Abstract
Objectives
Non-cardiovascular comorbidity is common in cardiovascular disease (CVD) populations but its influence on chest pain (CP) and shortness of breath (SOB) symptom-specific physical limitations is unknown. We wanted to test the a priori hypothesis that an unrelated comorbidity would influence symptom-specific physical limitations and to investigate this impact in different severities of CVD.
Method and results
The study was based on 5426 patients from ten family practices, organised into eight a priori exclusive severity groups: (i) no CVD or osteoarthritis (OA) (reference), (ii) index hypertension, ischaemic heart disease (IHD) and heart failure (HF) without OA, (iii) index OA without CVD and (iv) same CVD groups with comorbid OA. The measure of CP physical limitations was Seattle Angina Questionnaire and for SOB physical limitations was the Kansas City Cardiomyopathy Questionnaire. Adjusted baseline associations between the cohorts and symptom-specific physical limitations were assessed using linear regression methods. In the study population, 1443 (27%) reported CP and 2097 (39%) SOB. CP and SOB physical limitations increased with CVD severity in the index and comorbid groups. Compared with the respective index CVD group, the CP physical limitation scores for comorbid CVD groups with OA were lower by: − 14.7 (95% CI − 21.5, 7.8) for hypertension, − 5.5 (− 10.4, − 0.7) for IHD and − 22.1 (− 31.0, − 6.7) for HF. For SOB physical limitations, comorbid scores were lower by: − 9.2 (− 13.8, − 4.6) for hypertension, − 6.4 (− 11.1, − 1.8) for IHD and − 8.8 (− 19.3, 1.65) for HF.
Conclusions
CP and SOB are common symptoms, and OA increases the CVD symptom-specific physical limitations additively. Comorbidity interventions need to be developed for CVD specific health outcomes.
Abbreviations: CVD, cardiovascular disease; OA, osteoarthritis; IHD, ischaemic heart disease; HF, heart failure; CP, chest pain; SOB, shortness of breath; PL, physical limitation; IMD, Index Multiple Deprivation; HAD, hospital anxiety and depression
Keywords: Cardiovascular diseases, Comorbidity, Osteoarthritis, Health status
Highlights
-
•
The study was based on 5426 patients from 10 general practices in the UK.
-
•
A priori exclusive groups included index CVD severity groups with and without OA.
-
•
Increasing CVD severity and comorbid OA are associated with CVD symptom limitation.
-
•
Comorbid OA in CVD groups increases symptom physical limitations additively.
-
•
Comorbidity interventions need to be developed for CVD specific health outcomes.
1. Introduction
Cardiovascular diseases (CVD) and osteoarthritis (OA) often co-occur [1] and individually impact adversely on disease-specific symptoms and poor health [2,3]. Yet, their common co-occurrence suggests that there might be shared causal links [4–6], as well as shared consequences in relation to overall health [7,8]. Individual studies, for example of CVD or OA, tend to focus on specific outcomes, which often use a main symptom characteristic as a rationale for distinguishing from broader limitations of disease and health. In ischaemic heart disease, the symptom focus is localised to chest pain as a presenting feature of angina [9], whilst in heart failure, the symptom focus is on shortness of breath limitations [10]. Yet, pain is also a focus for osteoarthritis, but localised to the joint [11], and shortness of breath is a feature of activity limitation in the older population often associated with the comorbid presence of osteoarthritis [12].
Evidence for shared symptoms has shown that generalised pain may influence cardiovascular chest-specific pain [13] and obesity is a shared factor for limitations of cardiovascular or osteoarthritis-related activity [14,15]. Other studies link interventions for understanding shared consequences, such as the use of anti-inflammatory drugs in osteoarthritis in the management of patients with CVD [16]. Such in-direct evidence on comorbid links raises the specific hypothesis, as to whether and how comorbidity influences disease specific outcomes, but here the evidence is limited.
The stage of a disease provides a mechanism for identifying disease severity and possible outcomes. In HF, for example, the stage of disease as recognized by increasing symptoms, has been associated with poor quality of life, increased hospital admissions and mortality [17], and in OA, the severity of disease has been associated with increased mortality risk [18]. CVD represents a biological spectrum of linked conditions, from hypertension to end-stage HF and the individual diseases that develop as CVD progresses can provide a notion of disease spectrum severity which our previous work has shown associated with physical limitations [19]. What is less well known is how individual diseases within the CVD spectrum are influenced by the comorbid addition of another chronic disease such as OA.
In CVD, the comorbid addition of another chronic disease may impact on symptoms differently. ‘Biological interaction’ between two diseases which is measured by comparing their combined effects on a specific outcome with the sum of their independent effects is an approach which has the potential for understanding about how one disease impacts on another [23]. In this study we focus on biological interaction, using an a priori epidemiological design, on disease-specific outcomes which are an important consideration for clinical management and treatment decisions.
In UK family practice, through 95% population registration, surveys act as a population-level measure of symptoms and linked computer clinical records act as an epidemiological measure of the development of chronic disease [24]. We designed a cohort study, to test two specific hypotheses: (i) increasing CVD severity and comorbid OA is associated with increasing chest pain and shortness of breath physical limitations and (ii) CVD and OA comorbidity increase symptom specific physical limitations additively when compared to index groups.
2. Methods
2.1. Study population
Patients aged 40 years and over were recruited from 10 family practices in England, which routinely record morbidity in computer consultations using standard diagnostic and drug classifications. As a result of national pay-for-performance policies, high quality computer disease registers particularly cardiovascular diseases have become an established part of routine clinical practice [25].
2.2. Study design
The Comorbidity Cohort (2C) study was designed to investigate the interaction between CVD and OA, using clinical-survey linkage methods [26]. This study had a denominator population registered with ten family practices, which links recorded clinical data for specified CVD cohorts over a 5 year period to investigate healthcare outcomes and a sub-cohort who took part in a survey to provide linkage with patient reported health outcomes. In this study, the 2C survey population was used to investigate the association between CVD and OA comorbidity in relation to CVD-specific symptoms, which were measured using a postal questionnaire to obtain information on general health, pain and specific measures of CVD. Ethical approval for the 2C study was granted by a Research Ethics Committee (reference number: 09/H1017/40).
2.3. Study groups
The study groups were sampled from family practice computerised clinical records covering a three year time-period (November 2006–January 2010). In UK family practice, Read codes [27] as a standard classification are used to classify the morbidity of patients when they present in consultation or when clinical data is coded, and CVD registers based on these Read codes were identified in the three years before the baseline survey. In the same 3-year time period before the baseline survey, patients with OA diagnosis in their clinical records were also identified. Using the presence or absence of CVD or OA diagnosis, a total of 8 exclusive groups were constructed: (i) a random reference group of patients without CVD or OA, (ii) three index CVD groups without OA (hypertension, IHD, HF), (iii) a random index OA group without CVD, and (iv) three CVD groups with comorbid OA.
2.4. Study definitions
Three CVD definitions were chosen to reflect a spectrum of population severity [19]. Exclusive a priori ordering of CVD categories ranged in ‘severity’ from hypertension (least severe) (Read and daughter codes beginning with G20) to IHD (Read and daughter codes beginning with G3) to HF (most severe) (Read and daughter codes beginning with G58 and heart failure codes related to NYHA classification). Therefore, if a patient had a record for more than one of the three CVD conditions they were placed into the most ‘severe’ group, for example, a patient who had a record for hypertension and HF over the three year time-period would be placed in the HF group.
The OA definition was based on any coded clinical data, and included either diagnostic labels for any OA-related joint problem or radiographic-related diagnosis (Read and daughter codes beginning with N05 and codes related to OA joint replacement 7 K2 or 7 K3). Previous research also shows that the experience of osteoarthritis, which is usually presented as a local joint problem, is in fact commonly associated with multiple pain sites [28]. This approach to define three CVD groups for population severity and a single broader definition of OA enabled the hypothesis testing of specific comorbid groups, without over complicating the design further with sub-groups of OA. Our previous work and other evidence support the use of clinical records to measure both CVD population severity and OA [19].
2.5. Self-reported measurements
The Seattle Angina Questionnaire (SAQ) and the Kansas City Cardiomyopathy Questionnaire (KCCQ) were used as symptom measures of chest pain (CP) and shortness of breath (SOB) status respectively [29,30], and are widely used validated instruments including for use in the UK population. The SAQ and KCCQ questionnaires were used in family practice population samples, so a minor adaptation to the questionnaires was made to focus on the symptom of chest pain or shortness of breath as experienced by wider populations as opposed to the use of the clinical term such as ‘angina’ or ‘heart failure’ [31,32]. The SAQ has 3 sub-component scores and KCCQ has 5 sub-component scores and in this analysis, survey participants were included if they had completed all the components within each questionnaire. The physical limitation (PL) summary score, which is the component shared by both questionnaires was used as a comparable measure of symptom-based limitation. In addition, study data available included age, gender, deprivation, body mass index (BMI), smoking status, alcohol, and hospital anxiety and depression (HAD) questionnaire on psychological status [33]. The Index of Multiple Deprivation (IMD) uses the individual patient postcode to indicate deprivation, and is a weighted score relating to income; employment; health; education, skills and training; barriers to housing, and access to local services; crime; and living environment [34].
2.6. Statistical analysis
Study groups were categorised by age, gender, deprivation, BMI, smoking and alcohol status. Age was categorised into five groups and IMD score into four quartiles, with quartile 1 (least deprived) to quartile 4 (most deprived). Smoking was categorised into three groups (never, ex-smoker, smoker) and alcohol into six groups (never, special occasions, monthly, weekly (1–2), weekly (3–4), daily). The physical limitation score, generated from the SAQ and KCCQ was used as the primary ‘outcome’ measure in this analysis, with a high score indicating low physical limitations and a low score indicating higher physical limitations.
There were four stages to the analyses. First, symptoms and related mean physical limitations are presented by age bands, gender, deprivation quartiles, BMI, smoking and alcohol. Second, for each study group, the mean physical limitation score with 95% confidence intervals was assessed. The associations between the index and comorbid study groups and physical limitations compared with the reference group were estimated using linear regression methods, and expressed as the difference in physical limitation scores. Age, BMI, IMD, smoking and alcohol were included as continuous variables in the linear regression models. These analyses are presented as unadjusted values with 95% CI, then adjusted first for age, gender, deprivation, smoking and alcohol status, and finally adjusted in addition by BMI. Separately we also adjusted for the psychological status as measured by the HAD scale as a continuous variable (see Supplementary Table 1). Of total participants 7% were missing data on BMI. All other variables were missing < 5% of values. For BMI we did not exclude subjects with missing data but created a ‘dummy variable’ for the missing values. Third, we estimated how the observed associations between CVD and OA comorbid study groups and PLs differed from the expected estimates for the comorbid groups compared to the reference group. We calculated the observed estimates for the index and comorbid groups in linear regression, and then calculated the expected figures by adding the estimates for the index CVD and index OA groups. The difference between the observed and expected estimates for the CVD comorbid groups in this additive approach would allow the assessment of the potential interaction between CVD and OA. Finally, we compared each comorbid group directly with the respective index group, to assess the significance of the OA impact on symptom-specific physical limitation, and each comparison was adjusted for age, gender, deprivation, alcohol, smoking and BMI. We used Stata version 12 to conduct all analyses.
There were four stages to the analyses. First, symptoms and related mean physical limitations are presented by age bands, gender, deprivation quartiles, BMI, smoking and alcohol. Second, for each study group, the mean physical limitation score with 95% confidence intervals was assessed. The associations between the index and comorbid study groups and physical limitations compared with the reference group were estimated using linear regression methods, and expressed as the difference in physical limitation scores. Age, BMI, IMD, smoking and alcohol were included as continuous variables in the linear regression models. These analyses are presented as unadjusted values with 95% CI, then adjusted first for age, gender, deprivation, smoking and alcohol status, and finally adjusted in addition by BMI. Separately we also adjusted for the psychological status as measured by the HAD scale as a continuous variable (see Supplementary Table 1). Of total participants 7% were missing data on BMI. All other variables were missing < 5% of values. For BMI we did not exclude subjects with missing data but created a ‘dummy variable’ for the missing values. Third, we estimated how the observed associations between CVD and OA comorbid study groups and PLs differed from the expected estimates for the comorbid groups compared to the reference group. We calculated the observed estimates for the index and comorbid groups in linear regression, and then calculated the expected figures by adding the estimates for the index CVD and index OA groups. The difference between the observed and expected estimates for the CVD comorbid groups in this additive approach would allow the assessment of the potential interaction between CVD and OA. Finally, we compared each comorbid group directly with the respective index group, to assess the significance of the OA impact on symptom-specific physical limitation, and each comparison was adjusted for age, gender, deprivation, alcohol, smoking and BMI. We used Stata version 12 to conduct all analyses.
3. Results
3.1. CVD symptoms and PL in the study population
Of the 5426 study population, 1443 (27%) reported chest pain and 2098 (39%) shortness of breath symptom. Of the population with either symptom, 395 (16%) patients had chest pain without SOB, 1049 (42%) had SOB without chest pain, and 1048 (42%) had both symptoms.
Chest pain and shortness of breath symptoms and limitations increased in prevalence with age, higher deprivation and smoking and decreased in all but the highest alcohol intake group. Symptom limitation was higher in women, but chest pain symptom prevalence was lower (Table 1). The highest prevalence estimates were in the obese group (32% for chest pain, 50% for SOB) and smokers (30% chest pain and 44% SOB). Both symptoms and physical limitations were highest in the group who did not consume any alcohol (chest pain 32% and SOB 48%).
Table 1.
CVD symptoms and physical limitations in the study population.
| Study factors | Total | Chest paina |
Shortness of breatha |
||
|---|---|---|---|---|---|
| No. (%) | Mean score (SD) | No. (%) | Mean score (SD) | ||
| Age | |||||
| 40–49 | 439 | 61 (13.9) | 81.9 (22.6) | 102 (23.2) | 78.0 (24.4) |
| 50–59 | 966 | 212 (21.9) | 71.2 (28.9) | 303 (31.4) | 68.4 (27.9) |
| 60–69 | 1611 | 400 (24.8) | 63.5 (28.5) | 554 (34.4) | 58.1 (28.2) |
| 70–79 | 1541 | 495 (32.1) | 58.8 (27.8) | 707 (45.9) | 51.5 (26.9) |
| 80 years and over | 869 | 275 (31.6) | 52.0 (27.8) | 431 (49.6) | 44.5 (27.9) |
| Gender | |||||
| Men | 2653 | 780 (29.4) | 64.6 (28.8) | 1029 (38.8) | 58.8 (29.3) |
| Women | 2773 | 663 (23.9) | 58.1 (28.4) | 1068 (38.5) | 53.8 (28.1) |
| Deprivation status | |||||
| Quartile 1 — least | 1412 | 314 (22.2) | 69.1 (25.9) | 483 (34.2) | 62.4 (26.5) |
| Quartile 2 | 1430 | 360 (25.2) | 65.2 (28.4) | 507 (35.5) | 59.0 (28.0) |
| Quartile 3 | 1350 | 368 (27.3) | 60.6 (29.3) | 543 (40.2) | 54.5 (29.7) |
| Quartile 4 — most | 1207 | 392 (32.5) | 53.4 (29.0) | 548 (45.4) | 50.0 (29.2) |
| Body mass index | |||||
| Normal | 1479 | 329 (22.2) | 65.7 (27.6) | 469 (31.7) | 60.1 (29.0) |
| Underweight | 63 | 17 (27.0) | 47.3 (31.0) | 22 (34.9) | 46.3 (31.2) |
| Overweight | 2094 | 529 (25.3) | 65.1 (28.2) | 748 (35.7) | 60.4 (28.0) |
| Obese | 1407 | 449 (31.9) | 57.6 (29.8) | 704 (50.0) | 51.9 (28.4) |
| Unknown | 383 | 119 (31.1) | 52.4 (26.8) | 154 (40.2) | 45.8 (27.5) |
| Smoking | |||||
| Never | 2456 | 563 (22.9) | 69.9 (28.9) | 843 (34.3) | 58.2 (28.5) |
| Ex-smoker | 2357 | 698 (29.6) | 63.6 (29.8) | 988 (41.9) | 54.7 (28.7) |
| Smoker | 487 | 147 (30.2) | 64.8 (31.3) | 213 (43.7) | 53.8 (30.3) |
| Alcohol | |||||
| Never | 865 | 280 (32.4) | 56.6 (30.7) | 413 (47.7) | 46.9 (28.0) |
| Special occasions | 1096 | 314 (28.6) | 63.5 (29.7) | 490 (44.7) | 53.1 (28.9) |
| Monthly | 539 | 134 (24.9) | 70.2 (27.6) | 189 (35.1) | 57.2 (28.6) |
| Weekly (1–2) | 1144 | 273 (23.9) | 69.0 (28.4) | 414 (36.2) | 61.1 (28.0) |
| Weekly (3–4) | 785 | 179 (22.9) | 73.2 (28.8) | 237 (30.3) | 65.2 (27.9) |
| Daily | 748 | 184 (24.6) | 72.6 (28.0) | 262 (35.0) | 58.7 (27.8) |
Chest physical limitations from Seattle Angina Questionnaire and shortness of breath physical limitations from Kansas City Cardiomyopathy Questionnaire.
3.2. Associations between study groups and chest pain physical limitations
The highest mean chest pain physical limitation score was in the reference study group (80.5; SD 24.9), and the lowest score was in the HF and OA comorbid group (29.7; 21.8) (Table 2). With the exception of the index hypertension and OA groups and the comorbid IHD group, all other groups had significant associations with symptom related physical limitations compared to the reference group and these associations increased with CVD severity. The mean differences in physical limitation scores, adjusting for age, gender, deprivation, smoking, alcohol and BMI, for the index CVD groups were: 2.7 (95% CI − 3.6, 9.1) for hypertension, − 8.1 (− 13.7, − 2.5) for IHD and − 17.5 (− 26.9, − 8.1) for HF. The mean difference in physical limitation score for the index OA group was − 5.6 (− 12.7, 1.5).
Table 2.
Associations between study groups and chest pain physical limitations.
| Study groupsa | Chest pain PLb (n = 1443) |
Unadjusted |
Adjustedc |
Adjustedd |
|
|---|---|---|---|---|---|
| No. (%) | Mean score (SD) | Difference (95% CI) | Difference (95% CI) | Difference (95% CI) | |
| Reference n = 1165 | 128 (11.0) | 80.5 (24.9) | 0 | 0 | 0 |
| Hypertension n = 720 | 123 (17.1) | 72.3 (24.4) | − 8.2 (− 14.3, − 2.0) | − 0.9 (− 7.0, 5.2) | 2.7 (− 3.6, 9.1) |
| Ischaemic Heart Disease n = 1196 | 614 (51.3) | 60.9 (27.7) | − 19.5 (− 24.7, − 14.3) | − 11.9 (− 17.4, − 6.3) | − 8.1 (− 13.7, − 2.5) |
| Heart failure n = 149 | 70 (47.0) | 47.8 (27.0) | − 32.7 (− 40.2, − 25.2) | − 20.0 (− 29.3, − 10.8) | − 17.5 (− 26.9, − 8.1) |
| Osteoarthritis (OA) n = 828 | 131 (15.8) | 65.6 (28.1) | − 14.9 (− 21.4, − 8.4) | − 8.5 (− 15.4, − 1.7) | − 5.6 (− 12.7, 1.5) |
| Hypertension and OA n = 1017 | 194 (19.1) | 57.7 (29.6) | − 22.7 (− 29.0, − 16.5) | − 16.0 (− 23.8, − 8.2) | − 11.6 (− 20.0, − 3.2) |
| Ischaemic heart disease and OA n = 305 | 160 (52.5) | 52.0 (29.1) | − 28.5 (− 34.9, − 22.1) | − 11.0 (− 18.7, − 3.3) | − 7.6 (− 15.5, 0.3) |
| Heart failure and OA n = 46 | 23 (50) | 29.7 (21.8) | − 50.8 (− 61.7, − 39.8) | − 30.2 (− 43.0, − 17.5) | − 25.4 (− 37.6, − 13.1) |
Based on computer clinical records in 3-years before baseline — reference group without CVD or OA, index CVD groups without OA (hypertension, ischaemic heart disease and chronic heart failure), index OA group without CVD categories and comorbid (CVD and OA) groups.
Chest pain physical limitation from Seattle Angina Questionnaire.
Adjusted for age, gender, deprivation measured by Index of Multiple Deprivation, smoking and alcohol intake.
Adjusted additionally for Body Mass Index.
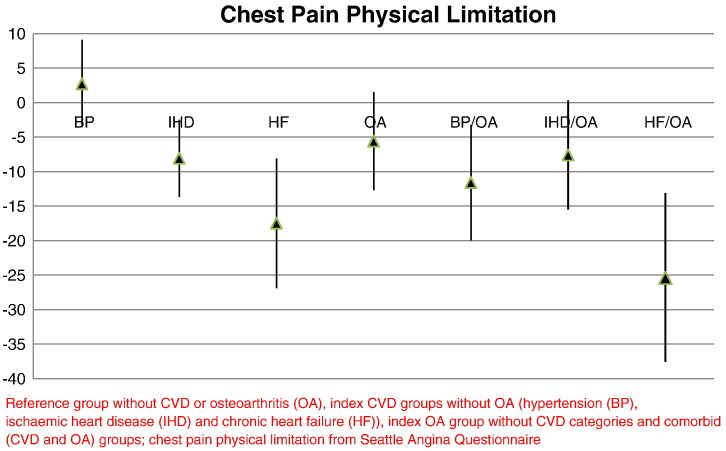
The mean differences in the physical limitation score for the CVD and OA comorbid groups compared to the reference group were larger than for the index groups and showed a similar observed trend with increasing CVD severity (Fig. 1). Adjusting for age, gender, deprivation, smoking, alcohol and BMI, the differences in CVD comorbid estimates were: − 11.6 (95% CI − 20.0, − 3.2) for hypertension, − 7.6 (− 15.5, 0.3) for IHD and − 25.4 (− 37.6, − 13.1) for HF. The adjustment for BMI, diminished the strength of associations between the disease-defined groups and chest pain physical limitations compared to the reference group, but the associations remained significant in all CVD comorbid groups except for the IHD and OA group.
Fig. 1.
Chest pain physical limitation score difference of the study groups compared to reference group.
Reference group without CVD or osteoarthritis (OA), index CVD groups without OA (hypertension (BP), ischaemic heart disease (IHD) and chronic heart failure (HF)), index OA group without CVD categories and comorbid (CVD and OA) groups; chest pain physical limitation from Seattle Angina Questionnaire.
3.3. Associations between study groups and shortness of breath physical limitations
The respective mean shortness of breath physical limitation scores were all lower than the respective chest pain PL scores. The highest mean shortness of breath physical limitation score was in the reference study group (75.4; SD 25.8), and the lowest score was in the HF and OA comorbid group (27.5; 20.8) (Table 3). With the exception of the index hypertension group, all other groups had significant associations with physical limitations compared to the reference group and these associations increased with CVD severity. The mean differences in physical limitation score, adjusting for age, gender, deprivation, smoking, alcohol and BMI, for the index CVD groups were: − 0.6 (95% CI − 5.4, 4.3) for hypertension, − 12.9 (− 17.4, − 8.4) for IHD and − 22.3 (− 29.1, − 15.6) for HF. The mean difference in physical limitation score for the index OA group was − 10.5 (− 15.2, − 5.9).
Table 3.
Associations between study groups and shortness of breath physical limitations.
| Study groupsa | Shortness of breath PLb (n = 2097) |
Unadjusted |
Adjustedc |
Adjustedd |
|
|---|---|---|---|---|---|
| No. (%) | Mean score (SD) | Difference (95% CI) | Difference (95% CI) | Difference (95% CI) | |
| Reference n = 1165 | 257 (22.1) | 75.4 (25.8) | 0 | 0 | 0 |
| Hypertension n = 720 | 244 (33.9) | 65.0 (27.3) | − 10.4 (− 15.0, − 5.8) | − 2.4 (− 7.2,2.3) | − 0.6 (− 5.4, 4.3) |
| Ischaemic Heart Disease n = 1196 | 650 (54.3) | 53.7 (27.8) | − 21.7 (− 25.6, − 17.7) | − 15.7 (− 20.1, − 11.2) | − 12.9 (− 17.4, − 8.4) |
| Heart failure n = 149 | 108 (72.5) | 39.7 (27.3) | − 35.7 (− 41.6, − 29.8) | − 22.9 (− 29.7, − 16.1) | − 22.3 (− 29.1, − 15.6) |
| Osteoarthritis (OA) n = 828 | 247 (29.8) | 59.1 (27.0) | − 16.3 (− 20.9, − 11.7) | − 12.6 (− 17.1, − 8.0) | − 10.5 (− 15.2, − 5.9) |
| Hypertension and OA n = 1017 | 381 (37.5) | 52.5 (27.0) | − 22.9 (− 27.1, − 18.7) | − 14.6 (− 19.6, − 9.5) | − 11.6 (− 16.9, − 6.4) |
| Ischaemic Heart Disease and OA n = 305 | 176 (57.7) | 44.0 (26.8) | − 31.4 (− 36.5, − 26.4) | − 17.7 (− 23.8, − 11.8) | − 16.8 (− 22.9, − 10.7) |
| Heart failure and OA n = 46 | 34 (73.9) | 27.5 (25.9) | − 47.8 (− 57.1, − 38.6) | − 31.7 (− 41.8, − 21.5) | − 29.5 (− 39.7, − 19.3) |
Based on computer clinical records in 3-years before baseline — reference group without CVD or OA, index CVD groups without OA (hypertension, ischaemic heart disease and chronic heart failure), index OA group without CVD categories and comorbid (CVD and OA) groups.
Shortness of breast physical limitation from Kansas City Cardiomyopathy Questionnaire.
Adjusted for age, gender, deprivation measured by Index of Multiple Deprivation, smoking and alcohol intake.
Adjusted additionally for body mass index.
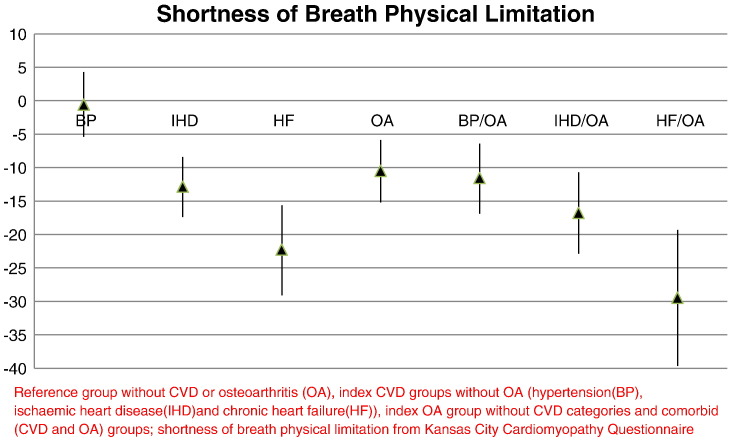
The mean differences in the shortness of breath physical limitation score for the CVD/OA comorbid groups compared to the reference group were larger than for the index groups and as with chest pain physical limitations, increased with CVD severity (Fig. 2). The mean differences for the CVD comorbid groups, adjusting for age, gender, deprivation, smoking, alcohol and BMI, were: − 11.6 (95% CI − 16.9, − 6.4) for hypertension and OA, − 16.8 (− 22.9, − 10.7) for IHD and OA and − 29.5 (− 39.7, − 19.3) for HF and OA. Adjustment for BMI again, diminished the strength of associations between the study groups compared to the reference group. Additional adjustment for psychological status did not change the estimates for either symptom (Supplementary Table 1).
Fig. 2.
Shortness of breath physical limitation score difference of the study groups compared to reference group.
Reference group without CVD or osteoarthritis (OA), index CVD groups without OA (hypertension (BP), ischaemic heart disease (IHD) and chronic heart failure (HF)), index OA group without CVD categories and comorbid (CVD and OA) groups; shortness of breath physical limitation from Kansas City Cardiomyopathy Questionnaire.
The mean differences in the shortness of breath physical limitation score for the CVD/OA comorbid groups compared to the reference group were larger than for the index groups and as with chest pain physical limitations, increased with CVD severity (Fig. 2). The mean differences for the CVD comorbid groups, adjusting for age, gender, deprivation, smoking, alcohol and BMI, were: − 11.6 (95% CI − 16.9, − 6.4) for hypertension and OA, − 16.8 (− 22.9, − 10.7) for IHD and OA and − 29.5 (− 39.7, − 19.3) for HF and OA. Adjustment for BMI again, diminished the strength of associations between the study groups compared to the reference group. Additional adjustment for psychological status did not change the estimates for either symptom (Supplementary Table 1).
3.4. Assessing interaction: expected and observed estimates
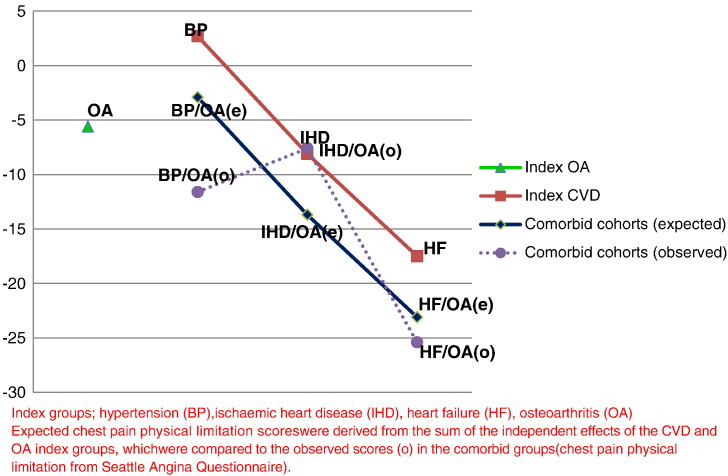
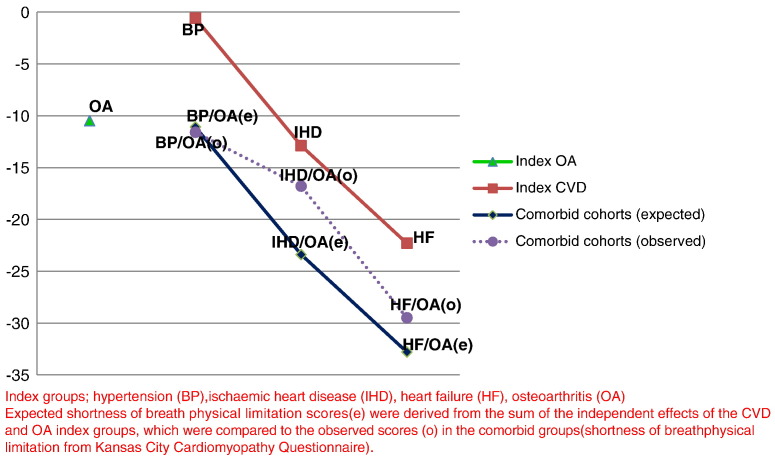
The expected estimates for the adjusted associations (age, gender, deprivation, smoking, alcohol and BMI) between CVD and OA comorbid groups and chest pain physical limitations were as follows: hypertension (2.7) and OA (− 5.6) = − 2.9, IHD (− 8.1) and OA (− 5.6) = − 13.7, and HF (− 17.5) and OA (− 5.6) = − 23.1. The respective observed estimates were: − 11.6, − 7.6 and − 25.4, which means that the associations between CVD and OA comorbidity with CP limitations were more than additive for hypertension and HF, but less than additive for IHD (Fig. 3). The expected estimates for the adjusted associations between CVD and OA comorbid groups and SOB physical limitations were as follows: hypertension (− 0.6) and OA (− 10.5) = − 11.1, IHD (− 12.9) and OA (− 10.5) = − 23.4, and HF (− 22.3) and OA (− 10.5) = − 32.8. The respective observed estimates were: − 11.6, − 16.8 and − 29.5, which means that the associations between CVD and OA comorbidity with SOB limitations were around additive for hypertension and HF, but less than additive for IHD (Fig. 4).
Fig. 3.
Observed and expected estimates for chest pain physical limitations.
Index groups; hypertension (BP), ischaemic heart disease (IHD), heart failure (HF), osteoarthritis (OA) expected chest pain physical limitation scores were derived from the sum of the independent effects of the CVD and OA index groups, which were compared to the observed scores (o) in the comorbid groups (chest pain physical limitation from Seattle Angina Questionnaire).
Fig. 4.
Observed and expected estimates for shortness of breath physical limitation.
Index groups; hypertension (BP), ischaemic heart disease (IHD), heart failure (HF), osteoarthritis (OA) expected shortness of breath physical limitation scores (e) were derived from the sum of the independent effects of the CVD and OA index groups, which were compared to the observed scores (o) in the comorbid groups (shortness of breath physical limitation from Kansas City Cardiomyopathy Questionnaire).
3.5. Associations between OA and symptom-specific limitations
The CVD comorbid groups were compared to the respective index CVD groups, to assess the OA ‘effect’ on chest pain and SOB physical limitations (Table 4). For all three CVD groups, there were still significant associations with chest pain physical limitations and for hypertension and IHD groups with SOB physical limitations. After adjustment for age, gender, deprivation, smoking, alcohol and BMI, the difference in the chest pain physical limitation score was: − 14.7 (95% CI − 21.5, − 7.8) for hypertension, − 5.5 (− 10.4, − 0.7) for IHD and − 22.1 (− 31.0, − 6.7) for HF. The CVD comorbid differences for shortness of breath physical limitation estimates were − 9.2 (− 13.8, − 4.6) for hypertension, − 6.4 (− 11.1, − 1.8) for IHD and − 8.8 (− 19.3, 1.65) for HF. Adjustment for BMI made little difference to the estimates of association for either the CP or SOB physical limitations.
Table 4.
Associations between CVD comorbid groups and symptom related physical limitations compared to index groups.
| Study groups | Chest pain PL differenced |
Shortness of breath PL differenced |
||||
|---|---|---|---|---|---|---|
| Unadjusted (95% CI) | Adjustedb (95% CI) | Adjustedc (95% CI) | Unadjusted (95% CI) | Adjustedb (95% CI) | Adjustedc (95% CI) | |
| Index CVD groupa | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypertension and OA n = 1017 | − 14.6 (− 20.8, − 8.3) | − 14.7 (− 21.3, − 8.1) | − 14.7 (− 21.5, − 7.8) | − 12.5 (− 16.8, − 8.1) | − 10.4 (− 14.9, − 5.9) | − 9.2 (− 13.8, − 4.6) |
| Ischaemic heart disease and OA n = 305 | − 8.9 (− 13.8, − 4.1) | − 6.2 (− 11.0, − 1.4) | − 5.5 (− 10.4, − 0.7) | − 9.8 (− 14.4, − 5.2) | − 7.1 (− 11.7, − 2.5) | − 6.4 (− 11.1, − 1.8) |
| Heart failure and OA n = 46 | − 18.1 (− 30.4, − 5.7) | − 17.5 (− 30.1, − 4.9) | − 18.5 (− 31.0, − 6.0) | − 12.1 (− 22.6, − 1.7) | − 8.9 (− 19.1, 1.4) | − 8.8 (− 19.3, 1.65) |
Respective index group: i.e. hypertension, ischaemic heart disease and heart failure.
Adjusted for age, gender, deprivation measured by Index of Multiple Deprivation, smoking and alcohol intake.
Adjusted additionally for body mass index.
Chest pain physical limitation from Seattle Angina Questionnaire; shortness of breast physical limitation from Kansas City Cardiomyopathy Questionnaire.
4. Discussion
The findings provide the evidence for the two study hypotheses. First, increasing CVD severity was associated with increased chest pain and SOB physical limitations in the index and comorbid groups. Second, OA comorbidity added to the symptom-specific physical limitations, irrespective of CVD severity. Additionally, we found that OA significantly influenced symptom specific physical limitations in all CVD severity groups. These associations were not explained by age, gender, deprivation, smoking, alcohol and BMI.
There was a dose–response relationship between increasing CVD severity and chest pain or SOB physical limitations. The CVD comorbid associations were mostly additive i.e. the combined ‘effect’ was the sum of individual parts, but there were differences between the two symptom limitations. In all CVD severity groups, the mean physical limitation score in relation to SOB was lower than for chest pain yet OA had a greater comorbid ‘effect’ on chest pain physical limitations than SOB when the comorbid group was compared directly with the index CVD group. This implies that the impact of comorbidity on physical limitation depends on the symptoms experienced by patients and requires careful consideration within disease management consultations.
This study shows uniquely that an unrelated chronic disease of OA has an independent effect in different cardiovascular diseases and on CVD-specific outcomes. Much of the current evidence has shown that comorbidity is associated with poor overall health and healthcare outcomes [35,36], but very few primary comorbidity studies have investigated the associations with disease-specific common symptoms and limitations. Whilst comorbidity may have been considered as an alternative explanation and adjustment in disease-specific outcomes, there has been a lack of focus on what the potential combined ‘effects’ of common chronic diseases are on specific limitations. This approach is crucial if one is to understand how new interventions for improving health outcomes might be developed and whether they are targeted at the index disease or the comorbidity. The clearest analogy here, where there is a substantial evidence base, is the identification and treatment of co-morbid depression in chronic diseases, to improve disease-specific outcomes [37].
The other key findings were that despite CVD severity, OA comorbidity was independently associated with symptom-specific physical limitations. Previous research has shown that hypertension and IHD are associated with relatively better health than HF [19]. So an expectation would have been that there was more scope for comorbidity to influence limitations in these CVD groups, than in the most severe HF group. Yet, the results show otherwise and there does not appear to be floor effects that are observed in severe HF. Also it has been postulated that people with severe disease may become accustomed to poor health and reconfigure their personal conceptualisations of ill-health and its impact [38]. This so-called ‘response shift’ can mean that the perception of symptom limitations might reduce in the presence of more severe disease and comorbidity but we found that in HF the association with specific-limitations was greater with comorbid OA and not less than what might have been expected. There was also the strong effect of BMI on the CVD associations with symptom-specific physical limitations. High BMI is associated with both CVD and OA [39] and separately for lower functional ability [40]. BMI may provide one explanation for the mechanism between the association between CVD severity, comorbid OA and symptom-specific limitations.
The study design allowed the a priori assessment of the biological interaction between two common and important chronic diseases. This allowed us to characterise the combined ‘effects’ and magnitude of two chronic diseases on outcomes. Using this design approach we were able to identify that the observed estimates of CVD and comorbid OA compared to the expected estimates were greater for chest pain limitations than SOB limitations. The increased influence of OA on chest pain physical limitations for the different CVD groups was further supported by the comparison of the comorbid groups with their respective index CVD groups. The physical limitation score differences when OA was present were greater for chest pain than for shortness of breath. Previous studies have identified variations in the statistical level, but not magnitude, of interaction of combined diseases on overall physical health [20–22]. The study findings show the magnitude of combined effects, by CVD severity and comorbidity as applied to two specific symptoms. The clinical implications are that in CVD management the severity or stage of disease is important in self-reporting of specific symptoms, which are common in the population, but that effective CVD-specific symptom management requires inclusion of comorbidity such as OA.
The study design incorporated exclusive CVD groups constructed on the basis of a priori hypothesis, in a large population-based setting. Exclusive groups were constructed using chronic disease cardiovascular and osteoarthritis registers. As such the definitions for the CVD groups were based on prevalent cases, but the hypothesis can still test whether the comorbid addition to an index condition is associated with greater symptom limitations. The design with the reference and index groups provides the basis for assessing the comorbid chronic disease interaction, and for the matching potentially of other comorbidity in the study groups. The symptom and physical limitations were measured using validated instruments, and the design provides the method for applying the same instruments to non-index conditions. Whilst most disease-specific instruments identify the key symptom or diagnosis in the population of interest, the underlying measure of physical limitation is often the same. Both the CVD measures used in the study in terms of the physical limitations had the same question and related it to common symptoms (chest pain and SOB) which occur not only in the CVD population, but also in the non-CVD population. Participation selection issues for this study have been previously reported and is in line with similar large population survey studies [26,41], but the design still allows for internal hypothesis testing.
5. Conclusions
In CVD severity populations, chest pain and SOB are common symptoms and the comorbid addition of OA influences the specific-symptom limitations. Clinically it provides evidence that pairs of common chronic disease combinations indicate worse health, the impact of comorbidity remains across different disease severities and the combined effect on health is often the sum of the independent effects even in the most severe disease. The clinical implication is whether such changes as a result of comorbidity influence clinical presentation and clinical decisions. This evidence highlights the importance of comorbidity for specific symptoms and that novel interventions for comorbidity need to be developed for both CVD specific health outcomes.
The following are the supplementary data related to this article.
Associations between study groups and symptom physical limitations.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ijcard.2014.05.001.
Acknowledgements
CAR was supported by a National Institute for Health Research (NIHR) Doctoral Fellowship (grant number NIHR-DRF-2012-05-288) (UK) and 2C study and UTK was supported by a National Institute for Health Research (NIHR) Post-Doctoral Fellowship (UK). We thank the practice and patient participants of the 2C study.
Role of the funding source
The study sponsors had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR (UK).
References
- 1.Ong K., Wu B., Cheung B., Barter P., Rye K. Arthritis: its prevalence, risk factors, and association with cardiovascular diseases in the United States, 1999 to 2008. Ann Epidemiol. 2013;23(2):80–86. doi: 10.1016/j.annepidem.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Alonso J., Ferrer M., Gandek B. Health-related quality of life associated with chronic conditions in eight countries: results from the International Quality of Life Assessment (IQOLA) Project. Qual Life Res. 2004;13(2):283–298. doi: 10.1023/b:qure.0000018472.46236.05. [DOI] [PubMed] [Google Scholar]
- 3.Kadam U., Croft P. Clinical comorbidity in osteoarthritis: associations with physical function in older patients in family practice. J Rheumatol. 2007;34(9):1899–1904. [PubMed] [Google Scholar]
- 4.Jonsson H., Helgadottir G.P., Aspelund T. Hand osteoarthritis in older women is associated with carotid and coronary atherosclerosis: the AGES Reykjavik Study. Ann Rheum Dis. 2009;68(11):1696–1700. doi: 10.1136/ard.2008.096289. [DOI] [PubMed] [Google Scholar]
- 5.Kadam U.T., Holmberg A., Blagojevic M., Nilsson P.M., Akesson K. Risk factors for cardiovascular disease and future osteoarthritis-related arthroplasty: a population-based cohort study in men and women from Malmo, Sweden. Scand J Rheumatol. 2011;40(6):478–485. doi: 10.3109/03009742.2011.585619. [DOI] [PubMed] [Google Scholar]
- 6.Suri P., Katz J.N., Rainville J., Kalichman L., Guermazi A., Hunter D.J. Vascular disease is associated with facet joint osteoarthritis. Osteoarthr Cartil. 2010;18(9):1127–1132. doi: 10.1016/j.joca.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried L., Bandeen-Roche K., Kasper J., Guralnik J. Association of comorbidity with disability in older women: the Women's Health and Aging Study. J Clin Epidemiol. 1999;52(1):27–37. doi: 10.1016/s0895-4356(98)00124-3. [DOI] [PubMed] [Google Scholar]
- 8.Ettinger W.H., Davis M.A., Neuhaus J.M., Mallon K.P. Long-term physical functioning in persons with knee osteoarthritis from NHANES. I: effects of comorbid medical conditions. J Clin Epidemiol. 1994;47(7):809–815. doi: 10.1016/0895-4356(94)90178-3. [DOI] [PubMed] [Google Scholar]
- 9.Swap C.J., Nagurney J.T. Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes. JAMA. 2005;294(20):2623–2629. doi: 10.1001/jama.294.20.2623. [DOI] [PubMed] [Google Scholar]
- 10.Walke L., Byers A., Tinetti M., Dubin J., McCorkle R., Fried T. Range and severity of symptoms over time among older adults with chronic obstructive pulmonary disease and heart failure. Arch Intern Med. 2007;167(22):2503–2508. doi: 10.1001/archinte.167.22.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoogeboom T.J., den Broeder A.A., de Bie R.A., van den Ende C.H. Longitudinal impact of joint pain comorbidity on quality of life and activity levels in knee osteoarthritis: data from the Osteoarthritis Initiative. Rheumatology (Oxford) 2013;52(3):543–546. doi: 10.1093/rheumatology/kes314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tinetti M.E., McAvay G., Chang S. Effect of chronic disease-related symptoms and impairments on universal health outcomes in older adults. J Am Geriatr Soc. 2011;59(9):1618–1627. doi: 10.1111/j.1532-5415.2011.03576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McBeth J., Symmons D.P., Silman A.J. Musculoskeletal pain is associated with a long-term increased risk of cancer and cardiovascular-related mortality. Rheumatology (Oxford) 2009;48(1):74–77. doi: 10.1093/rheumatology/ken424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oldridge N.B., Stump T.E., Nothwehr F.K., Clark D.O. Prevalence and outcomes of comorbid metabolic and cardiovascular conditions in middle- and older-age adults. J Clin Epidemiol. 2001;54(9):928–934. doi: 10.1016/s0895-4356(01)00350-x. [DOI] [PubMed] [Google Scholar]
- 15.Must A., Spadano J., Coakley E., Field A., Colditz G., Dietz W. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 16.Farkouh M.E., Greenberg J.D., Jeger R. Cardiovascular outcomes in high risk patients with osteoarthritis treated with ibuprofen, naproxen or lumiracoxib. Ann Rheum Dis. 2007;66(6):764–770. doi: 10.1136/ard.2006.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holland R., Rechel B., Stepien K., Harvey I., Brooksby I. Patients' self-assessed functional status in heart failure by New York Heart Association class: a prognostic predictor of hospitalizations, quality of life and death. J Card Fail. 2010;16(2):150–156. doi: 10.1016/j.cardfail.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamsen E., Puolakka T., Eskelinen A. Predictors of mortality following primary hip and knee replacement in the aged. Acta Orthop. 2013;84(1):44–53. doi: 10.3109/17453674.2012.752691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prior J.A., Kadam U.T. Cardiovascular disease and musculoskeletal disorder labels in family practice acted as markers of physical health severity. J Clin Epidemiol. 2011;64(5):547–555. doi: 10.1016/j.jclinepi.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Gaynes B.N., Burns B.J., Tweed D.L., Erickson P. Depression and health-related quality of life. J Nerv Ment Dis. 2002;190(12):799–806. doi: 10.1097/00005053-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Hunger M., Thorand B., Schunk M. Multimorbidity and health-related quality of life in the older population: results from the German KORA-Age Study. Health Qual Life Outcomes. 2011;9:53. doi: 10.1186/1477-7525-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darroch J. Biologic synergism and parallelism. Am J Epidemiol. 1997;145:661–668. doi: 10.1093/oxfordjournals.aje.a009164. [DOI] [PubMed] [Google Scholar]
- 23.Rothman K.J., Greenland S., Lash T.L. 3rd ed. Lippincott Williams and Wilkins; Philadelphia, USA: 2008. Modern epidemiology. [Google Scholar]
- 24.Lis Y., Mann R.D. The VAMP research multi-purpose database in the U.K. J Clin Epidemiol. 1995;48:431–443. doi: 10.1016/0895-4356(94)00137-f. [DOI] [PubMed] [Google Scholar]
- 25.Campbell S., Reeves D., Kontopantelis E., Middleton E., Sibbald B., Roland M. Quality of primary care in England with the introduction of pay for performance. N Engl J Med. 2007;357(2):181–190. doi: 10.1056/NEJMsr065990. [DOI] [PubMed] [Google Scholar]
- 26.Prior J.A., Rushton C.A., Jordan K.P., Kadam U.T. Comorbidity Cohort (2C) study: cardiovascular disease severity and comorbid osteoarthritis in primary care. BMC Health Serv Res. 2012;12(1):295. doi: 10.1186/1472-6963-12-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harding A., Stuart-Buttle C. The development and role of the Read codes. J AHIMA. 1998;69(5):34–38. [PubMed] [Google Scholar]
- 28.Croft P., Jordan K., Jinks C. “Pain elsewhere” and the impact of knee pain in older people. Arthritis Rheum. Aug 2005;52(8):2350–2354. doi: 10.1002/art.21218. [DOI] [PubMed] [Google Scholar]
- 29.Spertus J.A., Winder J.A., Dewhurst T. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25(2):333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 30.Green C.P., Porter C.B., Bresnahan D.R., Spertus J.A. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 31.Ruigómez A., Rodríguez L.A., Wallander M.A., Johansson S., Jones R. Chest pain in general practice: incidence, comorbidity and mortality. Fam Pract. 2006;23(2):167–174. doi: 10.1093/fampra/cmi124. [DOI] [PubMed] [Google Scholar]
- 32.Figarska S.M., Boezen H.M., Vonk J.M. Dyspnea severity, changes in dyspnea status and mortality in the general population: the Vlagtwedde/Vlaardingen Study. Eur J Epidemiol. 2012;27(11):867–876. doi: 10.1007/s10654-012-9736-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadam U.T., Croft P., Lewis M. Use of a cross-sectional survey to estimate outcome of health care: the example of anxiety and depression. J Clin Epidemiol. 2001;54(11):1112–1119. doi: 10.1016/s0895-4356(01)00379-1. [DOI] [PubMed] [Google Scholar]
- 34.Office of the Deputy Prime Minister The English indices of deprivation 2004—summary (revised) 2004. http://www.communities.gov.uk/documents/communities/pdf/131206.pdf Link:
- 35.Fortin M., Dubois M.F., Hudon C., Soubhi H., Almirall J. Multimorbidity and quality of life: a closer look. Health Qual Life Outcomes. 2007;5:52. doi: 10.1186/1477-7525-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vos H.M., Bor H.H., Rangelrooij-Minkels M.J., Schellevis F.G., Lagro-Janssen A.L. Multimorbidity in older women: the negative impact of specific combinations of chronic conditions on self-rated health. Eur J Gen Pract. 2013;19(2):117–122. doi: 10.3109/13814788.2012.755511. [DOI] [PubMed] [Google Scholar]
- 37.Katon W.J., Lin E.H., Von Korff M. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363(27):2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sprangers M.A., Schwartz C.E. Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med. 1999;48(11):1507–1515. doi: 10.1016/s0277-9536(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 39.Singh G., Miller J.D., Lee F.H., Pettitt D., Russell M.W. Prevalence of cardiovascular disease risk factors among US adults with self-reported osteoarthritis: data from the Third National Health and Nutrition Examination Survey. Am J Manag Care. 2002;8(15 Suppl.):S383–S391. [PubMed] [Google Scholar]
- 40.Alley D.E., Chang V.W. The changing relationship of obesity and disability, 1988–2004. JAMA. 2007;298(17):2020–2027. doi: 10.1001/jama.298.17.2020. [DOI] [PubMed] [Google Scholar]
- 41.Dunn K.M., Jordan K., Lacey R.J., Shapley M., Jinks C. Patterns of consent in epidemiologic research: evidence from over 25,000 responders. Am J Epidemiol. 2005;159(11):1087–1094. doi: 10.1093/aje/kwh141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Associations between study groups and symptom physical limitations.