Abstract
Evidence suggests that thrombin, a blood coagulation serine protease, mediates neuronal injury in experimental cerebral ischemia. Here, we test the hypothesis that nafamostat mesilate, a serine protease inhibitor, may ameliorate ischemia-induced neuronal damage through thrombin inhibition after ischemic stroke. Focal ischemia was induced in adult Sprague-Dawley rats by occlusion of the middle cerebral artery for 2 hours followed by 22 hours of reperfusion. The administration of nafamostat mesilate during ischemia and reperfusion reduced the brain infarct volume, edema volume and neurological deficit. Thrombin expression and activity in the ipsilateral striatum were increased after ischemia, whereas the administration of nafamostat mesilate significantly inhibited thrombin expression and activity. Immunostaining showed that the majority of thrombin was expressed in neurons. TUNEL staining showed that nafamostat mesilate reduced the number of dying cells during ischemia. A rat behavioral test showed that nafamostat mesilate treatment significantly improved the learning ability of ischemic rats. These results suggest that nafamostat mesilate may have a potential therapeutic role for neuroprotection against focal cerebral ischemia through thrombin inhibition.
Stroke can elicit many damaging events, such as blood-brain barrier (BBB) disruption, brain edema, and especially the induction of deleterious factors, such as leakage of thrombin from peripheral blood to the brain, which can cause brain injury1,2,3,4. Thrombin is a multifunctional serine protease generated by prothrombin cleavage, which can convert fibrinogen to fibrin during blood coagulation5,6. However, mounting evidence indicates that thrombin is also involved in a variety of pathological processes of brain diseases, including brain trauma, severe epilepsy, neurodegenerative disease and stroke, and others7. It has been reported that thrombin levels are increased in the ischemic core zone8,9. Following ischemia, the BBB becomes more permeable to high-molecular-weight proteins, such as the thrombin precursor prothrombin8. The brain itself may also be a source of prothrombin. This hypothesis is supported by the fact that prothrombin mRNA is upregulated after cerebral ischemia10. In addition, Factor Xa mRNA has been identified in the brain11, which is required for the cleavage of prothrombin to thrombin, suggesting that thrombin may be released even if the BBB is intact, leading to brain injury. Both in vivo and in vitro evidence have demonstrated that high levels of thrombin in the brain are deleterious2,9,10,12. Therefore, thrombin can not only enter the brain during ischemia in which the blood–brain barrier is compromised, but can also be synthesized in the brain and eventually exerts damage to brain tissue and induces a cognitive function deficit.
Nafamostat mesilate (NM), a synthetic serine protease inhibitor, is widely used in patients with disseminated intravascular coagulopathy or pancreatitis. It is also reported that this agent suppressed ischemia-induced injury in the myocardium and subarachnoid hemorrhage-induced cerebral vasospasm13. NM inhibits several serine proteases including thrombin, which is inhibited in a competitive manner with an inhibition constant (Ki) value of 10−7–10−6 M14,15. As thrombin plays an important role in brain damage during ischemia16, we evaluated the effect of nafamostat mesilate in a rat model of acute cerebral ischemia in the present study.
Results
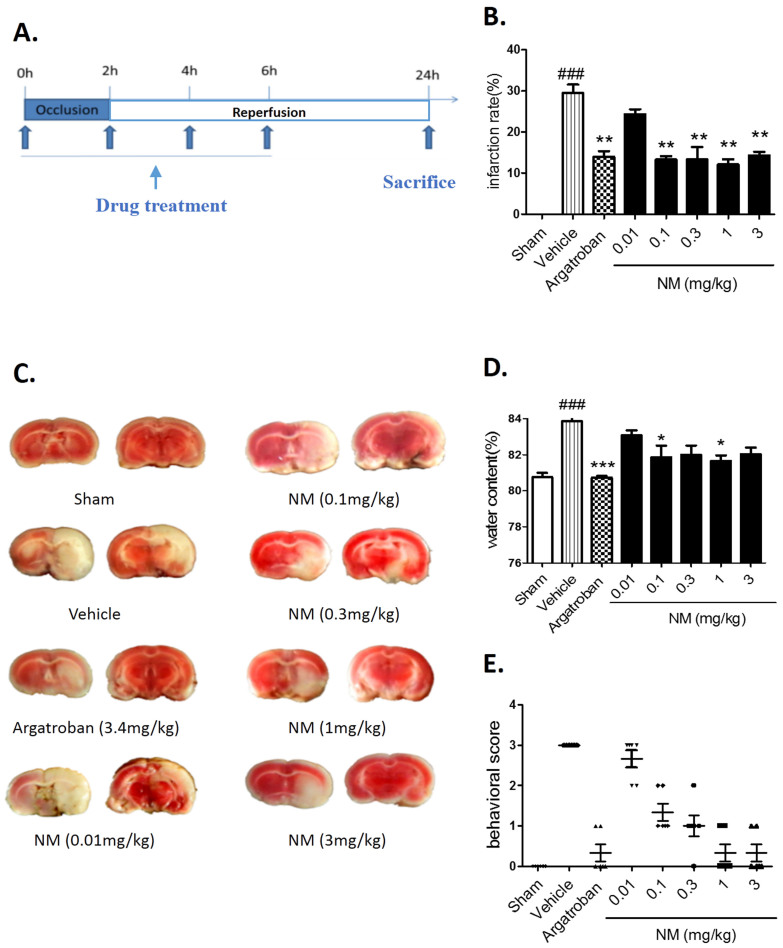
The effects of NM on infarct volume, edema and neurologic deficit in rats after MCAO
The procedure for the tMCAO model and various drug treatments were performed according to the methods shown in Fig. 1A. The NM-treated and argatroban-treated groups showed decreased infarct volumes compared with the vehicle group (Fig. 1B and C). A significant increase in the brain water content was revealed in rats 24 hours after MCAO when compared with the sham group (Fig. 1D), while NM treatment (0.1 mg/kg or 1 mg/kg) significantly decreased the ischemia-induced water content increase (Fig. 1D). Moreover, NM treatment significantly ameliorated the total neurological deficit score at four doses examined (0.1, 0.3, 1 or 3 mg/kg) (Fig. 1E). These results show that NM can improve the infarct volume, brain edema and neurological deficit in rats after MCAO.
Figure 1. The effect of nafamostat mesilate (NM) on acute cerebral ischemia in rats at 24 h after MCAO.
(A) The drug administration schedule and experimental protocol are illustrated schematically. (B–D) NM dose-dependently ameliorated the ischemia-induced infarct volume (B, C), brain edema (D) and neurological deficits (E). *P < 0.05, **P < 0.01, ***P < 0.001 vs. Vehicle (MCAO group); ###P < 0.001 vs. sham group, n = 8–10.
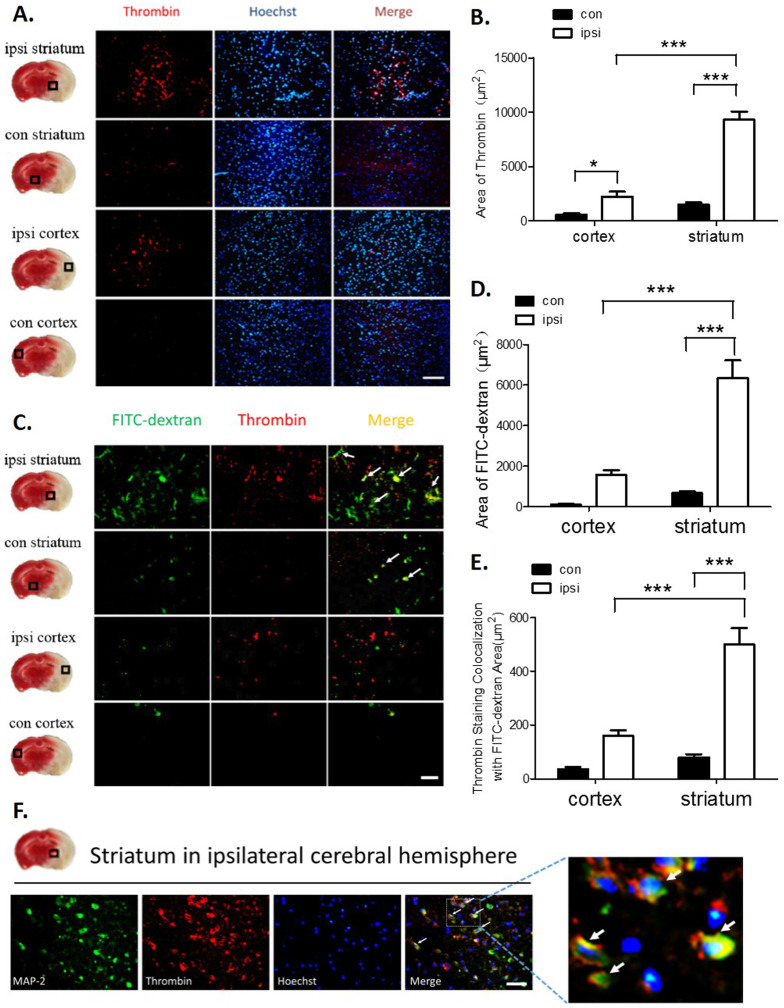
The expression of thrombin increased in the ischemic regions of rats
It has been shown that thrombin activation plays an important role during ischemia-induced brain injury8,10. The topographic distribution of thrombin was investigated by double fluorescent staining in an ischemic/nonischemic cortical and striatum lesion at 24 hours after MCAO. Significant thrombin antigen was found in the regions of the ipsilateral hemisphere striatum; however, little thrombin antigen was detectable in the ipsilateral hemisphere cortex or contralateral striatum (Fig. 2A and B).
Figure 2. The expression of thrombin was increased in the ischemic regions of MCAO rats.
(A–B) The immunoreactivity of thrombin in the cortex and striatum of ischemic brain tissue. Scale bar = 100 μm. Significant thrombin antigen was found in regions of the ipsilateral hemisphere striatum. (C) Double staining examining the colocalization of thrombin with FITC-dextran staining in the striatum and cortex. Scale bar = 50 μm. (D) Significant FITC-dextran leakage present in the ipsilateral hemisphere striatum. (E) Significant amounts of thrombin were expressed in areas with severe FITC-dextran leakage regions. (F) Double staining examining the colocalization of thrombin with MAP-2-positive neurons. Scale bar = 50 μm. Immunohistochemistry with an anti-thrombin antibody and a neuronal marker anti-MAP-2 antibody revealed thrombin expression mostly in ischemic neurons. The white arrow indicates the colocalization of thrombin staining with FITC-dextran or neurons. ***P < 0.001, n = 6–8.
To determine whether the source of the increased thrombin expression in the ischemic brain regions was from the cerebral parenchyma or from vascular disruption, high-molecular-weight FITC-dextran was used to detect severe vascular disruption combined with a thrombin antibody for double staining. As shown in Fig. 2C–E, FITC-dextran staining alone and FITC-dextran double staining with a thrombin antibody were markedly increased in the ischemic striatum compared with the ischemic cortex, and there was a significant difference between the amount of staining in the ipsilateral and contralateral striatum. Double staining with the neuronal marker MAP-2 showed that the majority of thrombin was expressed in neurons (Fig. 2F). These results suggest that thrombin expression in the ischemic regions is either from vascular leakage or neurons.
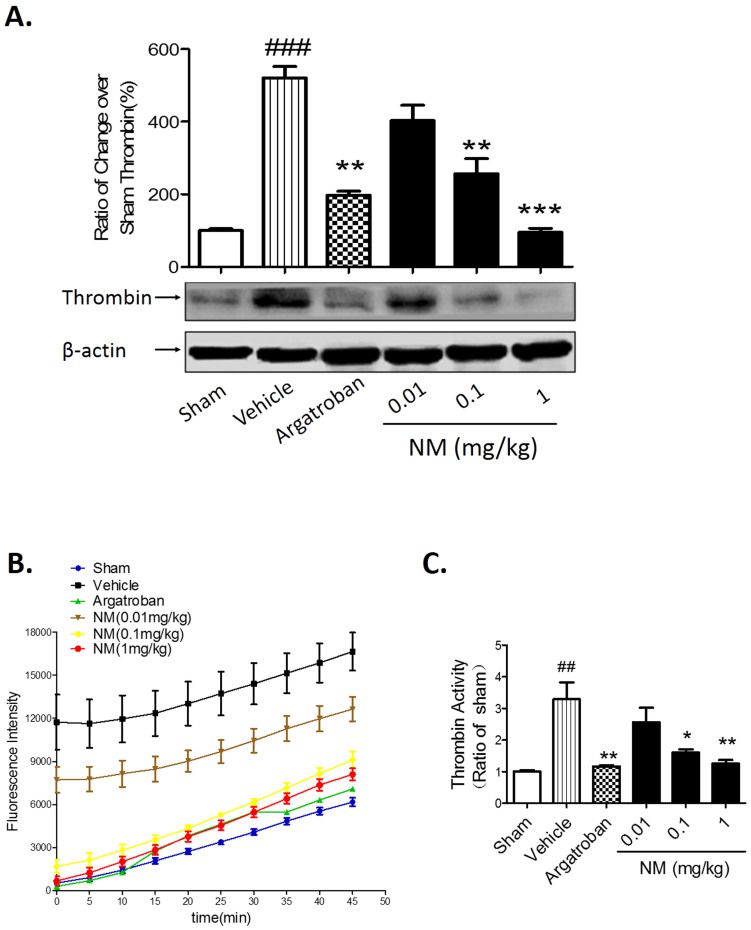
NM treatment decreased the expression and activity of thrombin in rats after MCAO
A high expression level and activity of thrombin are associated with brain damage after ischemic stroke9,12,17,18. Owing to NM's activity as a serine protease inhibitor, we determined whether NM treatment could inhibit thrombin expression and activity in the striatum lesion at 24 hours after MCAO. The results from Western blotting showed that NM treatment dose-dependently decreased the expression of thrombin from an ischemic striatum lesion (Fig. 3A). We also found that brain thrombin activity in the vehicle group at 24 hours after MCAO was significantly higher than that in the sham group; however, NM treatment (0.1 mg/kg and 1 mg/kg) significantly reduced brain thrombin activity at 24 hours after MCAO (Fig. 3B and C). These results show that NM treatment can decrease thrombin expression and activity in the brains of rats after MCAO.
Figure 3. Nafamostat mesilate (NM) treatment decreased the thrombin expression and activity induced by ischemia.
(A) NM dose-dependently reduced thrombin expression in the ischemic regions as determined by Western blotting. (B) NM significantly inhibited ischemia-induced thrombin activity in the ischemic striatum. (C) NM dose-dependently inhibited ischemia-induced thrombin activity at 45 min of fluorescence determinate progression. *P < 0.05, **P < 0.01, ***P < 0.001 vs. Vehicle (MCAO group); ##P < 0.01, ###P < 0.001 vs. sham group, n = 6–8.
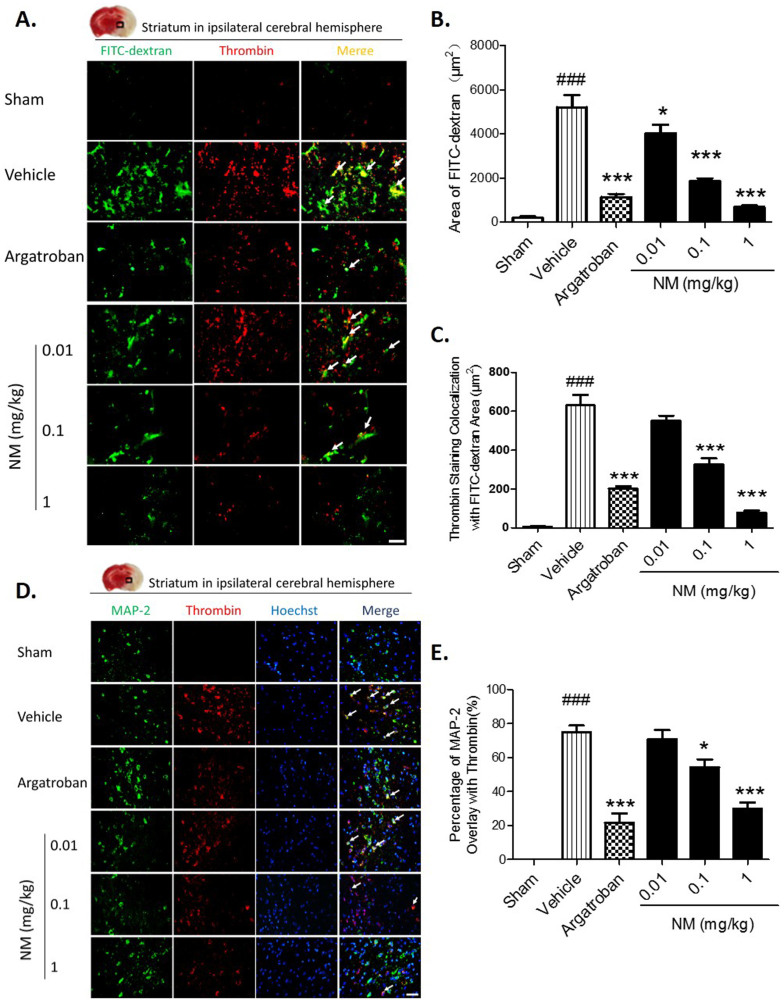
Evidence shows that thrombin can enter into the brain parenchyma through an opened BBB after cerebral ischemia2,8. At the same time, prothrombin is synthesized and significantly increased in the cerebral parenchyma during ischemia, which can be cleaved by Factor Xa to active thrombin in the brain11,19,20. NM treatment (0.1 mg/kg and 1 mg/kg) significantly alleviated the progression of vascular disruption in the ipsilateral hemisphere striatum and reduced thrombin expression as measured by double staining with FITC-dextran (Fig. 4A–C), which was particularly decreased in the neurons of the ischemia striatum lesion (Fig. 4D and E).
Figure 4. Nafamostat mesilate (NM) treatment decreased ischemia-induced expression of thrombin from blood leakage and neurons.
(A, B) Thrombin is colocalized with FITC-dextran staining as determined by double staining in the ipsilateral striatum. NM (0.1, 1 mg/kg) significantly alleviated FITC-dextran leakage. (C) NM (0.01, 0.1, 1 mg/kg) significantly alleviated FITC-dextran colocalization with thrombin. (D,E) Double staining showed the colocalization of thrombin with MAP-2-positive neurons, and NM (0.1, 1 mg/kg) significantly reduced this effect. Scale bar = 50 μm. The white arrow indicates the colocalization of thrombin staining with FITC-dextran or neurons. *P < 0.05, ***P < 0.001 vs. Vehicle (MCAO group); ###P < 0.001 vs. sham group, n = 6–8.
NM protected from neuronal death through thrombin inhibition in rats after MCAO
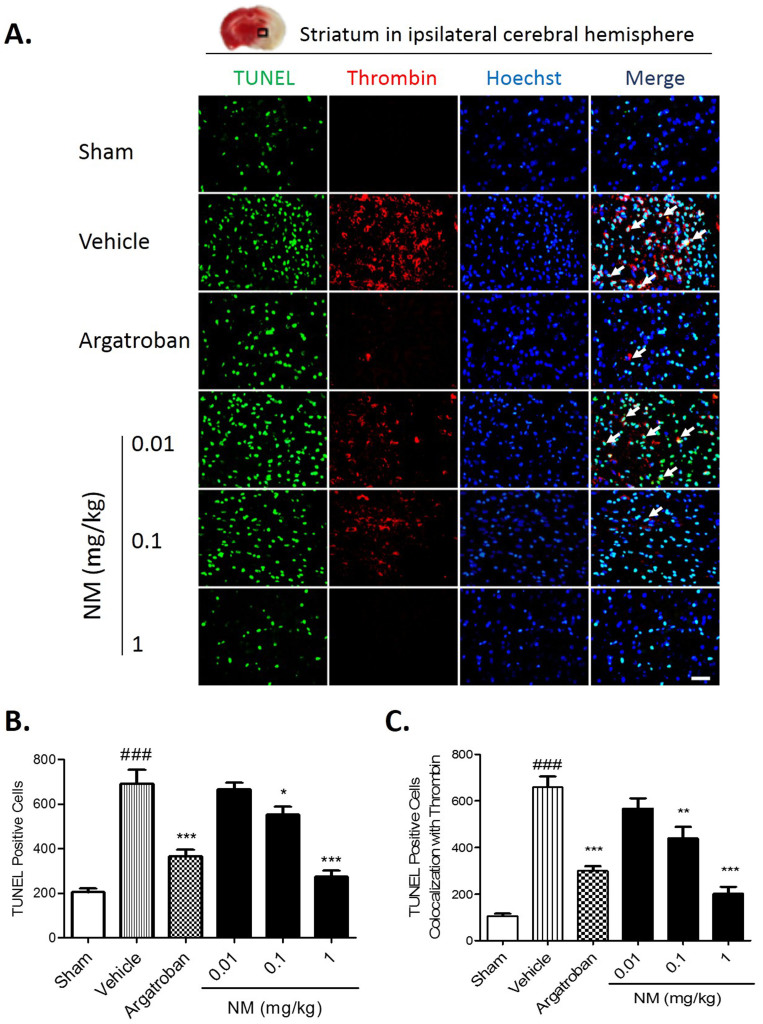
Ischemic cell death was detected with TUNEL staining. TUNEL-stained positive cells were significantly increased at 24 hours after the tMCAO; however, NM (0.1 mg/kg, 1 mg/kg) treatment significantly reduced the numbers of TUNEL-stained positive cells and significantly ameliorated thrombin expression (Fig. 5A and B). Furthermore, the number of TUNEL-positive cells colocalized with thrombin was significantly decreased with NM (0.1 mg/kg, 1 mg/kg) treatment (Fig. 5C). These results indicate that NM has a neuroprotective effect via the inhibition of brain thrombin.
Figure 5. Nafamostat mesilate (NM) treatment protected ischemia-induced cell death through thrombin inhibition.
(A) Cell death was determined by TUNEL staining. TUNEL-stained positive cells mostly localized in thrombin-expressing cells, and NM dose-dependently reduced ischemia-induced TUNEL-stained positive cells and thrombin expression as determined by immunostaining. (B) The number of TUNEL-stained positive cells was significantly increased after MCAO, and NM (0.1, 1 mg/kg) treatment significantly reduced this effect. (C) The number of TUNEL-stained positive cells colocalized with thrombin was significantly reduced with NM (0.1, 1 mg/kg) treatment. Scale bar = 50 μm. The white arrow indicates the colocalization of thrombin staining with apoptotic cells. *P < 0.05, **P < 0.01, ***P < 0.001 vs. Vehicle (MCAO group); ###P < 0.001 vs. sham group, n = 5–6.
NM treatment improved the neurological function in MCAO rats
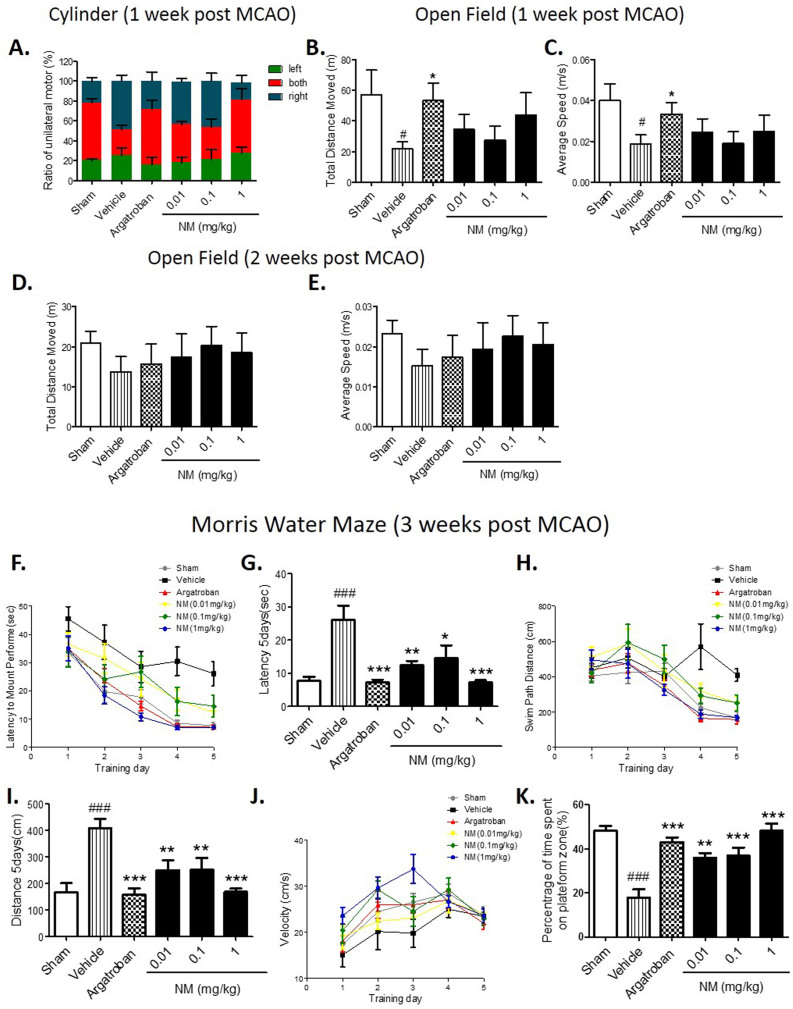
To further demonstrate the translational value of the NM neuroprotective effects in stroke, we assessed the NM effect on neurological function in rats after ischemia by behavioral tests. We found no differences in the behavior among all of the groups of animals before ischemia. The data from the cylinder test showed that there was no significant difference in the unilateral motor function between the NM-treated group and the MCAO group on day 7 after MCAO (Fig. 6A). Moreover, no significant difference was found in the spontaneous motor behavior of each group on day 7 or day 14 after MCAO as determined by the Open field test (Fig. 6B–E).
Figure 6. Nafamostat mesilate (NM) treatment improved an ischemia-induced cognitive deficit.
NM treatment had no beneficial effects on unilateral motor function at 1 week after ischemia evaluated by the cylinder test (A), or on spontaneous motor behavior at 1 and 2 weeks after ischemia evaluated by the Open field test (B–E). (F–K) The cognitive functions of the animals were assessed by the Morris Water Maze test. After ischemia (Vehicle group), the animals had a longer latency time (F,G) and a longer swim path distance (H, I) during the test. No significant difference in the velocity was found among all of the groups (J). In the following probe test, the NM-treated rats spent a significantly longer time in the platform zone (K), indicating that NM treatment improves the ability of a performance task associated with long-term memory in ischemic rats. *P < 0.05, **P < 0.01, ***P < 0.001 vs. Vehicle (MCAO group); #P < 0.05, ###P < 0.001 vs. sham group, n = 8–10.
However, the Morris Water Maze test showed that NM-treated rats exhibited enhanced learning ability with more latencies compared with the MCAO group (Fig. 6F–I). No significant differences in velocity were found among all of the groups (Fig. 6J). In the following probe test, the NM group spent significantly more time in the platform quadrant (Fig. 6K), indicating that NM treatment can improve the ability of performance tasks associated with long-term memory in ischemic rats.
Discussion
In this study, we evaluated the effects of NM treatment in a rat model of transient focal cerebral ischemia for the first time and found that NM treatment during ischemia improved neurological outcomes and reduced brain infarct volume and brain edema.
The molecular mechanism of the neuroprotective effects of NM in the brain tissue of rats after MCAO was investigated. Our data showed that NM treatment suppressed the elevation of thrombin either from BBB leakage or from neurons themselves in the brain parenchyma. NM treatment also alleviated brain damage through the inhibition of thrombin activity, which resulted in protection from neuronal injury, as indicated by a decrease in TUNEL-stained positive cells in the brain. Furthermore, our results indicate that there may be a relationship between thrombin toxicity and the long-term cognitive deficits after stroke. In this study, NM treatment improved cognitive function after acute cerebral ischemia, suggesting that NM has beneficial effects during ischemia, at least in part, through the inhibition of thrombin expression and activity.
Thrombin has been reported to modulate ischemic, hemorrhagic and traumatic brain injury8,21,22,23. The ischemic brain is characterized by high levels of thrombin expressed in the brain parenchyma or infused from the bloodstream as a result of BBB disruption. First, NM treatment decreased thrombin leakage from the neurovasculature. Factor Xa can cleave prothrombin to active thrombin in the blood. However, Factor Xa, prothrombin and thrombin are serine proteases. NM, as a serine proteases inhibitor, reduced MCAO-induced thrombin leakage, possibly by the inhibition of thrombin cleavage proteases, resulting in decreased thrombin originating from the blood after MCAO. In hippocampal slice cultures of an in vitro model of ischemia, another thrombin inhibitor, hirudin, has been shown to attenuate neuronal death in the CA1 region12. Second, it has been reported that ischemia-induced brain injury can further enhance the expression of prothrombin and thrombin in the brain parenchyma, particularly in neurons8,19,20. Our data showed that ischemia-induced cellular injury, represented as counts of TUNEL-stained positive cells, was increased with thrombin expression and decreased with NM treatment, suggesting that severe cellular damage is influenced by thrombin activation, and NM treatment can block this effect to show a neuroprotective effect in vivo.
Thrombin exerts several extravascular effects mediated by the activation of a family of protease-activated receptors (PARs). Until now, four PARs have been discovered, PAR-1, PAR-2, PAR-3 and PAR-424, among which PAR-1, PAR-3 and PAR-4 can be activated by thrombin, whereas PAR-2 is activated by trypsin25. Both neurons and glial cells express the PAR-1 protein19,26. Accumulating evidence demonstrates that thrombin and its receptors play important roles in neuroinflammatory and neurodegenerative processes in the central and peripheral nervous system27,28,29. PAR-1 is upregulated after anoxia in hippocampal slice cultures in vitro and in acute cerebral ischemia in vivo12. Thrombin can bind to the PAR-1 receptor to activate PAR-1 leading to neuronal injury. PAR-1 activation has been reported to show several neuropathological effects30, including neurite retraction31 and cell death in hippocampal cultures and motoneurons32,33. PAR-1 knockout mice had less brain edema formation, neuronal death, and behavioral impairment after bilateral common carotid artery occlusion17. Treatment with SCH79797, a PAR-1 antagonist, significantly reduced the average area of severe vascular disruption and TUNEL-stained positive cells after focal ischemia9. It might be possible that the neuroprotective effects of NM during ischemia are mediated not only by the inhibition of thrombin expression and activity but also by the prevention of PAR-1 receptor activation. Additional experiments are needed to clarify whether the PAR-1-activated signaling pathway is involved in NM-mediated neuroprotection.
Thrombin expression in the brain has also been associated with deleterious effects on cognitive function9,34,35. When treated with an intracerebroventricular infusion of thrombin, rats committed significantly more reference memory errors and had higher task completion latencies in the eight-arm radial maze35. Our results showed that brain ischemia caused long-term spatial memory impairment in the Morris Water Maze test, whereas postischemic NM treatment significantly attenuated this memory impairment, suggesting that NM may attenuate the long-term spatial memory impairment induced by ischemia through thrombin inhibition.
In conclusion, our results provide evidence that NM had a neuroprotective role during ischemia-induced brain injury via the inhibition of thrombin expression and activity in the brain, particularly in neurons, which suggests that NM could be a potential drug for the treatment of ischemic stroke patients.
Methods
Chemicals and reagents
Nafamostat mesilate (C19H17N5O2.2CH403S, 6-amino-2-naphthyl p-guanidinobenzoate dimethanesulfonate), a serine protease inhibitor, was obtained from NANJING D&R PHARMACEUTICAL COMPANY (China). Argatroban (C23H36N6O5S•H2O, 1-[5-[(aminoiminomethyl)amino]-1-oxo-2 -[[(1,2,3,4-tetrahydro-3-methyl-8-quinolinyl)sulfonyl]amino]pentyl]-4-methyl-2-piperidinecarboxylic acid) was purchased from Enzo Life Sciences (BML-PI146, USA). Fluorescein isothiocyanate (FITC) dextran was purchased from Sigma-Aldrich (USA), goat anti-thrombin antibody was purchased from Santa Cruz Biotechnology (USA), rabbit anti-MAP-2 antibody was purchased from Chemicon (USA), β-actin monoclonal antibody was purchased from Sigma-Aldrich (USA), Alexa Fluo-488 conjugated goat anti-rabbit IgG antibody and Alexa Fluo-594 conjugated donkey anti-goat IgG antibody were purchased from Invitrogen (CA). RIPA buffer and MicroBCA kit were purchased from Beyotime (China). Protease inhibitor cocktail was purchased from Roche (Indianapolis, IN, USA). Thrombin substrate Benzoyl-Phe-Val-Arg-AMC·HCl and Prolyl Endopeptidase Inhibitor II were purchased from Merck (Germany).
Animals
Adult male Sprague-Dawley (SD) rats weighing 260–280 g were purchased from Zhejiang Laboratory Animals Center (Hangzhou, China) and kept under standard housing conditions at a temperature between 20°C and 23°C, with a 12 h light–dark cycle and a relative humidity of 50%. Animal housing and handling were carried out in accordance with the US National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals published by the US National Academy of Sciences (http://oacu.od.nih.gov/regs/index.htm). All animal tests and experimental procedures were approved by the Administration Committee of Experimental Animals in Jiangsu Province and the Ethics Committee of China Pharmaceutical University.
Animal surgery
Transient middle cerebral artery occlusion (tMCAO) was performed as reported previously36. The external carotid artery was isolated and coagulated, a monofilament nylon suture (diameter of approximately 0.26 mm) with a round tip was inserted into the internal carotid artery through the external carotid artery stump, occluding the middle cerebral artery for 2 hours. After occlusion, the animals were reanesthetized and the filament was withdrawn to restore blood flow. Body temperature was regulated at 37.0°C with a temperature control system. All animals had free access to food and water. The neurological scores were evaluated 24 hours after the MCAO using a scoring system reported36. At the end of the reperfusion period, animals were euthanized with ketamine/xylazine (150:10 mg/kg) mixture, and then intracardially perfused with 250 ml of saline followed by 300 ml of 4% paraformaldehyde. Brains were removed, postfixed in 4% paraformaldehyde, cryoprotected in 30% sucrose in phosphate buffer, and then sliced into 20 μm sections on a freezing microtome. FITC-dextran (1 ml of 50 mg/ml) was administered intravenously (IV) to rats at 20 h after MCAO when needed.
Drug treatments
Animals were randomly divided into four groups, such as sham, vehicle, argatroban and nafamostat mesilate groups, respectively. Rats were intravenously treated with nafamostat mesilate diluted in 10% glucose (0.01, 0.1, 0.3, 1, 3 mg/kg) at 0 h, 2 h, 4 h and 6 h after ischemia onset as illustrated in Fig. 1A. Argatroban, a direct thrombin inhibitor, was proved that can reduce brain injury after ischemic stroke2,37,38. Rats were administrated by IV injection of argatroban (3.4 mg/kg) at 0 h, 2 h, 4 h and 6 h after ischemia onset as a positive control.
Assessment of neurological deficit, cerebral infarct volume and brain edema
Animals were carried with neurological scoring at 24 hours after MCAO followed by euthanization, then brains were harvested and sectioned into 2 mm coronal sections. Sections were inspected for the presence of macroscopic intracranial hemorrhage, then stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC) for 20 minutes at 37°C, fixed with 10% formalin, and evaluated for infarct size by morphometric analysis (image-pro plus) as described39. Total infarct volume was expressed as a percentage of the volume of the all sectional area to the lesion. The hemispheres were weighed after removal (wet weight) and weighed again after drying in an oven at 105°C for 24 hours (dry weight). Edema was calculated according to the following formula: [(wet weight-dry weight)/wet weight] × 100%40.
Immunohistochemistry
Immunohistochemistry was performed as previously described with some modifications41. Briefly, brain sections were incubated in a blocking solution (10% normal goat serum, 0.3% Triton X-100 in PBS) at room temperature for 1 h and then incubated with primary antibodies overnight at 4°C. The following primary antibodies were used: goat anti-thrombin antibody (1:100), rabbit anti-MAP2 antibody (1:800). After 3 rinses with PBS, sections were incubated with secondary antibodies: Alexa Fluo-488 conjugated goat anti-rabbit IgG antibody (1:400), Alexa Fluo-594 conjugated donkey anti-goat IgG antibody (1:400). The nuclei were counterstained with Hoechst 33342 when necessary. The fluorescent imaging was visualized by using Olympus fluorescence microscope (Japan).
Western blotting
Brain samples from ischemic and nonischemic brain tissue were homogenized with RIPA buffer supplemented with a protease inhibitor cocktail. Protein concentrations were measured with MicroBCA kit. Protein samples (30 μg) were separated by 10% SDS-PAGE and transferred onto the PVDF membranes which were immunoblotted with a polyclonal goat anti-thrombin antibody (1:1000). β-actin monoclonal antibody (1:1000) was used for detecting β-actin served as a loading control.
Thrombin activity determination
Thrombin activity was measured by use of a fluorometric assay as described previously with minor modifications42. Briefly, animals were reanesthetized and perfused with saline. Brains were removed and cut into 6–8 coronal sections (1 mm thickness) using a mouse brain slicer matrix after weighing. Each brain section was transferred to a well in a 96-well black microplate which was prefilled with 50 μl assay buffer (50 mM Tris·HCl, pH = 8, 150 mM NaCl, 1 mM CaCl2, 0.1% BSA, Benzoyl-Phe-Val-Arg-AMC·HCl (13 μM), protease inhibitor prolyl endopeptidase inhibitor II (20 μM)) in each well. Another 100 μl assay buffer was added to each well before measurement. The kinetic fluorescence was determined for 45 minutes at 25°C using a fluorescence detection system (Magellan V6.6; Safire2, Tecan; excitation 360–380 nm, emission 440–460 nm).
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining
At 24 h after MCAO, mice were deeply anesthetized with pentobarbital sodium and perfused with 4% paraformaldehyde. Coronal cryostat sections (20 μm) were obtained from frozen brains by serial sectioning. Sections through the striatum were chosen for TUNEL staining. The TUNEL staining was performed according to the manufacturer's instructions (Roche Molecular Bio-chemicals, Swiss). The brain sections were counterstained with Hoechst 33342 to visualize nuclei. We detected TUNEL-positive cells in the striatum of ischemic brain hemisphere in random areas, and the number of TUNEL-positive cells was counted at 20× magnification photograph.
Behavioral tests
Behavioral tests were conducted to assess the motor function as well as memory and learning ability of the ischemic rats. Ischemic rats were randomly assigned to receive drug treatments during ischemia: Nafamostat mesilate (0.01, 0.1 and 1 mg/kg), argatroban (3.4 mg/kg). One week after ischemia onset, these animals were tested using the cylinder test9. Briefly, the animals were placed in a 20 cm wide transparent glass cylinder and a video camera was used to record a minimum of 20 contacts of each forepaw with the wall of the glass cylinder. Data was recorded as left, right or both paws simultaneously. Each placement was counted and divided by 20 to obtain percentage of paw usage.
One and two weeks after ischemia onset, rats were placed in the open field chamber (100 × 100 cm) for 15 minutes in each test. Locomotive behavior was monitored and analyzed by an autotracking system, which quantified the total distance traveled. All rats were brought to the testing room 10 minutes before the beginning of the test and the activity was recorded immediately after the mice were placed in the open field apparatus43.
Spatial learning/memory was tested using a Morris water maze as described previously44. Briefly, Morris water maze task was carried out between days 21 and 30 after MCAO. A platform to escape (10 cm in diameter) is hidden under the surface. Rats received one trial per day for 6 days. For each trial, rats were facing the pool side placed into a small pool of water and were allowed to swim in water for 60 s (it took up to 6 days to train the rats). When the rats swam around the pool to find the platform, various parameters such as the time taken to reach the platform (latency), velocity and swimming paths were recorded by a video imaging analysis system (EthoVision®3, Noldus Information Technology). Rats that failed to locate the platform at the end of 60 s were helped to find the platform for 30 s. At day 7, a probe test was performed to measure the retention of spatial memory when the platform was removed. The time spent in the target quadrant was recorded.
Statistical analysis
Statistical analysis was carried out with GraphPad Prism 5 software (San Diego, CA). Values were tested using the student's t-test or ANOVA as appropriate. All data were expressed as mean ± SEM. Multivariate analysis of variation (MANOVA) was conducted by statistical software StatView (SAS) in Morris Water Maze test. P < 0.05 was defined to indicate a statistically significant difference.
Author Contributions
T.C., J.W., X.S., T.P. and H.L. designed the experiments. T.C., J.W., C.L. and W.Z. performed the experiments. T.C., L.Z. and L.A. analyzed and discussed the data. T.C., X.S., T.P. and H.L. wrote the paper. All authors contributed to the editing of the paper and to scientific discussions.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81271338; 81070967), Specialized Research Fund for the Doctoral Program of Higher Education of China (20130096110011), Natural Science Foundation of Jiangsu Province (BK20130653), and the Fundamental Research Funds for the Central Universities (JKZD2013006).
References
- Latour L. L., Kang D. W., Ezzeddine M. A., Chalela J. A. & Warach S. Early blood–brain barrier disruption in human focal brain ischemia. Ann. Neurol. 56, 468–477 (2004). [DOI] [PubMed] [Google Scholar]
- Chen B., Cheng Q., Yang K. & Lyden P. D. Thrombin mediates severe neurovascular injury during ischemia. Stroke 41, 2348–2352 (2010). [DOI] [PubMed] [Google Scholar]
- Xi G., Keep R. F., Hua Y., Xiang J. & Hoff J. T. Attenuation of thrombin-induced brain edema by cerebral thrombin preconditioning. Stroke 30, 1247–1255 (1999). [DOI] [PubMed] [Google Scholar]
- Lee K. R., Kawai N., Kim S., Sagher O. & Hoff J. T. Mechanisms of edema formation after intracerebral hemorrhage: effects of thrombin on cerebral blood flow, blood-brain barrier permeability, and cell survival in a rat model. J. Neurosurg. 86, 272–278 (1997). [DOI] [PubMed] [Google Scholar]
- Brummel K. E., Paradis S. G., Butenas S. & Mann K. G. Thrombin functions during tissue factor–induced blood coagulation. Blood 100, 148–152 (2002). [DOI] [PubMed] [Google Scholar]
- Nesheim M. et al. Thrombin, thrombomodulin and TAFI in the molecular link between coagulation and fibrinolysis. Thromb. Haemostasis. 78, 386–391 (1997). [PubMed] [Google Scholar]
- Schoenmakers S. H., Reitsma P. H. & Spek C. A. Blood coagulation factors as inflammatory mediators. Blood Cell Mol. Dis. 34, 30–37 (2005). [DOI] [PubMed] [Google Scholar]
- Xi G., Reiser G. & Keep R. F. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: deleterious or protective? J. Neurochem. 84, 3–9 (2003). [DOI] [PubMed] [Google Scholar]
- Chen B. et al. Thrombin activity associated with neuronal damage during acute focal ischemia. J. Neurosci. 32, 7622–7631 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y., Wu J., Keep R., Hoff J. & Xi G. in Brain Edema XII 163–166 (Springer, 2003). [Google Scholar]
- Shikamoto Y. & Morita T. Expression of factor X in both the rat brain and cells of the central nervous system. FEBS Lett. 463, 387–389 (1999). [DOI] [PubMed] [Google Scholar]
- de Castro Ribeiro M. et al. Thrombin in ischemic neuronal death. Exp. Neurol. 198, 199–203 (2006). [DOI] [PubMed] [Google Scholar]
- Okajima K., Uchiba M. & Murakami K. Nafamostat mesilate. Cardiovasc. Drug Rev. 13, 51–65 (1995). [Google Scholar]
- Hitomi Y., Ikari N. & Fujii S. Inhibitory effect of a new synthetic protease inhibitor (FUT-175) on the coagulation system. Pathophysiol. Haemo. T. 15, 164–168 (1985). [DOI] [PubMed] [Google Scholar]
- Uchiba M., Okajima K., Abe H., Okabe H. & Takatsuki K. Effect of nafamostat mesilate, a synthetic protease inhibitor, on tissue factor-factor VIIa complex activity. Thromb. Res. 74, 155–161 (1994). [DOI] [PubMed] [Google Scholar]
- Vivien D. & Buisson A. Serine protease inhibitors: novel therapeutic targets for stroke? J. Cereb. Blood Flow Metab. 20, 755–764 (2000). [DOI] [PubMed] [Google Scholar]
- Wang J., Jin H., Hua Y., Keep R. F. & Xi G. Role of protease-activated receptor-1 in brain injury after experimental global cerebral ischemia. Stroke 43, 2476–2482 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuomo O. et al. Antithrombin reduces ischemic volume, ameliorates neurologic deficits, and prolongs animal survival in both transient and permanent focal ischemia. Stroke 38, 3272–3279 (2007). [DOI] [PubMed] [Google Scholar]
- Dihanich M., Kaser M., Reinhard E., Cunningham D. & Monard D. Prothrombin mRNA is expressed by cells of the nervous system. Neuron 6, 575–581 (1991). [DOI] [PubMed] [Google Scholar]
- Riek-Burchardt M., Striggow F., Henrich-Noack P., Reiser G. & Reymann K. Increase of prothrombin-mRNA after global cerebral ischemia in rats, with constant expression of protease nexin-1 and protease-activated receptors. Neurosci. Lett. 329, 181–184 (2002). [DOI] [PubMed] [Google Scholar]
- Sanchez A. et al. Neurovascular unit and the effects of dosage in VEGF toxicity: role for oxidative stress and thrombin. J. Alzheimers Dis. 34, 281–291 (2013). [DOI] [PubMed] [Google Scholar]
- Hua Y., Keep R. F., Gu Y. & Xi G. Thrombin and brain recovery after intracerebral hemorrhage. Stroke 40, S88–89 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy D. et al. Thrombin, a mediator of cerebrovascular inflammation in AD and hypoxia. Front. Aging Neurosci. 5, 19 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane S. R., Seatter M. J., Kanke T., Hunter G. D. & Plevin R. Proteinase-activated receptors. Pharmacol. Rev. 53, 245–282 (2001). [PubMed] [Google Scholar]
- Coughlin S. R. Thrombin signalling and protease-activated receptors. Nature 407, 258–264 (2000). [DOI] [PubMed] [Google Scholar]
- Sokolova E. & Reiser G. Prothrombin/thrombin and the thrombin receptors PAR-1 and PAR-4 in the brain: Localization, expression and participation in neurodegenerative diseases. Thromb. Haemostasis. 100, 576–581 (2008). [PubMed] [Google Scholar]
- Wang H. & Reiser G. Thrombin signaling in the brain: the role of protease-activated receptors. Biol. Chem. 384, 193–202 (2003). [DOI] [PubMed] [Google Scholar]
- Rohatgi T., Sedehizade F., Reymann K. G. & Reiser G. Protease-activated receptors in neuronal development, neurodegeneration, and neuroprotection: thrombin as signaling molecule in the brain. Neuroscientist 10, 501–512 (2004). [DOI] [PubMed] [Google Scholar]
- Luo W., Wang Y. & Reiser G. Protease-activated receptors in the brain: receptor expression, activation, and functions in neurodegeneration and neuroprotection. Brain Res. Rev. 56, 331–345 (2007). [DOI] [PubMed] [Google Scholar]
- Junge C. E. et al. The contribution of protease-activated receptor 1 to neuronal damage caused by transient focal cerebral ischemia. Proc. Natl. Acad. Sci. U.S.A. 100, 13019–13024 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon V. L., Lloyd E. D., Wang S., Festoff B. W. & Houenou L. J. Thrombin perturbs neurite outgrowth and induces apoptotic cell death in enriched chick spinal motoneuron cultures through caspase activation. J. Neurosci. 18, 6882–6891 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan F. M., Pike C. J., Cotman C. W. & Cunningham D. D. Thrombin induces apoptosis in cultured neurons and astrocytes via a pathway requiring tyrosine kinase and RhoA activities. J. Neurosci. 17, 5316–5326 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon V. L., Milligan C. E. & Houenou L. J. Activation of the protease-activated thrombin receptor (PAR)-1 induces motoneuron degeneration in the developing avian embryo. J. Neuropath. Exp. Neur. 58, 499–504 (1999). [DOI] [PubMed] [Google Scholar]
- Almonte A. G. & Sweatt J. D. Serine proteases, serine protease inhibitors, and protease-activated receptors: roles in synaptic function and behavior. Brain Res. 1407, 107–122 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhatre M. et al. Thrombin, a mediator of neurotoxicity and memory impairment. Neurobiol. Aging 25, 783–793 (2004). [DOI] [PubMed] [Google Scholar]
- Longa E. Z., Weinstein P. R., Carlson S. & Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20, 84–91 (1989). [DOI] [PubMed] [Google Scholar]
- Kawai H., Yuki S., Sugimoto J. & Tamao Y. Effects of a thrombin inhibitor, argatroban, on ischemic brain damage in the rat distal middle cerebral artery occlusion model. J. Pharmacol. Exp. Ther. 278, 780–785 (1996). [PubMed] [Google Scholar]
- Lyden P. et al. Direct thrombin inhibitor argatroban reduces stroke damage in 2 different models. Stroke 45, 896–899 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic E., Özdemir Y. G., Hayrunnisa Bolay H. K. & scedil. Pinealectomy aggravates and melatonin administration attenuates brain damage in focal ischemia. J. Cereb. Blood Flow Metab. 19, 511–516 (1999). [DOI] [PubMed] [Google Scholar]
- Tsubokawa T. et al. Cathepsin and calpain inhibitor E64d attenuates matrix metalloproteinase-9 activity after focal cerebral ischemia in rats. Stroke 37, 1888–1894 (2006). [DOI] [PubMed] [Google Scholar]
- Galvão R. P., Garcia-Verdugo J. M. & Alvarez-Buylla A. Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in adult mice and rats. J. Neurosci. 28, 13368–13383 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushi D. et al. Quantitative detection of thrombin activity in an ischemic stroke model. J. Mol. Neurosci. 51, 844–850 (2013). [DOI] [PubMed] [Google Scholar]
- Mu J., Ostrowski R. P., Krafft P. R., Tang J. & Zhang J. H. Serum leptin levels decrease after permanent MCAo in the rat and remain unaffected by delayed hyperbaric oxygen therapy. Med. Gas Res. 3, 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. B. et al. Phosphoinositide 3-kinase enhancer regulates neuronal dendritogenesis and survival in neocortex. J. Neurosci. 31, 8083–8092 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]