentrations and storage was as above. For western blotting, cell pellets following PBS washing were added directly to Tris–glycine–SDS sample buffer (Novex) and loaded directly onto gels.
Western blotting
Whole mitochondria, nuclear extracts or whole cells were diluted 1:1 in SDS protein loading buffer supplemented with 50 mM 2-mercaptoethanol, sonicated, heated at 90°C for 10 min and cleared by centrifugation at 13 000 g for 5 min. The samples were loaded onto 12% Tris–glycine–SDS gels and electrophoresed at 130 V for 1.5 h. Gels were transferred to PVDF membranes (0.2 µm pore size; Invitrogen) at 250 mA for 2 h in transfer buffer containing 20% methanol. Membranes were blocked either 1 h at room temperature or overnight at 4°C in PBST (0.1% Tween-20) + 5% milk protein. Incubation with primary antibodies was in PBST + 5% milk protein, either 1 h at room temperature or overnight at 4°C, with the following antibodies and conditions:
APE1 (Trevigen 4415-PC-100; 1:1000); DNA polymerase β (Trevigen 4445-MC-100; 1:1000); αOGG1 (Novus human OGG1; 1:1000); PCNA (Santa Cruz; 1:1000); mtTFA (Santa Cruz; 1:100). Secondary anti-mouse, anti-rabbit, or anti-goat antibodies were applied at 1:5000 and membranes incubated at room temperature for 1 h. Membranes were then washed repeatedly with PBST, and developed using an ECL-plus kit (Amersham).
DNA repair assays
Mitochondria were permeabilized by addition of 1% Triton X-100 and incubation on ice for 30 min. DNA repair assays were done directly on these samples. Oligonucleotides used in these assays are presented in Table 1.
Table 1. Oligonucleotide substrates used.
| Name | Sequence |
|---|---|
| dsC | 5′-ATA TAC CGC GGC CGG CCG ATC AAG CTT ATT-3′ |
| 3′-TAT ATG GCG CCG GCC GGC TAG TTC GAA TAA-5′ | |
| ssC | 5′-ATA TAC CGC GGC CGG CCG ATC AAG CTT ATT-3′ |
| dsU | 5′-ATA TAC CGC GG(U) CGG CCG ATC AAG CTT ATT-3′ |
| 3′-TAT ATG GCG CC(G) GCC GGC TAG TTC GAA TAA-5′ | |
| ssU | 5′-ATA TAC CGC GG(U) CGG CCG ATC AAG CTT ATT-3′ |
| GAP | 5′-CGG ATC TGC AGC TGA TGC GC-OH P-GTA CGG ATC CCC GGG TAC-3′ |
| 3′-GCC TAG ACG TCG ACT ACG CGG CAT GCC TAG GGG CCC ATG-5′ | |
| 34U | 5′-CTG CAG CTG ATG CGC (U)GT ACG GAT CCC CGG GTA C-3′ |
| 3′-GAC GTC GAC TAC GCG GCA TGC CTA GGG GCC CAT G-5′ | |
| OG | 5′-GAA CGA CTG T(OG)A CTT GAC TGC TAC TGA T-3′ |
| 3′-CTT GCT GAC A (C)T GAA CTG ACG ATG ACT A-5′ | |
| THF | 5′-AAT TCA CCG GTA CG(F) TGA ATT CG-3′ |
| 3′-TTA AGT GGC CAT GG(C) TCT TAA GC-5′ |
The important features are underlined. dsC = double-stranded control (no damage); ssC = single-stranded control; ssU = single-stranded, containing a uracil; dsU = double-stranded, containing a uracil; OG = double-stranded, containing an 8-oxodG; THF = double-stranded, containing a THF abasic site analog (F).
UDG activities were determined by incubation of 1 µg (mitochondria), 100 ng (nuclear extracts) or 200 ng (WCEs) protein with 90 fmol of 32P-end labeled uracil-containing oligonucleotide (ssU) (Table 1) for 30 min (mitochondria and WCEs) or 20–60 min (nuclear extracts) at 37°C, in a 10 µl reaction containing 70 mM HEPES–KOH (pH 7.5), 1 mM EDTA, 1 mM DTT, 75 mM NaCl, 0.05% BSA and 4 ng of recombinant endonuclease IV, to ensure complete cleavage of abasic sites. Reactions were terminated by addition of 20 µl formamide loading buffer (80% formamide, 10 mM EDTA, 1 mg/ml xylene cyanol FF, and 1 mg/ml Bromophenol Blue) and heating at 90°C for 10 min.
OGG1 activity was measured as incision of 8-oxo-deoxyguanine (8-oxodG) from a 28mer oligonucleotide essentially as in (14). Ten microliter reactions contained 40 mM HEPES (pH 7.6), 5 mM EDTA, 1 mM DTT, 75 mM KCl, 10% glycerol, 88.8 fmol of oligonucleotide and 10 µg mitochondrial or 3.5 µg WCE protein. Reactions were incubated for 4 h (mitochondria) or 2 h (WCEs) at 32°C then terminated by adding 5 µg of proteinase K (PNK) and 1 µl of 10% SDS and incubating at 55°C for 30 min. DNA was precipitated by addition of 1 µg glycogen, 4 µl of 11 M ammonium acetate, 60 µl of ethanol and overnight incubation at –20°C. Samples were centrifuged, dried and suspended in 10 µl of formamide loading dye.
AP endonuclease activities of mitochondria, nuclear or WCEs were determined by incubation of 500 ng (mitochondria), 50 ng (nuclear) or 10–100 ng (nuclear and WCEs) protein with 1 pmol of a 32P-end labeled tetrahydrofuran (THF)-containing double-stranded 26mer oligonucleotide (Table 1) for 10 min at 37°C, in a 10 µl reaction containing 50 mM HEPES–KOH (pH 7.5), 50 mM KCl, 100 µg/ml BSA, 10 mM MgCl2, 10% glycerol and 0.05% Triton X-100. Reactions were terminated by the addition of 10 µl formamide loading buffer and heating at 90°C for 10 min.
For all incision assays, substrate and products were resolved by electrophoresis at 15 W for 1 h 10 min on 20% polyacrylamide gels containing 7 M urea. Bands were visualized by Phosphor Imager and analyzed using ImageQuant™ (Molecular Dynamics). Incision activity was determined as the intensity of product bands relative to the combined intensities of substrate and product bands.
Repair synthesis of a uracil-containing double-stranded oligonucleotide was measured essentially as described (15). Reactions contained 10 µg of mitochondrial protein in 10 µl of reaction buffer (40 mM HEPES, 0.1 mM EDTA, 5 mM MgCl2, 0.2 mg/ml BSA, 50 mM KCl, 1 mM DTT, 40 mM phosphocreatine, 100 µg/ml phosphocreatine kinase, 2 mM ATP, 40 µM dNTPs, 4 µCi [α-32P]dCTP, 3% glycerol) and 120 ng of oligonucleotide substrate. Complementation of uracil-initiated BER assays was done as above, but 5 µg mitochondrial protein and addition of pure proteins as indicated. The polymerase γ gap-filling assay was also done using the above conditions, but with 0.5 or 2.0 µg mitochondrial protein. Uracil-initiated BER with the 34U oligonucleotide (Table 1) was assayed essentially as above, but with 2.5 µg mitochondrial protein, 10 µM dCTP and no other dNTPs. Reactions were incubated for 1 h at 37°C (2 h at 37°C for complementation and 34U repair experiments) and terminated by addition of 5 µg PNK and 1 µl of 10% SDS followed by incubation at 55°C for 30 min. DNA was precipitated as described above, suspended in 10 µl formamide loading dye, heated at 90°C for 10 min and electrophoresed and visualized as above. Uracil-initiated BER activities were quantified by comparing ρ0 repair product signal intensity with that of wt (100%).
Hydrogen peroxide exposure
wt and ρ0 cells were grown to 80–90% confluence. Medium was removed and replaced with the same medium without fetal bovine serum. H2O2 was added to 250 µM final concentration and cells were incubated at 37°C for 1 h. Following incubation, the medium was removed and cells were either washed in PBS and harvested or placed in new medium containing fetal bovine serum and incubated at 37°C for 6 h. These cells were then washed and harvested as above. Isolation of mitochondria or preparation of WCEs were done as described above.
RESULTS
Verification of ρ0 status and characterization of mitochondrial fractions
The absence of mtDNA in ρ0 cells was verified by PCR amplification of mtDNA- and nuclear DNA-encoded nucleotide sequences in wt and ρ0 143B TK– cells. The PCR products of nuclear-encoded polymerase γ were present in both wt and ρ0 cells, and the tRNA-leu product was present in wt cells, but absent in ρ0 cells (not shown), confirming the presence of nuclear DNA but absence of mtDNA in the ρ0 cells. mtTFA, which correlates strongly with mtDNA amount (5) and is used as a marker of mtDNA levels, was also absent in mitochondria isolated from ρ0 cells (Fig. 1a).
Figure 1.
Verification of the absence of mtDNA and purity of isolated mitochondrial fractions. (a) Western blot showing the absence of mtTFA in mitochondrial fractions of ρ0 cells. (b) Western blot for Lamin B, a marker of nuclear contamination, in wt nuclear pellets, and wt and ρ0 mitochondria.
In all mitochondrial preparations, contamination with nuclear proteins was assessed by western blot detection of Lamin B2, a protein that is abundant in the nucleus. Lamin B2 was readily detected in nuclear fractions, but absent in mitochondrial fractions loaded at identical levels (60 µg), indicating that mitochondria isolated from the wt and ρ0 cells contained no significant nuclear contamination (Fig. 1b). To assess the comparative enrichment of wt and ρ0 mitochondrial fractions for mitochondrial proteins (i.e. the proportional abundance of mitochondrial proteins versus cellular contaminants), we measured the activity of CS, a tricarboxylic acid cycle protein present in the mitochondrial matrix. The specific activity of CS was marginally higher in ρ0 mitochondrial fractions relative to wt (not shown), indicating that enrichment of the fraction for mitochondria was at least as great in ρ0 preparations as in wt. CS activity measured in WCEs was also similar in wt and ρ0 cells (not shown), which validates this use of CS activity to gauge mitochondrial enrichment.
Mitochondrial BER in wt and ρ0 cells
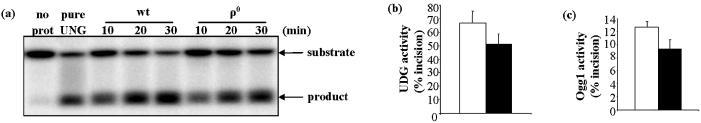
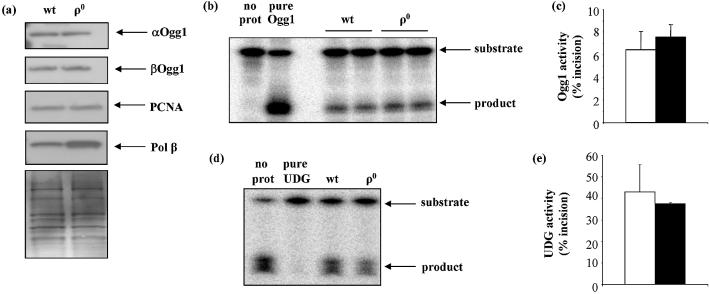
mtDNA repair protein activities were determined individually using in vitro assays in which mitochondrial lysates were incubated with oligonucleotide substrates containing specific lesions at defined positions (Table 1). Where possible, protein levels were also determined by western blot. All mtDNA repair proteins and activities examined were present in ρ0 cells, although generally at reduced levels (Figs 2 and 3). The magnitudes of differences in BER protein levels between wt and ρ0 were highly dependent upon the individual protein. DNA glycosylase activities (UDG, OGG1) were only slightly reduced in ρ0 mitochondria. UDG activity, measured as incision of ssU, was about 25% lower in ρ0 mitochondria relative to wt (Fig. 2a and b). OGG1 activity, measured as incision of an 8-oxodG-containing oligonucleotide (OG), was also about 25% lower in ρ0 mitochondria (Fig. 2c).
Figure 2.
mtDNA glycosylase activities were marginally lower in ρ0 cells. (a) Representative gel showing substrate and reaction products of UDG assay. (b) Mean UDG activity (% ssU incised) in wt and ρ0 mitochondria following 30 min incubation. Data are means ± S.D. of duplicate measurements on two different samples. (c) Mean OGG1 activity in wt and ρ0 mitochondria. Values are means of duplicate measurements on separately prepared samples. In all graphs, open bars = wt, filled bars = ρ0.
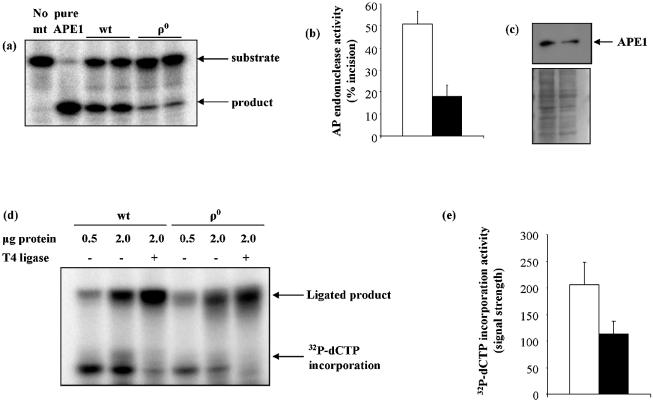
Figure 3.
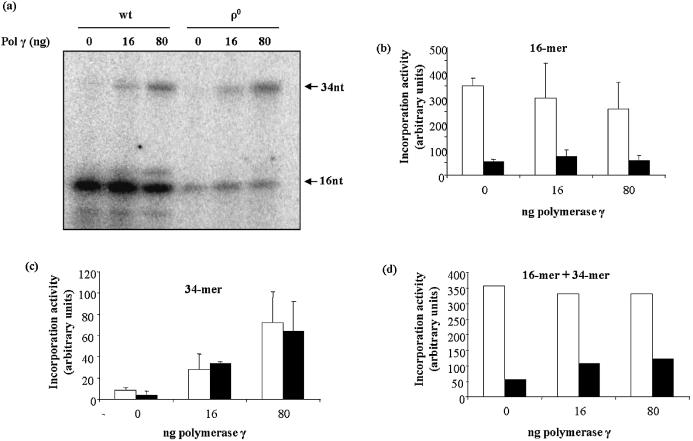
AP endonuclease and polymerase γ gap-filling activities were reduced in ρ0 mitochondria. (a) Representative gel showing results of duplicate assays of THF incision (AP endonuclease activity). (b) Mean AP endonuclease activity in wt and ρ0 mitochondria. Values are means ± S.D. of duplicate measurements made on each of two independently prepared samples. (c) Western blot showing APE1 protein levels in wt and ρ0 mitochondria. Lower panel is Amido Black stained membrane. (d) Representative gel from polymerase γ gap-filling assay, showing products of [32P]dCTP incorporation into the GAP oligonucleotide (Table 1). The gel shows the products of incorporation and incorporation plus ligation following incubation with 0.5 or 2 µg of mitochondrial lysate. In lanes 3 and 6, T4 DNA ligase has been added to ensure full ligation of the incorporation intermediate. The graph in (e) compares mean band intensities determined as in lanes 3 and 6. Values are means ± S.D. of two measurements. For both graphs, open bars = wt, filled bars = ρ0.
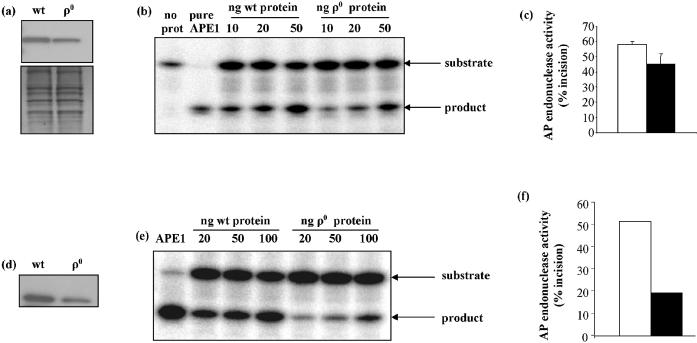
In contrast, mitochondrial AP endonuclease activity and APE1 protein levels were much more affected by the absence of mtDNA. Mitochondrial AP endonuclease activity was assayed as incision of an oligonucleotide containing a THF abasic site analog (Table 1). Figure 3a shows a representative gel with the migration of substrates and products as indicated by the arrows. Incision activity in ρ0 mitochondria was approximately 65% lower than in wt (Fig. 3b). Though it has not been clearly established that APE1 is the major mitochondrial AP endonuclease, a reduction in APE1 protein levels was seen in ρ0 mitochondria (Fig. 3c), indicating good agreement between AP endonuclease activity and APE1 protein.
mtDNA polymerase γ, as the only mtDNA polymerase in human cells, is an essential component of the BER pathway. Polymerase γ gap-filling activity was measured using an assay that monitored [32P]dCTP incorporation into an oligonucleotide containing a single nucleotide gap with processed 5′ and 3′ termini (GAP oligo, Table 1). To ensure that a possible deficiency in mtDNA ligase activity would not limit the nucleotide incorporation activity of polymerase γ, exogenous pure T4 DNA ligase was added as indicated. Figure 3d shows a representative gel in which the 21mer unligated intermediate and the ligated full-length 39mer can be discerned. Polymerase γ activity was quantified both by measuring signal intensity of the 21mer and 39mer (–T4 ligase), and by measuring only the signal intensity of the 39mer (+T4 ligase). The graph in Figure 3e shows the mean signal intensities using the latter method. Polymerase γ activity was approximately 45% lower in ρ0 mitochondria, compared to wt. Comparison of the sum of 21mer and 39mer bands between wt and ρ0 mitochondria produced a similar result (not shown).
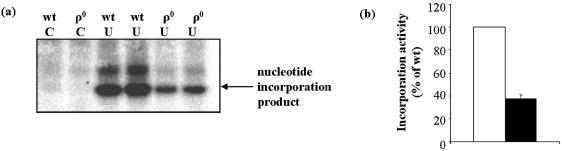
The mitochondrial BER pathway requires the coordinated activities of at least four proteins, a glycosylase, an AP endonuclease, polymerase γ and DNA ligase III (16). We assessed the overall activity of the BER pathway in mitochondrial lysates using a uracil-initiated BER assay where [32P]dCTP incorporation into a double-stranded oligonucleotide containing a single uracil at position 12 in the sense strand (dsU; Table 1) was monitored. In this assay, following uracil removal, processing of the abasic site and [32P]dCTP incorporation, ligation of the incised strand occurs at a relatively slow rate in vitro, with almost all [32P]dCTP signal being detected in the 12mer polymerase product, and virtually no detectable ligated 30mer. The representative gel in Figure 4a shows the 12-nucleotide intermediate of [32P]dCTP incorporation without ligation. Comparisons of nucleotide incorporation were made by quantifying the [32P]dCTP signal in the 12mer. The uracil-initiated BER activity of ρ0 mitochondria was approximately 60% less than that of wt (Fig. 4b).
Figure 4.
Uracil-initiated BER activity was reduced in ρ0 mitochondria. (a) Representative gel of BER incorporation activity, showing the product of [32P]dCTP incorporation associated with uracil-initiated repair synthesis. (b) Mean levels of [32P]dCTP containing product, expressed as a percentage of wt levels. Open bars = wt; filled bars = ρ0. Values are means ± S.D. of duplicate measurements on each of two individually prepared samples.
Identification of sites of BER activity reduction in ρ0 mitochondria
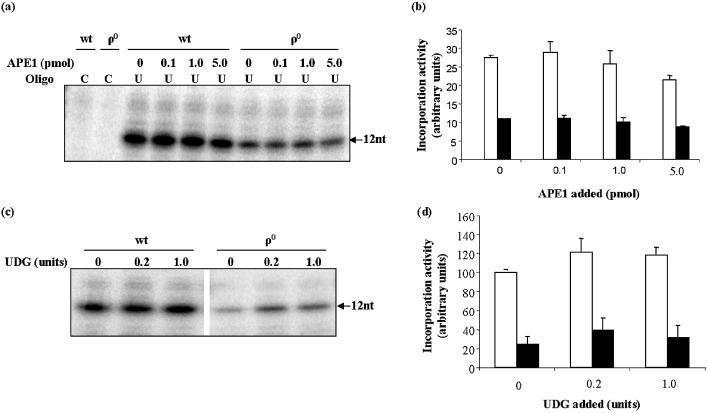
The activities of AP endonuclease and uracil-initiated BER were reduced by similar amounts in ρ0 mitochondria relative to wt, which suggested that AP endonuclease may play a central role in reducing BER flux. This hypothesis was tested by complementing ρ0 uracil BER assays with exogenous recombinant APE1. If AP endonuclease catalyzed the rate-limiting step in mitochondrial BER, restoration of this activity by addition of pure APE1 should have restored wt BER activity. However, additions of 0.1–5.0 pmol APE1 failed to stimulate uracil-initiated BER activity in ρ0 mitochondria (Fig. 5), though the protein was highly active in this range in other assays (for example, see Fig. 3). Thus, reduced AP endonuclease activity did not appear to limit BER pathway activity in ρ0 mitochondria.
Figure 5.
Complementation with UDG or APE1 does not recover uracil-initiated BER activity in ρ0 mitochondria to wt levels. Pure UDG or APE1 were added to the uracil-initiated BER assay in the amounts indicated. (a) Representative gel showing the effect of assay complementation with APE1 on levels of 16 nucleotide (16nt) incorporation product. The amount of fully ligated product under these conditions was negligible and is not shown. (b) Mean repair incorporation activities in wt and ρ0 mitochondria incubated in the presence of increasing amounts of APE1. (c) Representative gel showing the 12 nucleotide (12nt) product of [32P]dCTP incorporation in assays with wt and ρ0 mitochondria complemented with pure UDG. (d) Mean uracil-initiated BER activities with or without UDG. All values are means ± S.D. of two measurements. Open bars = wt; filled bars = ρ0.
We used a similar approach to assess other possible site(s) of BER pathway flux reduction in ρ0 mitochondria. Supplementation of the uracil-initiated BER assay with 0.2 or 1.0 U of pure UDG stimulated BER activity, though only by approximately 20%, and in both wt and ρ0 mitochondria (Fig. 5). However, uracil-initiated BER activity of ρ0 mitochondria remained well below wt levels. When pure polymerase γ catalytic subunit was added to the BER assay, we observed considerable extension activity, in both wt and ρ0 mitochondria, complicating the interpretation of results. We therefore used a different uracil-containing oligonucleotide (34U; Table 1), and eliminated dNTPs other than dCTP from this assay. Complementation with polymerase γ increased the amount of full-length product in both wt and ρ0 mitochondria (Fig. 6). Indeed, the amounts of ligated product were approximately equal in wt and ρ0 mitochondria. However, the levels of 16mer, representing incorporation without ligation, remained much lower in ρ0 mitochondria, relative to wt. Quantification of both bands in wt and ρ0 mitochondria showed that polymerase γ complementation did stimulate uracil-initiated BER activity in ρ0 mitochondria, by about 100%, though this activity still remained well below wt levels.
Figure 6.
Complementation with polymerase γ catalytic subunit increased production of ligated product, but not incorporation intermediate. (a) Representative gel showing 16- and 34-nucleotide products of [32P]dCTP incorporation and incorporation plus ligation. Assays were complemented with the amounts of polymerase γ catalytic subunit shown. Mean band intensities for (b) 16mer incorporation product, (c) 34mer ligation product and (d) total 16mer and 34mer signals. Values are means ± S.D. of two measurements. Open bars = wt; filled bars = ρ0.
Finally, we compared uracil-initiated BER activity in wt and ρ0 mitochondria supplemented with UDG, APE1 and polymerase γ catalytic subunit. Even with this simultaneous complementation of the first three steps in uracil-initiated BER, ρ0 activity remained lower than wt (data not shown).
Nuclear BER in wt and ρ0 cells
To confirm that differences in ρ0 and wt mitochondrial BER were not simply due to general perturbations of nuclear BER in ρ0 cells, we measured BER activities in WCEs. Mitochondrial volume density of cultured cells is very low (typically ∼3–5%), and mitochondria therefore make a negligible contribution to measurements of BER protein activities made in whole cells. We found that whole cell DNA glycosylase (OGG1 and UDG) activities were largely unaffected by the absence of mtDNA. OGG1 incision activity was approximately the same in wt and ρ0 cells, and UDG activity was only 13% lower (Fig. 7). Similarly, OGG1 protein levels, measured by immunoblot of αOGG1 and βOGG1 proteins did not appear to be different in WCEs of wt versus ρ0 cells (Fig. 7a). The levels of other BER proteins, PCNA and polymerase β were also not lower in ρ0, relative to wt, cells. Indeed, DNA polymerase β levels appeared to be slightly elevated in ρ0 cells. Thus, there was no evidence of a general impairment of DNA repair in the ρ0 cells.
Figure 7.
DNA glycosylase levels and activities in WCEs of wt and ρ0 cells are similar. (a) Western blot showing αOGG1, βOGG1, PCNA and polymerase β levels in WCEs. (b) Representative gel showing substrates and products of OGG1 assay. (c) Mean OGG1 activity in wt and ρ0 WCEs. (d) Representative gel showing substrates and products of UDG assay. (e) Mean UDG activity in wt and ρ0 WCEs. Values are means ± S.D. of duplicate measurements on each of two samples. Open bars = wt; filled bars = ρ0.
In contrast, AP endonuclease protein levels and activities were significantly lower in ρ0 cells. APE1 protein levels were visibly decreased, as determined by western blot (Fig. 8a), and AP endonuclease activity was approximately 25% (data taken at 30 min incubation time) lower (Fig. 8b and c). Interestingly, a more pronounced decrease in APE1 protein and AP endonuclease activities was found in nuclear extracts from ρ0 cells, in which the reduction of AP endonuclease activity was about 65% (Fig. 8d–f).
Figure 8.
AP endonuclease levels and activities are reduced in WCEs and nuclear extracts of ρ0 cells. (a) Western blot of APE1. Bottom panel shows Amido Black stained membrane. (b) Representative gel showing substrates and products of AP endonuclease assay, following incubation with 10, 20 or 50 ng WCE protein. (c) Mean AP endonuclease activities in 50 ng WCEs. Values are means of duplicate measurements made on each of two samples. (d) Western blot of APE1 in wt and ρ0 nuclear extracts. (e) Representative gel of AP endonuclease assay in nuclear extracts. (f) Mean AP endonuclease activity in 100 ng of wt and ρ0 nuclear extracts. Open bars = wt, filled bars = ρ0.
Effects of oxidative stress on mitochondrial BER in ρ0 cells
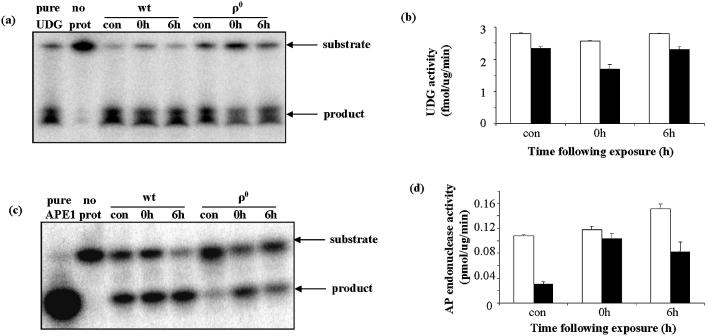
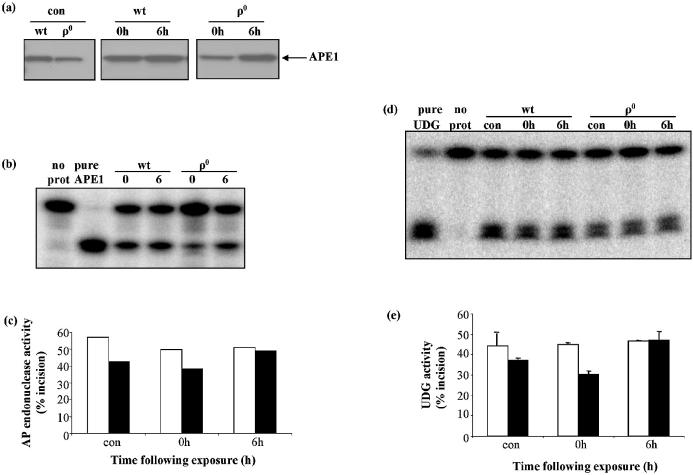
Some mtDNA repair protein activities were generally reduced in ρ0 WCEs, but their presence indicates that they continue to be synthesized and translocated into the mitochondrial compartment in the absence of mtDNA. This provides an opportunity to investigate the nature of BER protein regulation by oxidative stress. Specifically, APE1 has been reported to translocate to mitochondria in human cells after oxidative stress (17). We investigated whether this could be elicited in the absence of mtDNA, directly by reactive oxygen species (ROS), or whether mtDNA damage was necessary for the response. We addressed this issue by focusing on the mitochondrial activities of two proteins: APE1, which translocates to mitochondria following hydrogen peroxide exposure (17), and UDG, for which there is no evidence of an oxidative stress response. Cells were exposed to 250 µM H2O2 for 1 h, then either harvested immediately or allowed to recover in complete medium for 6 h before harvesting. Control cells were handled identically, but with no H2O2 treatment. Mitochondrial UDG activity showed an initial decline following H2O2 treatment in both wt and ρ0 cells (Fig. 9a and b). At 6 h post-exposure, UDG activity in wt had recovered to control levels, but remained lower in ρ0 mitochondria. On the other hand, H2O2 induced a substantial increase in mitochondrial AP endonuclease activity in both wt and ρ0 cells (Fig. 9c and d). At 6 h post-exposure, mitochondrial AP endonuclease activity in wt cells was approximately 40% higher than in controls. AP endonuclease activity in ρ0 mitochondria increased 3-fold at 0 h post-exposure, when ρ0 AP endonuclease activity was approximately equal to that of wt. However, at 6 h post-exposure, ρ0 AP endonuclease activity had declined and was approximately 50% lower than in wt mitochondria.
Figure 9.
Effect of H2O2 exposure on mitochondrial UDG and AP endonuclease. Measurements were made with wt and ρ0 mitochondria isolated immediately following (0 h) or 6 h following (6 h) exposure. (a) Representative gel showing substrates and products of UDG assay. (b) Mean UDG activities in wt (open bars) and ρ0 (filled bars) mitochondria. Values are means of two measurements. (c) Representative gel showing substrates and products of AP endonuclease assay. (d) Mean AP endonuclease activities in wt (open bars) and ρ0 (filled bars) mitochondria. Values are means of two measurements.
Effects of oxidative stress on BER in ρ0 WCEs
The effect of H2O2 exposure on BER proteins was examined in WCEs under the same experimental conditions. Whole cell levels of APE1 in ρ0 cells were lower in control (untreated) cells and at 0 h following H2O2 treatment (Fig. 10a). However, at 6 h following exposure, APE1 protein in ρ0 cells was approximately equal to wt levels. This was paralleled by an increase to wt levels of AP endonuclease activity in ρ0 cells at 6 h post-exposure (Fig. 10b and c). Interestingly, UDG activity was also slightly increased in ρ0 cells by H2O2 treatment (Fig. 10d and e).
Figure 10.
Induction of AP endonuclease activity and APE1 protein levels in ρ0 cells by H2O2. Measurements were made with wt and ρ0 WCEs prepared immediately following (0 h) or 6 h following (6 h) H2O2 exposure. (a) Western blot showing APE1 protein levels. (b) Representative gel showing substrates and products of AP endonuclease assay. (c) Mean AP endonuclease activities in wt and ρ0 WCEs. Values are means of three measurements. (d) Representative gel showing substrates and products of UDG assay. (e) Mean UDG activities in wt and ρ0 WCEs. Controls (con) were not exposed to H2O2. Values are means of duplicate measurements on each of two samples. Open bars = wt; filled bars = ρ0.
DISCUSSION
The continued localization of DNA BER proteins to the mitochondria of cells lacking mtDNA indicates that the signals involved in maintaining basal constitutive levels of some of these proteins are independent of actual mtDNA. In this respect, the regulation of mtDNA BER proteins differs from that of some other proteins involved in mtDNA transactions. The expression and/or protein levels of mtTFA (4,5), mtRNA polymerase (5) and mitochondrial single-stranded DNA binding protein (mtSSB) (18) are all tightly regulated with respect to mtDNA levels. Indeed, in the absence of mtDNA, we were unable to detect mtTFA in mitochondria of ρ0 cells by western blot.
mtDNA glycosylase activities were particularly insensitive to the absence of mtDNA, with UDG and OGG1 activities only about 25% lower in the mitochondria of ρ0 cells, relative to wt. This apparent unresponsiveness to mtDNA status is consistent with the regulatory features of the OGG1 gene. The hOGG1 gene promoter lacks TATA or CAAT boxes, consistent with a housekeeping function (19). Indeed, OGG1 expression and enzyme activity are invariant during the cell cycle and not altered in response to oxidative stress (20). Mitochondrial UDG, on the other hand, has a dedicated promoter distinct from that used to express the nuclear isoform and the expression of both UDG isoforms is cell-cycle regulated, and highest in S-phase (21). It is therefore possible that the lower mitochondrial UDG activity of ρ0 cells is related to their slower replication rate. Nonetheless, taken together, our results suggest that both DNA glycosylase activities in mitochondria are expressed largely constitutively and not tightly regulated with respect to either mtDNA amount or oxidative stress.
In contrast to the DNA glycosylase activities, other steps in the mitochondrial BER pathway were more significantly altered in the absence of mtDNA. The most substantial reductions observed were in AP endonuclease activity, APE1 protein levels and polymerase γ activity in mitochondria of ρ0 cells. This latter observation is particularly interesting in light of reports that polymerase γ protein levels are unchanged in ρ0 relative to wt mitochondria (4), and suggests a lower specific activity of polymerase γ in ρ0 mitochondria. This result may indicate that polymerase γ gap-filling activity is not regulated exclusively by the amount of the catalytic subunit. Indeed, other factors including the polymerase γ accessory subunit affect polymerase γ’s interaction with oligonucleotides (22,23). It is possible that, though the catalytic subunit is apparently present in ρ0 mitochondria (24), it is in a non-optimal conformation that decreases its specific activity. The reason for this apparent discordance between polymerase γ protein levels (4) and its measured gap-filling activity is not known, but may be interesting to investigate further.
While overall BER activity was substantially lower in ρ0 mitochondria relative to wt, some individual activities were little affected. To identify those activities which contributed most significantly to the reduced BER pathway activity, we supplemented the in vitro BER assay with exogenous proteins. While supplementation with UDG or APE1 elicited marginal or no increases in BER activity, addition of the polymerase γ catalytic subunit stimulated activity appreciably. Importantly, however, the amount of the fully ligated product, but not the 16 nucleotide incorporation product, was increased. This interesting result suggests that an activity associated with polymerase γ limits the rate of the ligation step both in wt and ρ0 mitochondria. It is likely that the dRp lyase activity of polymerase γ, which immediately succeeds nucleotide incorporation and has a notably low kcat (25), is a limiting step in mitochondrial BER. The kcat for polymerase γ dRp lyase is approximately 40-fold lower than that of DNA polymerase β, which is similarly thought to be rate-limiting in nuclear BER (26). The fact that the amount of full-length, ligated product was equal in polymerase γ-complemented wt and ρ0 mitochondria further suggests that, under these conditions, DNA ligase activity becomes limiting and that the ligase activity is approximately equal in wt and ρ0 mitochondria. Taken together, these data suggest a rate-limiting role for the polymerase γ dRp lyase step in mitochondrial BER. These results support the hypothesis outlined above, that despite sufficient levels of the polymerase γ catalytic subunit, its gap-filling activity in BER is reduced. Even a large excess of the catalytic subunit was unable to stimulate wt levels of BER activity.
Large differences in APE1 protein levels and activities were found between wt and ρ0 cells, both in mitochondrial and in nuclear compartments. Our data suggest that low endogenous ROS production was the underlying cause. Regulation of APE1 by oxidative stress is well documented (27), and ROS affects both cellular APE1 protein levels and translocation of APE1 to the nucleus (28). ρ0 cells, which lack mitochondrial respiration, the major endogenous source of ROS, have reduced levels of endogenous ROS (29–31). Here we have shown that they also have lower cellular, nuclear and mitochondrial levels of APE1. When we introduced oxidative stress to ρ0 cells, APE1 levels and AP endonuclease activity increased rapidly (within 6 h) to wt levels, indicating that ρ0 cells retain the ability to modulate APE1 in response to oxidative stress. While we did not study cellular APE1 regulation exhaustively in the present work, our data do suggest that ρ0 cells may be a useful model with which to further investigate interactions between ROS and APE1.
AP endonuclease activity was the single most altered BER activity in the mitochondria of ρ0 cells. APE1 protein levels appeared to be concomitantly reduced. However, it remains unknown whether APE1 is the major mitochondrial AP endonuclease, although our data favors this idea. Nonetheless, we used measurements of AP endonuclease activity to investigate ROS affects on mitochondrial AP endonuclease. H2O2 treatment increased mitochondrial AP endonuclease activity in wt cells by 40% relative to untreated cells, at 6 h following hydrogen peroxide treatment. This result is similar to that of Frossi et al. (17), who demonstrated ROS-induced translocation of APE1 into mitochondria in a human B lymphocyte cell line. Taken together, the data suggest that ROS-induced regulation of mitochondrial AP endonuclease is a general feature of mitochondrial BER in human cells. The significance of this phenomenon, especially given the failure of exogenous APE1 to stimulate uracil-initiated mitochondrial BER in vitro, is unclear.
In summary, mtDNA glycosylase activities were relatively insensitive to the complete absence of mtDNA, consistent with a constitutive and not tightly regulated expression of these proteins in mitochondria. Nonetheless, mitochondrial BER activity was substantially reduced in ρ0 cells, although the reasons for this reduction are not completely clear. Moreover, while the addition of polymerase γ catalytic subunit, and to a lesser extent UDG, stimulated BER activity, net BER activity in ρ0 mitochondria could not be increased to wt levels. ρ0 cells also had dramatically lower AP endonuclease protein levels and activities than wt cells, and our data are consistent with the interpretation that this phenomenon is due to lower endogenous oxidative stress in the absence of mitochondrial respiration in ρ0 cells. The ρ0 cells appear to be an interesting and useful model for identifying details of how mitochondrial respiration and mtDNA affect both mitochondrial and nuclear DNA damage and repair.
Acknowledgments
ACKNOWLEDGEMENTS
The authors thank G. Attardi, for providing ρ0 cells and Jingping Hu and Syed Imam for critical review of the manuscript.
REFERENCES
- 1.Bohr V.A. (2002) Repair of oxidative DNA damage in nuclear and mitochondrial DNA and some changes with aging in mammalian cells. Free Radic. Biol. Med., 32, 804–812. [DOI] [PubMed] [Google Scholar]
- 2.Wallace D.C. (2002) Animal models for mitochondrial disease. In Copeland,W.C. (ed.), Mitochondrial DNA: Methods and Protocols. Humana Press, Totowa, NJ, Vol. 197, pp. 3–54. [DOI] [PubMed] [Google Scholar]
- 3.Attardi G., Yoneda,M. and Chomyn,A. (1995) Complementation and segregation behavior of disease-causing mitochondrial-DNA mutations in cellular-model systems. Biochim. Biophys. Acta, 1271, 241–248. [DOI] [PubMed] [Google Scholar]
- 4.Davis A.F., Ropp,P.A., Clayton,D.A. and Copeland,W.C. (1996) Mitochondrial DNA polymerase gamma is expressed and translated in the absence of mitochondrial DNA maintenance and replication. Nucleic Acids Res., 24, 2753–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seidel-Rogol B.L. and Shadel,G.S. (2002) Modulation of mitochondrial transcription in response to mtDNA depletion and repletion in HeLa cells. Nucleic Acids Res., 30, 1929–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King M.P. and Attardi,G. (1989) Human cells lacking mtDNA — repopulation with exogenous mitochondria by complementation. Science, 246, 500–503. [DOI] [PubMed] [Google Scholar]
- 7.Appleby R.D., Porteous,W.K., Hughes,G., James,A.M., Shannon,D., Wei,Y.H. and Murphy,M.P. (1999) Quantitation and origin of the mitochondrial membrane potential in human cells lacking mitochondrial DNA. Eur. J. Biochem., 262, 108–116. [DOI] [PubMed] [Google Scholar]
- 8.Buchet K. and Godinot,C. (1998) Functional F1-ATPase essential in maintaining growth and membrane potential of human mitochondrial DNA-depleted rho0 cells. J. Biol. Chem., 273, 22983–22989. [DOI] [PubMed] [Google Scholar]
- 9.Chandel N.S. and Schumacker,P.T. (1999) Cells depleted of mitochondrial DNA (rho(0)) yield insight into physiological mechanisms. FEBS Lett., 454, 173–176. [DOI] [PubMed] [Google Scholar]
- 10.King M.P. and Attardi,G. (1996) Isolation of human cell lines lacking mitochondrial DNA. Methods Enzymol., 264, 304–313. [DOI] [PubMed] [Google Scholar]
- 11.Erzberger J.P., Barsky,D., Scharer,O.D., Colvin,M.E. and Wilson,D.M. (1998) Elements in abasic site recognition by the major human and Escherichia coli apurinic/apyrimidinic endonucleases. Nucleic Acids Res., 26, 2771–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longley M.J., Ropp,P.A., Lim,S.E. and Copeland,W.C. (1998) Characterization of the native and recombinant catalytic subunit of human AND polymerase gamma: identification of residues critical for exonuclease activity and dideoxynucleotide sensitivity. Biochemistry, 37, 10529–10539. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka M., Lai,J.S. and Herr,W. (1992) Promoter-selective activation domains in Oct-1 and Oct-2 direct differential activation of an snRNA and messenger-RNA promoter. Cell, 68, 755–767. [DOI] [PubMed] [Google Scholar]
- 14.Karahalil B., Hogue,B.A., Souza-Pinto,N.C. and Bohr,V.A. (2002) Base excision repair capacity in mitochondria and nuclei: tissue-specific variations. FASEB J., 16, 1895–1902. [DOI] [PubMed] [Google Scholar]
- 15.Nyaga S.G. and Bohr,V.A. (2002) Characterization of specialized mtDNA glycosylases. In Copeland,W.C. (ed.), Mitochondrial DNA: Methods and Protocols. Humana Press, Totowa, NJ, Vol. 197, pp. 227–244. [DOI] [PubMed] [Google Scholar]
- 16.Lakshmipathy U. and Campbell,C. (2001) Antisense-mediated decrease in DNA ligase III expression results in reduced mitochondrial DNA integrity. Nucleic Acids Res., 29, 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frossi B., Tell,G., Spessotto,P., Colombatti,A., Vitale,G. and Pucillo,C. (2002) H2O2 induces translocation of APE/Ref-1 to mitochondria in the Raji B-cell line. J. Cell. Physiol., 193, 180–186. [DOI] [PubMed] [Google Scholar]
- 18.Schultz R.A., Swoap,S.J., McDaniel,L.D., Zhang,B.Q., Koon,E.C., Garry,D.J., Li,K. and Williams,R.S. (1998) Differential expression of mitochondrial DNA replication factors in mammalian tissues. J. Biol. Chem., 273, 3447–3451. [DOI] [PubMed] [Google Scholar]
- 19.Dhenaut A., Boiteux,S. and Radicella,J.P. (2000) Characterization of the hOGG1 promoter and its expression during the cell cycle. Mutat. Res., 461, 109–118. [DOI] [PubMed] [Google Scholar]
- 20.Mistry P. and Herbert,K.E. (2003) Modulation of hOgg1 DNA repair enzyme in human cultured cells in response to pre-oxidant and antioxidant challenge. Free Radic. Biol. Med., 35, 397–405. [DOI] [PubMed] [Google Scholar]
- 21.Haug T., Skorpen,F., Aas,P.A., Malm,V., Skjelbred,C. and Krokan,H.E. (1998) Regulation of expression of nuclear and mitochondrial forms of human uracil-DNA glycosylase. Nucleic Acids Res., 26, 1449–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim S.E., Longley,M.J. and Copeland,W.C. (1999) The mitochondrial p55 accessory subunit of human DNA polymerase gamma enhances DNA binding, promotes processive DNA synthesis and confers N-ethylmaleimide resistance. J. Biol. Chem., 274, 38197–38203. [DOI] [PubMed] [Google Scholar]
- 23.Carrodeguas J.A., Pinz,K.G. and Bogenhagen,D.F. (2002) DNA binding properties of human pol gamma B. J. Biol. Chem., 277, 50008–50014. [DOI] [PubMed] [Google Scholar]
- 24.Garrido N., Griparic,L., Jokitalo,E., Wartiovaara,J., van der Bliek,A.M. and Spelbrink,J.N. (2003) Composition and dynamics of human mitochondrial nucleoids. Mol. Biol. Cell, 14, 1583–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longley M.J., Prasad,R., Srivastava,D.K., Wilson,S.H. and Copeland,W.C. (1998) Identification of 5′-deoxyribose phosphate lyase activity in human DNA polymerase gamma and its role in mitochondrial base excision repair in vitro. Proc. Natl Acad. Sci. USA, 95, 12244–12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sokhansanj B.A., Rodrigue,G.R., Fitch,J.P. and Wilson,D.M. (2002) A quantitative model of human DNA base excision repair. I. Mechanistic insights. Nucleic Acids Res., 30, 1817–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans A.R., Limp-Foster,M. and Kelley,M.R. (2000) Going APE over ref-1. Mutat. Res., 461, 83–108. [DOI] [PubMed] [Google Scholar]
- 28.Ramana C.V., Boldogh,I., Izumi,T. and Mitra,S. (1998) Activation of apurinic/apyrimidinic endonuclease in human cells by reactive oxygen species and its correlation with their adaptive response to genotoxicity of free radicals. Proc. Natl Acad. Sci. USA, 95, 5061–5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaux E.C., Metzen,E., Yeates,K.M. and Ratcliffe,P.J. (2001) Regulation of hypoxia-inducible factor is preserved in the absence of a functioning mitochondrial respiratory chain. Blood, 98, 296–302. [DOI] [PubMed] [Google Scholar]
- 30.Budinger G.R.S., Tso,M., McClintock,D.S., Dean,D.A., Sznajder,J.I. and Chandel,N.S. (2002) Hyperoxia-induced apoptosis does not require mitochondrial reactive oxygen species and is regulated by Bcl-2 proteins. J. Biol. Chem., 277, 15654–15660. [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen A.K., Chatterjee,A., Rasmussen,L.J. and Singh,K.K. (2003) Mitochondria-mediated nuclear mutator phenotype in Saccharomyces cerevisiae. Nucleic Acids Res., 31, 3909–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]