Abstract
Smooth muscle cell (SMC) proliferation and migration are key processes that occur in the pathogenesis of atherosclerosis and post-angioplasty restenosis. In the present study, we designed locked nucleic acid (LNA)-modified DNAzymes targeting a specific region spanning the translational start site of human EGR-1, an immediate-early gene, wherein two of the nucleotides in each of the 9+9 hybridizing arms of the DNAzyme were substituted with LNA monomers. In vitro cleavage experiments revealed that the LNA- modified DNAzyme (LzF4) cleaved a 32P-labelled 388 nt EGR-1 transcript with greater efficacy than its native unmodified phosphodiester counterpart, DzF. The scrambled versions of these molecules, LzF4SCR and DzFSCR, did not display any ability to cleave the transcript. Western blot analysis revealed that both active molecules abrogated serum-inducible EGR-1 protein expression in primary human aortic SMCs and inhibited serum-inducible SMC proliferation in a dose-dependent and non-toxic manner. SMC proliferation was inhibited by >50% with LzF4 at concentrations as low as 20 nM, whereas inhibition by DzF at this concentration was not evident. Finally, LzF4 and DzF inhibited SMC regrowth from the wound edge after mechanical injury in vitro. In contrast, neither DzFSCR nor LzF4SCR had any influence on EGR-1 protein expression, SMC proliferation or regrowth. These findings provide the first functional demonstration of LNA-modified DNAzyme efficacy in a biological setting of any kind. These studies also demonstrate that LNA modification increases DNAzyme potency without necessarily compromising specificity.
INTRODUCTION
Antisense oligonucleotide strategies have been used to silence genes that play key roles in the progression of a varied range of disease models through their ability to bind target sequences by Watson–Crick base pairing (1,2). Phosphorothioate (PS) modifications have been employed in the backbones of normally phosphodiester-linked oligonucleotides to increase resistance against nucleolytic degradation (3). However, the introduction of PS linkages may result in non-specific interactions with other proteins causing non-specific phenotypic effects (4,5).
A new class of DNA-based gene-targeting agent has been developed, through an in vitro selection technique (6), which binds RNA and cleaves between an unpaired purine and a paired pyrimidine. These agents, termed DNAzymes, display low toxicity and need not rely on RNase H for destruction of mRNA, unlike classic antisense oligonucleotides. One of the most attractive features of DNAzymes is their ability to turn over, whereby a given DNAzyme molecule cleaves multiple target transcripts with reasonable efficacy. Modifications to DNAzymes, such as phosphorothioate backbones or the addition of a 3′–3′-inverted T have been used to prolong the stability of these molecules (7–9). DNAzymes are useful as molecular tools to investigate the functions of the targeted gene in a biological system, or potentially useful as therapeutic tools in different disease states (10).
Recently a new type of ribonucleotide analogue, known as locked nucleic acids (LNAs), have been introduced into both antisense oligonucleotides (11,12) and DNAzymes (9,13). LNA bases contain a 2′-O, 4-C-methylene bridge that lock in a C3′-endo conformation (11,12). Constraint on the ribose ring increases binding affinity for complementary sequences and increases the melting temperature of the LNA-incorporated oligonucleotide (14,15). Recent studies have demonstrated that LNA-incorporated oligonucleotides can be transiently transfected into cells using cationic lipid-based transfection reagents (16,17). However, there are presently no studies that have evaluated the biological efficiency of LNA-modified DNAzymes.
Smooth muscle cell (SMC) proliferation and migration are key processes that occur in response to injury (such as after balloon angioplasty) resulting in intimal thickening. The promoter regions of genes that regulate SMC growth contain recognition elements for the immediate-early gene, early growth response-1 (EGR-1) (18). EGR-1 is a prototypic member of the zinc finger family of transcription factors (19), and is rapidly upregulated following mechanical injury both in vitro and in vivo and by numerous other agonists such as growth factors, cytokines, hormones and environmental stimuli (20,21). We have demonstrated that DNAzymes targeting EGR-1 can serve as useful therapeutic agents in the context of restenosis by their capacity to inhibit intimal thickening in rodent (22,23) and porcine (24) models.
In the present study we compared LNA-modified DNAzymes targeting EGR-1 with their native phosphodiester counterparts in the context of in vitro substrate cleavage, protein expression, SMC proliferation and regrowth after mechanical injury.
MATERIALS AND METHODS
Oligonucleotides
LNA-modified DNAzymes (LzF4 and LzF4SCR) were synthesized by Proligo France SAS and the unmodified DNAzymes were synthesized by Trilink Biotechnologies (San Diego, USA). The sequences of the DNAzymes (DzF and DzFSCR) and LNA-modified DNAzymes (LzF4 and LzF4SCR) are as follows: 5′-GCGGGGACAGGCTAG CTACAACGACAGCTGCAT-(3′–3′ T)-3′, DzF; 5′-GGAG CTGACGGCTAGCTACAACGAGATCGACGC-(3′–3′ T)-3′, DzFSCR; 5′-GCGgGGaCAGGCTAGCTACAACGAC AGCtGCaT-(3′–3′ T)-3′, LzF4 and 5′-GGAgCTgACGG CTAGCTACAACGAGATcGAcGC-(3′–3′ T)-3′, DzFSCR, where capitalized letters represent unmodified monomers and lower case letters represent LNA-modified monomers.
Cell culture and transfection
Primary human aortic SMCs were obtained from American Type Culture Collection (Manassas, VA) and cultured in Waymouth’s medium pH 7.4, supplemented with 10% fetal bovine serum (FBS), 10 µg/ml streptomycin and 10 U/ml penicillin at 37°C and 5% CO2. Cells were passaged by washing twice in PBS followed by trypsinization. Unless otherwise stated, subconfluent SMCs (75%) were growth-arrested in serum-free conditions for 6 h before transfecting with DNAzyme or LNA-modified DNAzyme using FuGENE6 (Roche Diagnostics GmbH, Mannheim, Germany). Cells were transfected a second time in the presence of 5% FBS 18 h following the initial transfection.
In vitro transcription and cleavage of RNA substrate
A 32P-labelled, 388-nucleotide EGR-1 RNA transcript was prepared by in vitro transcription (T7 RNA polymerase) of the plasmid construct pBlueScript2 and linearized before transcription with BamHI. Reactions were performed in a final volume of 20 µl containing 10 mM MgCl2, 5 mM Tris pH 7.5, 150 mM NaCl, wherein the substrate to DNAzyme or LNA-modified DNAzyme ratios were 1:1, 1:10, 1:20 and 1:50. Reactions proceeded at 37°C for 3 h then were quenched by transfer of aliquots to tubes containing formamide-loading buffer (5). Samples were denatured at 95°C for 5 min, placed on ice for 1 min and separated on 12% polyacrylamide denaturing gels followed by detection by phosphor imager.
SMC proliferation assay
SMCs were seeded into 96 well plates (3000 cells/well). For comparative studies, subconfluent SMCs were transfected with either 20 or 50 nM DNAzyme or LNA-modified DNAzyme. Seventy-two hours following the second transfection, cells were trypsinized and resuspended in 10 ml of isotone solution for counting. Cells were then counted using an automated Coulter counter (Coulter Z Series, Miami, USA).
Western blot analysis
SMCs were cultured in 10 mm tissue culture plates (Falcon, Becton Dickinson, Franklin Lakes, USA) and transfected with 0.2 µM DNAzyme or LNA-modified DNAzyme. One hour following the second transfection in the presence of 5% FBS, cells were washed twice in 1× PBS and total protein was extracted in 150 mM NaCl, 50 mM Tris–HCl (pH 7.5), 1% sodium deoxycholate, 0.1% SDS, 1% Triton X-100, 5 mM EDTA, 10 µg/ml leupeptin, 1% aprotinin and 2 mM PMSF. Ten micrograms of protein sample was loaded onto a 10% SDS–PAGE and electroblotted onto a PVDF nylon membrane (Millipore, Bedford, USA). Membranes were blocked in 0.05% Tween 20 (v/v) PBS containing 5% skim milk then incubated with rabbit polyclonal EGR-1 antibodies (Santa Cruz, CA) before being stripped and reprobed with rabbit polyclonal YY1 antibodies (Santa Cruz, CA) at a concentration of 2 µg/ml. Membranes were then incubated with a HRP-linked swine anti-rabbit IgG secondary antibody (Dako, Carpinteria, USA). Protein bands of interest were visualized by chemiluminescent detection (NEN, Boston, USA).
SMC injury/migration assay
SMCs were grown to confluence in 8 well chamber slides (Nunc, Copenhagen, Denmark) and transfected with 0.1 µM DNAzyme or LNA-modified DNAzyme. After the second transfection in 5% FBS, injury was performed with a single scratch using a sterile toothpick. Forty-eight hours following injury, cells were fixed in 5% formaldehyde (v/v), stained with haematoxylin and eosin, and analyzed by light microscopy. Cells in the denuded zone were counted at two different fields and expressed as the mean ± standard error of the mean (SEM).
RESULTS
LNA-modified DNAzyme cleaves EGR-1 mRNA with greater potency than DNAzyme
Prior to evaluating the effect of LzF4 on cellular activity, we determined the ability of this molecule to cleave its EGR-1 mRNA substrate compared to its all-phosphodiester cleaving counterpart, DzF. Both DzF and LzF4 target the A301U junction in human EGR-1 mRNA. The 388 nt 32P-labelled EGR-1 transcript was produced from a linearized plasmid template using T7 RNA polymerase. DNAzyme cleavage of this substrate is expected to produce 349 and 39 nt fragments; the latter is resolved by 12% denaturing PAGE. Cleavage conditions with RNA:DNAzyme/LNA-modified DNAzyme ratios of 1:1, 1:10, 1:20 and 1:50 demonstrated that LzF4 cleaved with greater potency compared to the unmodified DzF after 3 h (Fig. 1). LNA incorporation constrains the ribose ring of the LNA-modified nucleotide which, in turn, results in increased binding affinity (on-rate) for the complementary sequence (14,15).
Figure 1.
Cleavage of target RNA by DzF and LzF4. Each was incubated with a 32P-labelled 388 nt human EGR-1 transcript for 3 h at 37°C at the indicated stoichiometric ratios. Upon termination of the reaction, cleavage products were denatured and resolved by electrophoresis on 12% polyacrylamide denaturing gels. DzFSCR and LzF4SCR were used in parallel at the indicated stoichiometries.
DNAzyme and LNA-modified DNAzyme inhibition of SMC proliferation
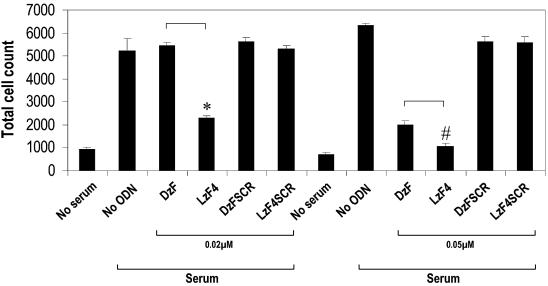
Having shown the capacity of LzF4 to cleave its substrate, we next determined whether it could influence SMC proliferation in the presence of serum (5%), an inducer of EGR-1 gene expression (20). We demonstrated that DzF and LzF4 both strongly inhibit SMC proliferation at 50 nM (Fig. 2). LzF4, at this concentration, inhibited serum-inducible SMC proliferation virtually to completion. Back-titrating the concentration of both molecules revealed greater potency by LzF4, which, at 20 nM, produced >50% inhibition whereas DzF was not effective at this concentration (Fig. 2). In contrast, neither DzFSCR nor LzF4SCR possessed any significant inhibitory effect on serum-inducible SMC proliferation at either concentration. These data demonstrate dose-dependent inhibition by both molecules, but greater cleavage and anti-proliferative activity by LzF4.
Figure 2.
Dose-dependent inhibition of human aortic SMC proliferation by DzF and LzF4 in vitro. Growth-arrested human aortic SMCs were transfected with DNAzymes in 96 well plates and exposed to 5% FBS for 72 h. Numbers of cells in each group were counted using an automated coulter counter. ODN denotes oligonucleotide. * indicates P < 0.001; # indicates P = 0.005 using Student’s t-test (two-tailed, unequal variance).
LNA-modified DNAzyme and DNAzyme inhibit induction of EGR-1 protein
We transfected growth-quiescent SMC with 0.2 µM of DzF or LzF4 prior to stimulation with serum (5%). Western blot analysis revealed that LzF4 and DzF inhibited EGR-1 protein induction 1 h after exposure to serum, with more potent inhibition by LzF4 (Fig. 3). DzFSCR and LzF4SCR had no influence on levels of EGR-1 (Fig. 3). Reprobing the blot for a second zinc finger transcription factor (YY1) demonstrated that neither serum, DzF or LzF4 had any influence over YY1 expression (Fig. 3).
Figure 3.
DNAzyme blockade of serum-inducible EGR-1 protein expression. Subconfluent growth-quiescent SMCs were transfected with 0.2 µM of either DzF or LzF4 in 100 mm tissue culture dishes. Protein lysates were harvested from cells 1 h following a second transfection in the presence of 5% FBS. Lysates were assessed for EGR-1 and YY1 immunoreactivity by western blot analysis using polyclonal antibodies to EGR-1 and YY1. ODN denotes oligonucleotide.
LNA-modified DNAzymes block SMC regrowth after injury in vitro
Injury by scraping, inflicted onto a confluent monolayer of SMCs triggers a regenerative response which results in complete regrowth from the wound edge into the denuded zone (22). LzF4 and DzF each inhibited this process, whereas both DzFSCR and LzF4SCR failed to influence this response (Fig. 4A and B). In line with our observations with SMC proliferation, EGR-1 protein expression, in vitro RNA cleavage activity and SMC repair, LzF4 was superior to DzF at the same molar concentration.
Figure 4.
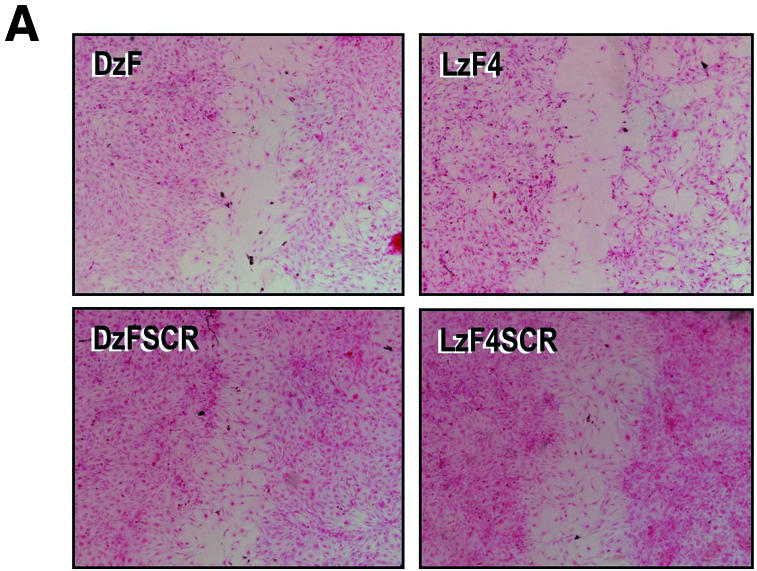
(A) DzF and LzF4 inhibit regrowth from the wound edge after mechanical injury of human aortic SMCs. Confluent monolayers of growth-arrested SMCs were transfected with 0.1 µM of either DzF or LzF4 in 8 well chamber slides, exposed to 5% FBS and injured by scratching with a sterile toothpick. Cells were incubated at 37°C for 48 h prior to fixing in 5% formaldehyde and staining in haematoxylin and eosin. (B) Assessment of population of cells in the denuded zone. Cells in the denuded zone were quantified under two different fields of view for each treatment and expressed as the mean ± SEM. The data is representative of two independent determinations. ODN denotes oligonucleotide. * indicates P < 0.033 using Student’s t-test (two-tailed, unequal variance).
DISCUSSION
In the present study, we generated LNA-modified DNAzymes targeting the human EGR-1 transcript, where two nucleotides in each of the hybridizing arms were substituted with LNA monomers. This is the first demonstration where a mixed LNA-modified DNAzyme is compared side by side to its unmodified counterpart in a biological setting. Our studies show that the LNA-modified DNAzyme is more superior at cleaving a 388 nt 32P-labelled EGR-1 transcript, inhibiting endogenous EGR-1 protein expression, SMC proliferation and regrowth from the wound edge after injury.
Recent studies have demonstrated that LNA monomers introduced into the substrate recognition arms of DNAzymes resulted in increased cleavage of substrate. Vester et al. (13) showed that a DNAzyme with two T nucleotides in each of its arms replaced with LNA-T monomers greatly increased the cleavage efficiency of these molecules, consistent with our findings demonstrating that the LNA-modified DNAzyme (LzF4) was more active than DNAzyme (DzF) under single turnover conditions. LNA-modified DNAzymes cleave their target sequences at significantly higher reaction rates in the presence of excess DNAzyme (9,13). Due to increased binding affinity of oligonucleotides incorporating LNAs, the number of LNA substitutions influences the enzymatic reaction rate. LNA-modified DNAzymes bearing four LNA substitutions on each of the substrate recognition arms have profoundly reduced reaction rates below those of the unmodified DNAzyme counterpart (9), whereas the reaction rate of a DNAzyme bearing two LNA substitutions on each of the hybridizing arms does not decelerate reaction rates under multiple turnover conditions (13).
DNAzymes, to date, have been generated toward a number of key genes that play critical roles in different disease states (22–29). The stability of these agents is an important factor, especially considering their potential use in vivo. Modifications such as PS backbones or 3′–3′-inverted Ts have been employed to increase stability and nuclease resistance. PS linkages have been widely used to increase stability, however, this modification can display affinity for various cellular proteins resulting in sequence-independent effects (4,5). A 3′–3′-inverted T is now a preferred modification to increase the half-life of a DNAzyme. We and others have shown that the addition of a 3′–3′-inverted T increases the half-life of a DNAzyme from <2 to >22 h (7,8,22). Recent findings indicate that a 3′–3′-inverted T does not alter the kinetic behaviour of the enzyme, whereas PS modification may affect cleavage efficiency by decreasing cleavage reaction rates to one fifth that of the unmodified DNAzyme under single turnover conditions and virtually to zero under multiple turnover conditions (9). Assessment of the intracellular cleavage activity and stability of DNAzymes revealed that a 3′–3′-inverted T-modified DNAzyme extracted from transfected cells, was still capable of cleaving its substrate, in vitro (8). In addition, an LNA–DNA mix-mer is more stable in serum than its all PS-oligonucleotide counterpart (17).
Studies, prior to the present, have not applied LNA-modified DNAzymes in a biological setting, although antisense LNA oligonucleotides have been used (16,17,30–32). LNA-modified oligonucleotides enter living cells efficiently using cationic lipid carriers similar to that used in our study (17,31). Comparative studies using PS- and LNA oligonucleotides demonstrate that PS-oligonucleotides cause toxicity (17) and are even capable of stimulating an immune response (32). Trypan Blue exclusion studies revealed that LNA modification to the DNAzyme bore no adverse toxic effects on cells in culture at 20 nM (data not shown). SMC proliferation assays show that both LzF4 or DzF produce dose-dependent, sequence-specific inhibition. The disadvantage of LNA-modified DNAzymes is that they are significantly more expensive to synthesize compared to unmodified DNAzymes, presently of the order of at least five times. Although LNA modification increases resistance to nucleolytic degradation, the melting temperature [Tm (°C)] of these oligonucleotides is high resulting in slower cleavage reaction rates when compared to their 2′-O-methyl oligoribonucleotide counterpart, which have lower Tm (°C) (9). Whether LNA-modified DNAzymes become clinically useful remains to be seen.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr Laurent Rivory for critical review of the manuscript. This work was supported by grants from Johnson and Johnson Research Pty Limited, National Health and Medical Research Council of Australia, and an R&D Infrastructure Grant from the NSW Department of Health. L.M.K. is a Principal Research Fellow of the N.H.M.R.C.
REFERENCES
- 1.Finkel T. and Epstein,S.E. (1995) Gene therapy for vascular disease. FASEB J., 9, 843–851. [DOI] [PubMed] [Google Scholar]
- 2.Zhang W.W. (1996) Antisense oncogene and tumor suppressor gene therapy of cancer. J. Mol. Med., 4, 191–204. [DOI] [PubMed] [Google Scholar]
- 3.Eppstein D.A., Schryver,B.B. and Marsh,Y.V. (1986) Stereoconfiguration markedly affects the biochemical and biological properties of phosphorothioate analogs of 2–5A core, (A2′p5′)2A. J. Biol. Chem., 261, 5999–6003. [PubMed] [Google Scholar]
- 4.Rockwell P., O’Connor,W.J., King,K., Goldstein,N.I., Zhang,L.M. and Stein,C.A. (1997) Cell-surface perturbations of the epidermal growth factor and vascular endothelial growth factor receptors by phosphorothioate oligodeoxynucleotides. Proc. Natl Acad. Sci. USA, 94, 6523–6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guvakova M.A., Yakubov,L.A., Vlodavsky,I., Tonkinson,J.L. and Stein,C.A. (1995) Phosphorothioate oligodeoxynucleotides bind to basic fibroblast growth factor, inhibit its binding to cell surface receptors, and remove it from low affinity binding sites on extracellular matrix. J. Biol. Chem., 270, 2620–2627. [DOI] [PubMed] [Google Scholar]
- 6.Santoro S.W. and Joyce,G.F. (1997) A general purpose RNA-cleaving DNA enzyme. Proc. Natl Acad. Sci. USA, 94, 4262–4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun L.Q., Cairns,M.J., Gerlach,W.L., Witherington,C., Wang,L. and King,A. (1999) Suppression of smooth muscle cell proliferation by a c-myc RNA-cleaving deoxyribozyme. J. Biol. Chem., 274, 17236–17241. [DOI] [PubMed] [Google Scholar]
- 8.Dass C.R., Saravolac,E.G., Li.Y. and Sun,L.Q. (2002) Cellular uptake, distribution, and stability of 10–23 deoxyribozymes. Antisense Nucleic Acid. Drug. Dev., 12, 289–299. [DOI] [PubMed] [Google Scholar]
- 9.Schubert S., Gul,D.C., Grunert,H.P., Zeichhardt,H., Erdmann,V.A. and Kurreck,J. (2003) RNA cleaving ‘10–23’ DNAzymes with enhanced stability and activity. Nucleic Acids Res., 31, 5982–5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khachigian L.M. (2002) DNAzymes: cutting a path to a new class of therapeutics. Curr. Opin. Mol. Ther., 4, 119–121. [PubMed] [Google Scholar]
- 11.Koshkin A.A., Singh,S.K., Nielsen,P., Rajwanshi,V.K., Kumar,R., Meldgaard,M., Olsen,C.E. and Wengel,J. (1998) LNA (locked nucleic acids): synthesis of the adenine, cytosine, guanine, 5-methylcytosine, thymine and uracil biocyclonucleoside monomers, oligomerisation and unprecendented nucleic acid recognition. Tetrahedron, 54, 3607–3630. [Google Scholar]
- 12.Singh S.K., Nielsen,P., Koshkin,A.A. and Wengel,J. (1998) LNA (locked nucleic acids): synthesis and high-affinity acid recognition. Chem. Commun., 455–456. [Google Scholar]
- 13.Vester B, Lundberg,L.B., Sorensen,M.D., Babu,B.R., Douthwaite,S. and Wengel,J. (2002) LNAzymes: incorporation of LNA-type monomers into DNAzymes markedly increases RNA cleavage. J. Am. Chem. Soc., 124, 13682–13683. [DOI] [PubMed] [Google Scholar]
- 14.Braasch D.A. and Corey,D.R. (2001) Locked nucleic acid (LNA): fine-tuning the recognition of DNA and RNA. Chem. Biol., 8, 1–7. [DOI] [PubMed] [Google Scholar]
- 15.Wengel J. (1998) Synthesis of 3′-C- and 4′-C-branched oligonucleotides and the development of locked nucleic acid (LNA). Acc. Chem. Res., 32, 301–302. [Google Scholar]
- 16.Arzumanov A., Walsh,A.P., Rajwanshi,V.K., Kumar,R., Wengel,J. and Gait,M.J. (2001) Inhibition of HIV-1 Tat-dependent trans activation by steric block chimeric 2′-O-methyl/LNA oligoribonucleotides. Biochemistry, 40, 14645–14654. [DOI] [PubMed] [Google Scholar]
- 17.Wahlestedt C., Salmi,P., Good,L., Kela,J., Johnsson,T., Hokfelt,T., Broberger,C., Porreca,F., Lai,J., Ren,K., Ossipov,M., Koshkin,A., Jakobsen,N., Skouv,J., Oerum,H., Jacobsen,M.H. and Wengel,J. (2000) Potent and nontoxic antisense oligonucleotides containing locked nucleic acids. Proc. Natl Acad. Sci. USA, 97, 5633–5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khachigian L.M. and Collins,T. (1997) Inducible expression of Egr-1-dependent genes. A paradigm of transcriptional activation in vascular endothelium. Circ. Res., 81, 457–461. [DOI] [PubMed] [Google Scholar]
- 19.Gashler A. and Sukhatme,V.P. (1995) Early growth response protein 1 (Egr-1): prototype of a zinc-finger family of transcription factors. Prog. Nucleic Acid. Res. Mol. Biol., 50, 191–224. [DOI] [PubMed] [Google Scholar]
- 20.Khachigian L.M., Lindner,V., Williams,A.J. and Collins,T. (1996) Egr-1-induced endothelial gene expression: a common theme in vascular injury. Science, 271, 1427–1431. [DOI] [PubMed] [Google Scholar]
- 21.Khachigian L.M. and Collins,T. (1998) Early growth response factor 1: a pleiotropic mediator of inducible gene expression. J. Mol. Med., 76, 613–616. [DOI] [PubMed] [Google Scholar]
- 22.Santiago F.S., Lowe,H.C., Kavurma,M.M., Chesterman,C.N., Baker,A., Atkins,D.G. and Khachigian,L.M. (1999) New DNA enzyme targeting Egr-1 mRNA inhibits vascular smooth muscle proliferation and regrowth after injury. Nat. Med., 5, 1264–1269. [DOI] [PubMed] [Google Scholar]
- 23.Khachigian L.M., Fahmy,R.G., Zhang,G., Bobryshev,Y.V. and Kaniaros,A. (2002) c-Jun regulates vascular smooth muscle cell growth and neointima formation after arterial injury. Inhibition by a novel DNA enzyme targeting c-Jun. J. Biol. Chem., 277, 22985–22991. [DOI] [PubMed] [Google Scholar]
- 24.Lowe H.C., Fahmy,R.G., Kavurma,M.M., Baker,A., Chesterman,C.N. and Khachigian,L.M. (2001) Catalytic oligodeoxynucleotides define a key regulatory role for early growth response factor-1 in the porcine model of coronary in-stent restenosis. Circ. Res., 89, 670–677. [DOI] [PubMed] [Google Scholar]
- 25.Fahmy R.G., Dass,C.R., Sun,L.Q., Chesterman,C.N. and Khachigian,L.M. (2003) Transcription factor Egr-1 supports FGF-dependent angiogenesis during neovascularization and tumor growth. Nat. Med., 9, 1026–1032. [DOI] [PubMed] [Google Scholar]
- 26.Unwalla H. and Banerjea,A.C. (2001) Inhibition of HIV-1 gene expression by novel macrophage-tropic DNA enzymes targeted to cleave HIV-1 TAT/Rev RNA. Biochem. J., 357, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y., Yu,L., McMahon,R., Rossi,J.J., Forman,S.J. and Snyder,D.S. (1999) Inhibition of bcr-abl oncogene expression by novel deoxyribozymes (DNAzymes). Hum. Gene Ther., 10, 2847–2857. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L., Gasper,W.J., Stass,S.A., Ioffe,O.B., Davis,M.A. and Mixson,A.J. (2002) Angiogenic inhibition mediated by a DNAzyme that targets vascular endothelial growth factor receptor 2. Cancer Res., 62, 5463–5469. [PubMed] [Google Scholar]
- 29.Yen L., Strittmatter,S.M. and Kalb,R.G. (1999) Sequence-specific cleavage of Huntingtin mRNA by catalytic DNA. Ann. Neurol., 46, 366–373. [DOI] [PubMed] [Google Scholar]
- 30.Crinelli R., Bianchi,M., Gentilini,L. and Magnani,M. (2002) Design and characterization of decoy oligonucleotides containing locked nucleic acids. Nucleic Acids Res., 30, 2435–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braasch D.A., Liu,Y. and Corey,D.R. (2002) Antisense inhibition of gene expression in cells by oligonucleotides incorporating locked nucleic acids: effect of mRNA target sequence and chimera design. Nucleic Acids Res., 30, 5160–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fluiter K., ten Asbroek,A.L., de Wissel,M.B., Jakobs,M.E., Wissenbach,M., Olsson,H., Olsen,O., Oerum,H. and Baas,F. (2003) In vivo tumor growth inhibition and biodistribution studies of locked nucleic acid (LNA) antisense oligonucleotides. Nucleic Acids Res., 31, 953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]