Children with brain injuries or cerebral palsy (CP) comprise a large percentage of pediatric clients served by physical therapists. There is no consensus on what the basic parameters should be for different treatment protocols. A very important parameter of intervention that is pivotal for treatment efficacy is dosing. Dosing decisions are complex. To date, the minimum doses for changing structure and function, activity, and participation in children with various disabilities are unknown. This article describes the process and outcomes of a research summit with the goals of: (1) fostering a critical debate that would result in recommendations for the development of large-scale, second-generation research proposals to address thresholds for effective dosing of interventions for children with brain injuries or CP and (2) enhancing the research capacity of pediatric physical therapists through collaborative research networks. The summit brought together an interdisciplinary cadre of researchers (physical therapists, basic and clinical scientists), representatives from funding agencies, and consumers to an intensive 2.5-day think tank. The summit targeted questions of treatment dosage related to 3 areas: practice and neuroplasticity, structure-behavior connections, and clinical trial design. The consensus was that the intervention must demonstrate some evidence of effectiveness before optimal dosing can be investigated. Constraint-induced movement therapy (CIMT) is used as an example of an intervention that has demonstrated effectiveness and that requires dosing-related research. Summit results, including factors that merit special consideration and recommendations for future dose-related studies, are highlighted.
Physical therapy is an important service for children with physical disabilities, particularly those with an injured brain resulting in neuromotor impairments and functional limitations.1 These children typically have multiple health complications that often result in complex functional limitations and require extensive health care, education, and vocational training. The costs of interventions result in substantial financial and social challenges for families and society.2 Although physical therapists frequently provide continuous interventions for children with physical disabilities, including CP,3 there remains limited empirical support for these services.4
Dosing represents a critical and pressing aspect of intervention that is central for treatment efficacy. In this article, dose is defined as the frequency, intensity, time, and type of an intervention.5 Frequency refers to how often, such as the number of sessions for a given intervention per day, week, or month. Intensity refers to how hard the child works within the intervention session and is recorded as the number of repetitions per minute, day, or week or amount of work (eg, 75% of maximal heart rate). Time refers to the duration of the intervention. Type refers to the kind of intervention and can be focused at any of the dimensions of the International Classification of Functioning, Disability and Health (ICF): body functions and structures, activity, or participation.6 Within types, variation exists. For example, task practice can vary in the type of behavioral shaping (ie, structured versus unstructured training) and amount of feedback or reward.
Type of intervention is central to the discussion because determining the “salient” or “active” ingredient of an intervention strategy is intimately related to the question of dosing. What is “it” that should be dosed? Intervention strategies also involve interactions with biomechanical, neuromuscular, and psychological elements of a child's behavior, necessitating insight into the mechanism leading to a change in behavior. Consequently, the nature of optimal dosage can be expected to vary with different types of interventions.
Intervention-related dosing is a source of disagreement among service providers, policy makers, insurers, and researchers, whose recent findings show the potential for high dosing intervention to be the catalyst for positive intervention outcomes.7 The cost related to high dosing is one impediment to implementation. Another is uncertainty about the minimal dose that is effective and sufficient. Some of the most promising targeted interventions that seek to promote plastic changes in the brain and muscle, such as training on a treadmill,8 CIMT,9–12 and strength training,13,14 have used varying doses. The findings from these studies have highlighted a complex dosing knowledge gap, namely, a disconnect between structural and behavioral changes, where changes that are seen at one dimension of the ICF are not always incorporated into others.
The disconnect between structural and behavioral changes15 provides a compelling argument for revisiting hypotheses regarding mechanisms for adaptive motor learning and for a critical examination of dosing at the cellular, structural, activity, and participation levels. Collectively, these findings and the question of dosing suggest that scientific exploration of the effect of dosing in pediatric physical therapy and its interaction with other aspects of intervention will be a challenging enterprise. The findings suggest that for the field of pediatric physical therapy or rehabilitation to move forward, the dosing question will require an interdisciplinary coordinated effort, using sound conceptual frameworks and a systems perspective.
Research Summits
A research summit offers a plausible launching pad for promoting an interdisciplinary coordinated effort and addressing some of these complex questions related to dosing. In this article, we define a research summit as a think-tank mechanism whereby different parties can contribute to the development of well-crafted research programs or action plans designed to move science on a specific topic to the next level. For the past 9 years, the Section on Pediatrics of the American Physical Therapy Association (APTA) has used interdisciplinary research summits developed by the first author and colleagues to foster development of large-scale research proposals on a topic of great importance to pediatric practice and simultaneously increase the research capacity of pediatric physical therapists. The results have shown that, with creative and meaningful collaborations, short-term intensive meetings can help increase the numbers of pediatric therapists with postprofessional doctoral training who are successful in securing funding to conduct research on a targeted topic that advances practice.
The first research summit (RS I), in 2004, addressed physical fitness in children with CP and secondary complications.16 To date, this summit has resulted in 71 publications, 36 presentations, and 29 funded grants, 6 of which were from the National Institutes of Health (NIH), totaling more than $2 million. The second summit (RS II), in 2007, focused on early intervention for children with or at risk for disabilities. It has resulted in 34 publications, 34 submitted grants, and 14 funded grants, 2 from NIH and 2 from the National Institute on Disability and Rehabilitation Research (NIDRR), totaling more than $900,000. Many of the grant proposals have involved collaboration between several physical therapists and non–physical therapist summit participants.
The current research summit (RS III) was modeled after these 2 highly successful summits to direct research attention to the critical issue of dosing of interventions for children with an injured brain. The goals of RS III were: (1) to foster a critical debate that would result in recommendations for the development of large-scale, second-generation research proposals to address thresholds for effective dosing for children with an injured brain or CP and (2) to enhance the research capacity of pediatric physical therapists by facilitating collaborations, information exchange (cross-fertilization), and networking among physical therapists, other researchers (basic, neuroscience, movement, and clinical scientists), consumers, and funding agencies in the area of dosing of interventions for children with an injured brain or CP. The summit was held in Alexandria, Virginia, in October 2011.
This article summarizes the highlights of the summit deliberations, factors that merited special consideration regarding dosing interventions, and recommendations for dosing studies that will help advance this area. The emphasis was on 3 body structures known to be affected by injury to the brain, rehabilitation, and dosing: muscle, bone, and brain.17–19 We used the ICF6 as an organizing framework. In addition to discussing issues related to direct intervention strategies, we also emphasized patient and family education and addressed the importance of activity, participation, and family dynamics. We illustrate the need for sequential and systematic studies of dosing and the salient features of intervention upon which dosing parameters should be based by summarizing the findings from an intervention with known efficacy (CIMT) that still requires dosing-related research.
Summit Organization
Participants
A total of 48 individuals (see Appendix) participated in the summit. The planning committee, with the help of other researchers and representatives of funding agencies, identified 12 scientists with relevant expertise in neuroscience, movement science, pediatrics, muscle and exercise physiology, imaging, bone health, neurology, occupational therapy, and statistics. Also invited were representatives from APTA, funding agencies and foundations, and 4 consumers. The committee identified 2 physical therapy researchers with established research agendas and a history of federal funding (Dr Stuart Binder-Macleod and Dr Diane Damiano) to serve as facilitators. Details of participants are listed in Table 1.
Table 1.
Characteristics of Research Summit Participants
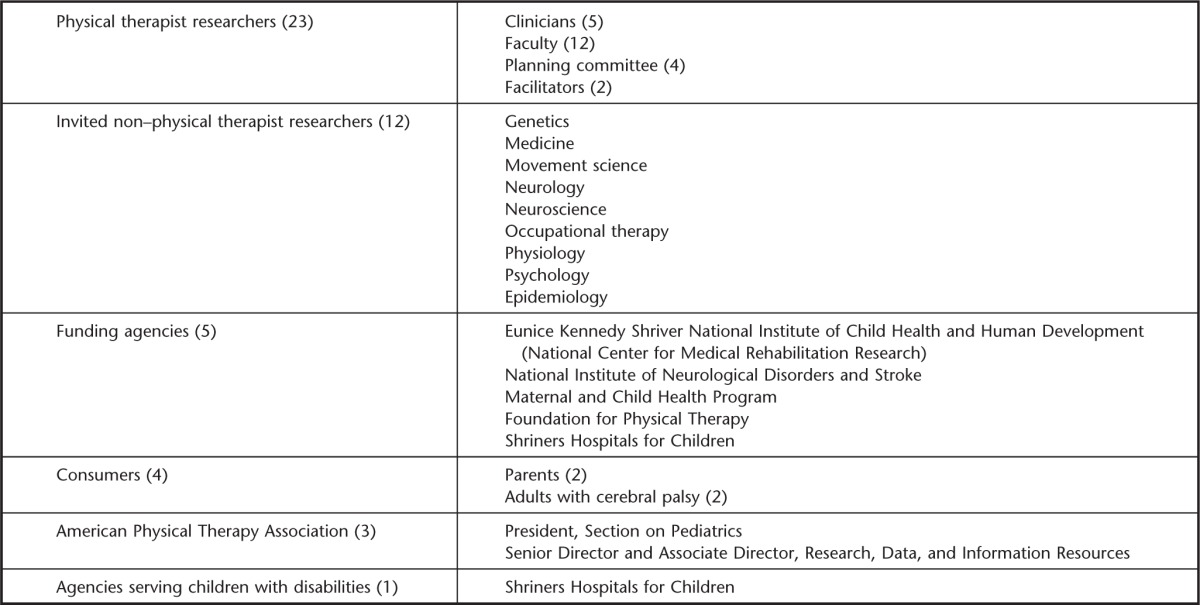
Participation by additional pediatric physical therapists was through an application process. An independent review committee selected 17 physical therapist participants who met one or more criteria: history of funded research in the area of interventions or measurement for children with neurological disorders, currently conducting research (funded or unfunded) in this area, or postprofessional physical therapist students who are mentored by researchers conducting clinical trials or intervention research.
All participants submitted a 3-page biographical sketch and a 2- to 4-page position or perspective paper that focused on the subject of dosing and expectations from the summit and highlighted their own research. The biographical sketches and position or perspective papers were disseminated to all participants before the summit to maximize information sharing and help participants become familiar with one another's research and expertise, thereby facilitating networking and communication during the summit.
The planning committee applied for and received funding from APTA's Section on Pediatrics, the National Institute of Child Health and Human Development, and the National Institute of Neurologic Disorders and Stroke to support the cost of the summit.
Format
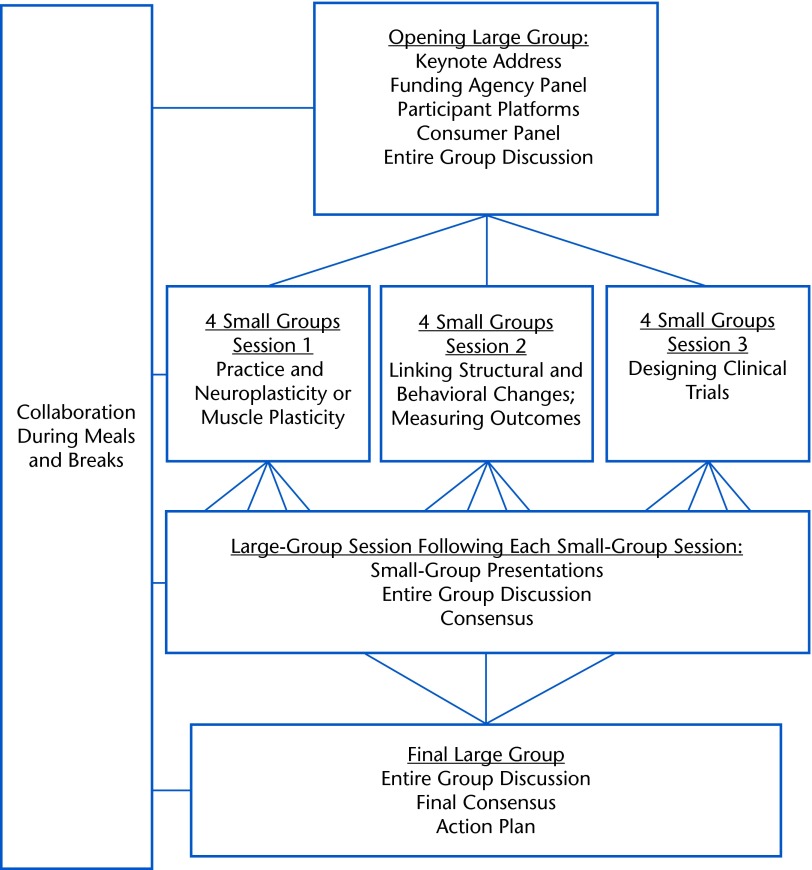
The RS III was a 3-day conference designed to systematically address the current state of clinical research in the area of intervention dosing and to create research networks and goals. The first day focused on information exchange and identification of knowledge gaps. The second day built on the information presented on the first day by formulating scientific questions and considering research designs to explore dosing. The third day was dedicated to the development of recommendations, action plans, and research priorities. The Figure presents the organization of the summit.
Figure.
Format and organization of Research Summit III.
The format included formal presentations and small- and large-group discussions. Formal presentations consisted of 2 keynote addresses, presentations by representatives from funding agencies, and 5- or 10-minute platform presentations by participants. The first keynote address was delivered by Dr Edward Taub, an established researcher experienced in multisite clinical trials. The goals of this keynote address were to “frame the summit” and to present “state of the science” in the area of intervention dosing. A panel of 2 family members and 2 individuals with CP gave the second keynote address. All were from the Washington, DC, metro area and diverse in terms of ethnicity, socioeconomic status, and age. The goals for the consumer panel were to highlight the realities of being on the receiving end of clinical practice and the implications of overdosing or underdosing for families.
Overall, the keynote presentations were intended to provide a catalyst for discussions and planning, expand recognition and appreciation of the impact of dosing and its complexity, and highlight potential research questions. Presentations by all participants were intended to define the scope of inquiry by exposing the group to the breadth and themes of research activities in which participants are engaged, exchange information on current and past research efforts, and highlight potential areas for collaborations and strategies for funding.
Small- and Large-Group Discussions
The goals of these group discussions were: (1) to promote the formation of partnerships, (2) to synthesize research findings, (3) to generate research questions that would lead to multisite research projects, and (4) to promote discussion of preliminary research proposals, action plans, and recommendations. Experienced researchers were grouped with less experienced investigators based on areas of interest or expertise. There were a total of 3 small-group discussion sessions, each consisting of at least 10 participants: (1) practice and neuroplasticity or muscle plasticity and recovery in children with injured brains or CP, (2) linking structural and behavioral changes and measuring outcomes, and (3) designing clinical trials. These 3 target areas were believed by the planning committee to be the crux for advancing the study of intervention dosing and emphasized muscle, bone, and brain. Participants were preassigned for the first 2 small-group sessions and allowed to self-select for the third small-group session based on research interests and potential areas of collaboration. Each small group discussed the same topic simultaneously. Reports of small-group discussions were presented to the large group for an entire group discussion and consensus for future collaborative research efforts.
The facilitators oriented the group to the charge and tasks at hand, maintained the focus and integrity of the summit goals, facilitated large-group discussions, helped the group adhere to time guidelines, provided guidance when needed, and summarized discussions. Four members of the planning committee served as small-group facilitators to maintain the integrity of the goals of the small-group discussions relative to the summit goals and to record the small- and large-group discussions for later transcription.
Proceedings
In the sections that follow, we highlight and summarize deliberations from small- and large-group discussions, specific aspects that merited critical consideration on the topic of dosing, and recommendations for research generated by the summit participants. Examples from the target areas of bone and brain behavior are used to offer models of dosage-related research that were discussed in the summit.
Opening Large Group
The consensus from the first entire group discussion that followed the keynote addresses and presentations by research participants and representatives from funding agencies was that dosing is a complex, multifactorial construct. Participants acknowledged that the minimal and optimal dosage effects of certain interventions would depend on the cognitive, neuromuscular, and biomechanical resources of the children served, the nature of the intervention strategy, the type of functional goals to be acquired and sustained, and environment influences. A major challenge cited by Dr Taub in his keynote address was to identify the salient components of the intervention itself that lead to changes in motor function and greater independence. Aspects to be considered included: repetitions of movement required per hour, hours of intervention per day, days per week, total number of days, and amount of intervention. From the point of view of clinical practice, questions of intensity of intervention, distribution of intervention, and total time of intervention are related but empirically separate issues, as are their interactions. The sentiment was that each of these variables would require its own set of quantitative studies or that the interaction effects of these variables should be considered separately in comparative effectiveness research. Another challenge, corroborated by the information presented by the panel of consumers, was to determine the interaction of dose with other environmental influences. Caregivers carry over many interventions used in pediatric rehabilitation, often within nonclinical or nonlaboratory settings. The group acknowledged that because dosing may need to be determined for each unique intervention, a systematic exploration of dosing would be a vast enterprise requiring research over many years.
Large- and Small-Group Discussion: Practice and Neuroplasticity or Muscle/Bone Plasticity and Recovery in Children With Injured Brains or CP
Reports from the first small-group discussions coalesced around the need to understand the effectiveness or mechanism of recovery for specific types of interventions within and across patients and disorders. Mechanistic explanations must seek to address the question of why some changes are sustainable and some are not. Dose-related mechanistic studies also must address exogenous and endogenous factors likely to interact with plastic changes in the nervous system, bone, and muscles. Studies of the integrity of the bone architecture of children with CP or injured brains offer a model for considering specific exogenous and endogenous factors.20
Dosing research questions targeting bone architecture in children with CP should consider exercise that primarily targets the bone-forming cells (osteoblasts) in the growing skeleton. Osteoblasts are most responsive to novel, high-impact activities compared with low-impact activities and are more responsive when rest is included between repetitions.21–24 The magnitude of impact loading may be modified based on a child's severity of impairment and may change as a child adapts to the exercise program. Because the skeleton prefers fewer repetitions with more rest between exercise sessions to maximize the skeletal benefits, frequency of dosing will be another important parameter. Bone tissue has a saturation point at which osteoblasts stop responding.25 Certain exogenous factors that have the potential to both enhance and restrict the osteogenic potential of a particular exercise, such as calcium and vitamin D, bisphosphonates, or anticonvulsants,26 also need to be considered. In addition, the skeleton requires a longer duration (typically a minimum of 3 months) to generate a measurable response to exercise compared with other organ systems such as cardiac and skeletal muscle tissue.27 Specific dose-related mechanistic questions were suggested to be different depending on the focus of the inquiry. Examples of mechanistic questions discussed are presented in Table 2.
Table 2.
Examples of Dose-Related Research Questionsa
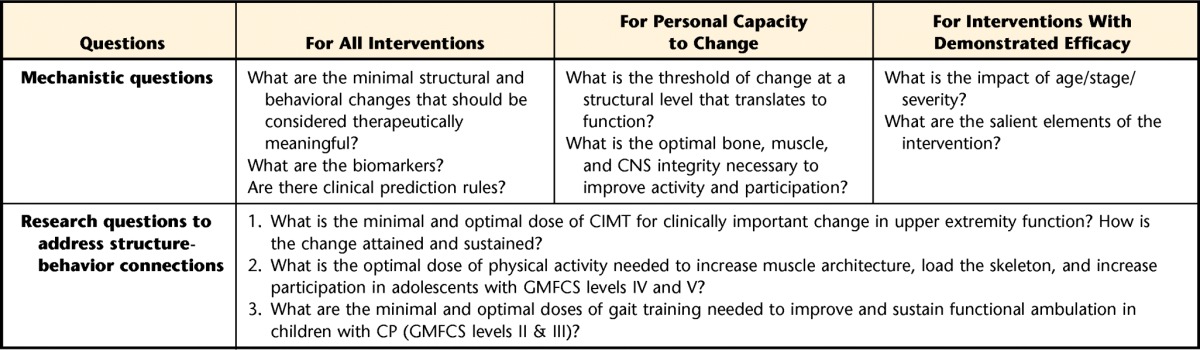
CNS=central nervous system, CIMT=constraint-induced movement therapy, GMFCS=Gross Motor Function Classification System, CP=cerebral palsy.
Large- and Small-Group Discussion: Linking Structural and Behavioral Changes and Measuring Outcomes
Three perspectives emerged from the entire group discussion based on reports from small-group discussions on structure-behavior relationship: (1) that current dose-response intervention studies have reported outcomes that do not always translate directly to valid life situations, (2) that changes reported appear to be short-lived, and (3) that the results attained through intervention are context specific (ie, you get what you train). Examples of these perceptions include reports that the cortical reorganization observed following high doses of upper limb sensorimotor stimulation for children with CP has not necessarily led to improvement in functional use of the affected arm.11 Changes in muscle structure have been associated with limited changes in gait.28,29 Other studies have demonstrated significant improvements in unimanual capacity in children who received unilateral training and improved bimanual performance in those who received bilateral upper extremity training.9,30 Table 2 presents examples of research questions independently identified by the small groups that, if addressed, would help to elucidate answers to dosing questions and structure-behavior disconnect.
Pertinent to the need to understand structure-behavior connections was the consideration that although outcomes measured in the laboratory are deemed important, the patient's life situation also must be measured to capture the intervention effect. Improvements in the laboratory do not matter if the patient's life situation does not change. Furthermore, outcomes must include determination of retention and whether permanent change has occurred. The conclusion was that valid studies of intervention dosing must discern the minimum and optimal doses that can achieve effects across all levels of the ICF that are sustainable and generalizable to new contexts. The group also highlighted the importance of using and analyzing measures of patient engagement and performance-based data in addition to global measures of intervention intensity and frequency.
The paucity of multilevel outcome measures that span ICF levels and the challenge of balancing the use of many measures with children's tolerance and cost were acknowledged. Given lessons gained from the few clinical trials, combined with greater knowledge of the neural mechanisms underlying brain organization or reorganization in children with CP,31,32 dose-related research also must involve use of valid markers of body structure and function that predict changes in activity and participation. The existence of many measures of performance at the activity level of the ICF was acknowledged. These measures could be correlated with data from recent advances in imaging techniques such as voxel-based morphometry33 and diffusion tensor imaging.34
The greatest paucity of measures is at the participation level. The group discussed newer, “smarter” outcomes such as computer adaptive testing (CAT), which tailors items that adapt to the child's ability level. Several CATs have been developed and validated to measure physical function.35,36 Discussion included the use of the Patient-Reported Outcomes Measurement Information System (PROMIS®), which was developed by NIH to provide a system of highly reliable, precise measurement of patient-reported health status for physical, mental, and social well-being.37 The use of CAT and PROMIS® with children with CP has been the focus of several recent investigations.36,38,39
Large- and Small-Group Discussion: Designing Clinical Trials
The small-group discussion session on clinical trials and other complementary research designs identified 3 areas of research: (1) minimal dose and optimal dose for clinically important upper extremity function; (2) optimal dose to improve and sustain functional ambulation in children with CP (Gross Motor Function Classification System [GMFCS] levels II and III), which included examining the optimal dose of physical activity to load the skeleton to increase muscle and bone architecture and bone health; and (3) the relationship between utilization and outcomes in longitudinal studies or comparative effectiveness research (ie, what works for whom and when and the potential benefits and harms of an intervention). A summary of the extensive discussions on dose-related study designs, challenges, and opportunities as they pertain to these 3 areas are outlined below. The summary also serves as recommendations for future dose-related research designs.
Multisite trials.
Collaborative multisite trials and longitudinal studies, including comparative effectiveness designs, were recommended as necessary strategies for attaining a critical mass of data to address the dosing questions and challenges described previously. Investigators should propose and only study interventions that have the potential to generate meaningful change for the child and family and that are clinically feasible, cost-effective, and both tolerable and safe.
Dosing threshold.
Determining effective dose must involve a sequential set of related hypotheses and studies. Demonstration of effectiveness in achieving a meaningful outcome should come first, followed by carefully planned studies to determine the minimal dose for eliciting or maintaining the same desired outcome. The sequential approach should further require identification of the salient components of the intervention itself that lead to changes in ultimate independence of the participants and questions of frequency, intensity, and timing of a type of intervention (dosing), which are related but empirically separate issues, as well as including covariates such as child characteristics.40
Sustainability.
For the intervention to be considered effective, gains achieved at the end of an intervention regimen must be sustainable, which would require at least one follow-up evaluation after more temporary effects would have dissipated. Promoting sustainability would need to incorporate strategies such as a “transfer package,” which is a detailed home program to facilitate transfer of therapeutic gains to daily life activities.41,42
Uptake of the intervention.
Related to sustainability is the concept of intervention uptake. This concept refers to tracking changes at different time points during an intervention to gather information on the dose-response relationship, determine at what points different outcomes were achieved, and establish whether continued intervention beyond that produced led to continued improvements. This recommendation suggests that dosing-related studies will require repeated-measures designs rather than pretest-posttest designs.
Child and family engagement.
Although all agreed that it would be useful to evaluate optimal dosing, it was recognized that other factors beyond the dose would affect the response and, therefore, would need to be measured as well. Because personal and environmental factors will moderate or mediate the dose-response relationship, they must be clearly defined, measured, and included in analyses. Personal factors must include motivation, self-efficacy, level of engagement, and environmental family resources. The group agreed that the onus should be on all investigators to study only interventions that have the potential to generate meaningful change for the child and family and that are feasible, cost-effective, tolerable, and safe. Because variability in response to dosed interventions is due, in part, to individual differences that are social, cultural, psychological, and genetic, the complexity of teasing out the influence of individual differences will be confounded by the interrelationships among these factors and the biological factors outlined previously. The question for researchers should be: What aspects of the family, beyond socioeconomic status and ethnicity, interact with intervention doses to influence outcomes? The consumer panel raised concerns about discontinuities of therapy, especially as children grow older, and elaborated on the importance of tailoring the intensity of therapy to the child's current age-related interests, such as sports or work. Therefore, dose-related studies will require use of multivariate designs and clear theoretical or conceptual frameworks. The panel strongly advocated for an increased level of consumer input in research and training projects and that such input should be solicited early in the process of grant proposal or program planning.
Common outcomes.
The group discussed the need to consider having an agreed-on minimal dataset or consistent set of outcomes for studies with similar aims, such as those with the goal of improving ambulation or upper limb function. Existing resources such as the NIH Toolbox43,44 and PROMIS®35 warrant consideration and further exploration as standard outcomes for dosing investigations. Because meta-analyses are one approach to determine the effectiveness of existing interventions, the use of common outcomes was viewed as being of great importance in addressing dosing questions.
Overall, the consensus was that the intervention must demonstrate some evidence of effectiveness before optimal dosing should be investigated. An example of an intervention that has demonstrated effectiveness and that requires dosing-related research is CIMT with children.45 This intervention also was selected as a future focus area by one group during the small-group discussions to address the question of a minimal dose and optimal dose for clinically important upper extremity function. What follows is a brief account of the modified CIMT findings, summarized in Table 3, that underscores dosing cannot be considered separately from the various other parameters of intervention that it interacts with closely.
Table 3.
Chronology of the Modified Constraint-Induced Movement Therapy (CIMT) Studies and Relation to Dosinga
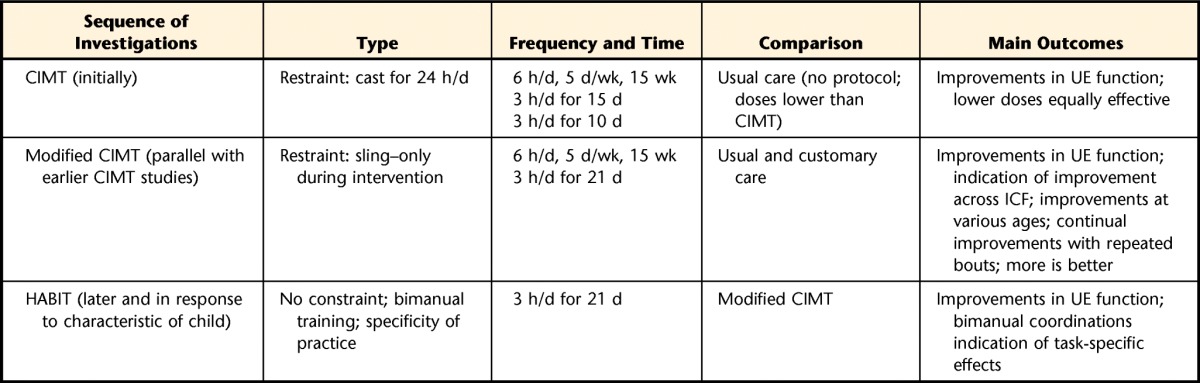
A retrospective analysis of more than 100 children undergoing either 60 or 90 hours of modified CIMT or hand-arm bimanual intensive therapy (HABIT)46 show that 90 hours of training resulted in better improvements or better retention of improvements at subsequent follow-up than 60 hours of training. UE=upper extremity, ICF=International Classification of Functioning, Disability and Health.
Initially, children with CP and hemiplegia (aged 7 months to 8 years) were given 6 hours of intervention per day involving functional task practice with shaping (ie, training with positive reinforcement) for 15 consecutive weekdays.47 Subsequently, in children with CP and hemiplegia aged 2 to 6 years, it was found that just 3 hours per day, for 15 days, yielded results that were equivalent.42 Further research showed that reducing the duration of training from 15 to 10 consecutive weekdays while keeping the number of hours of training per day at 3 hours did not reduce the therapeutic outcome.48,49 However, restraint of the less affected arm, accomplished by casting, was maintained 24 hours per day in each of the studies. Another early CIMT research model, developed in parallel, demonstrated motor learning after extensive practice.50 In this paradigm, modified restraints and age-appropriate activities (play) with the more affected upper extremity were used. It should be pointed out, however, that despite there being nearly 70 studies of pediatric CIMT, until recently almost all of these studies compared the intervention with a no-intervention or usual and customary care group at normal dosing schedules,51 suggesting that improvements following CIMT may be a reflection of time rather than the type of intervention itself. Following the development of hand-arm bimanual intensive therapy (HABIT),46,52 randomized controlled trials of HABIT and modified CIMT have shown that both groups demonstrated similar improvements in unimanual dexterity and use of the impaired upper extremity as a bimanual assist.30,53 Improvements in spatiotemporal coordination of the upper extremities during a bimanual task54 suggest the importance of specificity of practice (type). Overall findings30 show that 90 hours of training resulted in better improvements and better retention of improvements at subsequent follow-up than 60 hours of training. This example highlights the importance of research to understanding the specific therapeutic ingredients and their relationship to sustained changes.
Outcomes
The primary goal of this summit was to foster a critical debate that would result in recommendations for the development of large-scale research proposals to address dosing for children with injured brains or CP. Information presented in the preceding sections suggests that the summit was successful in fostering a critical debate on this topic and proposing recommendations for advancing research on this topic. The next steps for moving intervention dosing forward are summarized in Table 4 and outline what RS III participants, physical therapists, and different entities can do to advocate that intervention dosing be a research priority and to foster collaborations on a broad scale.
Table 4.
Recommendations for Advancing Research on Dosinga
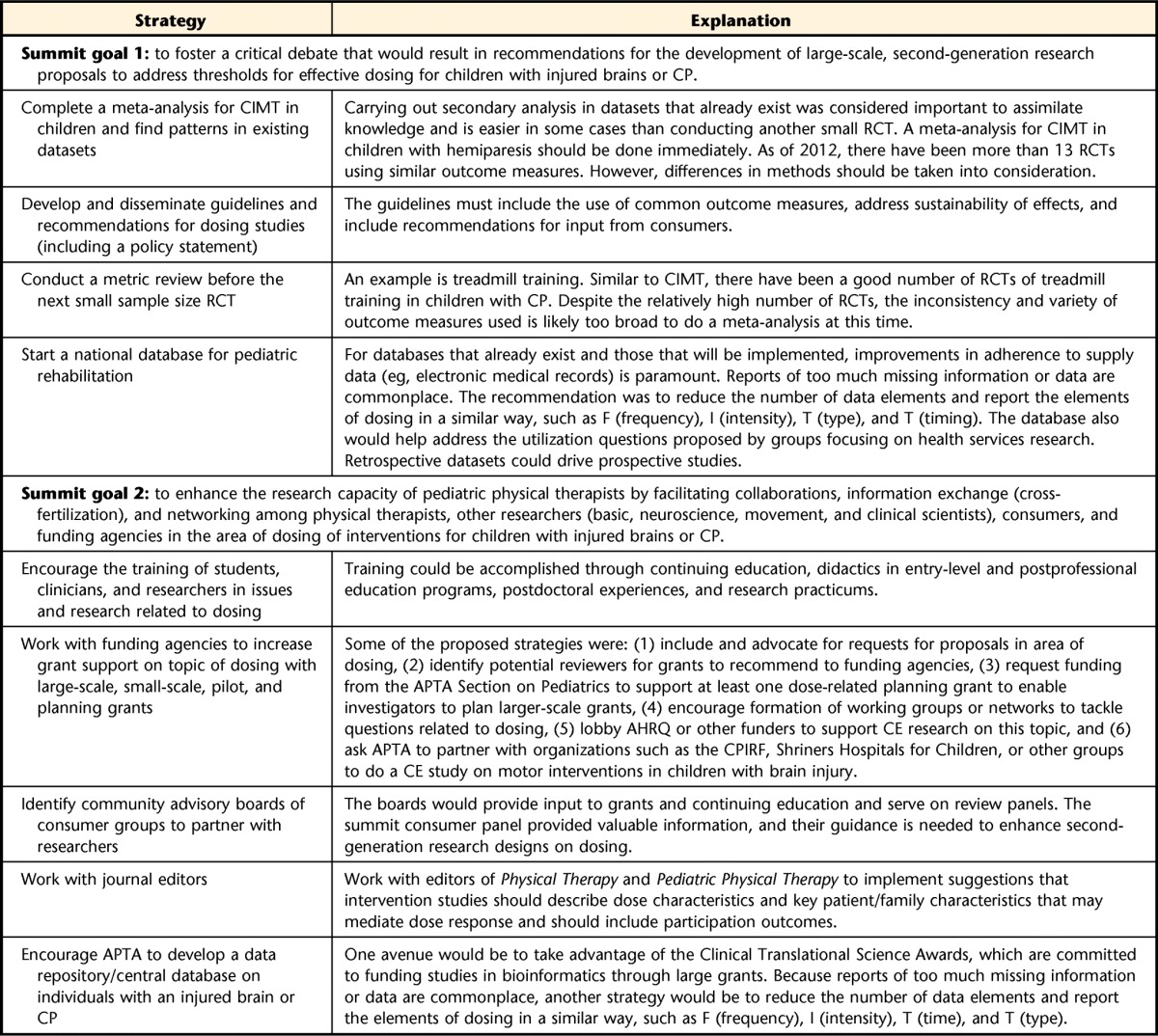
CP=cerebral palsy, CIMT=constraint-induced movement therapy, RCT=randomized controlled trial, AHRQ=Agency for Healthcare Research and Quality, CE=comparative effectiveness, APTA=American Physical Therapy Association, CPIRF=Cerebral Palsy International Research Foundation.
The second goal of enhancing the research capacity of pediatric physical therapists by facilitating collaborations and networking was partially realized, as evidenced by how the groups self-selected for the third small-group discussion. Some of the groups went on to plan and submit 2 interdisciplinary planning grants that were funded by the Section on Pediatrics in 2012. Pediatric physical therapists served as principal investigators. As observed in previous summits, we expect the benefits of this summit to increase with time. Summit participants will be surveyed every 2 years about submitted grants, funded research, and publications that resulted from this summit.
Conclusion
This research summit was considered the first and necessary step in a systematic and in-depth analysis of dosing questions and an interdisciplinary approach toward second-generation dose-related research. The overall consensus is that an intervention must demonstrate effectiveness before dose-related studies can be undertaken. In this article, we summarized the process and outcome of the group discussions, highlighted several factors that merit special consideration for dosing studies, and proposed recommendations for clinical trials and other strategies to advance research on this topic. The recommendations are intended to facilitate second-generation intervention research questions and targeted interdisciplinary multisite protocols, influence funding, foster understanding of dose-response rehabilitation protocols at all levels of the ICF, and ultimately improve the lives of children with an injured brain or CP.
Appendix.
Appendix.
Section on Pediatrics Research Summit III Participantsa
a The Research Summit III (Dosing and Motor Learning for Children With an Injured Brain or Cerebral Palsy) was sponsored by the American Physical Therapy Association (APTA) Section on Pediatrics, the National Institutes of Health's Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Neurological Disorders and Stroke (NINDS); October 28–30, 2011; Alexandria, Virginia. Participants' affiliations at the time of Research Summit III are listed.
Footnotes
Dr Kolobe, Dr Christy, Dr Gannotti, Dr Heathcock, Dr Damiano, Dr Taub, Dr Majsak, Dr Fuchs, Dr O'Neil, and Dr Caiozzo provided concept/idea/project design. Dr Kolobe, Dr Christy, Dr Gannotti, Dr Heathcock, Dr Damiano, Dr Taub, Dr Majsak, Dr Gordon, and Dr Fuchs provided writing. Dr Kolobe, Dr Christy, Dr Gannotti, and Dr Heathcock provided project management. Dr Kolobe, Dr Christy, Dr Gannotti, and Dr Heathcock provided fund procurement. All authors provided consultation (including review of manuscript before submission).
The authors thank the American Physical Therapy Association, Section on Pediatrics; the National Institutes of Health's Eunice Kennedy Shriver National Institute of Child Health and Human Development; and the National Institute of Neurological Disorders and Stroke (grant R13HD070615, principal investigator: Dr Kolobe) for funding the Research Summit.
References
- 1. Rosenbaum PL. Cerebral palsy: what parents and doctors want to know. BMJ. 2003;326:970–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Economic Costs Associated With Mental Retardation, Cerebral Palsy, Hearing Loss, and Vision Impairment. Atlanta, GA: Centers for Disease Control and Prevention; 2004 [Google Scholar]
- 3. Consortium for Youth and Children With Special Health Care Needs. Children With Special Health Care Needs and Access to Physical Therapy Services: A Fact Sheet on Findings. Washington, DC: Center for Child and Human Development, Georgetown University; 2002 [Google Scholar]
- 4. Damiano DL. Rehabilitative therapies in cerebral palsy: the good, the not as good, and the possible. J Child Neurol. 2009;24:1200–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American College of Sports Medicine. ASCM's Guidelines for Exercise Testing and Prescription. 9th ed Philadelphia, Pa: Lippincott Williams & Wilkins; 2013 [Google Scholar]
- 6. International Classification of Functioning, Disability and Health: ICF. Geneva, Switzerland: World Health Organization; 2001 [Google Scholar]
- 7. Institute of Medicine of the National Academies. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: National Academies Press; 2009 [Google Scholar]
- 8. Ulrich DA, Ulrich BD, Angulo-Kinzler RM, Yun J. Treadmill training of infants with Down syndrome: evidence-based developmental outcomes. Pediatrics. 2001;108:E84. [DOI] [PubMed] [Google Scholar]
- 9. Huang HH, Fetters L, Hale J, McBride A. Bound for success: a systematic review of constraint-induced movement therapy in children with cerebral palsy supports improved arm and hand use. Phys Ther. 2009;89:1126–1141 [DOI] [PubMed] [Google Scholar]
- 10. DeLuca SC, Echols K, Law CR, Ramey SL. Intensive pediatric constraint-induced therapy for children with cerebral palsy: randomized, controlled, crossover trial. J Child Neurol. 2006;21:931–938 [DOI] [PubMed] [Google Scholar]
- 11. Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104 [DOI] [PubMed] [Google Scholar]
- 12. Wallen M, Ziviani J, Herbert R, et al. Modified constraint-induced therapy for children with hemiplegic cerebral palsy: a feasibility study. Dev Neurorehabil. 2008;11:124–133 [DOI] [PubMed] [Google Scholar]
- 13. Damiano DL. Loaded sit-to-stand resistance exercise improves motor function in children with cerebral palsy. Aust J Physiother. 2007;53:201. [DOI] [PubMed] [Google Scholar]
- 14. Dodd KJ, Taylor NF, Damiano DL. A systematic review of the effectiveness of strength-training programs for people with cerebral palsy. Arch Phys Med Rehabil. 2002;83:1157–1164 [DOI] [PubMed] [Google Scholar]
- 15. Dobkin BH. Rehabilitation and functional neuroimaging dose-response trajectories for clinical trials. Neurorehabil Neural Repair. 2005;19:276–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fowler EG, Kolobe TH, Damiano DL, et al. ; Section on Pediatrics Research Summit Participants, Section on Pediatrics Research Committee Task Force. Promotion of physical fitness and prevention of secondary conditions for children with cerebral palsy: Section on Pediatrics Research Summit Proceedings. Phys Ther. 2007;87:1495–1510 [DOI] [PubMed] [Google Scholar]
- 17. Anderson V, Spencer-Smith M, Wood A. Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain. 2011;134(pt 8):2197–2221 [DOI] [PubMed] [Google Scholar]
- 18. Biewener AA, Bertram JE. Structural response of growing bone to exercise and disuse. J Appl Physiol. 1994;76:946–955 [DOI] [PubMed] [Google Scholar]
- 19. Navarrette R, Vrbova G. Activity-dependent interactions between motoneurones and muscles: their role in the development of the motor unit. Prog Neurobiol. 1993;41:93–124 [DOI] [PubMed] [Google Scholar]
- 20. Holihan CM, Stevenson RD. Bone density in children with cerebral palsy. Phys Med Rehabil Clin N Am. 2009;20:493–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fuchs RK, Bauer JJ, Snow CM. Jumping improves hip and lumbar spine bone mass in prepubescent children: a randomized controlled trial. J Bone Miner Res. 2001;16:148–156 [DOI] [PubMed] [Google Scholar]
- 22. Mackelvie KJ, McKay HA, Khan KM, Crocker PR. A school-based exercise intervention augments bone mineral accrual in early pubertal girls. J Pediatr. 2001;139:504–508 [DOI] [PubMed] [Google Scholar]
- 23. Gunter KB, Baxter-Jones AD, Mirwald RL, et al. Impact exercise increases BMC during growth: an 8-year longitudinal study. J Bone Miner Res. 2008;23:986–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gunter KB, Almstedt HC, Janz KF. Physical activity in childhood may be the key to optimizing lifespan skeletal health. Exerc Sport Sci Rev. 2012;40:13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robling AG, Bellido T, Turner CH. Mechanical stimulation in vivo reduces osteocyte expression of sclerostin. J Musculoskelet Neuronal Interact. 2006;6:354. [PubMed] [Google Scholar]
- 26. Lee RH, Lyles KW, Colon-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8:34–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Warden SJ, Fuchs RK. Exercise and bone health: optimising bone structure during growth is key, but all is not in vain during ageing. Br J Sports Med. 2009;43:885–887 [DOI] [PubMed] [Google Scholar]
- 28. Damiano DL, Arnold AS, Steele KM, Delp SL. Can strength training predictably improve gait kinematics? A pilot study on the effects of hip and knee extensor strengthening on lower-extremity alignment in cerebral palsy. Phys Ther. 2010;90:269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moreau NG, Holthaus K, Marlow N. Differential adaptations of muscle architecture to high-velocity versus traditional strength training in cerebral palsy. Neurorehabil Neural Repair. 2013;27:325–334 [DOI] [PubMed] [Google Scholar]
- 30. Gordon AM. To constrain or not to constrain, and other stories of intensive upper extremity training for children with unilateral cerebral palsy. Dev Med Child Neurol. 2011;53(suppl 4):56–61 [DOI] [PubMed] [Google Scholar]
- 31. Trivedi R, Gupta RK, Shah V, et al. Treatment-induced plasticity in cerebral palsy: a diffusion tensor imaging study. Pediatr Neurol. 2008;39:341–349 [DOI] [PubMed] [Google Scholar]
- 32. Sutcliffe TL, Gaetz WC, Logan WJ, et al. Cortical reorganization after modified constraint-induced movement therapy in pediatric hemiplegic cerebral palsy. J Child Neurol. 2007;22:1281–1287 [DOI] [PubMed] [Google Scholar]
- 33. Ashburner J, Friston KJ. Voxel-based morphometry: the methods. Neuroimage. 2000;11(6 pt 1):805–821 [DOI] [PubMed] [Google Scholar]
- 34. Yoshida S, Hayakawa K, Oishi K, et al. Athetotic and spastic cerebral palsy: anatomic characterization based on diffusion-tensor imaging. Radiology. 2011;260:511–520 [DOI] [PubMed] [Google Scholar]
- 35. Haley SM, Chafetz RS, Tian F, et al. Validity and reliability of physical functioning computer-adaptive tests for children with cerebral palsy. J Pediatr Orthop. 2010;30:71–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kratz AL, Slavin MD, Mulcahey MJ, et al. An examination of the PROMIS pediatric instruments to assess mobility in children with cerebral palsy. Qual Life Res. 2013;22:2865–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Forrest CB, Bevans KB, Tucker C, et al. Commentary: the Patient-Reported Outcome Measurement Information System (PROMIS®) for children and youth: application to pediatric psychology. J Pediatr Psychol. 2012;37:614–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haley SM, Fragala-Pinkham MA, Dumas HM, et al. Evaluation of an item bank for a computerized adaptive test of activity in children with cerebral palsy. Phys Ther. 2009;89:589–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haley SM, Ni P, Dumas HM, et al. Measuring global physical health in children with cerebral palsy: illustration of a multidimensional bi-factor model and computerized adaptive testing. Qual Life Res. 2009;18:359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosenbaum PL, Walter SD, Hanna SE, et al. Prognosis for gross motor function in cerebral palsy: creation of motor development curves. JAMA. 2002;288:1357–1363 [DOI] [PubMed] [Google Scholar]
- 41. Taub E, Griffin A, Nick J, et al. Pediatric CI therapy for stroke-induced hemiparesis in young children. Dev Neurorehabil. 2007;10:3–18 [DOI] [PubMed] [Google Scholar]
- 42. Taub E, Griffin A, Uswatte G, et al. Treatment of congenital hemiparesis with pediatric constraint-induced movement therapy. J Child Neurol. 2011;26:1163–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rine RM, Schubert MC, Whitney SL, et al. Vestibular function assessment using the NIH Toolbox. Neurology. 2013;80(11 suppl 3):S25–S31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reuben DB, Magasi S, McCreath HE, et al. Motor assessment using the NIH Toolbox. Neurology. 2013;80(11 suppl 3):S65–S75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taub E, Miller NE, Novack TA, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74(4):347–354 [PubMed] [Google Scholar]
- 46. Gordon AM, Schneider JA, Chinnan A, Charles JR. Efficacy of a hand-arm bimanual intensive therapy (HABIT) in children with hemiplegic cerebral palsy: a randomized control trial. Dev Med Child Neurol. 2007;49:830–838 [DOI] [PubMed] [Google Scholar]
- 47. Taub E, Ramey SL, DeLuca S, Echols K. Efficacy of constraint-induced movement therapy for children with cerebral palsy with asymmetric motor impairment. Pediatrics. 2004;113:305–312 [DOI] [PubMed] [Google Scholar]
- 48. Taub E, Ramey S, DeLuca S, Echols K. Efficacy of CI therapy for children with cerebral palsy. Program No. 106.111. Neuroscience Abstracts. Orlando, FL: Society for Neuroscience; 2002 [Google Scholar]
- 49. Taub E, Griffin A, Gammons K, Nick J, Law C. CI therapy for young children with congenital hemiparesis. Program No. 583.518/OO514. Neuroscience Abstacts. Atlanta, GA: Society for Neuroscience; 2006 [Google Scholar]
- 50. Gordon AM, Charles J, Duff SV. Fingertip forces during object manipulation in children with hemiplegic cerebral palsy. II: bilateral coordination. Dev Med Child Neurol. 1999;41:176–185 [DOI] [PubMed] [Google Scholar]
- 51. Sakzewski L, Ziviani J, Boyd R. Systematic review and meta-analysis of therapeutic management of upper-limb dysfunction in children with congenital hemiplegia. Pediatrics. 2009;123:e1111–e1122 [DOI] [PubMed] [Google Scholar]
- 52. Charles JR, Gordon AM. A repeated course of constraint-induced movement therapy results in further improvement. Dev Med Child Neurol. 2007;49:770–773 [DOI] [PubMed] [Google Scholar]
- 53. Gordon AM, Chinnan A, Gill S, et al. Both constraint-induced movement therapy and bimanual training lead to improved performance of upper extremity function in children with hemiplegia. Dev Med Child Neurol. 2008;50:957–958 [DOI] [PubMed] [Google Scholar]
- 54. Hung YC, Casertano L, Hillman A, Gordon AM. The effect of intensive bimanual training on coordination of the hands in children with congenital hemiplegia. Res Dev Disabil. 2011;32:2724–2731 [DOI] [PubMed] [Google Scholar]